Abstract
Admixture and recombination create populations and genomes with genetic ancestry from multiple source populations. Analyses of genetic ancestry in admixed populations are relevant for trait and disease mapping, studies of speciation, and conservation efforts. Consequently, many methods have been developed to infer genome-average ancestry and to deconvolute ancestry into continuous local ancestry blocks or tracts within individuals. Current methods for local ancestry inference perform well when admixture occurred recently or hybridization is ongoing, or when admixture occurred in the distant past such that local ancestry blocks have fixed in the admixed population. However, methods to infer local ancestry frequencies in isolated admixed populations still segregating for ancestry do not exist. In the current paper, I develop and test a continuous correlated beta process model to fill this analytical gap. The method explicitly models autocorrelations in ancestry frequencies at the population-level and uses discriminant analysis of SNP windows to take advantage of ancestry blocks within individuals. Analyses of simulated data sets show that the method is generally accurate such that ancestry frequency estimates exhibited low root-mean-square error and were highly correlated with the true values, particularly when large (±10 or ±20) SNP windows were used. Along these lines, the proposed method outperformed post hoc inference of ancestry frequencies from a traditional hidden Markov model (i.e., the linkage model in structure), particularly when admixture occurred more distantly in the past with little on-going gene flow or was followed by natural selection. The reliability and utility of the method was further assessed by analyzing genetic ancestry in an admixed human population (Uyghur) and three populations from a hybrid zone between Mus domesticus and M. musculus. Considerable variation in ancestry frequencies was detected within and among chromosomes in the Uyghur, with a large region of excess French ancestry harboring a gene with a known disease association. Similar variation was detected in the mouse hybrid zone, with notable constancy in regions of excess ancestry among admixed populations. By filling what has been an analytical gap, the proposed method should be a useful tool for many biologists. A computer program (popanc), written in C++, has been developed based on the proposed method and is available on-line at http://sourceforge.net/projects/popanc/.
Introduction
Genetic admixture between differentiated populations or species is common in plants and animals [1–6], including humans [7–10]. Admixture and recombination result in individuals whose genomes comprise a mosaic of chromosome segments with different genetic ancestry, that is to say, chromosome segments that have been inherited from different source populations. Given the prevalence of admixture, analyses of genetic ancestry are relevant in many areas of biology [11, 12]. For example, patterns of admixture and introgression in the wild show that species boundaries are often porous, and have been used to characterize the genetic basis of adaptation and reproductive isolation [13–19]. Patterns of genetic variation in admixed populations also provide information about past demographic events [11, 20–22]. Moreover, an accurate characterization of genetic ancestry is required when using genome-wide association or admixture mapping to identify genetic variants associated with trait variation or disease [11, 23–27]. Finally, admixture can be catalyzed by anthropogenic habitat alteration or species introductions, and can cause species collapse or extinction [28–31]. Thus, an understanding of admixture can be important for biodiversity conservation and wildlife management.
Numerous statistical methods have been developed to infer genetic ancestry from molecular data (reviewed in [12]). Early methods considered unlinked genetic markers and were primarily concerned with inference of genome-average ancestry, that is, the proportion of an individual’s genome inherited from each of K potential source populations (as in the admixture model in structure [23]). More recently, a variety of methods have been proposed to resolve genetic ancestry into a series of continuous blocks of DNA inherited from different source populations, and thereby infer local or locus-specific ancestry along chromosomes [32–37]. Local ancestry inference can be based on population allele frequencies or haplotypes. Hidden Markov models (HMMs) are commonly used to model correlations in local ancestry along chromosome (as in the linkage HMM in structure), and in some cases, background linkage disequilibrium (this includes Markov-HMMs and infinite-HMMs as in sabre and mspectrum) [32, 33, 36]. Local ancestry inference can be very accurate, particularly when samples from well-defined source populations and phased DNA sequence data are available [35–37]. Methods that summarize genetic ancestry for a population or lineage also exist. These include tree-based methods used to infer population admixture proportions [7, 38] and genomic cline models, which can be used to quantify differential introgression in hybrid zones [39, 40].
Different ancestry inference methods are better suited for different tasks or under different conditions. For example, estimates of genome-average ancestry from structure can be used to identify recent hybrids [41], whereas tree-based methods are better able to detect ancient introgression [7, 38]. My primary focus in this paper is on local ancestry inference at the population-level. Because of recombination, genetic drift and selection in admixed populations, population local ancestry frequencies can vary across the genome [15, 39, 42–44]. In other words, local ancestry from a given source population can be more common in some regions of the genome than others. Such variation in local ancestry frequencies precedes genome stabilization during hybrid speciation [11], and has been associated with adaptation in several systems, including maize [19], humans [45], and butterflies [17, 18]. Several approaches have been used to quantify variation in local ancestry frequencies. In particular, ancestry frequencies can be inferred post hoc from resolved local ancestry blocks [19], or in the case of very ancient admixture, using tree-based methods [17]. Similarly, genomic cline methods can provide derived summaries of local ancestry frequencies when hybridization is an ongoing process [39, 40].
Herein, I propose and evaluate a new statistical method to estimate local ancestry frequencies. The primary motivation for this method is a desire to infer ancestry frequencies in admixed populations when ongoing gene flow from source populations is rare or absent, but before genome stabilization is complete. Such situations exists in nature [6, 46], but do not meet the assumptions of existing methods (local ancestry inference generally assumes ancestry frequencies do not vary across the genome and tree-based methods ignore segregating variation within lineages). Moreover, analyses of ancestry frequencies in isolated admixed populations should provide novel insights about the relative roles of selection, drift and recombination in shaping genomes (e.g., [47]), and on the genetic basis of trait variation (e.g., [27]). I first describe the proposed method, which combines discriminant analysis with a continuous correlated beta process model to jointly estimate local ancestry within individuals and local ancestry frequencies at the population-level. I then assess the accuracy of the method by applying it and a traditional HMM approach to simulated data sets. The reliability and utility of the method is further demonstrated by using it to analyze genetic ancestry in an admixed human population (Uyghur), and three admixed populations from a house mouse hybrid zone. These analyses show that the method is both accurate and useful. Computer software implementing these methods (popanc) is available on-line at http://sourceforge.net/projects/popanc/.
Methods
Model
As a basis for the proposed statistical method, consider a model where admixture between two populations, A and B, occurs t generations in the past. The resulting admixed population then evolves until the present by recombination, drift, and selection, but with little or no ongoing gene flow from the source populations (as in [48, 49]). Under this model, genome-average ancestry should initially vary among individuals because of variation in the number of migrant ancestors (genealogy variance) and variation in the contribution of genetic material from each ancestor (assortment variance) (Fig 1, [21]). However, these sources of variation should decay rapidly, and be replaced by genome-wide variation in local ancestry frequencies among chromosome segments [44]. Eventually, chromosome segments will fix for local ancestry from population A or B. This process has been referred to as genome stabilization, particularly in the context of homoploid hybrid speciation [11, 50]. My current focus is on inference during the intermediate stages of this processes, that is, once variation in genome-average ancestry has mostly been removed, but while variation for local ancestry is still segregating in the admixed population (i.e., before genome stabilization; Fig 1). Once genome stabilization is complete or nearly complete, tree-based methods can be used to analyze local ancestry at a population or species-level, as intra-population variation in genetic ancestry can be ignored. However, during this intermediate period, methods are needed that allow for variable ancestry frequencies and that can account for genetic divergence in the admixed population (via drift or selection).
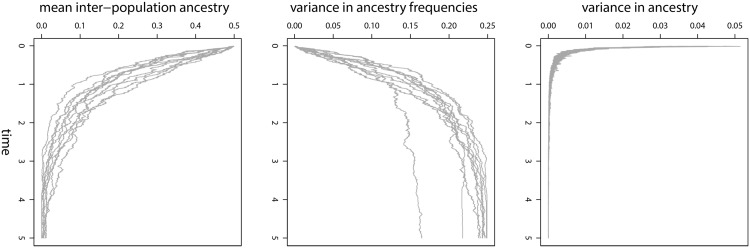
Fig 1. Variance in ancestry.
Plots depict summaries of genetic ancestry from 10 replicate simulations (gray lines). Each simulation followed a Wright-Fisher model starting with an admixed population composed entirely of F1s. The population then evolved by recombination and genetic drift. Population size was constant (2N = 200), and simulations ran for t = 1000 generation (time is reported relative to populations size as t/2N). Ancestry was followed at 100 genetic loci that were equally spaced on a 1 Morgan chromosome. Plots show the variance in genome-average ancestry among individuals (top pane), the variance in local ancestry frequencies among loci, and the proportion of loci where individuals are expected to have one gene copy from each source population (i.e., inter-population ancestry [6]). Note that variation in ancestry frequencies persists long-after variation in genome-average ancestry has decayed.
Most traditional methods for local ancestry inference use homogeneous HMMs or extensions of these [32, 33, 36]. HMMs are parameterized by a transition probability matrix that gives the probability of switching from one ancestry state (or ancestral haplotype) to another as one moves along a chromosome. Homogeneous HMMs assume that the transition probability matrix is constant and independent of the position in the genome. In essence, this assumes that local ancestry frequencies are the same everywhere in the genome. Although this assumption is reasonable when admixture occurred recently or when hybridization is ongoing, it becomes less tenable as progress towards genome stabilization occurs (Fig 1). This problem could be circumvented by defining a non-homogeneous HMM where the transition probability matrix varies along chromosomes to reflect variation in local ancestry frequencies, but computational methods for non-homogeneous HMMs are not well developed. Instead, my proposed method uses a continuous correlated beta process model (CCBPM; [51]) to co-estimate local ancestry within individuals and population-level local ancestry frequencies while explicitly modeling the genomically autocorrelated variation in the latter. Key model parameters can be estimated in a computationally efficient way via Gibbs sampling. An additional advantage of this method relative to many HMMs is that inference does not depend on phased data. While such data might be available for humans and some model systems, sequencing strategies commonly used in non-model organisms, such as genotyping-by-sequencing (GBS) methods [18, 52, 53], generate sparse, un-phased SNP data. My goal is to develop a method that can be used in these situations, as long as a draft reference genome is available.
First, I begin with a general description of a CCBPM. Consider a series of binomial experiments where the probability of success (θ) varies from experiment to experiment. In a Bayesian framework, it would be natural to place a conjugate beta prior on the probability of success for each experiment (θx), and thereby obtain the posterior probability distribution for θx (also a beta distribution), which would be , where yx denotes the number of successes out of nx trials, and α0 and β0 are shape parameters for the beta prior. Note that the posterior is a beta distribution with shape parameters α = yx + α0 and β = nx − yx + β0. Now assume that experiments are conducted one after another and that the probability of success is autocorrelated in time (or space) such that successive experiments have similar values of θ (i.e., θx and θx+1 tend to be similar). While the model described above could still be used for inference, it would be sub-optimal as it does not provide a means to share information about θ among experiments. An alternative solution is to estimate θ using a CCBPM [51], which is a graphical model that generalizes the Bayesian model above by allowing for information sharing among experiments. A kernel function K(x, x′) dictates the extent that information is shared among experiments. Different kernel functions are possible, but the kernel should be a decreasing function of the time or distance between a pair of experiments. Under this model an approximate posterior distribution for each θx can be generated by drawing samples from Pr(θx|y, n, α0, β0) = beta(α = (∑i yi k(x, i)) + α0, β = (∑i(ni − yi)k(x, i)) + β0).
