Abstract
Locating centromeres on genome sequences can be challenging. The high density of repetitive elements in these regions makes sequence assembly problematic, especially when using short-read sequencing technologies. It can also be difficult to distinguish between active and recently extinct centromeres through sequence analysis. An effective solution is to identify genetically active centromeres (functional in meiosis) by half-tetrad analysis. This genetic approach involves detecting heterozygosity along chromosomes in segregating populations derived from gametes (half-tetrads). Unreduced gametes produced by first division restitution mechanisms comprise complete sets of nonsister chromatids. Along these chromatids, heterozygosity is maximal at the centromeres, and homologous recombination events result in homozygosity toward the telomeres. We genotyped populations of half-tetrad-derived individuals (from Brassica interspecific hybrids) using a high-density array of physically anchored SNP markers (Illumina Brassica 60K Infinium array). Mapping the distribution of heterozygosity in these half-tetrad individuals allowed the genetic mapping of all 19 centromeres of the Brassica A and C genomes to the reference Brassica napus genome. Gene and transposable element density across the B. napus genome were also assessed and corresponded well to previously reported genetic map positions. Known centromere-specific sequences were located in the reference genome, but mostly matched unanchored sequences, suggesting that the core centromeric regions may not yet be assembled into the pseudochromosomes of the reference genome. The increasing availability of genetic markers physically anchored to reference genomes greatly simplifies the genetic and physical mapping of centromeres using half-tetrad analysis. We discuss possible applications of this approach, including in species where half-tetrads are currently difficult to isolate.
Keywords: recombination, molecular karyotyping, Brassica, centromeres, unreduced gametes
IN the age of next-generation sequencing, many new aspects of the genome are being revealed (Metzker 2010). However, certain genomic features still remain recalcitrant to sequence-based analysis. The most obvious of these are repeat sequences; assembly of large repetitive regions using the current generation of technologies is very difficult (Metzker 2010). Unfortunately, these repeat sequences also hide a chromosomal feature of great interest to many geneticists: the centromere. Assembly of centromeric sequences is notoriously problematic (Rudd and Willard 2004), and locating the centromeres on both genetic and physical maps is difficult to achieve (Copenhaver et al. 1999). Adding to the complexity, previously active centromeres have been demonstrated to become inactive (no longer functional in meiosis) after chromosome fusion or polyploidization events (Han et al. 2006), and active centromeres do not always have associated sequence-based motifs (Nasuda et al. 2005; Zhang et al. 2010).
Genetic mapping of centromeres using tetrads or half-tetrads avoids these sequence-based pitfalls. Tetrad analysis was developed in fungi (Mather and Beale 1942), but has been rarely applied to locate functional centromeres in plant genomes, with Arabidopsis thaliana being the most notable exception (Copenhaver et al. 1999). The physical location of functional centromeres on reference genomes can be inferred from genetic map positions via physically anchored markers (Copenhaver et al. 2000). Full-tetrad analysis in plants is contingent on the availability of mutants where tetrads fail to disassociate during pollen development (Copenhaver et al. 2000). Pollen tetrads have been reported in hundreds of species to date (Copenhaver 2005); however, manual isolation of individual tetrads can be challenging (Copenhaver et al. 2000; Ludlow et al. 2013). Half-tetrad analysis may be more readily applicable: unreduced (2n) gametes have been reported in a wide range of plant and animal species (Ramsey and Schemske 1998). The frequency of unreduced gametes is low in most species, but can be enriched in populations derived from interspecific, interploid or translocation hybrids (Ramsey and Schemske 1998; Ramanna and Jacobsen 2003; Mason et al. 2011b). Unreduced gamete production can also often be induced or enhanced by environmental stimuli (Ramsey and Schemske 1998; Mason et al. 2011b; De Storme et al. 2012). Half-tetrad analysis has been carried out in a number of animal species to map centromeres: zebrafish (Johnson et al. 1995), cape honeybee (Baudry et al. 2004), abalone (Nie et al. 2012), Japanese eel (Nomura et al. 2006), oyster (Hubert et al. 2009), carp (Liu et al. 2013), turbot flatfish (Martinez et al. 2008), and sea cucumber (Nie et al. 2011). Plant species centromeres have been genetically mapped using half-tetrad analysis in maize (Schneerman et al. 1998; Lin et al. 2001), alfalfa (Tavoletti et al. 1996), mandarin (Cuenca et al. 2011; Aleza et al. 2015), and potato (Park et al. 2007).
Many different mechanisms of unreduced gamete formation exist [reviewed by Bretagnolle and Thompson (1995), Brownfield and Köhler (2011), and Veilleux (1985)]. For half-tetrad analysis, unreduced gametes must be produced by either a first-division restitution-like mechanism (FDR) or a second-division restitution-like mechanism (SDR) that in both cases permits homologous chromosome pairing at metaphase I. In FDR, the first meiotic division fails to separate nonsister chromatids, resulting in a mitosis-like division (but often with homologous chromosome pairing). In SDR, the second meiotic division fails to separate sister chromatids. The principle of centromere mapping relies on the fact that in FDR, both centromeres of each pair of nonsister chromatids are transmitted to the resulting gamete, resulting in perfectly heterozygous centromere regions in every gamete. Recombination events convert distal chromosome locations from heterozygous to homozygous states. Therefore, across the FDR gamete population the frequency of heterozygosity decreases as the distance from the centromere increases, due to homologous recombination events. In half-tetrad analysis via SDR (failure of meiosis to separate sister chromatids), both centromeres of each pair of sister chromatids are transmitted to the resulting gamete. This results in perfectly homozygous centromere regions in every SDR gamete, but with increasing heterozygosity toward the telomeres across the SDR gamete population due to homologous recombination events.
In previous half-tetrad analyses, centromere locations were mapped using low-throughput marker (e.g., microsatellite) genotyping of populations derived from interploid crosses or chromosome translocation hybrids (Zhao and Speed 1998). In the absence of reference genome sequences, these studies used either highly sophisticated mapping algorithms or a two-step approach whereby markers were first allocated to positions on chromosomes and then heterozygosity levels were independently mapped to identify centromere locations (Zhao and Speed 1998). This technical complexity, along with the relatively few systems with reproducibly high frequencies of unreduced gametes, may in part explain why comparatively few species have currently undergone half-tetrad analysis.
Here we demonstrate how current genomic technologies can be applied for very simple mapping of centromeres, using markers of known genomic locations. Using the recently released Illumina Infinium 60K SNP chip for Brassica napus, we undertook centromere mapping in a population cultured from unreduced microspores of B. napus × B. carinata hybrids (genome configuration CCAB), and B. juncea × B. napus hybrids (genome configuration AABC). Our results for half-tetrad mapping of the C-genome chromosomes using a smaller population were reported previously in Parkin et al. (2014). Here, we describe our method in full and report its application to the A-genome and to larger C-genome populations (providing greater resolution) and the results of a sequence-based centromere placement approach. All active Brassica centromeres were physically mapped to the 10 A- and 9 C-genome chromosomes in the recently released B. napus reference genome sequence (Chalhoub et al. 2014) using half-tetrad analysis.
