Abstract
Objectives
The existing risk prediction model for patients requiring prolonged mechanical ventilation is not applicable until after 21 days of mechanical ventilation. We sought to develop and validate a mortality prediction model for patients earlier in the ICU course using data from day 14 of mechanical ventilation.
Study Design
Multi-center retrospective cohort study.
Patients
Adult patients receiving at least 14 days of mechanical ventilation at 5 medical centers (development cohort) or enrolled in the ARDS Network FACTT trial (validation cohort).
Measurements and Main Results
Predictor variables were measured on day 14 of mechanical ventilation in the development cohort and included in a logistic regression model with one-year mortality as the outcome. Variables were sequentially eliminated to develop the ProVent 14 model. This model was then generated in the validation cohort. A simplified prognostic scoring rule (ProVent 14 Score) using categorical variables was created in the development cohort and then tested in the validation cohort. Model discrimination was assessed by the area under the receiver-operator characteristic curve (AUC).
491 patients and 245 patients were included in the development and validation cohorts, respectively. The most parsimonious model included age, platelet count, requirement for vasopressors, requirement for hemodialysis, and non-trauma admission. The AUC for the ProVent 14 model using continuous variables was 0.80 (95% CI, 0.76–0.83) in the development cohort and 0.78 (95% CI, 0.72–0.83) in the validation cohort. The ProVent 14 Score categorized age at 50 and 65 years old, and categorized platelet count at 100 × 109/L, and had similar discrimination as the ProVent 14 model in both cohorts.
Conclusion
Using clinical variables available on day 14 of mechanical ventilation, the ProVent14 model can identify patients receiving prolonged mechanical ventilation with a high risk of mortality within one year.
Keywords: Prolonged mechanical ventilation, outcomes, prognosis, critical care, communication, multiple organ failure, decision making
For an increasing number of patients, critical illness or injury is neither self-limited nor imminently fatal1. As survival from life-threatening illness improves, pre-existing disease and newly-acquired organ dysfunction may conspire against recovery, leaving patients dependent on life supporting therapies for extended time periods. This syndrome, termed “chronic critical illness”, is commonly typified by persistent respiratory failure that requires prolonged mechanical ventilation (PMV). Long-term mortality is high, approaching rates of 40% to 60% at one-year in inclusive cohorts2–10. Patients have a very high symptom burden during the weeks of prolonged ventilation11,12 and chances of living at home with functional independence at the end of the year are as low as 10%9,13.
Most PMV patients are unable to participate in their own clinical decision making and must therefore rely on surrogates to guide their goals of care. These surrogate decision makers report that physicians rarely discuss prognosis, and studies have shown that physician and family estimates of one year outcome are highly discordant14,15. Traditional approaches to ICU mortality prediction, such as APACHE, are ill-suited to predicting longer term outcomes, particularly in this population16. Our research group developed and validated the ProVent mortality prediction model which estimates one-year mortality for patients receiving at least 21 days of mechanical ventilation to address prognostic uncertainty for these unique patients2,3,17.
However, many important decisions regarding the care of PMV patients occur before 21 days have elapsed, including electing to perform tracheotomy18 or transfer care to a long-term acute care hospital (LTACH), both of which in are in some form decisions to continue life sustaining therapies19,20. If compelling prognostic data were available sooner for the patient whose goals of care are known, these decisions might be made more easily and earlier in the course of illness21–23. Building on our previous work, we hypothesized that a simple mortality prediction model constructed from factors clinically available by day 14 in the course of mechanical ventilation could discriminate between PMV patients at high and low risk of death at one year. Using a heterogeneous multicenter cohort of PMV patients in addition to a contemporaneous cohort of patients with acute lung injury (ALI) who were enrolled in a randomized, controlled trial, we sought to develop and validate a new model using data collected on day 14 of mechanical ventilation rather than on day 21 as in our previous model.