As the general description above makes clear, a CCBPM can naturally be used to model local ancestry frequencies in an admixed population. In particular, at each locus (x) the number of gene copies with ancestry from source population A (zx) in a sample of nx diploid individuals can be modeled as the outcome of a binomial experiment with the probability of success given by the population ancestry frequencies at that locus (qx and 1 − qx). Because of recombination and the chromosomal nature of inheritance, ancestry frequencies should be autocorrelated along chromosomes. The CCBPM is used to share information across linked loci when estimating each qx. Thus, the approximate conditional posterior distribution for each qx is,
| (1) |
Here I use the squared exponential kernel , where σ is a scale parameter. While the scale parameter could be fixed, I instead propose to place an uniform prior on this parameter and estimate it from the data (though my main aim is to integrate over uncertainty in this nuisance parameter rather than make inferences about it).
In this model, local ancestry (z) is not an observed quantity, but instead represents a latent variable that must be inferred from the data. zx can be decomposed as zx = ∑j zxj where the sum is over individuals and zxj ∈ {0, 1, 2} denotes the number of gene copies at locus x that individual j inherited from source population A. Following Bayes’ rule, a posterior distribution for zxj can be specified as,
| (2) |
where sxj is the DNA sequence data (discussed more below). The first term on the right side of Eq (2) represents the probability of the observed sequence data for individual j conditional on that individual having 0, 1 or 2 gene copies derived from source population A, and the last term is the prior probability of inheriting zxj gene copies from source population A. The latter is clearly given by,
| (3) |
but specifying a probability distribution for the first term (i.e., the likelihood of zxj given the data) is more complicated. Thus, I will describe my approach for specifying Pr(sxj|zxj) in detail. Because of segregating allelic variation in the source populations and drift (or selection) in the admixed and source populations, sequence data from any particular nucleotide variant (i.e., SNP) can be rather uninformative about local ancestry. To overcome this limitation and to model expected autocorrelations in local ancestry within individuals, I propose to approximate Pr(sxj|zxj) using discriminant analysis (DA; a related approach was used by [54] for global ancestry inference).
Here DA is used to provide different weights to different SNPs such that they are maximally informative about local ancestry. The analysis proceeds one SNP or locus at a time. First, a window is defined around each genetic locus; a window includes a specific number of neighboring SNPs (this could be constrained by physical or recombination distance). Choosing a reasonable window size can be important (this is discussed more in the Discussion). In particular, larger windows will contain more information about local ancestry, but if windows become too large they will frequently span ancestry breakpoints, which is undesirable (see e.g., [34, 55]). Also, the window cannot include more SNPs than reference individuals (i.e., the number of observations must exceed the number of variables). DA is then used to generate a discriminant function and thereby distinguish between individuals with 0, 1 or 2 gene copies from source population A for each window (i.e., to infer zxj). A set of reference samples from each source population (i.e., populations A and B) is required to generate the discriminant function. These represent zx = 0 (source B) and zx = 2 (source A). Reference samples with one gene copy from each source population can then be simulated to represent zx = 1. My implementation of DA then proceeds as follows. Let Sx be a N × P matrix with reference individuals as rows and the genotypic data from the set of genetic variants within a window as columns. Here the genotypic data are centered counts of one of the two alleles for each SNP. The within group covariance matrix (Sw) is then calculated as,
| (4) |
where the summation is over the three groups (i.e., samples with 0, 1 or 2 gene copies from source population A), ng is the sample size for group g, and sxg is the sub-matrix containing only individuals from group g. Next, the between group scatter matrix (SB) is obtained as,
| (5) |
where μg and μ are the group and grand means of sxg and Sx, respectively. Eigenvalue decomposition of the canonical matrix can then be used to obtain the discriminant function. Specifically, the eigenvector associated with the largest eigenvalue of the canonical matrix contains the discriminant coefficients. These coefficients can be used to calculate a discriminant score for each reference sample and thereby transform the reference samples onto a new space that maximizes the genetic differences among the three groups relative to within group variation. Note that only the first discriminant function is required to separate the three groups because the zx = 1 reference group is intermediate between the two other groups.
The mean and variance of the discriminant scores for the reference individuals from each group are used to define Pr(sxj|zxj) such that,
| (6) |
where and var(dxg = zz) are the mean and variance of the discriminant scores for the set of reference samples with ancestry zx, f(sxj) is the discriminant function, and dxj is the discriminant score for an individual with unknown ancestry. Thus, after transforming the admixed individuals onto the new sample space with the discriminant function developed from the reference set, Eq (6) can be used to calculate the probability of the sequence data (or more precisely the probability of the discriminant score based on the sequence data) if the individual has 0, 1 or 2 gene copies from source population A.
A computer program (popanc), written in C++, has been developed to generate parameter estimates from the model described above. The program first performs the DA using linear algebra functions provided by the GNU Scientific Library [56]. Markov chain Monte Carlo (MCMC) is then used to obtain samples from the approximate posterior distributions for each of the model parameters (this is an approximation because Eq (1) represents a process-model generalization of Bayes’ rule [51]). MCMC includes Gibbs samplers for population ancestry frequencies (q) based on Eq (1) and individual local ancestry (z) based on Eqs (3) and (6). A Metropolis update is performed for the scale parameter σ. HDF5 is used for efficient storage and processing of the MCMC samples [57].
Simulations and data analysis
Multiple data sets were simulated and analyzed to assess the efficacy of the proposed method. Simulations were individual-based, and tracked ancestry segments rather than genetic markers (as in [11, 39, 40]). In each simulation an admixed population of N diploid individuals was initiated via hybridization between two source populations, A and B. This was followed by t discrete generations, where mating occurred within the admixed population with or without ongoing gene flow (m). Genomes consisted of 2, 1 Morgan chromosomes (i.e., 1 recombination event occurred per chromosome each generation). All simulations included genetic drift and some also included natural selection (details below). At the end of each simulation, 50 individuals were sampled from each source population and the admixed population, and genetic marker (SNP) data were generated for these individuals. Allele frequencies in the source populations were drawn from independent uniform distributions bounded by 0.05 and 0.95. Genotypes were then obtained by randomly sampling from the population allele frequencies. A similar procedure was used to generate genotypes for the admixed individuals, except alleles were sampled based on the source population allele frequencies and local ancestry. Moreover, allele frequencies within ancestry segments were modified to account for genetic drift (genetic drift affects ancestry frequencies, and also allele frequencies within ancestry segments). Specifically, for each genetic marker and ancestry type (source A and B) a new allele frequency was sampled from beta(α = pγ, (1 − p)γ), where and [58, 59]. Evolutionary dynamics depend on the ratio of the time since admixture and the population size (i.e., ). Allowing drift to affect ancestry and allele frequencies (rather than just ancestry frequencies) is realistic and important as it makes local ancestry inference considerably more difficult.
A series of data sets was simulated to determine how time since admixture affects the accuracy of ancestry frequency estimates. Ten replicate data sets were simulated with an admixed population size of N = 500 and t = 20, 50, or 200 generations since admixture (, 0.10, or 0.40) with m = 0 (no ongoing gene flow). Another series of simulations was used to quantify the effect of selection on ancestry inference. Here, each individual’s fitness was determined by its ancestry at L loci, with individuals having mixed ancestry at these loci suffering reduced fitness (i.e., underdominance was assumed). Fitness was multiplicative such that , where wj is the relative fitness of individual j and s is the selection coefficient, and lj is the number of loci (out of L) where individual j has one allele copy from each source population. Ten replicate data sets were generated with diffuse (s = 0.03 and L = 20 with 10 underdominant loci per chromosome) or strong (s = 0.3 and L = 2 with both underdominant loci on the same chromosome) selection. A third series of data sets was simulated with low (m = 0.005) or high (m = 0.05) rates of ongoing gene flow following the initial admixture event. Here m denotes the proportion of the admixed population composed of immigrants each generation. Ten replicate data sets were simulated for each migration rate and t = 200 generations with no selection.
The proposed method was then used to estimate population ancestry frequencies for each simulated data set. Data sets were analyzed using local ancestry windows that included 4, 10, or 20 SNPs on either side of the focal SNP. Two MCMC runs were conducted for each data set and window size, each with a 10,000 iteration burn-in, 30,000 total MCMC steps and a thinning interval of 5. Likely convergence to the stationary and adequate MCMC mixing were evaluated by calculating the Gelman and Rubin’s potential scale reduction factor and the effective sample size for each parameter. As a comparison, each data set was also analyzed using the correlated local ancestry HMM (i.e., linkage model) implemented in structure (version 2.3.4 [32]). Unlike many local ancestry inference approaches, the linkage model in structure can analyze un-phased genotypic data, and thus represents a valid comparison to the proposed method. Posterior estimates of local ancestry were based on two MCMC runs each with 10,000 iterations for sampling and 10,000 iteration burn-ins. Reference samples were specified, and were used as the sole source of information to infer source population allele frequencies. Population ancestry frequencies were then inferred post hoc from local ancestry estimates by equating the sample mean with the population ancestry frequency.
Approximate posterior distributions for population ancestry frequencies from the proposed CCBPM were summarized by calculating the posterior mean (point estimate) and 95% equal-tail probability intervals (ETPIs). Discrepancies between true ancestry frequencies and estimates from both the proposed method (posterior mean) and the linkage model in structure were quantified by calculating the root-mean-square deviation (RMSD). The coefficient of variation (CV) of the RMSD was then obtained by dividing the RMSD by the true parameter value. In addition, the CVRMSD was calculated for a null model where genome-average ancestry (calculated as the average of the local ancestry frequencies) was used as the estimate of the ancestry frequency for each locus. Model adequacy was also assessed by determining the frequency with which the true population ancestry frequency was included in the 95% ETPIs and by calculating the correlation between the true and estimated parameter values.
Application to human genetic data
The proposed method was also applied to the Uyghur, which are a human population in Xinjiang, China known to be historically admixed with western Eurasian and Asian ancestry [7, 60]. Published results suggest that admixture in this group occurred approximately 790 (±60) years ago, or about 27 generations ago (assuming 29 years per generation, [7]; an alternative approach suggests admixture occurred 126 generations ago [60]). This places the time since admixture within the realm where the proposed methods should be applicable. Previous results indicate that the population admixture proportion for Uyghur (i.e., genome-average ancestry at the population-level) is 45.2% to 52.5% west Eurasian [7, 60, 61].
