Introduction
It has long been recognized that B cells with Regulatory function (BREGs) play an important role in human diseases. However, the majority of the studies on BREGs have been conducted in murine models. The studies of BREGs in human disease are limited but of great importance since they provide valuable insights into the potential B cell-directed therapeutics in humans.
A number of reports described human B cells that produce IL-10, [1-3] as potential BREGs and findings suggest that these human BREGs are potent generators of regulatory T cells (Treg) [4, 5]. Spontaneous IL-10 production by blood mononuclear cells is dramatically higher in untreated autoimmune disease patients than in healthy controls [6]. Yet, current knowledge regarding human B-cell production of IL-10 provides diverse results that are difficult to unify in a coherent model.
IL-10 producing B cells were diffusely scattered throughout the B-cell lineage and not restricted to either the immature transitional B-cell compartment (CD24hiCD38hi) or the memory B-cell compartment (IgD+CD27+). These B cells, obtained from human peripheral blood or spleen, were induced by stimulation with oligonucleotides that contained CpG motifs and anti-Ig and that these cells inhibited T-cell proliferation in an IL-10–dependent manner. However, it is still unclear to what extent IL-10-producing B cells express other cytokines and how this may affect their BREG cell functionality [7].
Despite the reports that B cells in humans have functions analogous to those of BREGs described in various experimental mouse models of disease [8, 9], it remains unclear whether human BREGs exist naturally or they need to be induced following any immune stimulation.
This review is to summarize the current knowledge about the phenotypic markers of human BREGs and their function in autoimmune- and non-autoimmune diseases.
Phenotypic Markers of human BREGs
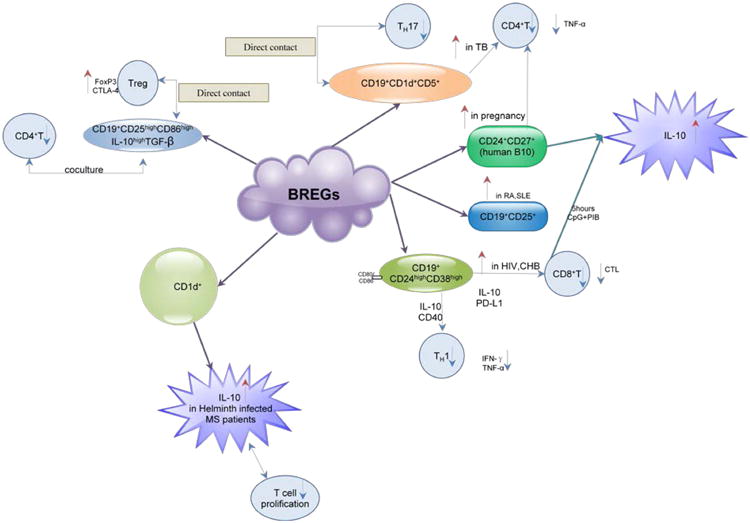
CD19+-CD25+B cells are the first subset of human B cells previously suggested to have a regulatory role. They were characterized as a phenotypically and functionally distinct subset by expressing high levels of immunoglobulin's compared with CD19+-.
CD25- B cells, while they lacked the ability to secrete them. CD27 and CD80 which have previously been shown to enhance antigen-presenting cell function are strongly expressed on this B-cell subpopulation. They could present antigen more efficiently to allogeneic CD4+T cells in Mixed Lymphocyte Reaction (MLR) compared with CD25−B cells, a function that was attributed to the high expression level of CD25. Finally the differential regulation of CD25 expression via selective TLR ligands suggests a role for CD25+B cells in bridging innate and acquired immune responses [10].
In addition, CD25+B cells secreted significantly higher levels of inhibitory cytokine IL-10 versus CD25- B cells. In contrast, TGF-β1 secretion was similar between the CD25+ and CD25+sub-populations [11]. CD25+B cells were found to display a high activated and mature phenotype in RA and SLE patients compared to healthy controls. This subset was sensitive to IL-2 which was found to affect auto-reactive responses and suggested to have a regulatory role in autoimmune diseases. CD19+CD25 high B cells were reported to be significantly higher in patients suffering from ANCA-related vasculitis in remission than in active patients [12]. However, the functional properties of this subset of B cells remained obscure.