METHODS
Development Cohort
Patients were enrolled from 5 tertiary care medical centers in geographically diverse regions of the U.S. (Seattle, WA, Denver, CO, San Francisco, CA, Philadelphia, PA, and Durham, NC). Patients who were discharged in 2005 and received at least 14 days of mechanical ventilation uninterrupted by more than 48 hours were eligible. Patients were excluded if they were less than 18 years old; diagnosed with acute or chronic neuromuscular disease, had extensive burns, or required chronic mechanical ventilation prior to hospital admission. Commitment to continued care with life-sustaining therapies was not an inclusion or exclusion criterion. Consecutive samples of patients were included from 4 centers, and a random sample of 176 patients was included from the largest center. The overall cohort included 260 patients who received 21 days or more of mechanical ventilation with whom we validated our previous model3.
Data on demographic variables, potential risk factors for death, and outcomes were abstracted from medical records by 2 investigators at each site. Risk factors were measured on day 14 of mechanical ventilation. The investigator abstracting data on risk factors was blinded to outcomes. The principal investigator at each site reviewed the first 10 charts and a random sample of 10 additional charts to confirm accuracy of data and identify errors that would prompt review and correction.
Potential risk variables for the model were chosen a priori based upon previous literature, clinical judgment and reliability of measurement. Each variable was measured on day 14 of mechanical ventilation. These included the four primary risk variables from the original ProVent model, including age, platelet count, requirement for vasopressors, and requirement for hemodialysis2,3. Requirement for hemodialysis was defined as renal replacement therapy provided on or within 48 hours before or after day 14 of mechanical ventilation. Additional variables included gender, a primary or secondary diagnosis of trauma, PEEP level, serum glucose, white blood cell count, and hemoglobin. The primary outcome, one year vital status, was obtained from medical records if available, or alternatively the National Death Index and Washington State Death Database. Work on the development cohort was approved by Institutional Review Boards (IRB) at all participating institutions, with waiver of informed consent for this minimal risk observational study.
Validation Cohort
The validation cohort was derived from patients enrolled in the NHLBI ARDS Network Fluids and Catheter Treatment Trial (FACTT)24,25 who consented to be concomitantly enrolled in a prospective economic outcomes study (EA-PAC)26 and who received at least 14 days of mechanical ventilation. Patients were enrolled in FACTT from 40 centers across the U.S. if they met consensus criteria for acute lung injury and were willing to consent to the trial and consent to a central venous catheter. Patients requiring hemodialysis or those having very advanced comorbid diseases such as severe cirrhosis at admission were excluded. While imminent plan to withdraw life support was an exclusion for enrollment in FACTT, there was no encouragement or enforcement of continued commitment to life-sustaining therapies beyond the enrollment window27. A subgroup of patients in FACTT was enrolled in EA-PAC when surrogate decision makers consented to both studies. Detailed characteristics of patients in both studies have been described elsewhere24–26. Overlap between patients in development and validation cohorts was not measurable due to de-identified datasets, however if it occurred at all, it was less than 2% due to differences in study periods and non-consecutive enrollment in the FACTT trial. Day 14 risk variables were extracted from the FACTT database. Missing risk variable data were assumed to be normal. One-year mortality was extracted from the FACTT and EA-PAC databases; all patients in the validation cohort had known vital status at one year.
Informed consent was obtained for participation in FACTT and EA-PAC studies, and IRB approval was obtained at all participating institutions. Additionally, IRB approval was obtained at the University of Washington for use of FACTT/EA-PAC data for this validation cohort, with waiver of additional informed consent.
Analysis
Descriptive statistics are presented using mean and standard deviation for continuous variables with normal distribution or median and interquartile range for variables with non-normal distribution. Categorical variables are presented as proportions. Using the derivation cohort, all of the preselected predictor variables were included in a logistic regression equation with one-year mortality as the outcome variable. The area under the Receiver Operator Characteristic curve (AUC) was used to assess model discrimination. Individual variables were dropped from the model in a stepwise fashion with a subsequent assessment of the AUC. Variables were returned to the model if the AUC changed by more than 0.02 from the original value3. Calibration of the final model was assessed by the Hosmer and Lemeshow goodness of fit statistic, comparing observed mortality to predicted mortality for each decile of risk. A bootstrap method repeating 1000 random samples consisting of 60% of the cohort was used to obtain a 95% confidence interval for the AUC of the final model.
We performed 2 additional steps to develop a points-based clinical scoring rule. Continuous variables from the final model above were categorized and included in a second logistic regression model using categorical variables only. Next, points were assigned to each variable based upon the beta coefficients in that model. To assess the performance of the scoring rule, cumulative scores (ProVent 14 Score) were tabulated for each patient, and a third logistic regression model for one-year mortality was generated using the cumulative score as the independent variable.