The data analyzed here come from the curated version of the Harvard HGDP-CEPH Genotypes for Population Genetics Analyses Supplement 10 that was released with admixtools [7]. Han (32 individuals) and French (26 individuals) were chosen as source populations for Uyghur (as suggested by [7]). The full data set of 621,038 SNPs was filtered to retain only those SNPs that were variable in the source populations with a minor allele frequency greater than 0.1, and to discard tightly linked, redundant SNPs (every third SNP was retained). This left a data set of 116,871 SNPs across the 22 human autosomes, which I analyzed with the proposed CCBPM. Each chromosome was analyzed separately for computational efficiency. Parameter estimates were obtained from two MCMC runs, each consisting of 30,000 iterations, a 10,000 iteration burn-in and a thinning interval of 5. I used a 15 SNP window, which previous results suggest should rarely span ancestry breakpoints (average ancestry block lengths are 2.43 to 4.07 cM [61]).
Application to a mouse hybrid zone
The house mouse species Mus domesticus and M. musculus diverged about 500,000 years ago [62, 63], but now hybridize along a narrow hybrid zone in central Europe that formed a few thousand years ago [64]. Weak assortative mating and reduced hybrid fertility, particularly in males, limit gene flow across the hybrid zone, although the severity of each varies among populations and individuals (e.g., [65–67]). Because this hybrid zone is wide relative to dispersal distance, the hybrid zone consists of numerous admixed populations that differ in their genome composition but exhibit relatively little variation in genome-average ancestry within populations [67, 68]. Given these low dispersal rates (i.e., limited ongoing gene flow), ancestry frequencies as inferred from the proposed CCBPM should be a useful summary of genetic ancestry in these admixed populations. I focused on three admixed populations from the Bavarian transect through this hybrid zone, which differ in the genomic contribution of M. domesticus (as measured by a hybrid index, h): Tütenhausen (TU: , range = [0.18 − 0.36]), Haindlfing (HA: , range = [0.17 − 0.38]), and Neufahrn bei Freising (FS: , range = [0.45 − 0.68]) [16, 67, 69].
The data analyzed here are from captive-bred first generation offspring of wild-caught mice (only mice with both parents from a single locality were included; [69]); this included 21 mice from TU, 32 from HA, and 31 from FS. Smaller sample sizes were available for source (i.e., reference) populations: five M. domesticus from SO and ST (Pelka and Pallhausen) and five M. musculus from GL, RE and RF (Emling, Neufahrn bei Erding, and Finsingermoos) [67, 69]. The genetic data comprised 93,699 SNPs (from the Mouse Diversity Genotyping Array) from the 23 autosomal chromosomes (SNPs with very high LD were not included in the data set [69]). I analyzed each admixed populations separately using the proposed CCBPM. Parameter estimates were obtained from two MCMC runs, each consisting of 30,000 iterations, a 10,000 iteration burn-in and a thinning interval of 5. A window size of 4 SNPs was used because of the age of the hybrid zone and to account for the low parental sample sizes (i.e., to ensure that the sample size exceeded the number of variables for the DA). A key strength of this data set was that it allowed me to contrast patterns of variation in ancestry frequencies across multiple admixed populations, and thus ask about the consistency of these patterns.
Results
Performance on simulated data
Variation in local ancestry increased with time and selection, as expected (because dynamics depend on , variation in ancestry would also scale with population size, but this was kept constant; [21, 44]). Specifically, ancestry frequencies varied most across the genome in the 200 generation simulations, followed by the 50 generation simulations with diffuse and strong selection; gene flow reduced variation in ancestry frequencies (Fig 2). However, the simulated populations were generally segregating for local ancestry across the genome. In particular the mean proportion of genetic loci where ancestry from one source population reached fixation varied from 0.0% (20 and 50 generation simulations without selection or with diffuse selection) to 0.3% (s.d. = 0.2%; 200 generation simulations) or 2.3% (s.d. = 1.5%; 50 generation simulations with strong selection).
Fig 2. Variation in ancestry frequencies.
Boxplots summarize the variance in local ancestry frequencies calculated across 20,002 genetic loci and 10 replicate simulations. This includes 20, 50 and 200 generation simulations without selection, 50 generation simulations with strong or diffuse selection, and 200 generations with low (m = 0.005) or high (m = 0.05) migration.
Accuracy was affected by window size and time since admixture, such that ancestry frequencies were estimated more accurately with larger windows. And there was a greater correlation between true and estimated ancestry frequencies with more time since admixture, but the CVRMSD was lower when admixture occurred more recently (e.g., t = 20, ±4 SNPs, r = 0.62, CVRMSD = 0.16 vs. t = 20, ±20 SNPs, r = 0.77, CVRMSD = 0.10 vs. t = 200, ±20 SNPs, r = 0.89, CVRMSD = 0.20; Figs 3, 4, & 5; Table 1). The HMM in structure performed similarly to or slightly better than the proposed method with a window size of ±4 SNPs, but the proposed CCBPM out-performed this method when windows of ±10 or ±20 SNPs were used, particularly when admixture occurred 200 generations ago (Fig 4; Table 1). Both the proposed CCBPM and the structure HMM were considerably more accurate than the null model of no ancestry frequency variation (Fig 4). Time since admixture and window size also affected coverage of the true parameter value by the 95% ETPIs, with better coverage for more recent admixture and smaller windows (e.g., t = 20, ±4 SNPs, coverage = 64% vs. t = 200, ±4 SNPs, coverage = 52% vs. t = 200, ±20 SNPs, coverage = 41%).
Fig 3. Model evaluation.
Boxplots show the distribution of correlations (Pearson correlation coefficient) between true and inferred ancestry frequencies from each of 10 replicate simulations analyzed with different methods, window sizes (for popanc), generations since admixture, selection regimes, and migration rates.
Fig 4. Model evaluation.

Boxplots show the average CVRMSD for local ancestry frequencies from each of 10 replicate simulations analyzed with different methods, generations since admixture, and window sizes (for popanc). The CVRMSD for a null model that equates the ancestry frequencies at each locus with the genome-average is also shown.
Fig 5. Genetic ancestry estimates from simulated data.
Plots show the true and inferred (posterior mean from popanc) ancestry frequencies from one representative data set from the 20, 50, and 200 generation simulations. Results with a window size of ±20 SNPs are shown. The dashed vertical lines delineate the two distinct chromosomes.
Table 1. Performance of methods on simulated data.
Results shown for local ancestry frequencies estimates in 20, 50, or 200 generation (gens.) simulations with or without selection or ongoing gene flow and analyzed with the proposed method (popanc) or the HMM in structure: Correlation between true and estimated parameter (correlation), normalized RMSD (CVRMSD), and proportion of cases where the true value was included in the 95% ETPIs (95$ ETPI cov.).
| gens. | selection | migration | method | correlation (r) | CVRSMD | 95% ETPI cov. |
|---|---|---|---|---|---|---|
| 20 | none | none | popanc ±4 SNPs | 0.62 | 0.16 | 0.64 |
| 20 | none | none | popanc ±10 SNPs | 0.73 | 0.12 | 0.56 |
| 20 | none | none | popanc ±20 SNPs | 0.77 | 0.10 | 0.60 |
| 20 | none | none | structure HMM | 0.60 | 0.11 | NA |
| 50 | none | none | popanc ±4 SNPs | 0.70 | 0.20 | 0.61 |
| 50 | none | none | popanc ±10 SNPs | 0.83 | 0.13 | 0.51 |
| 50 | none | none | popanc ±20 SNPs | 0.87 | 0.11 | 0.56 |
| 50 | none | none | structure HMM | 0.68 | 0.15 | NA |
| 200 | none | none | popanc ±4 SNPs | 0.74 | 0.33 | 0.52 |
| 200 | none | none | popanc ±10 SNPs | 0.87 | 0.22 | 0.46 |
| 200 | none | none | popanc ±20 SNPs | 0.89 | 0.20 | 0.41 |
| 200 | none | none | structure HMM | 0.72 | 0.29 | NA |
| 50 | diffuse | none | popanc ±4 SNPs | 0.77 | 0.17 | 0.61 |
| 50 | diffuse | none | popanc ±10 SNPs | 0.88 | 0.11 | 0.51 |
| 50 | diffuse | none | popanc ±20 SNPs | 0.91 | 0.10 | 0.56 |
| 50 | diffuse | none | structure HMM | 0.72 | 0.16 | NA |
| 50 | strong | none | popanc ±4 SNPs | 0.83 | 0.21 | 0.60 |
| 50 | strong | none | popanc ±10 SNPs | 0.92 | 0.14 | 0.48 |
| 50 | strong | none | popanc ±20 SNPs | 0.94 | 0.12 | 0.53 |
| 50 | strong | none | structure HMM | 0.76 | 0.23 | NA |
| 200 | none | 0.005 | popanc ±4 SNPs | 0.73 | 0.24 | 0.58 |
| 200 | none | 0.005 | popanc ±10 SNPs | 0.85 | 0.16 | 0.47 |
| 200 | none | 0.005 | popanc ±20 SNPs | 0.87 | 0.15 | 0.48 |
| 200 | none | 0.005 | structure HMM | 0.70 | 0.21 | NA |
| 200 | none | 0.05 | popanc ±4 SNPs | 0.51 | 0.14 | 0.67 |
| 200 | none | 0.05 | popanc ±10 SNPs | 0.62 | 0.11 | 0.61 |
| 200 | none | 0.05 | popanc ±20 SNPs | 0.66 | 0.10 | 0.64 |
| 200 | none | 0.05 | structure HMM | 0.50 | 0.09 | NA |
Results with selection were generally similar, but the improved performance of the proposed CCBPM relative to the linkage HMM in structure was more evident (Figs 6 & 7; Table 1). Once again, more accurate estimates were obtained with larger SNP windows. Parameter estimates were more strongly correlated with their true values when selection was strong (e.g., r: diffuse selection, ±4 SNPs = 0.77, ±20 SNPs = 0.91; strong selection, ±4 SNPs = 0.83, ±20 SNPs = 0.94; Fig 3; Table 1), but the CVRMSD was lower when selection was diffuse (e.g., CVRMSD: diffuse selection, ±4 SNPs = 0.17, ±20 SNPs = 0.097; strong selection, ±4 SNPs = 0.21, ±20 SNPs = 0.12; Fig 6; Table 1). Nearly identical results were obtained when the SNPs nearest to each selected locus were removed from the analysis (performance metrics were indistinguishable from those in Table 1), thus the improved performance of the CCBPM was not driven by these loci but rather by the overall effect of selection on variation in ancestry frequencies across the genome.
Fig 6. Model evaluation with selection.