Materials and Methods
Experimental material
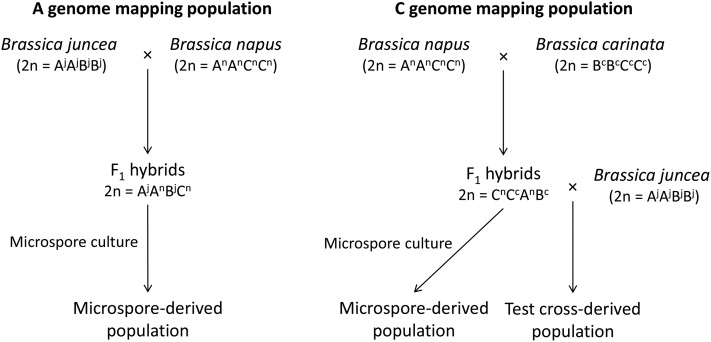
The experimental material comprised populations derived by two different methods: microspore culture (to map both A- and C- genome centromeres) and test crosses (C-genome centromeres only). An overview of the methods used to generate the experimental material is provided in Figure 1.
Figure 1.
Overview of the methods used to obtain unreduced gamete-derived individuals to map the Brassica A- and C-genome centromere locations.
To generate the novel A-genome mapping population, four different B. juncea × B. napus first generation interspecific hybrids (AABC genome configuration; Supporting Information, Table S1) were cultured to produce 86 total microspore-derived progeny. These progeny sets contained 20, 23, 23, and 20 individuals, respectively. Microspore culture was carried out according to protocols detailed in Takahira et al. (2011).
For C-genome mapping, microspore-derived progeny from CCAB hybrids were from two different groups. The first group comprised a population of 81 individuals derived from gametes of B. napus × B. carinata hybrids (CCAB genome) via microspore culture. Detailed information about the production of this population was provided by Nelson et al. (2009) and Mason et al. (2011a). In Mason et al. (2011a) SNP genotyping was carried out for DNA samples from the 57 plants in 10 progeny sets identified as (1) resulting from unreduced gametes and (2) having nonidentical haplotypes. The results from this set of individuals were previously reported in Parkin et al. (2014). The second group (new to this study) comprised a population of 75 individuals also derived from gametes of B. napus × B. carinata hybrids (CCAB genome) via microspore culture. This population was generated at The University of Western Australia following protocols detailed in Takahira et al. (2011) as above. Four different interspecific hybrid genotypes were cultured to produce 18, 16, 23, and 18 progeny, respectively (Table S1).
Also for C-genome mapping, a novel third population of 65 individuals was produced through test crossing of six B. napus × B. carinata interspecific hybrid genotypes (CCAB genome composition) to B. juncea (six progeny sets, Table S1). Crossing was carried out according to methods detailed in Mason et al. (2012), using the B. napus × B. carinata hybrid as the female parent in the cross.
Marker genotyping and half-tetrad analysis to identify centromere locations
Genomic DNA was extracted from embryos, cotyledons, or true leaves of the progeny sets following the method of Chen et al. (2008) for the microspore-derived embryos and following the microprep method of Fulton et al. (1995) for the cross-progeny. The Brassica 60K Infinium array (Illumina, San Diego) was used to generate SNP marker data for the experimental populations, parental species, and B. napus × B. carinata and B. juncea × B. napus interspecific hybrid controls (Mason et al. 2014a). Data were visualized in Genome Studio (Illumina). In total, 7,168 A-genome-specific SNP markers and 13,819 C-genome-specific SNP markers were polymorphic between the two parent genotypes of at least one progeny set in the experimental population and could be uniquely located on the B. napus “Darmor” reference sequence (Chalhoub et al. 2014). This resulted in an average SNP density of one SNP every 42 kbp in the A genome and one SNP every 36 kbp in the C genome. Putatively multilocus SNP markers (as evidenced by heterozygosity in the homozygous parent lines or by multiple genotype clusters in Genome Studio) and SNPs with haplotype patterns not matching the chromosome on which they were putatively located were removed from the analysis [see Mason et al. (2014a) for a detailed description of this method]. SNP markers that were monomorphic between parent genotypes within a progeny set were set as missing values. Missing values in haplotype blocks were imputed from flanking markers when available and to the ends of chromosomes. Resulting percentage of heterozygosity in each haplotype block across the population was plotted for each chromosome to identify “peaks” of high heterozygosity in FDR unreduced gamete-derived individuals putatively representing the genetic centromere locations. SNPs with the maximum heterozygosity for each chromosome were assumed to be within the centromere region. The first SNP marker to show decreased heterozygosity in the direction of each telomere was taken as a flanking marker for the centromere boundary. SNP marker locations were derived from the published B. napus Darmor genome sequence (Chalhoub et al. 2014) using the best match from BLAST alignments of the 50-bp probe sequences (95% sequence identity, no gaps permitted).
Location of B. napus centromere- and pericentromere-specific repeats
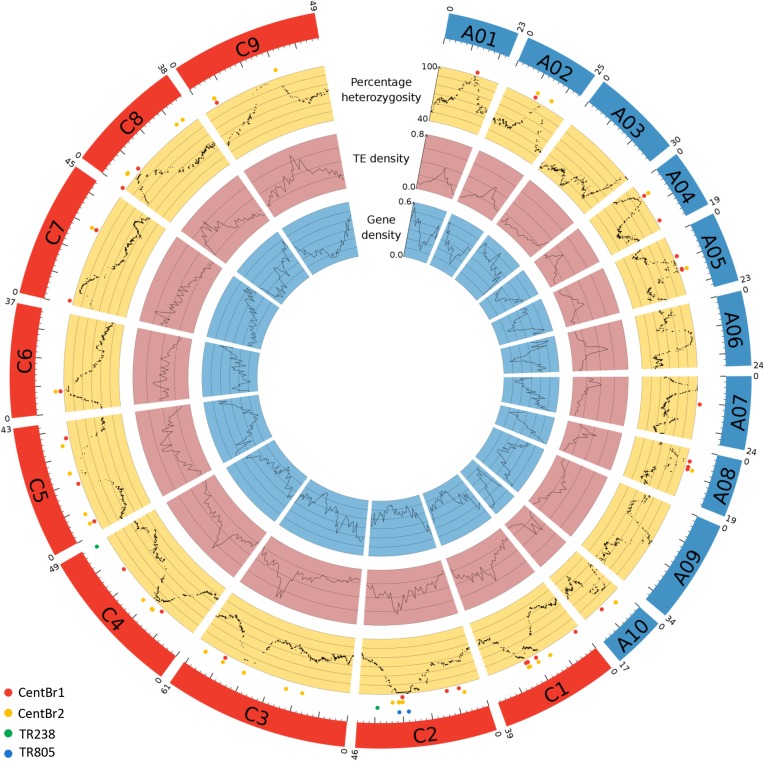
The (peri)centromere-specific repeat sequences CentBr1 and CentBr2 (176-bp centromere satellite repeats) were retrieved from the BAC end sequences of the B. rapa clones KBrH001B09 (GenBank accession CW978699) and KBrH001E07 (GenBank accession CW978837), respectively (Lim et al. 2005; Koo et al. 2011). These two sequences were blasted (e-value: 10−6) against the B. napus (Darmor v4.1) genome sequence (Chalhoub et al. 2014) using BLASTn (Altschul et al. 1990). Only BLAST results with at least 90% sequence similarity to the B. napus genome were kept for each CentBr sequence, as the different copies of each class have >90% sequence similarity, while CentBr1 and CentBr2 present ∼82% sequence similarity (Lim et al. 2005). In addition, BLASTn was used to align sequences (against the B. napus genome) that are found in the pericentromeric heterochromatin blocks of Brassica chromosomes (Lim et al. 2007). These comprised a centromere-specific Ty1/copia-like retrotransposon of Brassica (CRB, BAC KBrH00P13: GenBank accession AC166739); a 238-bp degenerate tandem repeat (TR238, BAC KBrH015B20: GenBank accession AC166740); and an 805-bp tandem repeat (TR805, BAC KBrH00P13: GenBank accession AC166739). Subsequently, only BLAST results presenting at least 90% identity and a minimal alignment length of 20% were considered [a low stringency was used to improve the chance of detecting these difficult-to-assemble repeat sequences, in line with the methodology of Cheng et al. (2013)]. Finally, attempts were made to physically localize centromere-specific histone H3 variant using the partial B. napus CenH3 cDNA clone sequences [GenBank accession HM582931, HM582932, HM582933, HM582934, HM582935, HM582936, HM582937, and HM582938; Wang et al. (2011)], by performing a BLASTp alignment against Darmor amino-acid gene-coding sequences (Chalhoub et al. 2014). The physical locations of these various (peri)centromere-specific sequences on the B. napus (Darmor) genome were represented graphically (Figure 2, outer circle) using Circos software (Krzywinski et al. 2009).