Using the validation cohort, we then fit a logistic regression model using the beta values of the risk variables from the derivation scoring rule model. Assessments of model discrimination and calibration for the validation cohort were performed similarly to the development set by calculating the AUC and Hosmer Lemeshow goodness of fit statistics (using observed levels of score). We compared ROC discrimination of the ProVent14 continuous model to APACHE III28 for prediction of one-year mortality using the DeLong method29. We created Kaplan-Meier plots to assess differential survival by ProVent 14 Score in both development and validation cohorts.
Analyses were performed using Stata version 11.0 (Stata, College Station, Texas), MedCalc Statistical Software version 14.10.2 (MedCalc Software, Ostend, Belgium) and SAS/STAT software, Version 9.4 of the SAS System for Windows (Cary, NC, USA).
RESULTS
Model Development
A total of 491 patients were included in the development cohort. Patient characteristics and hospital outcomes are presented in Table 1. The mean age of patients was 54 ± 17 years old, and 40% were female. Median (IQR) duration of mechanical ventilation was 22 (17–33) days. Hospital mortality was 29%, and one-year mortality was 45%. Univariate analysis of associations between predictor variables and one-year mortality are presented in Table 2. The AUC for the logistic regression model with all predictor variables was 0.80. Stepwise elimination of gender, glucose, white blood cell count, hemoglobin, and PEEP level resulted in a final model containing age, platelet count, vasopressors, hemodialysis, and non-trauma diagnosis. Enrollment site was not an independent predictor when added to this model and did not affect the AUC. The AUC for the final parsimonious model was 0.80 (95% CI, 0.76–0.83), and the Hosmer and Lemeshow goodness of fit statistic was 3.32 with 8 degrees of freedom, p=0.91. In contrast, the AUC using APACHE III to predict one-year mortality in this cohort was 0.60 (95% CI, 0.55–0.64), which differed significantly from the ProVent 14 model (p<0.0001).
TABLE 1.
Patient Characteristics and Outcomes
| Variable | Development (n = 491) | Validation (n = 245) | P |
|---|---|---|---|
| Age, yr, mean ± SD | 54±17 | 53±16 | 0.2090 |
| Female sex, n (%) | 185 (40) | 107 (44) | 0.3230 |
| Non white race, n (%) | 182 (37) | 94 (38) | 0.9754 |
| Trauma, n (%) | 118 (24) | 29 (12) | 0.0001 |
| Acute Physiology and Chronic Health Evaluation III score, mean ± SD | 81±28 | 101±28 | < 0.0001 |
| Platelets day 14, mean ± SD | 316±199 | 297±191 | 0.2303 |
| Platelets < 100,000/mL, n (%) | 63 (13) | 32 (13) | 0.9770 |
| Vasopressors/inotropes day 14, n (%) | 78 (16) | 35 (16) | 0.9634 |
| Hemodialysis day 14, n (%) | 51 (10) | 34 (14) | 0.2027 |
| Mechanical ventilation days, median (IQR) | 22 (17–33) | 23 (17–31) | 0.9277 |
| ICU length of stay, median (IQR) | 28 (21–38) | 25 (19–33) | 0.0015 |
| Hospital length of stay, median (IQR) | 38 (27–55) | 34 (26–47) | 0.0102 |
| Hospital mortality, n (%) | 141 (29) | 121 (49) | < 0.0001 |
| 1-yr mortality, n (%) | 221 (45) | 145 (59) | 0.0004 |
IQR = interquartile range.
TABLE 2.