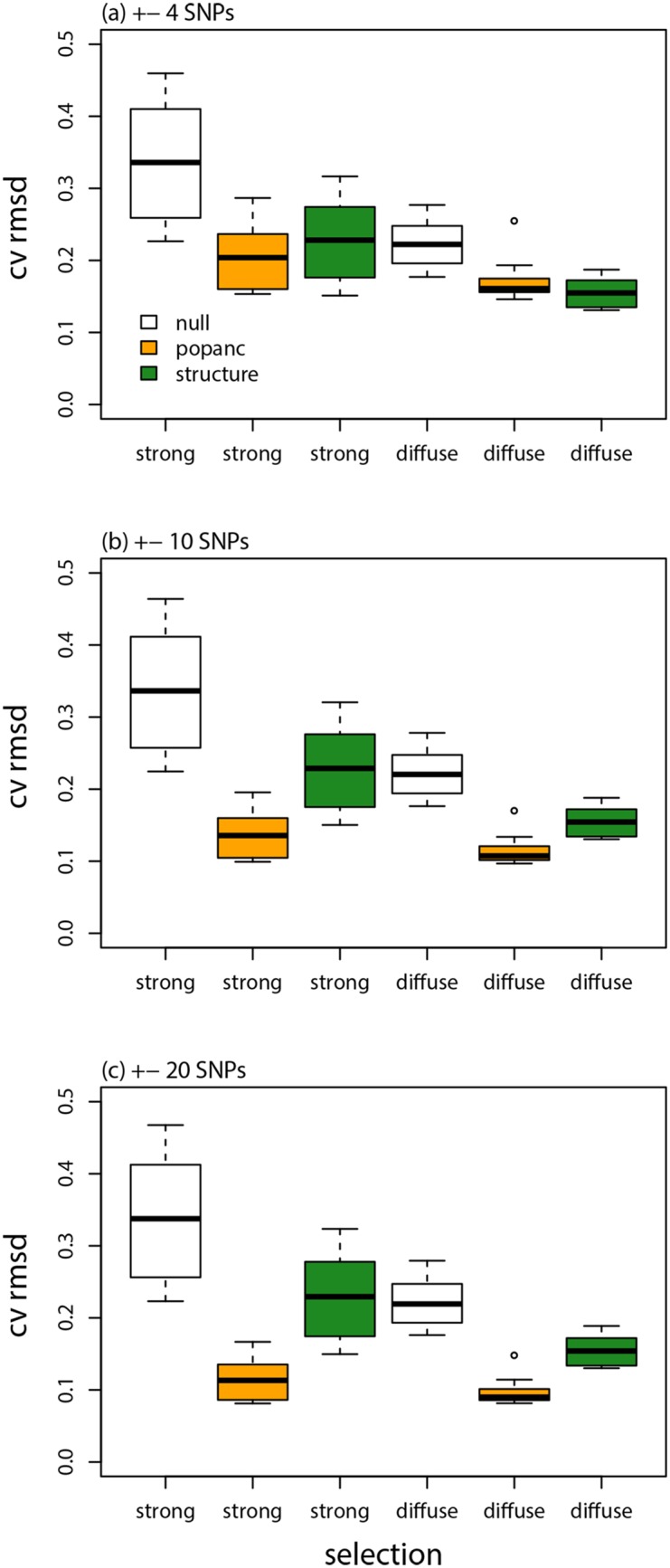
Boxplots show the average CVRMSD for local ancestry frequencies for each of 10 replicate simulations analyzed with different methods, selection regimes, and window sizes (for popanc). The CVRMSD for a null model that equates the ancestry frequencies at each locus with the genome-average is also shown.
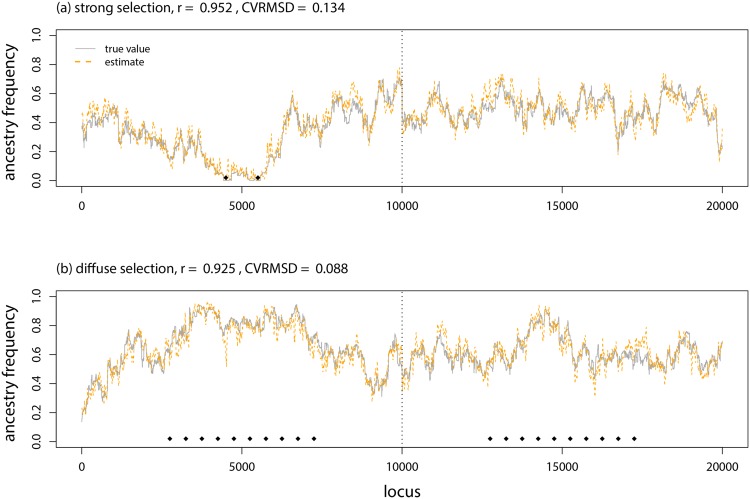
Fig 7. Genetic ancestry estimates from simulated data with selection.
Plots show the true and inferred (posterior mean from popanc) ancestry frequencies from one representative data set from the strong and diffuse selection simulations. Results with a window size of ±20 SNPs are shown. The dashed vertical lines delineate the two distinct chromosomes. Black diamonds indicate the genome positions that affected fitness (i.e., the direct targets of selection against inter-population ancestry).
A low rate of ongoing gene flow after admixture (i.e., m = 0.005) did not noticeably degrade the performance of the CCBPM in general or relative to the linkage HMM in structure (Figs 3, 8, & 9; Table 1). However, parameter estimates were less strongly correlated with their true values when ongoing gene flow occurred at a higher rate (r: ±4 SNPs = 0.51, ±10 SNPs = 0.62, ±20 SNPs = 0.66). But, even under these conditions, ancestry estimates from the CCBPM were more strongly correlated with their true values than the estimates from the HMM, particularly when a window size of ±10 or ±20 was used (Fig 3; Table 1).
Fig 8. Model evaluation with on-going gene flow.
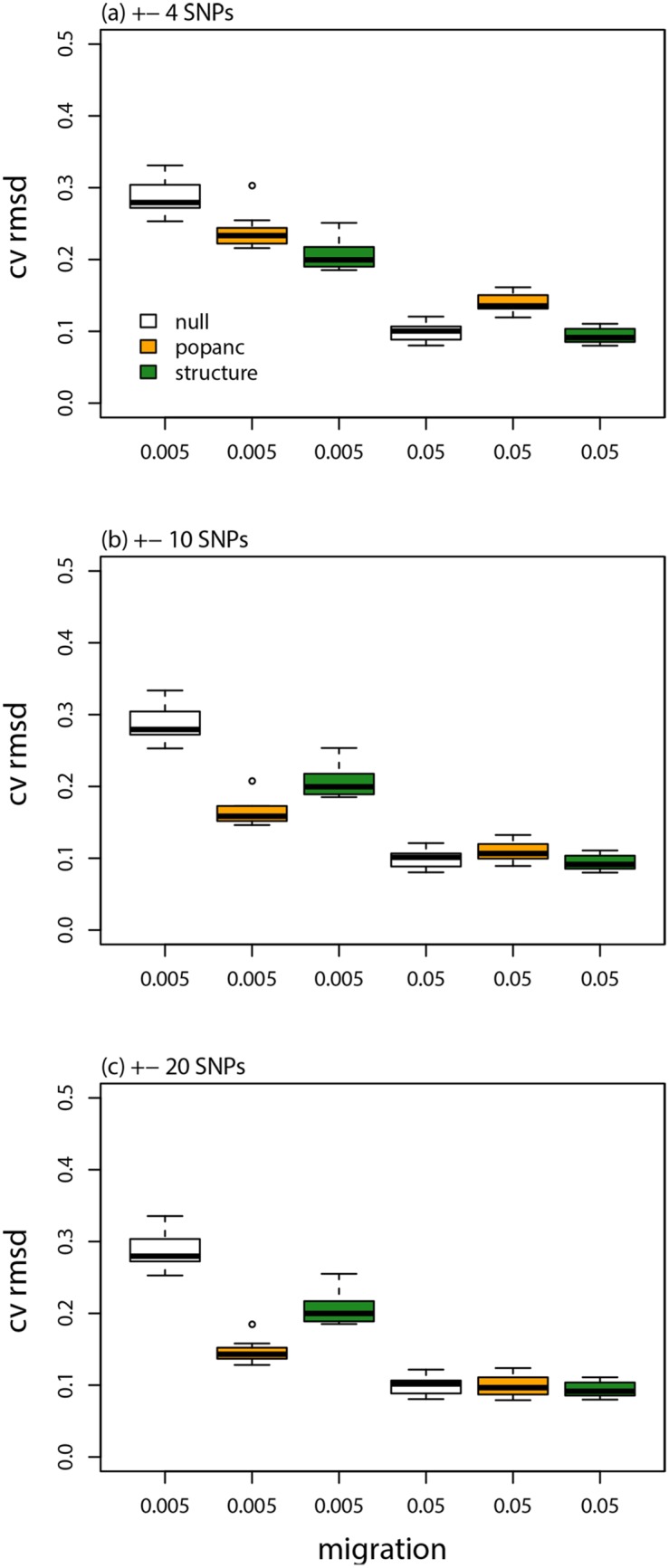
Boxplots show the average CVRMSD for local ancestry frequencies for each of 10 replicate simulations analyzed with different methods, migration rates, and window sizes (for popanc). The CVRMSD for a null model that equates the ancestry frequencies at each locus with the genome-average is also shown.
Fig 9. Genetic ancestry estimates from simulated data with on-going gene flow.
Plots show the true and inferred (posterior mean from popanc) ancestry frequencies from one representative data set from simulations with low or high gene flow. Results with a window size of ±20 SNPs are shown. The dashed vertical lines delineate the two distinct chromosomes.
Admixture in humans
The genome-average ancestry frequencies in the Uyghur population were 52.9% Han and 47.1% French, which is consistent with [7]. Average ancestry frequencies varied modestly among chromosomes, ranging from 47.0% Han on chromosome 18 to 58.6% Han on chromosome 20. Even more variation in ancestry frequencies was observed within chromosomes (average ancestry frequency variance within chromosomes = 0.016, s.d. = 0.003; Fig 10). Indeed, for 55.1% of loci, the chromosome-average ancestry frequency was outside of the local ancestry frequency. Although no regions of fixed ancestry were detected, ancestry frequencies as high as 96.1% Han or 95.6% French were observed.
Fig 10. Genetic ancestry in Uyghur.
Plots show ancestry frequency estimates from three human chromosomes. The posterior median (solid, orange line) and 95% ETPIs (dashed, tan line) for Han ancestry are given. The average ancestry frequency for each chromosome is shown by the dashed, black line.