Figure 2.
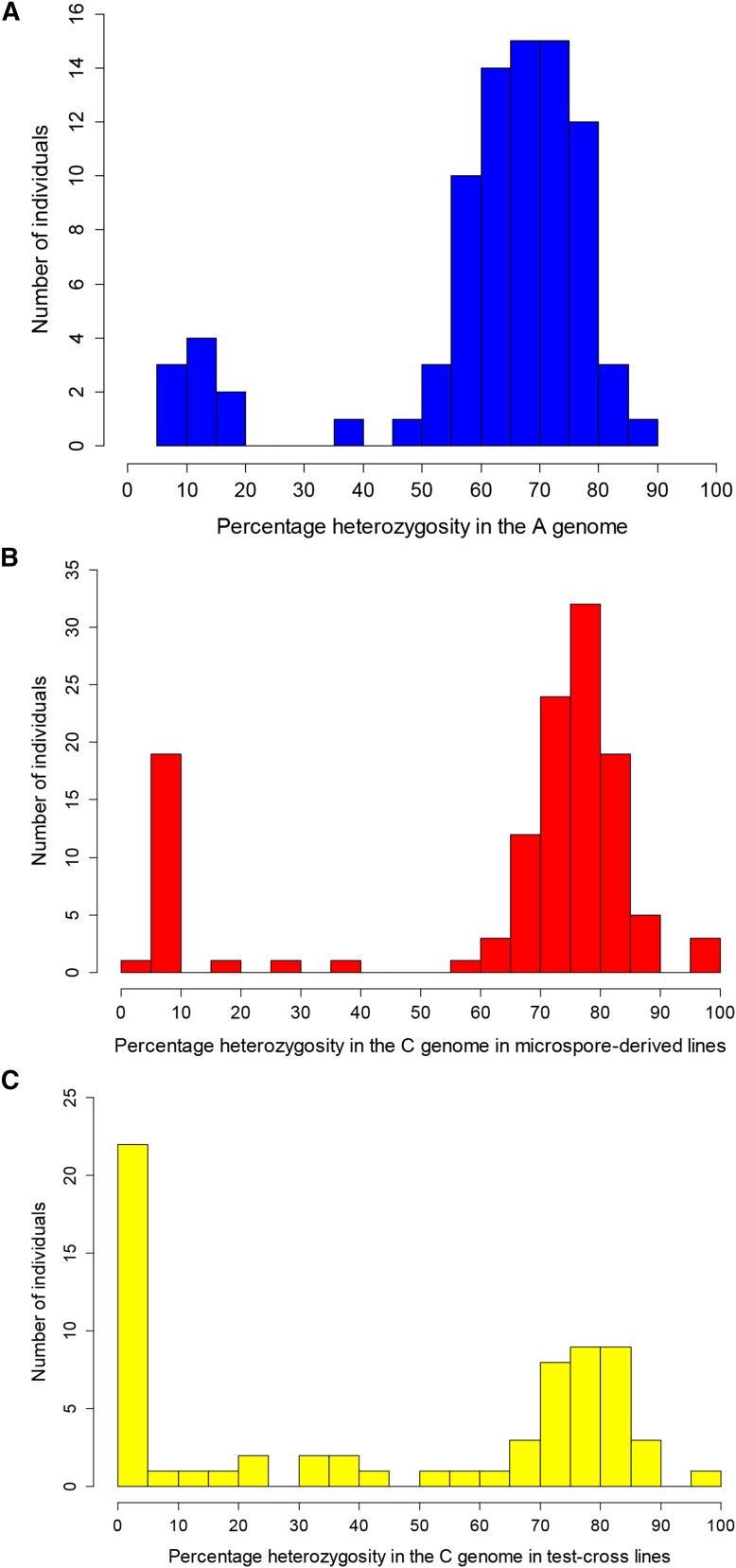
Percentage of heterozygosity as assessed by presence of both parental alleles at a single locus in the diploid genome in individuals derived from (A) microspore culture of B. juncea × B. napus (2n = AABC) interspecific hybrids; (B) microspore culture of B. napus × B. carinata (2n = CCAB) interspecific hybrids; and (C) test crosses between B. napus × B. carinata (2n = CCAB) interspecific hybrids and B. juncea.
Gene and transposable element density
To determine the gene density on each B. napus chromosome, the start and end position of each of the 101,0140 gene sequences (messenger RNA, mRNA) identified in B. napus Darmor by Chalhoub et al. (2014) and available from the Genoscope website (http://www.genoscope.cns.fr/brassicanapus/data/) were recovered. For each chromosome, the gene density was defined using a sliding 1-Mb window; for each window, the gene density was calculated by dividing the number of nucleotides annotated as gene sequences by the size of the window (1,000,000 bp). Similarly, the density of transposable elements (TEs) was calculated using the total number of nucleotides annotated as TE divided by the size of the window (1,000,000 bp) (Chalhoub et al. 2014).
Data availability
Genotypes and production methods to obtain 2n-gamete derived progeny are presented in Table S1. Detailed information related to the centromere positions and flanking SNP marker locations on the genome reference sequence is presented in Table S2. Sequence information produced by Chalhoub et al. 2014 used to generate the Circos plot is available from the Genoscope website (http://www.genoscope.cns.fr/brassicanapus/data/).
Results
Production of unreduced gamete-derived individuals through test crossing and microspore culture
A total of 86 individuals were generated from microspore culture of four different B. juncea × B. napus interspecific hybrids (same B. juncea parent, four different B. napus; AABC genome; Table S1). Two pairs of twins were observed from marker results. These plants may have resulted from secondary embryogenesis generating identically twinned embryos during the tissue culture process (Raemakers et al. 1995; Cousin and Nelson 2009); one of each pair was removed from the analysis. Of the remaining 84 individuals, 75 (89%) were heterozygous at 38–86% (average 68%) of loci in the A genome; heterozygosity was assessed as presence of both a B. juncea and a B. napus parental allele at a single A-genome locus (Figure 2A). These were considered to be derived from unreduced AABC gametes from the hybrid parent via first-division restitution-like mechanisms, where nonsister chromatids assorted into the same gamete after meiosis. The remaining nine individuals were heterozygous at 6–15% of loci in the A genome (Figure 2A). These were considered to be derived from reduced gametes (residual heterozygosity may have resulted from nonhomologous recombination events), or from second division restitution, and were spread evenly among the four progeny sets (Table S1). After removal of clones and reduced gamete-derived individuals, 75 unique experimental individuals were obtained for the A-genome mapping population (87% of microspore-derived progeny).