Univariate Analysis of Risk Variables and 1-Year Mortality in Development Cohort
| Variable | Alive, n = 270 | Dead, n = 221 | P |
|---|---|---|---|
| Age, yr | |||
| Mean (SD) | 48.9 (16.9) | 60.9 (14.6) | < 0.0001 |
| Median (IQR) | 49 (38–61) | 63 (52.8–72) | < 0.0001 |
|
| |||
| Gender (male), n (%) | 172 (64.7) | 111 (55.0) | 0.0421 |
|
| |||
| Race (nonwhite),a n (%) | 137 (50.7) | 64 (29.0) | 0.2411 |
|
| |||
| Platelets (day 14), × 109/L | |||
| Mean (SD) | 380 (211) | 238 (150) | < 0.0001 |
| Median (IQR) | 347 (215–507) | 234 (109.3–342) | < 0.0001 |
|
| |||
| Vasopressors (day 14), n (%) | 18 (6.7) | 60 (27.1) | < 0.0001 |
|
| |||
| Hemodialysis (day 14), n (%) | 12 (4.4) | 39 (17.6) | < 0.0001 |
|
| |||
| Nontrauma, n (%) | 174 (64.4) | 199 (90) | < 0.0001 |
|
| |||
| Positive end-expiratory pressure (day 14) | |||
| Mean (SD) | 7.1 (3.5) | 7.2 (3.6) | 0.8572 |
| Median (IQR) | 5 (5–8) | 5 (5–8) | 0.7508 |
|
| |||
| Glucose (day 14), mg/dL | |||
| Mean (SD) | 145 (46) | 167 (64) | < 0.0001 |
| Median (IQR) | 134 (112.5–171.5) | 156 (124–197.5) | 0.0001 |
|
| |||
| WBC (day 14), × 109/L | |||
| Mean (SD) | 14.3 (7.5) | 15.1 (12.3) | 0.4216 |
| Median (IQR) | 12.7 (9.7–17.3) | 12.5 (9.2–18.7) | 0.9188 |
|
| |||
| Hemoglobin (day 14), g/dL | |||
| Mean (SD) | 9.9 (7.3) | 9.5 (2.4) | 0.3610 |
| Median (IQR) | 9.1 (8.2–10.1) | 9.3 (8.6–10) | 0.1685 |
IQR= interquartile range.
Nonwhite category includes race coded as unknown.
Model Validation
Three hundred forty-two patients from FACTT received 14 or more days of mechanical ventilation, of whom 245 had known one-year outcomes through EA-PAC and were included in the validation cohort. Table 1 compares patient characteristics and outcomes between the development and validation cohorts. Patients in the two cohorts were similar in age, gender, and race, and had similar median days of mechanical ventilation. Patients in the validation cohort had higher acute illness severity at hospital admission as represented by APACHE III score, and they had higher hospital and one-year mortality. The AUC for the Provent 14 model in the validation cohort was 0.78 (95% CI 0.72–0.83), and the Hosmer Lemeshow statistic was 9.39, p=0.31. The AUC using APACHE III to predict one-year mortality in the validation cohort was 0.62 (95% CI, 0.55–0.68), which differed significantly from the ProVent 14 model (p<0.0001).
ProVent 14 Score
To develop the simplified prognostic scoring rule, age was cut at 50 and 65 years old as in the original ProVent model3. Platelet count was cut at 100 × 109, which was associated with a higher risk of mortality when measured at day 14 than a cutpoint of 150 × 109 as in the original 21 day model. In the development cohort, a logistic regression model was fit with these categorized variables as independent variables and one-year mortality as the dependent variable. Points assigned to each predictor according to the beta coefficients from the categorical model are shown in Table 3.
TABLE 3.
Model in Development Cohort With Categorized Risk Variables to Derive Simplified Scoring Rule (ProVent 14 Score)
| Categorical Variable | n (%) | OR (95% Cl) | β | Points |
|---|---|---|---|---|
| Age, ≥ 65 yr | 144 (29) | 6.5 (3.8, 11.2) | 1.86 | 2 |
| Age, 50–64 yr | 162 (33) | 2.7 (1.6, 4.4) | 0.95 | 1 |
| Platelets ≤ 100×109/L | 63 (13) | 3.0 (1.5, 6.1) | 1.11 | 1 |
| Vasopressors | 78 (16) | 3.8 (2.0, 72) | 1.32 | 1 |
| Hemodialysis | 51 (10) | 2.5 (1.1, 5.4) | 0.93 | 1 |
| Nontrauma | 373 (76) | 2.6 (1.5, 4.6) | 0.94 | 1 |
OR = odds ratio.