Genetic loci with the greatest excess or deficit of Han or French ancestry, that is, the 118 SNPs below the 0.05th (9.0% Han) or above the 99.95th (92.7% Han) empirical quantile for ancestry frequencies, were analyzed in more detail. These 118 SNPs formed three contiguous genetic regions with excess Han ancestry (two on chromosome 2 and one on chromosome 10) and four contiguous regions with excess French ancestry (one on chromosome 2 and three on chromosome 11; mean size of region = 408.9 kbp; Table 2). Most of these genetic regions contain one or more known genes. For example, a region of excess French ancestry on chromosome 11 includes bridging integrator 1 (BIN1), and variation in this gene has repeatedly been associated with Alzheimer’s disease [70–72]. We also found evidence consistent with the hypothesis of selection on this gene in the French: FST between French and Han was elevated for the two SNPs in BIN1 relative to the rest of chromosome 2 (FST for BIN1 = 0.23, mean for chromosome 2 = 0.073, randomization test, p = 0.0617), and genetic diversity was significantly reduced in the French population (heterozygosity for BIN1 = 0.00, mean for chromosome 2 = 0.37, randomization test, p < 0.0001).
Table 2. Top excess ancestry regions in the Uyghur.
Chromosome and position (start and end) for seven regions of excess Han or French ancestry in the Uyghur. Genes in these regions were identified using the UCSC Genome Browser on the Human Feb. 2009 (GRCh37/hg19) Assembly.
| chrom. | start (kbp) | end (kbp) | no. SNPs | excess | genes |
|---|---|---|---|---|---|
| 2 | 155508 | 157032 | 43 | Han | KCNJ3 |
| 2 | 182130 | 182175 | 3 | Han | AK125001 |
| 10 | 72176 | 72303 | 13 | Han | EIF4EBP2, NODAL, PALD1 |
| 2 | 127590 | 127875 | 17 | French | BIN1 |
| 11 | 26727 | 27268 | 24 | French | BBOX1, FIBIN, SLC5A12 |
| 11 | 28373 | 28657 | 16 | French | none |
| 11 | 89859 | 89915 | 2 | French | NAALAD2 |
Mouse hybrid zone
Genome-average ancestry frequencies varied among admixed mouse populations from 19.0% (s.d. = 9.5%) and 21.9% (s.d. = 10.4%) M. domesticus in TU and HA to 35.6% (s.d. = 13.7%) M. domesticus in FS (Fig 11). This differed somewhat from, but were correlated with previous estimates of hybrid index which were based on different reference samples and different SNP markers [67]. As with the Uyghur, average ancestry frequencies differed substantially among chromosomes in each admixed population (ranges: TU = 13.8% M. domesticus to 22.5%, HA 18.6% to 26.9%, FS = 25.1% to 45.4%). In each population, some genetic loci were fixed or nearly fixed for M. musculus ancestry (number of genetic loci with M. domesticus ancestry frequencies < 1%: TU = 202, HA = 96, FS = 2), while maximum frequencies of M. domesticus ancestry were high, but always less than one (TU = 84.5%, HA = 76.8%, FS = 92.1%).
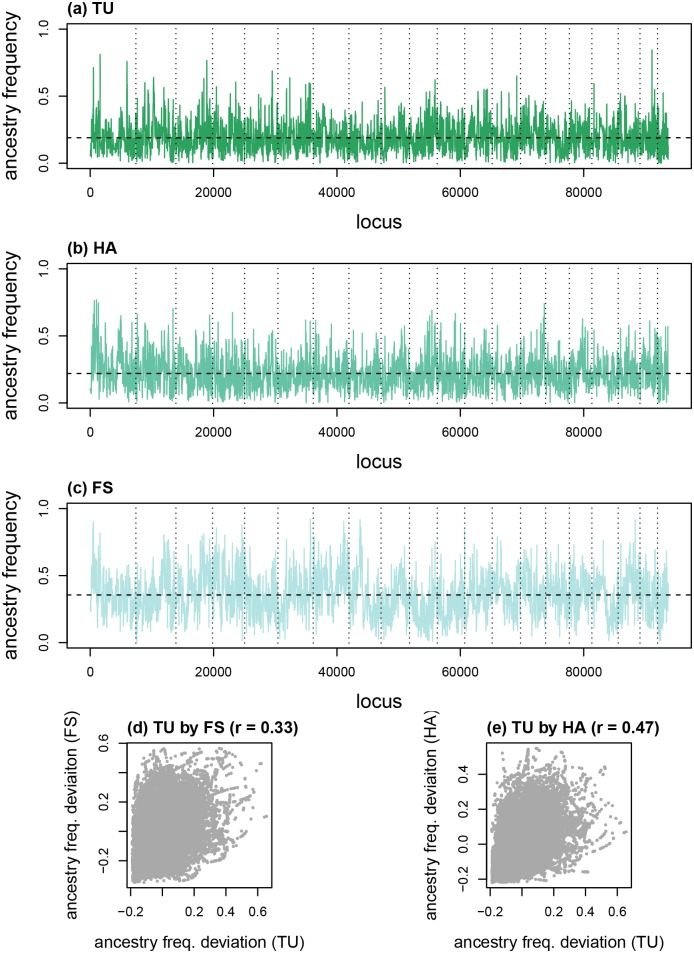
Fig 11. Genetic ancestry in a mouse hybrid zone.
Plots show ancestry frequency estimates from three admixed populations. Solid lines in (a-c) give ancestry frequencies along chromosomes for TU, HA, and FS populations. Vertical dotted lines delineate chromosomes and the average ancestry frequency for each chromosome is shown by the dashed, black line. Scatterplots in (e) and (f) depict correlations in ancestry frequencies (relative to the mean for each population) for pairs of populations.
Ancestry frequencies were correlated between pairs of admixed populations (TU × HA: r = 0.47, p < 0.001; TU × FS: r = 0.33, p < 0.001; HA × FS: r = 0.33, p < 0.001; Fig 11). Moreover, several of the same genetic loci had high M. domesticus ancestry in more than one admixed population. For example, considering the 0.5% of SNPs with the highest M. domesticus frequency in each admixed population (469 SNPs), 173 high M. domesticus ancestry SNPs were shared between two populations and six were shared among all three admixed populations. This is significantly more overlap than would be expected under a null hypothesis of independence among populations (null for two pops.: expected = 7.4, ×-fold enrichment [observed/expected] = 23.3×, p < 0.001; null for all three pops.” expected < 0.01, ×-fold enrichment = 1500×, p < 0.001). However, because of gene flow, strict independence would not be expected anyway. A similar analysis was not conducted for SNPs with excess M. musculus ancestry, because TU and HA had high frequencies of M. musculus ancestry overall and were fixed or nearly fixed for M. musculus ancestry at dozens of SNPs (as described in the previous paragraph). Consistent results were found when considering the 0.05% SNPs with the greatest excess of M. domesticus ancestry (47 SNPs above the 99.95th empirical quantile) in each population (Table 3). While none of these were shared among populations, neighboring regions of excess M. domesticus were detected in the three populations (chromosome 1, 21, 910–22, 026 kb in TU, 20, 0825–21, 726 kb in FS, and 24,848–25, 420 kb in HA). Moreover, regions with the greatest excess M. domesticus ancestry in any one population had elevated M. domesticus ancestry frequencies in the others (randomization test, TU SNPs: mean in other pops. = 0.455, ratio of observed to null expectation = 1.58×, p = 0.002; HA SNPs: mean in other pops. = 0.417, ratio of observed to null expectation = 1.54×, p = 0.012; FS SNPs: mean in other pops. = 0.392, ratio of observed to null expectation = 1.91×, p < 0.001).
Table 3. Top excess ancestry regions in house mice.
Chromosome and position (start and end) for regions with the greatest excess M. domesticus ancestry in each of the three admixed house mouse populations. Only regions comprised of more than one SNP were reported.
| population | chrom. | start (kbp) | end (kbp) | no. SNPs |
|---|---|---|---|---|
| TU | 1 | 21910 | 22026 | 8 |
| TU | 1 | 47056 | 47096 | 3 |
| TU | 1 | 168139 | 168693 | 18 |
| TU | 3 | 139005 | 139401 | 15 |
| TU | 19 | 44236 | 44237 | 2 |
| HA | 1 | 24848 | 25420 | 26 |
| HA | 1 | 35230 | 35298 | 6 |
| HA | 1 | 40639 | 40774 | 9 |
| HA | 2 | 170032 | 170038 | 3 |
| HA | 14 | 120403 | 120433 | 4 |
| FU* | 1 | 20825 | 21726 | 23 |
| FU | 6 | 141892 | 142305 | 10 |
| FU | 7 | 138382 | 138450 | 3 |
| FU | 8 | 46928 | 46988 | 9 |
| FU | 18 | 73871 | 73891 | 2 |
* this range represents a composite of a few nearly contiguous regions.
Discussion
Performance and utility
I have described a new method to infer local ancestry frequencies from un-phased genotypic data using a continuous correlated beta process model (CCBPM). The proposed method produced accurate estimates of ancestry frequencies, outperforming a traditional HMM (and a null model of no variation in ancestry frequencies) under most conditions examined (e.g., Figs 3, 4, 6, & 8, Table 1). As expected, the improved performance of this new approach relative to the HMM in structure was more pronounced when ancestry frequencies varied more across the genome, either because of selection, more ancient admixture, or little to no gene flow post admixture. The poorer performance of the homogeneous HMM under these conditions likely reflects the fact that the HMM makes the a priori assumption that ancestry frequencies do not vary. This should bias local ancestry inference towards the genome-average unless the genetic data are perfectly informative of ancestry. Moreover, the difference in performance between these methods was not trivial; for example, the CVRMSD for ancestry frequencies in the 200 generation simulations with ±20 SNP windows for the CCBPM was only that of the structure HMM. Another key distinction between the approaches is that CCBPM generates measures of uncertainty in the ancestry frequencies (as captured in the approximate posterior distribution), which account for uncertainty in local ancestry within individuals, whereas post hoc estimates of ancestry frequencies from deconvolutions of local ancestry within individuals do not. However, this benefit is lessened by the fact that the 95% ETPIs inferred from the CCBPM appear to routinely underestimate uncertainty in local ancestry (the cause and a potential solution for this are discussed more below; Table 1). Finally, computational methods used for the proposed method are relatively efficient, and thus run times should not be prohibitive for the analysis of large GBS, SNP, or even whole genome sequence data (for the results presented here runs on standard Linux compute nodes took 2-5 hours, and different chromosomes can be analyzed separately).
By applying the proposed method to a case of historical admixture in a human population, I further documented the reliability and utility of the approach. The method generated estimates of genome-average ancestry consistent with previous studies [7], but also showed that ancestry frequencies varied substantially both within and among chromosomes (Fig 10; [60] also reported chromosome-average ancestries which were similar to those obtained with popanc, r = 0.895, p < 0.0001). Ancestry frequency variation in the Uyghur likely reflects the effects of drift and selection, but parsing these effects remains difficult, as drift can have a substantial effect on ancestry frequencies given sufficient time (e.g., Fig 1). Here, comparisons with other admixed human populations could be useful, as selection would be more likely to generate consistent excess ancestry in the same regions of the genome (e.g., [73–75]). Analyzing such variation in ancestry can also be important for uncovering the basis for variation in traits and the prevalence of diseases among different human populations (e.g., [26, 27, 76–79]). Thus, ancestry frequency inference can be viewed as complementary to admixture mapping approaches used in recently admixed populations (e.g., African Americans or Hispanic or Latino populations), which utilize variation in local ancestry blocks and disease risk among individuals within a population (e.g., [26, 80, 81]). Along these lines, here I found a 285 kb region on chromosome 11 in the Uyghur population where greater than 90% of gene copies harbored French ancestry, whereas the genome-average frequency of French ancestry was only 47.1%. This region also contained a gene that has been repeatedly associated with Alzheimer’s risk [70, 71], and showed patterns of genetic differentiation and variation in the source populations consistent with a history of selection in the French. Thus, it is possible that risk for this or a related disease was affected by this gene and varied between the ancestral source populations of the Uyghur, though further work would be required to test this hypothesis.