Both microspore culture and test crosses were used to generate unreduced gamete-derived progeny from B. napus × B. carinata interspecific hybrids (CCAB genome). A total of 124 microspore-derived progeny and 65 test-cross progeny were produced from 14 different genotype combinations (Table S1).
Of the 57 microspore-derived plants in 10 progeny sets with nonidentical haplotypes in Mason et al. (2011a), an additional eight individuals were conservatively excluded for this study after SNP marker genotyping revealed that they may have been clones (>95% similar genetic identity) (Raemakers et al. 1995; Cousin and Nelson 2009) (Table S1). An additional four clone pairs were also identified in the new microspore-derived progeny, and one of each pair was excluded from further analysis. Of the remaining microspore-derived progeny, 74% (80/112) were heterozygous at 29–97% of C-genome loci, where heterozygosity was assessed as presence of both a B. napus and a B. carinata allele (average 76%) (Figure 2B). These individuals were therefore concluded to be derived from unreduced CCAB gametes via a first division restitution mechanism. The remaining microspore-derived progeny had 4.5–19% of loci heterozygous for B. napus and B. carinata alleles (average 7.6%) and were assumed to be derived from reduced gametes (or from second division restitution) [Figure 2B; the 13 reduced gamete-derived individuals from Mason et al. (2011a) were not regenotyped using the SNP array and are hence not included in the figure, but showed 0–16% heterozygosity based on SSR markers]. In total, 65% of microspore-derived progeny were unique and derived from unreduced gametes via a first-division restitution-like mechanism.
Of the test-cross progeny from B. napus × B. carinata interspecific hybrids (CCAB genome) crossed with B. juncea, 51% (34/67) were 45–100% heterozygous for parent B. napus and B. carinata alleles in the C genome (average 77%) (Figure 2C). These were therefore determined to be derived from CCAB unreduced gametes from the hybrid parent via a first-division restitution-like mechanism. Of the remaining test-cross progeny, 25/67 had 0–25% heterozygosity (average 4%) and were assumed to be derived from reduced gametes from the CCAB parent (Figure 2C). The residual heterozygosity was assumed to result from nonhomologous recombination events. Another 8 individuals had 34–58% heterozygosity (Figure 2C) but contained multiple whole chromosomes that were completely heterozygous or completely homozygous and were unable to be classified as derived from standard FDR, SDR, or reduced gametes (two may have resulted from SDR with minor additional abnormalities), and hence were not included in the half-tetrad analysis.
Locations of the active centromeres deduced by half-tetrad analysis
SNP markers with known physical locations in the B. napus genome and peaks of heterozygosity within each chromosome were used to determine the genetic locations of functional centromeres (Table 1, Figure 3, Table S2). Centromeric regions were delineated based on the SNP marker haplotypes containing the highest proportion of heterozygosity on each chromosome, and a conservative estimate of the chromosome region containing the centromere was obtained for each of the 19 Brassica chromosomes. The size of the centromere-containing region delineated on the reference genome sequence ranged from 20 kbp to 10.7 Mbp (average 1.8 Mbp; Table 1). These regions were strongly correlated with observable peaks and troughs in TE and gene density, respectively (Figure 3).
Table 1. Locations of the Brassica A- and C-genome centromeres.
| Chromosome | Start (Mbp) | End (Mbp) | Size of centromere-containing region (Mbp)* | Chromosome length (Mbp)** |
|---|---|---|---|---|
| A01 | 13.1 | 14.1 | 1.0 | 23.1 |
| A02 | 13.9 | 14.2 | 0.3 | 24.7 |
| A03 | 28.0 | 29.7 | 1.7 | 29.7 |
| A04 | 5.8 | 6.3 | 0.5 | 19.1 |
| A05 | 10.9 | 10.9 | 0.07 | 23.0 |
| A06 | 11.1 | 11.1 | 0.02 | 24.3 |
| A07 | 3.3 | 5.2 | 1.9 | 24.0 |
| A08 | 3.9 | 5.2 | 1.4 | 18.9 |
| A09 | 15.6 | 15.9 | 0.3 | 33.7 |
| A10 | 2.9 | 5.3 | 2.4 | 17.3 |
| C1 | 17.9 | 24.2 | 6.2 | 38.3 |
| C2 | 31.8 | 32.2 | 0.3 | 46.1 |
| C3 | 40.6 | 41.9 | 1.3 | 60.6 |
| C4 | 17.1 | 19.4 | 2.3 | 48.9 |
| C5 | 17.6 | 28.3 | 10.7 | 42.6 |
| C6 | 8.0 | 8.4 | 0.5 | 37.2 |
| C7 | 5.4 | 7.2 | 1.8 | 44.5 |
| C8 | 5.8 | 6.4 | 0.6 | 38.3 |
| C9 | 23.1 | 23.4 | 0.3 | 48.4 |
Represents the conservative outer boundaries within which the active centromeric region must fall. SNPs with the maximum heterozygosity for each chromosome were assumed to be within the centromere region. The first SNP marker to show decreased heterozygosity in the direction of each telomere was taken as a flanking marker for the centromere boundary.
As covered by polymorphic SNP markers in the assembled B. napus Darmor v4.1 reference genome sequence (Chalhoub et al. 2014).
Figure 3.
Gene density, TE density, and percentage of heterozygosity (the latter in a population derived from first-division restitution-type unreduced gametes) along each B. napus chromosome represented in a Circos plot (Krzywinski et al. 2009). The B. napus chromosomes belonging to the A and C subgenomes are in blue and red, respectively. The size of each chromosome in megabase pairs is indicated above each chromosome and a ruler is drawn underneath each chromosome, with larger tick marks every 10 Mbp and smaller tick marks every 2 Mbp. Locations of active centromeres are visible as peaks of increased heterozygosity, increased TE density, and decreased gene density. Dots indicate BLAST-located centromeric and pericentromere sequences: CentBr1 sequences (red), CentBr2 sequences (yellow), TR238 sequences (green), and TR805 sequences (blue).
Distribution of polymorphic SNP markers and haplotype-based inferences
SNP markers that were polymorphic and amplified only a single locus in the genotypes used in our study were distributed across the B. napus Darmor reference genome. A lower density of useable polymorphic SNP markers was found around the centromere regions for all A-genome chromosomes except A04 and A10 and for all C-genome chromosomes except C2, C4, and C6. Most chromosomes appeared to have small physical regions of reduced polymorphism around centromeres based on SNP marker distribution on the Illumina Infinium Brassica 60K array, with the exception of chromosomes A02 and C5.