CD19+CD24hiCD38hi (so-called immature transitional B cells) was identified by Blair and coworkers in the peripheral blood of healthy individuals as another human regulatory B-cell subset. The CD24hiCD38hiB-cell population was capable of suppressing IFN-γ and TNF- α secretion by anti-CD3–stimulated T helper cells, and this suppression was dependent on IL-10 and CD80/CD86 co-stimulation. This subset is able to suppress the differentiation of Th1 effector cells in a CD40 dependent way. In addition, it was also shown that their suppressive capacity was IL-10, but not TGF-β dependent. When analyzed in SLE patients, these cells were functionally impaired as incapable of suppressing Th1 cells compared to their ability in healthy individuals [8, 11].
CD19+CD25highCD86high IL-10high TGF-βhigh cells were defined by Kessel as “Breg cells”.
B cells were purified from human Peripheral Blood Mononuclear Cells (PBMCs) of healthy individuals and were stained with fluorescent mAbs to CD19, CD25, CD86 and mAbs to IL-10 and TGF-β. Gaiting on IL-10high expressing cells, phenotypic analyzes revealed that IL-10 high expressing cells were also CD25high, CD86high, and TGF-βhigh. The co-culture of these Breg cells with autologous stimulated CD4+T cells decreased significantly (in a dose-dependent way) the proliferative capacity of CD4+T cells. Furthermore, Foxp3 and CTLA-4 expression in Treg cells were enhanced by non-stimulated and further by ODN-CD40L stimulated Breg cells. The regulatory function of Breg cells on Treg cells was mainly dependent on a direct contact between Breg and Treg cells, but was also TGF-β but not IL-10 dependent. In conclusion, human Breg cells decrease the proliferation of CD4+T cells and also enhance the expression of Foxp3 and CTLA-4 in Treg cells by cell-to-cell contact [13].
CD24+CD27+ B cells were characterized as a rare IL-10–competent B-cell subset in humans that parallels mouse regulatory B10 cells by Iwata and named it as “human B10 and progenitor B10 cells”. B10 cells were functionally identified by their ability to express cytoplasmic IL-10 after 5 hours of ex vivo stimulation, whereas progenitor B10 (B10pro) cells required 48 hours of in vitro stimulation before they acquired the ability to express IL-10 [14].
CD19+ CD1d+ CD5+ B cell were found in the patients with tuberculosis with stronger suppressive activity than such cells from healthy donors. Furthermore, the frequency of CD19+CD1d+CD5+ B cells in peripheral blood was inversely correlated with that of Th17 cells in patients with tuberculosis [15].
CD1d+ B cells were reported by Correale and coworkers that this subset B cells were present in the peripheral blood of helminth-infected patients with Multiple Sclerosis (MS), producing high levels of IL-10 in response to CD40 ligation. B cells from healthy controls and helminth-infected patients with MS, were able to suppress T-cell proliferation in an IL-10-dependent manner in vitro while uninfected patients with MS didn't have this suppressive function [9].
BREGs in Autoimmune Diseases
So far, most of the data on role of human BREGs in autoimmune diseases have been obtained from patients with Systemic Lupus Erythematosus (SLE). Some research studies on BREGs have also been conducted in patients with Rheumatoid Arthritis (RA), Primary Sjögren's syndrome, Autoimmune bullous diseases, and Multiple sclerosis.