The proposed CCBPM was also applied to genetic data from three admixed populations that were part of the Bavarian transect through the central European M. domesticus × M. musculus hybrid zone. As with the human data, notable variation in ancestry frequencies was detected within and among chromosomes. Genetic regions with high or very high M. domesticus ancestry frequencies in one population tended to have higher M. domesticus ancestry frequencies in the other populations as well. Often such patterns of consistency or parallelism across replicate populations are interpreted as evidence of selection [14, 73]. While this could be correct here, gene flow among admixed populations could also explain this pattern. Indeed, comparative analyses of distant transects through this hybrid zone have shown that patterns of introgression vary considerably in different parts of this hybrid zone [68]. Of course, gene flow and selection are not mutually exclusive hypotheses. And additional data support the hypothesis that at least one of the excess M. domesticus ancestry regions (chromosome 1, 168, 139–168, 693 kb in TU) was likely affected by selection as it has been associated with a trait (aberrant RNA expression patterns in the testis) that likely contributes to reduced fertility in hybrids [69], and coincides with a marker with a putative epistatic effect on fitness in this hybrid zone [82].
Results from these empirical studies raise an important question: to what extent can genomic variation in ancestry frequencies be interpreted as evidence for past selection? Clearly, selection can drive extreme ancestry frequencies (Fig 7; [39, 44]). In particular, if selection in an admixed population favors a generally beneficial allele that had a higher frequency in one source population, the local ancestry frequency of the chromosomal segment containing that allele should increase. Underdominance and epistasis will have a similar effect, but the favored allele and ancestry type should exhibit positive frequency dependence, and thus, the outcome will depend on the initial conditions (e.g., with underdominance the marginal fitness of the more common ancestry type will be higher because it will occur proportionally less often in heterozygotes; [44]). However, genetic drift, particularly in small or old admixed populations, can also cause substantial variation in ancestry frequencies (Fig 5). The effects of drift and selection can be hard to disentangle, particularly when selection is weak. This could be further confounded by variation in recombination rates, which would cause some genetic regions to be more or less influenced by the indirect effects of selection [83–85]. This will be particularly pronounced in admixed populations, because of admixture linkage disequilibrium. Thus, while it would be possible to develop an explicit test for selection-based patterns of ancestry frequency variation (e.g., [39, 40]), I avoid that here. Rather, genetic regions with extreme ancestry frequencies (relative to the overall variance across the genome) should be viewed as potential regions of interest that are likely enriched for targets of selection, but one cannot assume that all or most of these have in fact experienced substantial selection. Contrasts among independent admixed populations (e.g., [68, 86, 87]) or between admixed and allopatric source populations (e.g., [18, 88]) could be used to further test the hypothesis of selection, but this often assumes selection acts similarly across populations and environments.
Methodological considerations
As demonstrated here, window size affects the accuracy of ancestry inference. In particular, because of drift in admixed populations and shared variation between source populations, information must be extracted from a series of SNPs to obtain accurate estimates of local ancestry and ancestry frequencies (hence the popularity of window-based methods for local ancestry inference, e.g., [34, 55, 89]). Thus, for the simulated data sets analyzed here, ancestry frequencies were better estimated with the larger ±10 and ±20 SNP windows than the ±4 SNP windows (Figs 4 & 6, Table 1). However, very large windows will also lead to errors, because windows will frequently span ancestry breakpoints, and thus will include a mixture of SNPs with different ancestry. Note that window size and the scale parameter from the kernel function are related but distinct. Window size reflects ancestry blocks within individuals, whereas the scale parameter captures autocorrelation in ancestry frequencies at the population-level and is inferred from the data.
A few different approaches could be used to select an appropriate window size. First, if the time since admixture is known, the expected density of ancestry breakpoints and thus size of ancestry blocks can be calculated. For example, if one assumes that recombination operates as a random Poisson process and that half of all recombination events cause ancestry breakpoints (ancestry breakpoints are only generated by recombination between chromosomes with different local ancestry [11]), then the density of ancestry breakpoints for a pair of homologous chromosomes (i.e., in a diploid individual) should be , where Lchrom is the map size of the chromosome in Morgans (M) and t is the time since admixture. Assuming one has genotypes from LSNP SNPs on each chromosome, and that each chromosome is 1 M in length, ancestry blocks should thus contain an average of SNPs. Therefore, window sizes smaller than SNPs should be used. When admixture is very old or the contributions of the source populations differ substantially, this equation will underestimate ancestry block size and thus the approach is conservative (this occurs because recombination is less likely to generate breakpoints when ancestry frequencies are farther from 0.5). Applying this equation to the 20, 50 and 200 generation simulations analyzed here, one would expect average ancestry block sizes of 500 (t = 20), 200 (t = 50), and 50 (t = 200) SNPs. As expected, these numbers slightly underestimate the true block sizes, which were 540.3 (t = 20), 213.0 (t = 50), and 59.4 (t = 200) SNPs. Alternatively, if the time since admixture is not known, one can obtain a reasonable estimate of the size of ancestry blocks by first inferring local ancestry using a HMM, such as the linkage model in structure [32], or one of the other available HMMs (e.g., [33, 36]). Moreover, data sets can be analyzed using a series of window sizes to evaluate the robustness of the results to this parameter. One should then be able to identify a range of reasonable window sizes that give consistent estimates of local ancestry frequencies.
The proposed method uses DA to calculate the likelihood of local ancestry given the genetic data, that is, Pr(sxj|zxj) (Eq 6). However, the proposed CCBPM could be combined with ancestry likelihoods obtained from other discriminant methods, such as the random forest algorithm implemented in RFMix [37]. With that said, DA is rapid and can be used for integer or non-integer valued genetic data. The latter is a particularly nice feature, as this means that posterior mean genotypes, such as those obtained from imputation or low to moderate coverage GBS data [6, 90, 91], can readily be handled by the proposed method with DA. Thus, this is an important feature to ensure that the proposed method can be applied to non-model systems.
A final issue of note is that the approximate 95% ETPIs for local ancestry were consistently too narrow, and thus often failed to contain true parameter value; this was true despite low overall errors and high correlations between parameter values and their estimates (Table 1). This is a predictable outcome of the generalized Bayesian update for the CCBPM specified in Eq (1). By combining information across genetic loci, the CCBPM generates more accurate estimates of ancestry frequencies that account for autocorrelations along chromosomes, but this also unduly narrows the posterior distribution for these parameters. This problem could be circumvented by obtaining point estimates from the CCBPM posterior distribution (i.e., by using Eq (1)), but also sampling from beta(α = zx + α0, β = 2nx − zx + β0) to obtain 95% ETPIs for that parameter. This option is available in the popanc software, and greatly increased the proportion of the time the 95% ETPIs contained the true parameter value for a strong selection data set that I re-analyzed (95% ETIP coverage: CCBPM = 0.48, simple beta-binomial model = 0.78).
Conclusions
Existing ancestry deconvolution methods are best suited for relatively recent or very ancient admixture. In the former case, ancestry frequencies should not vary much across the genome and instead variation in genome-average ancestry or local ancestry-blocks are of interest, whereas in the latter case one can assume that admixed population or species are fixed for ancestry blocks. However, admixed populations between these two extremes exist [6], and should exhibit substantial variation in ancestry frequencies (Fig 1). The method proposed and evaluated in this paper can be used to estimate ancestry frequencies under such conditions. Ancestry frequencies can then be examined to describe the genomic composition of admixed populations and to evaluate progress towards genome stabilization (i.e., the loss of segregating variation for local ancestry). Moreover, comparisons of admixture frequencies across multiple admixed populations could provide critical information on whether hybridization has repeatable, predictable outcomes, and thus on the relative roles of deterministic and stochastic processes in shaping genome composition [92]. Additionally, admixture mapping methods could condition on local ancestry frequencies and thereby gain power to map important trait variation in admixed populations. And finally, ancestry frequencies could help identify regions of the genome that have been targets of selection in hybrids, which is particularly relevant for understanding the genetic basis of reproductive isolation between incipient species [15, 39, 93]. Thus, by filling what has been an analytical gap, the proposed CCBPM should be a useful tool for a variety of biologists.
Acknowledgments
This manuscript was improved by comments from A. Buerkle, C. Nice, and an anonymous reviewer. A. Buerkle provided the program used to simulated the data sets (simadmix, available from http://www.uwyo.edu/buerkle/software/dfuse/). B. Harr kindly provided the data from the mouse hybrid zone. Computing, storage, and other resources from the Division of Research Computing in the Office of Research and Graduate Studies at Utah State University are gratefully acknowledged.
Data Availability
Computer code, compiled binaries, and example files are available from http://sourceforge.net/projects/popanc/.
Funding Statement
The author has no support or funding to report.