Surprisingly, one progeny set did not show inheritance of chromosome A10 expected from homologous recombination between B. napus and B. juncea. Instead, the unrecombined B. napus parent chromosome was present in 8/22 individuals with no B. juncea alleles present, and hence no centromere region could be identified in these individuals. As well, four large (>3 Mbp) putative inversions were observed based on haplotype analysis of all genotypes used in this study relative to the B. napus Darmor reference genome sequence. These ranged from 4.62 to 6.01 Mbp in size and were located over the centromere regions on chromosomes C1, C2, and C7 (Table S2). One additional putative inversion was present in only two progeny sets and comprised a 7.90-Mbp region on chromosome C5 (Table S2). Several other smaller putative inversions or rearrangements (<3 Mbp) were also identified and are listed in Table S2.
Distribution of known Brassica centromeric and pericentromeric sequences in the B. napus genome
A total of 206 sequences for CentBr1 and 710 sequences for CentBr2 were identified in the B. napus genome using the criteria of >90% identity and >20% alignment length. Of these, only 26% (56) and 58% (415) of CentBr1 and CentBr2 sequence locations, respectively, were on assembled chromosomes. Most of these CentBr2 signals were predominantly localized on chromosomes A10 (88% of the BLAST results specifically located on A pseudochromosomes as opposed to unanchored scaffolds) and C4 (71% of the BLAST results specifically located on C pseudochromosomes). A total of 14 of the 19 A- and C-genome chromosomes had either a CentBr1 or CentBr2 sequence identified in the putative genetic centromere region (Figure 3). However, only chromosomes C6 and A01 contained CentBr sequences solely in the active centromere region, rather than also being present in other locations along the chromosome (Figure 3).
A total of 197 TR238 sequences, 18 TR805 sequences, and 4 CRB sequences were identified, of which 2 (1%), 4 (22%), and none could be mapped to assembled chromosomes. The four TR805 sequences localized to the C2 genetic centromere region, whereas the two sequences from TR238 were not found in active centromere regions (Figure 2). The eight CENH3 query protein sequences all aligned to a single location in the B. napus genome that was on an unanchored C chromosome location. This B. napus protein sequence was identical to one of the CENH3 query proteins (GenBank accession HM582935).
Discussion
Based on genomic sequence information from B. napus, we located the 19 active Brassica A- and C-genome centromeres on the reference genome. This was achieved by high-resolution genotyping of populations of microspore- and test-cross-derived progeny generated from unreduced gametes of interspecific Brassica hybrids. With the increasing availability of high-throughput genotyping methodologies and reference genome sequences, this half-tetrad mapping approach can be readily carried out to locate centromeres in other species of interest known to produce unreduced gametes.
The physical size of the centromere regions in A. thaliana ranges from 0.5 to 1.79 Mbp (Copenhaver et al. 1999; Hosouchi et al. 2002). Assuming that centromere size is similar or greater in B. napus, and from the fact that the majority of centromere-specific sequences did not find matches in the assembled pseudochromosomes, we expect that the majority of the centromere regions in the B. napus reference genome sequence are not yet assembled and/or genetically anchored. This is not unexpected, due to the difficulty in assembling the complex repetitive elements that make up these regions using next-generation sequencing approaches. However, for all centromeric regions there were distinctive patterns in the surrounding genome sequence that provide additional landmarks indicating their presence, in particular, an observable drop in gene density and generally a concomitant increase in TE density (Figure 3). Centromeres C4 and A10 appear to be the most represented centromere regions in the current reference genome sequence, based on the number of localized repeats (the majority of CentBr sequences identified were on these two chromosomes). The observation of CentBr sequence alignments outside the predicted genetic centromeres may represent small-scale misplacements of these particular scaffolds relative to the reference genome sequence. Alternatively, these could represent defunct centromere regions remaining after the Brassica polyploidization events (Cheng et al. 2013), or the repeat sequences may simply not underlie the active centromeres, as centromeric repeats are not always necessary for centromere activity (Nasuda et al. 2005; Liu et al. 2015).
The physically anchored genetic centromere locations identified here correlated with the chromosome structures reported previously using cytogenetic methods (Olin-Fatih and Heneen 1992; Cheng et al. 1995; Xiong and Pires 2011). Chromosomes C7 and C8 had subterminal centromeres, and the remainder of the C-genome chromosomes approximately median or submedian centromeres, supporting the molecular cytogenetic karyotyping of Xiong and Pires (2011). The orientation of chromosomes in the C-genome reference sequence is based on the reference genetic map of Parkin et al. (1995) and Parkin et al. (2014). Here we confirm the previous proposal by Howell et al. (2002) that several C-genome linkage groups (e.g., C2 and C3) were inverted, relative to the convention of orienting chromosomes with the short arm on top and long arm at the bottom. Further validation of these results is pending a more complete genome assembly. Koo et al. (2004) identified two median, five submedian, two subtelocentric, and one acrocentric centromere in the B. rapa genome; Xiong and Pires (2011) show chromosome A07 as acrocentric and A04 and A10 as subtelocentric, reasonably congruent with our results (Table S2). Chromosomes A01, A02, and A03 in the current genome reference sequence were inverted relative to the “short arm on top” convention, most significantly for A03 with centromere positioned at the end of the chromosome assembly. Hence, our centromere-positioning analysis suggests that the short arm of chromosome A03 has not been assembled in the published Darmor reference genome. This is not surprising as the short arm of chromosome A03 is a known nucleolar organizing region (NOR), composed of repetitive elements and arrays; Mun et al. (2010) were also unable to identify any BACs localized to this chromosome arm in their sequence and assembly of A03.
Cheng et al. (2013) performed an extensive investigation of paleocentromeres in B. rapa using a sequence-based approach. Their placement of 8/10 A-genome centromeres corresponded perfectly to our identified genetic centromeres; only for chromosomes A02 and A09 was an inactive region selected as the active centromere (and for both A02 and A09 a “trace” centromere region was identified by Cheng et al. (2013), which aligned with our active genetic centromere region). This result supports the utility of half-tetrad analysis in identifying active vs. paleocentromeres, but also shows that combining both sequence-based and genetic mapping-based approaches can yield the most useful genomic information. This was also demonstrated by the close correlations between the placement of the centromeres using TE and gene density and the placement using the half-tetrad mapping approach in our study.
Several large inversions and a number of small rearrangements were observed in our study based on haplotype analysis relative to the B. napus Darmor reference genome. Chromosomes C1, C2, and C7 all had large inversions over the centromere region (Table S2); for chromosome C1 (and to a lesser extent C2) this was directly observable as the presence of two peaks of heterozygosity (instead of one peak) indicating the centromere region (Figure 3). These rearrangements may result from actual genotypic differences between the genotypes used in this experiment and Darmor or from misassembly of the B. napus genome sequence. The lack of resolution of the C5 centromere in our study may also be related to putative chromosome structural rearrangements between the parents of our mapping populations, as few polymorphic SNPs were identified between our parent lines for this region; but SNPs were present in this region on the array. Future validation using a wider range of genotypes could be helpful to confirm this and to improve the accuracy of the C5 centromere mapping. The accurate genetic anchoring and orienting of sequenced scaffolds closely associated with centromeres is often impeded by limited recombination in the proximity of the centromere. Differences between genotypes for SNP probe specificity may also cause differences between our results and the reference genome sequence. However, such differences are more likely to result in A- or C-genome probe aspecificity (e.g., amplification of a homeologous region in our lines, instead of the region in the reference identified through BLAST) than inversions and small-scale rearrangements. Further investigation of the genotypic variation for chromosome rearrangements present within B. napus would be useful in confirming our observations. Although a number of homeologous translocation events have been previously characterized in B. napus (Osborn et al. 2003), the recent availability of a reference genome sequence and high-throughput genotyping tools such as the Illumina Infinium Brassica 60K array is expected to shed light on the B. napus core and disposable genome.