Cumulative points based upon the number of predictor variables present for a patient constitute the ProVent 14 Score. We combined the seven possible scores into five categories: 0, 1, 2, 3 and 4 or greater. Figure 1 contains Kaplan Meier plots with long-term survival by ProVent 14 Score in the development and validation cohorts. ProVent 14 Score models performed well both in the development cohort (AUC 0.78, 95% CI 0.74–82; Hosmer-Lemeshow statistic 9.57, p=0.02) and in the validation cohort (AUC 0.76, 95% CI 0.70–0.81; Hosmer-Lemeshow statistic 1.45, p=0.69).
Figure 1.
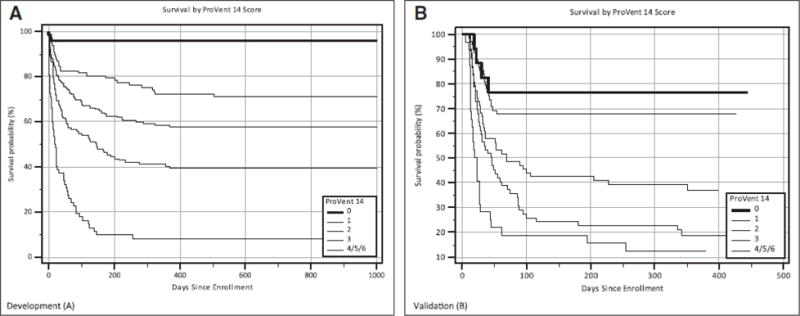
A, Kaplan-Meier plots of survival for development cohort by ProVent 14 Score. B, Kaplan-Meier plots of survival for validation cohort by ProVent 14 Score.
DISCUSSION
In this two-cohort multi-center study, we successfully developed and validated a long-term mortality prediction model for patients requiring 14 days of mechanical ventilation after acute illness or injury. The model performed well for discrimination and calibration in the 5 centers constituting the development cohort, and the model has similar discrimination and calibration in the validation cohort. Calibration was best in the validation cohort for patients who are at highest and lowest risk for death—the patient subgroups groups for whom objective mortality prediction is likely to have the most impact on decision making30.
The good performance of the model in the validation cohort is notable given that the cohort is a distinctly different group of patients derived from 40 hospitals in the U.S. who were enrolled in a clinical trial for patients with ARDS. This compares to the development cohort which enrolled consecutive patients with any diagnosis who received 14 days of mechanical ventilation. The validation cohort had higher overall hospital and one-year mortality, likely reflecting higher acute illness severity associated with the presence of ARDS in all patients. Overall mortality also was increased because vital status in the EA-PAC database was known for all patients who died in the hospital, whereas unknown vital status due to loss to follow up affected hospital survivors only. Other factors differentiating the validation cohort from the development cohort include various exclusion criteria of the FACTT trial such as low expected 6-month survival, morbid obesity, and requirement for hemodialysis at trial enrollment. Despite the overall differences between patients in the 2 cohorts and any variations in management, the mortality prediction model had good performance in the external validation cohort, which supports the broad based value of the model31,32.
Multiple studies have shown that family members of patients in the ICU desire prognostic information, including families of patients requiring PMV, but this information is not always forthcoming33,34. This deficiency results in significant discordance between expectations of clinicians and surrogate decision-makers, with typically overly optimistic expectations by families14. There are numerous barriers to sharing of prognostic information, including uncertainty and worries about sharing incorrect prognoses, concerns about upsetting family members or defeating hope, and available clinician time35,36. Like the MELD score in common use for patients with cirrhosis37, the ProVent 14 model uses a small number of readily available clinical variables that can be collected in minutes at the bedside or remotely from the electronic medical record. Surrogate decision makers for patients with very high likelihood of death can be prepared for that eventuality and prompted to consider the role of ongoing life support in the context of the patient’s values and wishes. For patients at very low risk of long-term mortality, family members and surrogate decision makers can be reassured that their loved one is unlikely to meet the usual grim outcome of the average patient requiring prolonged mechanical ventilation. Even for surrogates of patients with intermediate risk (e.g. 30% to 70% mortality estimate), typically overly optimistic family expectations may be modified14, and families can be better prepared for the potential of poor outcome.