References
- 1. Stebbins GL. Variation and Evolution in Plants. New York: Columbia University Press; 1950. [Google Scholar]
- 2. Whittemore AT, Schaal BA. Interspecific gene flow in sympatric oaks. Proceedings of the National Academy of Sciences. 1991;88(6):2540–2544. 10.1073/pnas.88.6.2540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arnold ML. Natural Hybridization and Evolution. Oxford University Press, New York; 1997. [Google Scholar]
- 4. Mallet J. Hybridization as an invasion of the genome. Trends in Ecology and Evolution. 2005. May;20(5):229–237. 10.1016/j.tree.2005.02.010 [DOI] [PubMed] [Google Scholar]
- 5. Hermansen JS, Sæther SA, Elgvin TO, Borge T, Hjelle E, Sætre GP. Hybrid speciation in sparrows I: phenotypic intermediacy, genetic admixture and barriers to gene flow. Molecular Ecology. 2011;20:3812–3822. 10.1111/j.1365-294X.2011.05183.x [DOI] [PubMed] [Google Scholar]
- 6. Gompert Z, Lucas LK, Buerkle CA, Forister ML, Fordyce JA, Nice CC. Admixture and the organization of genetic diversity in a butterfly species complex revealed through common and rare genetic variants. Molecular Ecology. 2014;23(18):4555–4573. 10.1111/mec.12811 [DOI] [PubMed] [Google Scholar]
- 7. Patterson N, Moorjani P, Luo Y, Mallick S, Rohland N, Zhan Y, et al. Ancient Admixture in Human History. Genetics. 2012;192(3):1065–1093. 10.1534/genetics.112.145037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moorjani P, Thangaraj K, Patterson N, Lipson M, Loh PR, Govindaraj P, et al. Genetic evidence for recent population mixture in India. The American Journal of Human Genetics. 2013;93(3):422–438. 10.1016/j.ajhg.2013.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vernot B, Akey JM. Human Evolution: Genomic Gifts from Archaic Hominins. Current Biology. 2014;24(18):R845–R848. 10.1016/j.cub.2014.07.079 [DOI] [PubMed] [Google Scholar]
- 10. Racimo F, Sankararaman S, Nielsen R, Huerta-Sánchez E. Evidence for archaic adaptive introgression in humans. Nature Reviews Genetics. 2015;16(6):359–371. 10.1038/nrg3936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Buerkle CA, Rieseberg LH. The rate of genome stabilization in homoploid hybrid species. Evolution. 2008;62(2):266–275. 10.1111/j.1558-5646.2007.00267.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gompert Z, Buerkle CA. Analyses of genetic ancestry enable key insights for molecular ecology. Molecular Ecology. 2013;22:5278–5294. Available from: 10.1111/mec.12488 10.1111/mec.12488 [DOI] [PubMed] [Google Scholar]
- 13. Barton NH, Hewitt GM. Analysis of hybrid zones. Annual Review of Ecology and Systematics. 1985;16:113–148. 10.1146/annurev.es.16.110185.000553 [DOI] [Google Scholar]
- 14. Buerkle CA, Rieseberg LH. Low intraspecific variation for genomic isolation between hybridizing sunflower species. Evolution. 2001;55:684–691. 10.1111/j.0014-3820.2001.tb00804.x [DOI] [PubMed] [Google Scholar]
- 15. Payseur BA, Krenz JG, Nachman MW. Differential patterns of introgression across the X chromosome in a hybrid zone between two species of house mice. Evolution. 2004;58:2064–2078. 10.1111/j.0014-3820.2004.tb00490.x [DOI] [PubMed] [Google Scholar]
- 16. Teeter KC, Payseur BA, Harris LW, Bakewell MA, Thibodeau LM, O’Brien JE, et al. Genome-wide patterns of gene flow across a house mouse hybrid zone. Genome Research. 2008;18(1):67–76. 10.1101/gr.6757907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dasmahapatra KK, Walters JR, Briscoe AD, Davey JW, Whibley A, Nadeau NJ, et al. Butterfly genome reveals promiscuous exchange of mimicry adaptations among species. Nature. 2012;487:94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gompert Z, Lucas LK, Nice CC, Fordyce JA, Forister ML, Buerkle CA. Genomic regions with a history of divergent selection affect fitness of hybrids between two butterfly species. Evolution. 2012;66(7):2167–2181. 10.1111/j.1558-5646.2012.01587.x [DOI] [PubMed] [Google Scholar]
- 19. Hufford MB, Lubinksy P, Pyhäjärvi T, Devengenzo MT, Ellstrand NC, Ross-Ibarra J. The Genomic Signature of Crop-Wild Introgression in Maize. PLoS Genetics. 2013. May;9(5):e1003477 10.1371/journal.pgen.1003477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chapman NH, Thompson EA. The effect of population history on the lengths of ancestral chromosome segments. Genetics. 2002;162:449–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gravel S. Population Genetics Models of Local Ancestry. Genetics. 2012;191:607–619. 10.1534/genetics.112.139808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liang M, Nielsen R. The lengths of admixture tracts. Genetics. 2014;197(3):953–967. 10.1534/genetics.114.162362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hoggart CJ, Parra EJ, Shriver MD, Bonilla C, Kittles RA, Clayton DG, et al. Control of confounding of genetic associations in stratified populations [Article]. American Journal of Human Genetics. 2003. June;72(6):1492–1504. 10.1086/375613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nature Genetics. 2006;38(8):904–909. 10.1038/ng1847 [DOI] [PubMed] [Google Scholar]
- 26. Shriner D, Adeyemo A, Rotimi CN. Joint Ancestry and Association Testing in Admixed Individuals [Article]. PLoS Computational Biology. 2011. December;7(12):e1002325 10.1371/journal.pcbi.1002325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zaitlen N, Pasaniuc B, Sankararaman S, Bhatia G, Zhang J, Gusev A, et al. Leveraging population admixture to characterize the heritability of complex traits. Nature Genetics. 2014;46(12):1356–1362. 10.1038/ng.3139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Seehausen O, Van Alphen JJ, Witte F. Cichlid fish diversity threatened by eutrophication that curbs sexual selection. Science. 1997;277(5333):1808–1811. 10.1126/science.277.5333.1808 [DOI] [Google Scholar]
- 29. Taylor EB, Boughman JW, Groenenboom M, Sniatynski M, Schluter D, Gow JL. Speciation in reverse: morphological and genetic evidence of the collapse of a three-spined stickleback (Gasterosteus aculeatus) species pair. Molecular Ecology. 2006;15(2):343–355. 10.1111/j.1365-294X.2005.02794.x [DOI] [PubMed] [Google Scholar]
- 30. Fitzpatrick BM, Johnson JR, Kump DK, Smith JJ, Voss SR, Shaffer HB. Rapid spread of invasive genes into a threatened native species [Article]. Proceedings of National Academy of Sciences. 2010. February 23;107(8):3606–3610. 10.1073/pnas.0911802107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gese EM, Knowlton FF, Adams JR, Beck K, Fuller TK, Murray DL, et al. Managing hybridization of a recovering endangered species: The red wolf Canis rufus as a case study. Curr Zool. 2015;61:191–203. 10.1093/czoolo/61.1.191 [DOI] [Google Scholar]
- 32. Falush D, Stephens M, Pritchard JK. Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics. 2003;164:1567–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tang H, Coram M, Wang P, Zhu XF, Risch N. Reconstructing genetic ancestry blocks in admixed individuals [Article]. American Journal of Human Genetics. 2006. July;79(1):1–12. 10.1086/504302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sankararaman S, Sridhar S, Kimmel G, Halperin E. Estimating Local Ancestry in Admixed Populations. American Journal of Human Genetics. 2008;82:290–303. 10.1016/j.ajhg.2007.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Price AL, Tandon A, Patterson N, Barnes KC, Rafaels N, Ruczinski I, et al. Sensitive Detection of Chromosomal Segments of Distinct Ancestry in Admixed Populations. PLoS Genetics. 2009. June;5(6):e1000519 10.1371/journal.pgen.1000519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sohn KA, Ghahramani Z, Xing EP. Robust Estimation of Local Genetic Ancestry in Admixed Populations using a Non-parametric Bayesian Approach. Genetics. 2012;. [DOI] [PMC free article] [PubMed]
- 37. Maples BK, Gravel S, Kenny EE, Bustamante CD. RFMix: A Discriminative Modeling Approach for Rapid and Robust Local-Ancestry Inference. The American Journal of Human Genetics. 2013;93(2):278–288. 10.1016/j.ajhg.2013.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lawson DJ, Hellenthal G, Myers S, Falush D. Inference of Population Structure using Dense Haplotype Data. PLoS Genetics. 2012. January;8(1):e1002453 10.1371/journal.pgen.1002453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gompert Z, Buerkle CA. A powerful regression-based method for admixture mapping of isolation across the genome of hybrids. Molecular Ecology. 2009;18:1207–1224. 10.1111/j.1365-294X.2009.04098.x [DOI] [PubMed] [Google Scholar]
- 40. Gompert Z, Buerkle CA. Bayesian estimation of genomic clines [Article]. Molecular Ecology. 2011. May;20(10):2111–2127. 10.1111/j.1365-294X.2011.05074.x [DOI] [PubMed] [Google Scholar]
- 41. Vaha J, Primmer C. Efficiency of model-based Bayesian methods for detecting hybrid individuals under different hybridization scenarios and with different numbers of loci [Article]. Molecular Ecology. 2006. January;15(1):63–72. 10.1111/j.1365-294X.2005.02773.x [DOI] [PubMed] [Google Scholar]
- 42. Barton NH, Hewitt GM. Adaptation, speciation and hybrid zones. Nature. 1989;341:497–503. 10.1038/341497a0 [DOI] [PubMed] [Google Scholar]
- 43. Rieseberg LH, Whitton J, Gardner K. Hybrid zones and the genetic architecture of a barrier to gene flow between two sunflower species. Genetics. 1999;152:713–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gompert Z, Parchman TL, Buerkle CA. Genomics of isolation in hybrids. Philosophical Transactions of the Royal Society B: Biological Sciences. 2012;367(1587):439–450. 10.1098/rstb.2011.0196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sankararaman S, Mallick S, Dannemann M, Prüfer K, Kelso J, Pääbo S, et al. The genomic landscape of Neanderthal ancestry in present-day humans. Nature. 2014;507(7492):354–357. 10.1038/nature12961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. da Barbiano LA, Gompert Z, Aspbury AS, Gabor CR, Nice CC. Population genomics reveals a possible history of backcrossing and recombination in the gynogenetic fish Poecilia formosa. Proceedings of the National Academy of Sciences. 2013;110(34):13797–13802. 10.1073/pnas.1303730110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rieseberg LH, Raymond O, Rosenthal DM, Lai Z, Livingstone K, Nakazato T, et al. Major ecological transitions in wild sunflowers facilitated by hybridization. Science. 2003;301:1211–1216. 10.1126/science.1086949 [DOI] [PubMed] [Google Scholar]
- 48. Bertorelle G, Excoffier L. Inferring admixture proportions from molecular data. Molecular Biology and Evolution. 1998;15(10):1298–1311. 10.1093/oxfordjournals.molbev.a025858 [DOI] [PubMed] [Google Scholar]
- 49. Nice CC, Gompert Z, Fordyce JA, Forister ML, Lucas LK, Buerkle CA. Hybrid speciation and independent evolution in lineages of alpine butterflies. Evolution. 2013;67(4):1055–1068. 10.1111/evo.12019 [DOI] [PubMed] [Google Scholar]
- 50. Ungerer MC, Baird SJE, Pan J, Rieseberg LH. Rapid hybrid speciation in wild sunflowers. Proceedings of the National Academy of Sciences. 1998;95:11757–11762. 10.1073/pnas.95.20.11757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goetschalckx R, Poupart P, Hoey J. Continuous correlated beta processes. In: IJCAI Proceedings-International Joint Conference on Artificial Intelligence. vol. 22. Citeseer; 2011. p. 1269.