We used both microspore culture and test crosses to produce unreduced gamete-derived individuals for half-tetrad analysis (Nelson et al. 2009; Mason et al. 2011a). Although microspore culture is not practicable in most species, unreduced gametes are also commonly observed in interspecific and interploid hybrids (Ramsey and Schemske 1998; De Storme and Mason 2014; Mason and Pires 2015), which can be used to generate suitable populations in a wider range of species through test-cross approaches. In our study, both test-crossing and microspore culture yielded >50% unreduced gamete-derived progeny using a number of different interspecific hybrid genotypes. It is interesting to note that test crossing of a single genotype of B. juncea × B. napus interspecific hybrid to two genotypes of B. carinata in a previous study yielded only reduced gamete-derived individuals (Mason et al. 2012). In this study, the same hybrid genotype of B. juncea × B. napus was successfully used to generate 87% unreduced gamete-derived progeny through microspore culture (Table S1, A_MD_03). This supports previous research that microspore culture preferentially selects unreduced gametes in interspecific Brassica hybrids (Nelson et al. 2009; Mason et al. 2011a).
Progeny were most commonly derived from first division restitution-like mechanisms: an example mechanism is parallel spindles at meiosis II, which has previously been observed in these Brassica hybrids (Nelson et al. (2009); however, many such mechanisms exist [see Bretagnolle and Thompson (1995); De Storme and Geelen (2013), and De Storme and Mason (2014)]. The common observation of FDR rather than SDR is not surprising: in these AABC and CCAB hybrid types, FDR produces gametes with a similar chromosome composition to the parents, but SDR results in either zero or two copies of single copy (univalent) chromosomes, with probable detrimental effects on viability. The observation of what appears to be indeterminate meiotic restitution [where some homologous chromosomes separate and some sister chromatids separate, but not all; see Lim et al. (2001) for a description of this phenomenon] in some of the test-cross progeny was surprising, but this form of meiotic restitution has also been observed previously in other species and hybrid types (Lim et al. 2001; Ramanna and Jacobsen 2003). Another explanation for the failure to detect heterozygous regions for some centromeres may be the undetected occurrence of double cross-over events (a cross-over on either side of the centromere) very close to the centromere region, where SNP marker coverage was poor and the genome assembly more likely to contain gaps.
The Brassica model is unusually amenable for generating interspecific hybrids in many combinations of A, B, and C genomes (Chen et al. 2011), with largely regular chromosome pairing and segregation between homologous chromosomes derived from different species (Leflon et al. 2006; Mason et al. 2014b). However, even in this system we found an unusual example of failure of homologous Brassica chromosomes to segregate normally. The microspore-derived progeny of hybrid genotype A_MD_02 (Table S1) failed to show expected segregation patterns and recombination between chromosome A10 from B. napus and chromosome A10 from B. juncea. Missegregation of homologous chromosomes is thought to be uncommon but has been observed occasionally in Brassica interspecific hybrid types (Mason et al. 2015), and such meiotic abnormalities may occur more frequently in interspecific hybrids of other plant genera (De Storme and Mason 2014). The failure to detect heterozygosity associated with the A10 centromere in several individuals from one genotype group in this study may also be related to the presence of a genotype-specific chromosome rearrangement; this could either hinder pairing between the homologous chromosomes or hinder detection of cross-over events over the centromere region.
Interspecific hybrids are an extremely common phenomenon in many genera (Mallet 2007). The tendency for interspecific hybrids to produce high frequencies of unreduced gametes is not only frequently observed (Ramsey and Schemske 1998), but is predicted to be moderately universal, due to the common chromosome mechanics involved [for a review see De Storme and Mason (2014) and De Storme and Geelen (2013)]. This study demonstrates the utility of both test crosses and microspore culture in generating unreduced gamete-derived progeny from interspecific hybrids: such approaches should also be feasible in other genera. In future, as single-molecule genome sequencing technologies become available, it may also become possible to sequence unreduced gametes sorted by size using flow cytometry (Pan et al. 2004; De Storme et al. 2013), bypassing the often technically challenging stage of developing an adult population and offering a potential avenue for half-tetrad analysis in an even broader range of species.
Acknowledgments
We thank Shyam Sundar Dey for assistance with microspore culture. Rowan Bunch is gratefully acknowledged for Illumina HiScan SNP chip scanning. This work was supported by an Australian Research Council Discovery Early Career Researcher Award (DE120100668); Emmy Noether Deutsche Forschungsgemeinschaft grant MA 6473/1-1; and Australian Research Council Project grants LP0883642, DP0985953, and LP110100200.
Footnotes
Communicating editor: A. Houben
Supporting information is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.115.183210/-/DC1.
Literature Cited
- Aleza P., Cuenca J., Hernandez M., Juarez J., Navarro L., et al. , 2015. Genetic mapping of centromeres in the nine Citrus clementina chromosomes using half-tetrad analysis and recombination patterns in unreduced and haploid gametes. BMC Plant Biol. 15: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J., 1990. Basic Local Alignment Search Tool. J. Mol. Biol. 215: 403–410. [DOI] [PubMed] [Google Scholar]
- Baudry E., Kryger P., Allsopp M., Koeniger N., Vautrin D., et al. , 2004. Whole-genome scan in thelytokous-laying workers of the cape honeybee (Apis mellifera capensis): central fusion, reduced recombination rates and centromere mapping using half-tetrad analysis. Genetics 167: 243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretagnolle F., Thompson J. D., 1995. Tansley review No. 78. Gametes with the stomatic (sic) chromosome number: mechanisms of their formation and role in the evolution of autopolypoid plants. New Phytol. 129: 1–22. [DOI] [PubMed] [Google Scholar]
- Brownfield L., Köhler C., 2011. Unreduced gamete formation in plants: mechanisms and prospects. J. Exp. Bot. 62: 1659–1668. [DOI] [PubMed] [Google Scholar]
- Chalhoub B., Denoeud F., Liu S. Y., Parkin I. A. P., Tang H. B., et al. , 2014. Early allopolyploid evolution in the post-Neolithic Brassica napus oilseed genome. Science 345: 950–953. [DOI] [PubMed] [Google Scholar]