Over twenty years ago, the SUPPORT trial failed to show an impact of providing objective prognostic information to clinicians, perhaps because clinicians did not share the information in 80% of cases38. Yet, when incorporated into formal decision aids, data from prognostic models can facilitate the presentation of objective prognostic information to surrogates, reduce decisional conflict, reduce use of low value health care, and better align choice with values21–23. Preliminary studies incorporating the ProVent model in an innovative decision aid to inform surrogate decision makers of PMV patients indicate that significant discordance in expectations of outcome between clinicians and decision-makers can be minimized39. The ProVent model also differs from SUPPORT in its simplicity and timing, intending to provide information more than a week after the onset of critical illness—a time when surrogates remain uncertain of prognosis and anxious for information. However, objective prognostic information should not replace clinical judgment that incorporates unique patient characteristics, but such models are useful in the clinical setting to support clinical judgment and anchor it in objective data40.
Our model has several limitations. The ProVent 14 model was developed in cohorts of patients requiring PMV at tertiary care research centers and may not be generalizable to other settings. However the majority of patients requiring PMV are managed in tertiary centers which have a high number of patients at risk for PMV and accept transfers of complicated patients from smaller centers. Additionally, some of the hospitals in the validation cohort were smaller community hospitals affiliated with research centers. The development cohort consisted of patients who received PMV in 2005, however there have been no empirical data published in the intervening period that suggests that long-term outcomes of PMV patients have improved in any appreciable way. In this study, variables were chosen for analysis as predictors based upon associations with outcomes in acute or chronic critical illness, ease of measurement, and relatively complete availability in medical records. We deliberately limited the number of variables assessed in order to create a simple, usable model that would stand up to external validation. It is possible that inclusion of additional variables could have improved the discrimination of the model, but this would be a subject for future prospective studies. Finally, the ProVent 14 model predicts one-year mortality, but it does not provide information on long-term physical or cognitive function, which would also be of significant importance in decision making and should be an objective of future research41.
Conclusion
The ProVent 14 mortality prediction model has good discrimination and calibration for one-year mortality in patients who require mechanical ventilation for at least 14 days. This simple prognostic model can support clinical judgment of prognosis and can inform clinical decision aids to facilitate discussions of goals of care in the setting of PMV, where long-term outcomes are patient specific and often not immediately evident.
TABLE 4.
ProVent 14 Score and Observed 1-Year Mortality
| ProVent 14 Score | Development Cohort | Validation Cohort | ||
|---|---|---|---|---|
| n | Observed Mortality, % (95% Cl) | n | Observed Mortality, % (95% Cl) | |
| 0 | 70 | 4 (0, 9) | 17 | 24 (3, 44) |
| 1 | 99 | 28 (19, 37) | 68 | 32 (21, 44) |
| 2 | 142 | 43 (35, 51) | 66 | 62 (50, 74) |
| 3 | 117 | 61 (52, 70) | 62 | 81 (71, 90) |
| 4–6 | 63 | 92 (84, 100) | 32 | 88 (75, 100) |
The PnoVent 14 Score is calculated by summing the point values assigned according to the presence of risk variables listed in Table 3 when measured on day 14 of mechanical ventilation.
Acknowledgments
Financial support: National Institutes of Health (1R21HL094975, 1R01HL109823).