- 52. Elshire RJ, Glaubitz JC, Sun Q, Poland JA, Kawamoto K, Buckler ES, et al. A Robust, Simple Genotyping-by-Sequencing (GBS) Approach for High Diversity Species. PLoS ONE. 2011. May;6(5):e19379 10.1371/journal.pone.0019379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Parchman TL, Benkman CW, Jenkins B, Buerkle CA. Low levels of population genetic structure in Pinus contorta (Pinaceae) across a geographic mosaic of co-evolution. American Journal of Botany. 2011;98(4):669–679. 10.3732/ajb.1000378 [DOI] [PubMed] [Google Scholar]
- 54. Jombart T, Devillard S, Balloux F. Discriminant analysis of principal components: a new method for the analysis of genetically structured populations. BMC Genetics. 2010;11(1):94 Available from: http://www.biomedcentral.com/1471-2156/11/94 10.1186/1471-2156-11-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Paşaniuc B, Sankararaman S, Kimmel G, Halperin E. Inference of locus-specific ancestry in closely related populations. Bioinformatics. 2009;25(12):i213–i221. 10.1093/bioinformatics/btp197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Galassi M, Davies J, Theiler J, Gough B, Jungman G, Booth M, et al. GNU Scientific Library: Reference Manual. Network Theory Ltd.; 2009.
- 57.The HDF5 Group. Hierarchical data format version 5, 2000-2010; 2010. http://www.hdfgroup.org/HDF5
- 58. Nicholson G, Smith AV, Jonsson F, Gustafsson O, Stefansson K, Donnelly P. Assessing population differentiation and isolation from single-nucleotide polymorphism data [Proceedings Paper]. Journal of the Royal Statistical Society Series B-Methodological. 2002;64(Part 4):695–715. 10.1111/1467-9868.00357 [DOI] [Google Scholar]
- 59. Ewens WJ. Mathematical Population Genetics: I Theoretical Introduction. vol. 27 Springer Science & Business Media; 2004. [Google Scholar]
- 60. Xu S, Huang W, Qian J, Jin L. Analysis of genomic admixture in Uyghur and its implication in mapping strategy. The American Journal of Human Genetics. 2008;82(4):883–894. 10.1016/j.ajhg.2008.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Xu S, Jin L. A genome-wide analysis of admixture in Uyghurs and a high-density admixture map for disease-gene discovery. The American Journal of Human Genetics. 2008;83(3):322–336. 10.1016/j.ajhg.2008.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Salcedo T, Geraldes A, Nachman MW. Nucleotide variation in wild and inbred mice. Genetics. 2007;177(4):2277–2291. 10.1534/genetics.107.079988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Geraldes A, Basset P, Gibson B, Smith KL, Harr B, Yu HT, et al. Inferring the history of speciation in house mice from autosomal, X-linked, Y-linked and mitochondrial genes. Molecular Ecology. 2008;17(24):5349–5363. 10.1111/j.1365-294X.2008.04005.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cucchi T, Vigne JD, Auffray JC. First occurrence of the house mouse (Mus musculus domesticus Schwarz & Schwarz, 1943) in the Western Mediterranean: a zooarchaeological revision of subfossil occurrences. Biological Journal of the Linnean Society. 2005;84(3):429–445. 10.1111/j.1095-8312.2005.00445.x [DOI] [Google Scholar]
- 65. Smadja C, Catalan J, Ganem G. Strong premating divergence in a unimodal hybrid zone between two subspecies of the house mouse. Journal of evolutionary biology. 2004;17(1):165–176. 10.1046/j.1420-9101.2003.00647.x [DOI] [PubMed] [Google Scholar]
- 66. Good JM, Dean MD, Nachman MW. A complex genetic basis to X-linked hybrid male sterility between two species of house mice. Genetics. 2008;179(4):2213–2228. 10.1534/genetics.107.085340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Turner LM, Schwahn DJ, Harr B. Reduced male fertility is common but highly variable in form and severity in a natural house mouse hybrid zone. Evolution. 2012;66(2):443–458. 10.1111/j.1558-5646.2011.01445.x [DOI] [PubMed] [Google Scholar]
- 68. Teeter KC, Thibodeau LM, Gompert Z, Buerkle CA, Nachman MW, Tucker PK. The variable genomic architecture of isolation between hybridizing species of house mouse. Evolution. 2010;64(2):472–485. 10.1111/j.1558-5646.2009.00846.x [DOI] [PubMed] [Google Scholar]
- 69. Turner LM, Harr B. Genome-wide mapping in a house mouse hybrid zone reveals hybrid sterility loci and Dobzhansky-Muller interactions. eLife. 2014;3:e02504 10.7554/eLife.02504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hu X, Pickering E, Liu YC, Hall S, Fournier H, Katz E, et al. Meta-analysis for genome-wide association study identifies multiple variants at the BIN1 locus associated with late-onset Alzheimer’s disease. PloS ONE. 2011;6(2):e16616 10.1371/journal.pone.0016616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wijsman EM, Pankratz ND, Choi Y, Rothstein JH, Faber KM, Cheng R, et al. Genome-wide association of familial late-onset Alzheimer’s disease replicates BIN1 and CLU and nominates CUGBP2 in interaction with APOE. PLoS Genetics. 2011;7(2):e1001308 10.1371/journal.pgen.1001308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Pin̄ero J, Queralt-Rosinach N, Bravo A, Deu-Pons J, Bauer-Mehren A, Baron M, et al. DisGeNET: a discovery platform for the dynamical exploration of human diseases and their genes. Database. 2015;2015. Available from: http://database.oxfordjournals.org/content/2015/bav028.abstract 10.1093/database/bav028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Schluter D, Nagel LM. Parallel speciation by natural selection. American Naturalist. 1995;146:292–301. 10.1086/285799 [DOI] [Google Scholar]
- 74. Colosimo PF, Hosemann KE, Balabhadra S, Villarreal G, Dickson M, Grimwood J, et al. Widespread parallel evolution in sticklebacks by repeated fixation of ectodysplasin alleles [Article]. Science. 2005. March 25;307(5717):1928–1933. 10.1126/science.1107239 [DOI] [PubMed] [Google Scholar]
- 75. Soria-Carrasco V, Gompert Z, Comeault AA, Farkas TE, Parchman TL, Johnston JS, et al. Stick Insect Genomes Reveal Natural Selection’s Role in Parallel Speciation. Science. 2014;344(6185):738–742. 10.1126/science.1252136 [DOI] [PubMed] [Google Scholar]
- 76. Freedman ML, Haiman CA, Patterson N, McDonald GJ, Tandon A, Waliszewska A, et al. Admixture mapping identifies 8q24 as a prostate cancer risk locus in African-American men. PNAS. 2006;103:14068–14073. 10.1073/pnas.0605832103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Deo RC, Patterson N, Tandon A, McDonald GJ, Haiman CA, Ardlie K, et al. A High-Density Admixture Scan in 1,670 African Americans with Hypertension. PLoS Genetics. 2007;3:e196 10.1371/journal.pgen.0030196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Nalls MA, Wilson JG, Patterson NJ, Tandon A, Zmuda JM, Huntsman S, et al. Admixture Mapping of White Cell Count: Genetic Locus Responsible for Lower White Blood Cell Count in the Health ABC and Jackson Heart Studies. American Journal of Human Genetics. 2008;82(1):81–87. 10.1016/j.ajhg.2007.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Molineros JE, Maiti AK, Sun C, Looger LL, Han S, Kim-Howard X, et al. Admixture Mapping in Lupus Identifies Multiple Functional Variants within IFIH1 Associated with Apoptosis, Inflammation, and Autoantibody Production. PLoS Genetics. 2013. February;9(2):e1003222 10.1371/journal.pgen.1003222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. McKeigue PM. Prospects for Admixture Mapping of Complex Traits. The American Journal of Human Genetics. 2005;76(1):1–7. 10.1086/426949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Buerkle CA, Lexer C. Admixture as the basis for genetic mapping. Trends in Ecology & Evolution. 2008;23(12):686–694. 10.1016/j.tree.2008.07.008 [DOI] [PubMed] [Google Scholar]
- 82. Janoušek V, Wang L, Luzynski K, Dufková P, Vyskočilová MM, Nachman MW, et al. Genome-wide architecture of reproductive isolation in a naturally occurring hybrid zone between Mus musculus musculus and M. m. domesticus. Molecular Ecology. 2012;21(12):3032–3047. 10.1111/j.1365-294X.2012.05583.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Charlesworth D, Charlesworth B, Morgan MT. The pattern of neutral molecular variation under the background selection model. Genetics. 1995;141:1619–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Charlesworth B. Background selection and patterns of genetic diversity in Drosophila melanogaster. Genetical Research. 1996;68(2):131–150. 10.1017/S0016672300034029 [DOI] [PubMed] [Google Scholar]
- 85. Hahn MW. Toward a selection theory of molecular evolution. Evolution. 2008;62(2):255–265. 10.1111/j.1558-5646.2007.00308.x [DOI] [PubMed] [Google Scholar]
- 86. Nolte AW, Gompert Z, Buerkle CA. Variable patterns of introgression in two sculpin hybrid zones suggest that genomic isolation differs among populations. Molecular Ecology. 2009;18:2615–2627. 10.1111/j.1365-294X.2009.04208.x [DOI] [PubMed] [Google Scholar]
- 87. Dufková P, Macholan M, Pialek J. Inference of selection and stochastic effects in the house mouse hybrid zone. Evolution. 2011;65(4):993–1010. 10.1111/j.1558-5646.2011.01222.x [DOI] [PubMed] [Google Scholar]
- 88. Parchman TL, Gompert Z, Braun MJ, Brumfield RT, McDonald DB, Uy JAC, et al. The genomic consequences of adaptive divergence and reproductive isolation between species of manakins. Molecular Ecology. 2013;22:3304–3317. 10.1111/mec.12201 [DOI] [PubMed] [Google Scholar]
- 89. Baran Y, Pasaniuc B, Sankararaman S, Torgerson DG, Gignoux C, Eng C, et al. Fast and accurate inference of local ancestry in Latino populations. Bioinformatics. 2012;28(10):1359–1367. 10.1093/bioinformatics/bts144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zhou X, Stephens M. Genome-wide efficient mixed-model analysis for association studies. Nature Genetics. 2012;44:821–824. 10.1038/ng.2310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Gompert Z, Jahner JP, Scholl CF, Wilson JS, Lucas LK, Soria-Carrasco V, et al. The evolution of novel host use is unlikely to be constrained by trade-offs or a lack of genetic variation. Molecular Ecology. 2015;24(11):2777–2793. 10.1111/mec.13199 [DOI] [PubMed] [Google Scholar]
- 92. Rieseberg LH, Widmer A, Arntz AM, Burke JM. The genetic architecture necessary for transgressive segregation is common in both natural and domesticated populations. Philosophical Transactions of the Royal Society London B. 2003;358:1141–1147. 10.1098/rstb.2003.1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Barton N, Bengtsson B. The barrier to genetic exchange between hybridizing populations. Heredity. 1986. December;57(3):357–376. 10.1038/hdy.1986.135 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Computer code, compiled binaries, and example files are available from http://sourceforge.net/projects/popanc/.