- Chen S., Nelson M. N., Ghamkhar K., Fu T.-d., Cowling W. A., 2008. Divergent patterns of allelic diversity from similar origins: the case of oilseed rape (Brassica napus L.) in China and Australia. Genome 51: 1–10. [DOI] [PubMed] [Google Scholar]
- Chen S., Nelson M. N., Chèvre A.-M., Jenczewski E., Li Z., et al. , 2011. Trigenomic bridges for Brassica improvement. Crit. Rev. Plant Sci. 30: 524–547. [Google Scholar]
- Cheng B. F., Heneen W. K., Chen B. Y., 1995. Mitotic karyotypes of Brassica campestris and Brassica alboglabra and identification of the B. alboglabra chromosome in an addition line. Genome 38: 313–319. [DOI] [PubMed] [Google Scholar]
- Cheng F., Mandakova T., Wu J., Xie Q., Lysak M. A., et al. , 2013. Deciphering the diploid ancestral genome of the mesohexaploid Brassica rapa. Plant Cell 25: 1541–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copenhaver G. P., 2005. A compendium of plant species producing pollen tetrads. J. N. C. Acad. Sci. 121: 17–35. [Google Scholar]
- Copenhaver G. P., Nickel K., Kuromori T., Benito M. I., Kaul S., et al. , 1999. Genetic definition and sequence analysis of Arabidopsis centromeres. Science 286: 2468–2474. [DOI] [PubMed] [Google Scholar]
- Copenhaver G. P., Keith K. C., Preuss D., 2000. Tetrad analysis in higher plants. A budding technology. Plant Physiol. 124: 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousin A., Nelson M., 2009. Twinned microspore-derived embryos of canola (Brassica napus L.) are genetically identical. Plant Cell Rep. 28: 831–835. [DOI] [PubMed] [Google Scholar]
- Cuenca J., Froelicher Y., Aleza P., Juarez J., Navarro L., et al. , 2011. Multilocus half-tetrad analysis and centromere mapping in citrus: evidence of SDR mechanism for 2n megagametophyte production and partial chiasma interference in mandarin cv ‘Fortune’. Heredity 107: 462–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Storme N., Geelen D., 2013. Sexual polyploidization in plants–cytological mechanisms and molecular regulation. New Phytol. 198: 670–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Storme N., Mason A. S., 2014. Plant speciation through chromosome instability and ploidy change: Cellular mechanisms, molecular factors and evolutionary relevance. Current Plant Biology 1: 10–33. [Google Scholar]
- De Storme N., Copenhaver G. P., Geelen D., 2012. Production of diploid male gametes in Arabidopsis by cold-induced destabilization of postmeiotic radial microtubule arrays. Plant Physiol. 160: 1808–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Storme N., Zamariola L., Mau M., Sharbel T. F., Geelen D., 2013. Volume-based pollen size analysis: an advanced method to assess somatic and gametophytic ploidy in flowering plants. Plant Reprod. 26: 65–81. [DOI] [PubMed] [Google Scholar]
- Fulton T. M., Chunwongse J., Tanksley S. D., 1995. Microprep protocol for extraction of DNA from tomato and other herbaceous plants. Plant Mol. Biol. Rep. 13: 207–209. [Google Scholar]
- Han F. P., Lamb J. C., Birchler J. A., 2006. High frequency of centromere inactivation resulting in stable dicentric chromosomes of maize. Proc. Natl. Acad. Sci. USA 103: 3238–3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosouchi T., Kumekawa N., Tsuruoka H., Kotani H., 2002. Physical map-based sizes of the centromeric regions of Arabidopsis thaliana chromosomes 1, 2, and 3. DNA Res. 9: 117–121. [DOI] [PubMed] [Google Scholar]
- Howell E. C., Barker G., Jones G. H., Kearsey M. J., King G., et al. , 2002. Integration of the cytogenetic and genetic linkage maps of Brassica oleracea. Genetics 161: 1225–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert S., Cognard E., Hedgecock D., 2009. Centromere mapping in triploid families of the Pacific oyster Crassostrea gigas (Thunberg). Aquaculture 288: 172–183. [Google Scholar]
- Johnson S. L., Africa D., Horne S., Postlethwait J. H., 1995. Half-tetrad analysis in zebrafish: mapping the ros mutation and the centromere of linkage group I. Genetics 139: 1727–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo D. H., Plaha P., Lim Y. P., Hur Y., Bang J. W., 2004. A high-resolution karyotype of Brassica rapa ssp pekinensis revealed by pachytene analysis and multicolor fluorescence in situ hybridization. Theor. Appl. Genet. 109: 1346–1352. [DOI] [PubMed] [Google Scholar]
- Koo D. H., Hong C. P., Batley J., Chung Y. S., Edwards D., et al. , 2011. Rapid divergence of repetitive DNAs in Brassica relatives. Genomics 97: 173–185. [DOI] [PubMed] [Google Scholar]
- Krzywinski M., Schein J., Birol I., Connors J., Gascoyne R., et al. , 2009. Circos: an information aesthetic for comparative genomics. Genome Res. 19: 1639–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leflon M., Eber F., Letanneur J. C., Chelysheva L., Coriton O., et al. , 2006. Pairing and recombination at meiosis of Brassica rapa (AA) × Brassica napus (AACC) hybrids. Theor. Appl. Genet. 113: 1467–1480. [DOI] [PubMed] [Google Scholar]
- Lim K.-B., Ramanna M. S., de Jong J. H., Jacobsen E., van Tuyl J. M., 2001. Indeterminate meiotic restitution (IMR): a novel type of meiotic nuclear restitution mechanism detected in interspecific lily hybrids by GISH. Theor. Appl. Genet. 103: 219–230. [Google Scholar]
- Lim K. B., de Jong H., Yang T. J., Park J. Y., Kwon S. J., et al. , 2005. Characterization of rDNAs and tandem repeats in the heterochromatin of Brassica rapa. Mol. Cells 19: 436–444. [PubMed] [Google Scholar]
- Lim K. B., Yang T. J., Hwang Y. J., Kim J. S., Park J. Y., et al. , 2007. Characterization of the centromere and peri-centromere retrotransposons in Brassica rapa and their distribution in related Brassica species. Plant J. 49: 173–183. [DOI] [PubMed] [Google Scholar]
- Lin B. Y., Chang S. J., Lin H. M., 2001. RFLP mapping of the centromere of chromosomes 1, 6 and 9 by B-A translocations in maize. Bot. Bull. Acad. Sin. 42: 273–279. [Google Scholar]
- Liu L. S., Tong J. O., Guo W. J., Yu X. M., 2013. Microsatellite-centromere mapping in bighead carp (Aristichthys nobilis) using gynogenetic diploid families. Aquacult. Res. 44: 1470–1488. [Google Scholar]
- Liu Y. L., Su H. D., Pang J. L., Goo Z., Wang X. J., et al. , 2015. Sequential de novo centromere formation and inactivation on a chromosomal fragment in maize. Proc. Natl. Acad. Sci. USA 112: E1263–E1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow C. L., Scott A. C., Cromie G. A., Jeffery E. W., Sirr A., et al. , 2013. High-throughput tetrad analysis. Nat. Methods 10: 671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet J., 2007. Hybrid speciation. Natl. Rev. 446: 279–283. [DOI] [PubMed] [Google Scholar]
- Martinez P., Hermida M., Pardo B. G., Fernandez C., Castro J., et al. , 2008. Centromere-linkage in the turbot (Scophthalmus maximus) through half-tetrad analysis in diploid meiogynogenetics. Aquaculture 280: 81–88. [Google Scholar]
- Mason A. S., Pires J. C., 2015. Unreduced gametes: meiotic mishap or evolutionary mechanism? Trends Genet. 31: 5–10. [DOI] [PubMed] [Google Scholar]
- Mason A. S., Nelson M. N., Castello M.-C., Yan G., Cowling W. A., 2011a Genotypic effects on the frequency of homoeologous and homologous recombination in Brassica napus × B. carinata hybrids. Theor. Appl. Genet. 122: 543–553. [DOI] [PubMed] [Google Scholar]