References
- 1.Kahn JM, Le T, Angus DC, et al. The Epidemiology of Chronic Critical Illness in the United States. Crit Care Med. 2014 doi: 10.1097/CCM.0000000000000710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carson SS, Garrett J, Hanson LC, et al. A prognostic model for one-year mortality in patients requiring prolonged mechanical ventilation. Crit Care Med. 2008;36:2061–2069. doi: 10.1097/CCM.0b013e31817b8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carson SS, Kahn JM, Hough CL, et al. A multicenter mortality prediction model for patients receiving prolonged mechanical ventilation. Crit Care Med. 2012;40:1171–1176. doi: 10.1097/CCM.0b013e3182387d43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chelluri L, Im KA, Belle SH, et al. Long-term mortality and quality of life after prolonged mechanical ventilation. Crit Care Med. 2004;32:61–69. doi: 10.1097/01.CCM.0000098029.65347.F9. [DOI] [PubMed] [Google Scholar]
- 5.Combes A, Costa MA, Trouillet JL, et al. Morbidity, mortality, and quality-of-life outcomes of patients requiring >or=14 days of mechanical ventilation. Crit Care Med. 2003;31:1373–1381. doi: 10.1097/01.CCM.0000065188.87029.C3. [DOI] [PubMed] [Google Scholar]
- 6.Cox CE, Carson SS, Lindquist JH, et al. Differences in one-year health outcomes and resource utilization by definition of prolonged mechanical ventilation: a prospective cohort study. Crit Care. 2007;11:R9. doi: 10.1186/cc5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gracey DR, Naessens JM, Krishan I, et al. Hospital and posthospital survival in patients mechanically ventilated for more than 29 days. Chest. 1992;101:211–214. doi: 10.1378/chest.101.1.211. [DOI] [PubMed] [Google Scholar]
- 8.Martin CM, Hill AD, Burns K, et al. Characteristics and outcomes for critically ill patients with prolonged intensive care unit stays. Crit Care Med. 2005;33:1922–1927. doi: 10.1097/01.ccm.0000178184.97813.52. quiz 1936. [DOI] [PubMed] [Google Scholar]
- 9.Unroe M, Kahn JM, Carson SS, et al. One-year trajectories of care and resource utilization for recipients of prolonged mechanical ventilation: a cohort study. Ann Intern Med. 153:167–175. doi: 10.1059/0003-4819-153-3-201008030-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engoren M, Arslanian-Engoren C, Fenn-Buderer N. Hospital and long-term outcome after tracheostomy for respiratory failure. Chest. 2004;125:220–227. doi: 10.1378/chest.125.1.220. [DOI] [PubMed] [Google Scholar]
- 11.Nelson JE, Meier DE, Litke A, et al. The symptom burden of chronic critical illness. Crit Care Med. 2004;32:1527–1534. doi: 10.1097/01.ccm.0000129485.08835.5a. [DOI] [PubMed] [Google Scholar]
- 12.Nelson JE, Tandon N, Mercado AF, et al. Brain dysfunction. Another burden for the chronically critically ill. Archives of Internal Medicine. 2006;166:1993–1999. doi: 10.1001/archinte.166.18.1993. [DOI] [PubMed] [Google Scholar]
- 13.Nasraway SA, Button GJ, Rand WM, et al. Survivors of catastrophic illness: outcome after direct transfer from intensive care to extended care facilities. Crit Care Med. 2000;28:19–25. doi: 10.1097/00003246-200001000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Cox CE, Martinu T, Sathy SJ, et al. Expectations and outcomes of prolonged mechanical ventilation. Crit Care Med. 2009;37:2888–2894. doi: 10.1097/CCM.0b013e3181ab86ed. quiz 2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelson JE, Mercado AF, Camhi SL, et al. Communication about chronic critical illness. Arch Intern Med. 2007;167:2509–2515. doi: 10.1001/archinte.167.22.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carson SS, Bach PB. Predicting mortality in patients suffering from prolonged critical illness: an assessment of four severity-of-illness measures. Chest. 2001;120:928–933. doi: 10.1378/chest.120.3.928. [DOI] [PubMed] [Google Scholar]
- 17.Leroy G, Devos P, Lambiotte F, et al. One-year mortality in patients requiring prolonged mechanical ventilation: multicenter evaluation of the ProVent score. Crit Care. 2014;18:R155. doi: 10.1186/cc13994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freeman BD, Morris PE. Tracheostomy practice in adults with acute respiratory failure. Critical Care Medicine. 2012;40:2890–2896. doi: 10.1097/CCM.0b013e31825bc948. [DOI] [PubMed] [Google Scholar]
- 19.Kahn JM, Benson NM, Appleby D, et al. Long-term acute care hospital utilization after critical illness. JAMA. 2010;303:2253–2259. doi: 10.1001/jama.2010.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kahn JM, Werner RM, Carson SS, et al. Variation in long-term acute care hospital use after intensive care. Medical care research and review: MCRR. 2012;69:339–350. doi: 10.1177/1077558711432889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El-Jawahri A, Mitchell SL, Paasche-Orlow MK, et al. A Randomized Controlled Trial of a CPR and Intubation Video Decision Support Tool for Hospitalized Patients. J Gen Intern Med. 2015 doi: 10.1007/s11606-015-3200-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCannon JB, O’Donnell WJ, Thompson BT, et al. Augmenting communication and decision making in the intensive care unit with a cardiopulmonary resuscitation video decision support tool: a temporal intervention study. J Palliat Med. 2012;15:1382–1387. doi: 10.1089/jpm.2012.0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Butler M, Ratner E, McCreedy E, et al. Decision aids for advance care planning: an overview of the state of the science. Ann Intern Med. 2014;161:408–418. doi: 10.7326/M14-0644. [DOI] [PubMed] [Google Scholar]
- 24.The National Heart, Lung, and Blood Institute ARDS Clinical Trials Network. Pulmonaryartery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med. 2006;354:2213–2224. doi: 10.1056/NEJMoa061895. [DOI] [PubMed] [Google Scholar]
- 25.The National Heart, Lung, and Blood Institute ARDS Clinical Trials Network. Comparison of two fluid-management strategies in acute lung injury. New Engl J Med. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 26.Clermont G, Kong L, Weissfeld LA, et al. The effect of pulmonary artery catheter use on costs and long-term outcomes of acute lung injury. PLoS One. 2011;6:e22512. doi: 10.1371/journal.pone.0022512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 28.Knaus WA, Wagner DP, Draper EA, et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100:1619–1636. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- 29.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 30.Lloyd CB, Nietert PJ, Silvestri GA. Intensive care decision making in the seriously ill and elderly. Crit Care Med. 2004;32:649–654. doi: 10.1097/01.ccm.0000115636.29294.2f. [DOI] [PubMed] [Google Scholar]
- 31.McGinn TG, Guyatt GH, Wyer PC, et al. Users’ guides to the medical literature: XXII: how to use articles about clinical decision rules. Evidence-Based Medicine Working Group. Jama. 2000;284:79–84. doi: 10.1001/jama.284.1.79. [DOI] [PubMed] [Google Scholar]
- 32.Christakis NA. Death Foretold: Prophecy and Prognosis in Medical Care. Chicago, IL: University of Chicago Press; 1999. [Google Scholar]
- 33.Nelson JE, Kinjo K, Meier DE, et al. When critical illness becomes chronic: informational needs of patients and families. J Crit Care. 2005;20:79–89. doi: 10.1016/j.jcrc.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 34.White DB, Engelberg RA, Wenrich MD, et al. Prognostication during physician-family discussions about limiting life support in intensive care units. Crit Care Med. 2007;35:442–448. doi: 10.1097/01.CCM.0000254723.28270.14. [DOI] [PubMed] [Google Scholar]
- 35.Christakis NA, Iwashyna TJ. Attitude and self-reported practice regarding prognostication in a national sample of internists. Arch Intern Med. 1998;158:2389–2395. doi: 10.1001/archinte.158.21.2389. [DOI] [PubMed] [Google Scholar]
- 36.White DB, Evans LR, Bautista CA, et al. Are physicians’ recommendations to limit life support beneficial or burdensome? Bringing empirical data to the debate. Am J Respir Crit Care Med. 2009;180:320–325. doi: 10.1164/rccm.200811-1776OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kamath PS, Kim WR. The model for end-stage liver disease (MELD) Hepatology. 2007;45:797–805. doi: 10.1002/hep.21563. [DOI] [PubMed] [Google Scholar]
- 38.A controlled trial to improve care for seriously ill hospitalized patients. The study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT). The SUPPORT Principal Investigators. JAMA. 1995;274:1591–1598. [PubMed] [Google Scholar]
- 39.Cox CE, Lewis CL, Hanson LC, et al. Development and pilot testing of a decision aid for surrogates of patients with prolonged mechanical ventilation. Crit Care Med. 2012;40:2327–2334. doi: 10.1097/CCM.0b013e3182536a63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lynn J, Teno JM, Harrell FE., Jr Accurate prognostications of death. Opportunities and challenges for clinicians. West J Med. 1995;163:250–257. [PMC free article] [PubMed] [Google Scholar]
- 41.Turnbull AE, Krall JR, Ruhl AP, et al. A scenario-based, randomized trial of patient values and functional prognosis on intensivist intent to discuss withdrawing life support. Crit Care Med. 2014;42:1455–1462. doi: 10.1097/CCM.0000000000000227. [DOI] [PMC free article] [PubMed] [Google Scholar]