- Mason A. S., Nelson M. N., Yan G. J., Cowling W. A., 2011b Production of viable male unreduced gametes in Brassica interspecific hybrids is genotype specific and stimulated by cold temperatures. BMC Plant Biol. 11: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason A. S., Yan G. J., Cowling W. A., Nelson M. N., 2012. A new method for producing allohexaploid Brassica through unreduced gametes. Euphytica 186: 277–287. [Google Scholar]
- Mason A. S., Batley J., Bayer P. E., Hayward A., Cowling W. A., et al. , 2014a High-resolution molecular karyotyping uncovers pairing between ancestrally related Brassica chromosomes. New Phytol. 202: 964–974. [DOI] [PubMed] [Google Scholar]
- Mason A. S., Nelson M. N., Takahira J., Cowling W. A., Moreira Alves G., et al. , 2014b The fate of chromosomes and alleles in an allohexaploid Brassica population. Genetics 197: 273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason A. S., Takahira J., Atri C., Samans B., Hayward A., et al. , 2015. Microspore culture reveals complex meiotic behaviour in a trigenomic Brassica hybrid. BMC Plant Biol. 15: 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather K., Beale G. H., 1942. The calculation and precision of linkage values from tetrad analysis. J. Genet. 43: 1–30. [Google Scholar]
- Metzker M. L., 2010. Sequencing technologies: the next generation. Nat. Rev. Genet. 11: 31–46. [DOI] [PubMed] [Google Scholar]
- Mun J. H., Kwon S. J., Seol Y. J., Kim J. A., Jin M., et al. , 2010. Sequence and structure of Brassica rapa chromosome A3. Genome Biol. 11: R94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasuda S., Hudakova S., Schubert I., Houben A., Endo T. R., 2005. Stable barley chromosomes without centromeric repeats. Proc. Natl. Acad. Sci. USA 102: 9842–9847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M. N., Mason A. S., Castello M. C., Thomson L., Yan G. J., et al. , 2009. Microspore culture preferentially selects unreduced (2n) gametes from an interspecific hybrid of Brassica napus L. x Brassica carinata Braun. Theor. Appl. Genet. 119: 497–505. [DOI] [PubMed] [Google Scholar]
- Nie H. T., Li Q., Kong L. F., 2011. Microsatellite-centromere mapping in sea cucumber (Apostichopus japonicus) using gynogenetic diploid families. Aquaculture 319: 67–71. [Google Scholar]
- Nie H. T., Li Q., Kong L. F., 2012. Centromere mapping in the Pacific abalone (Haliotis discus hannai) through half-tetrad analysis in gynogenetic diploid families. Anim. Genet. 43: 290–297. [DOI] [PubMed] [Google Scholar]
- Nomura K., Morishima K., Tanaka H., Unuma T., Okuzawa K., et al. , 2006. Microsatellite-centromere mapping in the Japanese eel (Anguilla japonica) by half-tetrad analysis using induced triploid families. Aquaculture 257: 53–67. [Google Scholar]
- Olin-Fatih M., Heneen W. K., 1992. C-banded karyotypes of Brassica campestris, Brassica oleracea, and Brassica napus. Genome 35: 583–589. [DOI] [PubMed] [Google Scholar]
- Osborn T. C., Butrulle D. V., Sharpe A. G., Pickering K. J., Parkin I. A., et al. , 2003. Detection and effects of a homeologous reciprocal transposition in Brassica napus. Genetics 165: 1569–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan G., Zhou Y., Fowke L. C., Wang H., 2004. An efficient method for flow cytometric analysis of pollen and detection of 2n nuclei in Brassica napus pollen. Plant Cell Rep. 23: 196–202. [DOI] [PubMed] [Google Scholar]
- Park T. H., Kim J. B., Hutten R. C. B., van Eck H. J., Jacobsen E., et al. , 2007. Genetic positioning of centromeres using half-tetrad analysis in a 4x-2x cross population of potato. Genetics 176: 85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin I. A. P., Sharpe A. G., Keith D. J., Lydiate D. J., 1995. Identification of the A and C genomes of amphidiploid Brassica napus (oilseed rape). Genome 38: 1122–1131. [DOI] [PubMed] [Google Scholar]
- Parkin I. A., Koh C., Tang H., Robinson S. J., Kagale S., et al. , 2014. Transcriptome and methylome profiling reveals relics of genome dominance in the mesopolyploid Brassica oleracea. Genome Biol. 15: R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raemakers C. J. J. M., Jacobsen E., Visser R. G. F., 1995. Secondary somatic embryogenesis and applications in plant breeding. Euphytica 81: 93–107. [Google Scholar]
- Ramanna M. S., Jacobsen E., 2003. Relevance of sexual polyploidization for crop improvement: a review. Euphytica 133: 3–18. [Google Scholar]
- Ramsey J., Schemske D. W., 1998. Pathways, mechanisms, and rates of polyploid formation in flowering plants. Annu. Rev. Ecol. Syst. 29: 467–501. [Google Scholar]
- Rudd M. K., Willard H. F., 2004. Analysis of the centromeric regions of the human genome assembly. Trends Genet. 20: 529–533. [DOI] [PubMed] [Google Scholar]
- Schneerman M. C., Lee W. S., Doyle G., Weber D. F., 1998. RFLP mapping of the centromere of chromosome 4 in maize using isochromosomes for 4S. Theor. Appl. Genet. 96: 361–366. [DOI] [PubMed] [Google Scholar]
- Takahira J., Cousin A., Nelson M. N., Cowling W. A., 2011. Improvement in efficiency of microspore culture to produce doubled haploid canola (Brassica napus L.) by flow cytometry. Plant Cell Tissue Organ Cult. 104: 51–59. [Google Scholar]
- Tavoletti S., Bingham E. T., Yandell B. S., Veronesi F., Osborn T. C., 1996. Half tetrad analysis in alfalfa using multiple restriction fragment length polymorphism markers. Proc. Natl. Acad. Sci. USA 93: 10918–10922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veilleux R., 1985. Diploid and polyploid gametes in crop plants: mechanisms of formation and utilization in plant breeding. Plant Breed. Rev. 3: 253–288. [Google Scholar]
- Wang G. X., He Q. Y., Liu F., Cheng Z. K., Talbert P. B., et al. , 2011. Characterization of CENH3 proteins and centromere-associated DNA sequences in diploid and allotetraploid Brassica species. Chromosoma 120: 353–365. [DOI] [PubMed] [Google Scholar]
- Xiong Z., Pires J. C., 2011. Karyotype and identification of all homoeologous chromosomes of allopolyploid Brassica napus and its diploid progenitors. Genetics 187: 37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W. L., Friebe B., Gill B. S., Jiang J. M., 2010. Centromere inactivation and epigenetic modifications of a plant chromosome with three functional centromeres. Chromosoma 119: 553–563. [DOI] [PubMed] [Google Scholar]
- Zhao H. Y., Speed T. P., 1998. Statistical analysis of half-tetrads. Genetics 150: 473–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Genotypes and production methods to obtain 2n-gamete derived progeny are presented in Table S1. Detailed information related to the centromere positions and flanking SNP marker locations on the genome reference sequence is presented in Table S2. Sequence information produced by Chalhoub et al. 2014 used to generate the Circos plot is available from the Genoscope website (http://www.genoscope.cns.fr/brassicanapus/data/).