SLE
Systemic Lupus Erythematosus (SLE) is a chronic autoimmune disease whose hallmark is B cell hyperactivity with autoantibody production [16]. Cytoplasmic IL-10 productions of B cells were tested from SLE patients and normal controls by flow cytometry. SLE B cells spontaneously produced more cytoplasmic IL-10 (1.1%) than controls (0.6%), while more SLE B cells (1.3%) and controls (1.5%) expressed IL-10 after P+I stimulation. LPS stimulation could not increase the frequencies of intracellular IL-10-producing B cells in comparison with unstimulated cells. Because of the inherent difficulties associated with the analysis of such rare cells, rigorously examined and further verified is still needed although stimulation of CD5− B cells from normal controls induced higher levels of IL-10 production than stimulation of CD5+ cells from normal controls [17,18].
In another experiment, Surface CD154 and intracytoplasmic IL-10 expression were quantified with flow cytometry of the blood from 11 SLE patients and 10 healthy volunteers. The results showed that IL-10 production was intimately linked to CD154 expression in B cells, and that the IL-10+CD154+ B cell subset increases abnormally when SLE-derived cells are stimulated with CSS (Cowan I Strain Staphylococcus) [16].
A human study focused on characterizing the phenotype of regulatory B cells conducted on the peripheral blood of healthy subjects along with patients with a confirmed diagnosis of Systemic Lupus Erythematosus (SLE), Sjogern syndrome, and Vesiculobullous skin disease. The authors in this study identified CD24hiCD27+ as IL-10–competence human B10 and progenitor B10 (B10pro) cells. Mean B10 B10pro-cell frequencies were also significantly higher in patients with autoimmune disease compared with healthy controls [14]. However the functional abilities of human B10 cells from autoimmune patients compared with those from healthy controls was not measured in this study with respect to their capacity to reduce T-cell cytokine responses or whether other cytokines were simultaneously expressed in B10 cells from the patients.
BREGs in Non-Autoimmune Diseases
BREGs in Tuberculosis
Studies from different groups showed controversial results over the frequency of B cells in peripheral blood of the patients with tuberculosis [19, 20]. Nevertheless, recent studies on human tuberculous lung tissue demonstrated that B cells were one of the major components of tuberculous granulomas which suggested that the cross-talk between B and T cells is critical for containment of Mtb (Mycobacterium tuberculosis). In a human study, Zhang found that primary CD19+B cells isolated from patients with tuberculosis significantly inhibited Th17, but not Th1, cell activation. Moreover, the suppressive activity was mediated by a CD19+CD1d+CD5+ B cell population. Notably, patients with tuberculosis were found to have significantly higher frequencies of CD19+ CD1d+ CD5+ B cells with stronger suppressive activity than such cells from healthy donors. Furthermore, the frequency of CD19+CD1d+CD5+ B cells in peripheral blood was inversely correlated with that of Th17 cells in patients with tuberculosis. This finding that B cells negatively regulate Th17 responses provides a novel mechanism in the regulation of CD4+T cell responses aside from regulatory T cells during M. tuberculosis infection, which may impact the clinical outcome of tuberculosis [15].
BREGs in HIV
HIV infection is associated with elevated expression of IL-10 and PD-L1, contributing to impairment of T cell effector functions [21]. IL-10 expression is up-regulated in multiple cell types during HIV infection [22], but animal model studies indicate that B cells are a key source of IL-10 [23], and these IL-10-expressing B cells are enriched in the BREG (CD19+CD24hiCD38hi) subset [24, 25]. Siewe present the first report on B cell-mediated regulation of T cell function during HIV infection. It is indicated that BREGs contribute to HIV-infection associated immune dysfunction by T cell impairment, via IL-10 and possibly PD-L1 expression [21]. In vitro BREGs depletion leads to enhanced HIV-specific, CD8+T cell CTL activity.
BREGs in Adipose Tissue Inflammation
Recent studies have shown that obesity induces chronic inflammation within adipose tissue, which leads to metabolic abnormalities and inflammation in distant tissues. Macrophages and T cells play crucial roles in adipose inflammation [26]. By far, the mechanisms by which the immune cell network controls adipose tissue inflammation are poorly understood. Nishimura et al., describe a subset of BREG cells that are abundant within adipose tissue and constitutively produce IL-10, with which they restrain adipose tissue inflammation and maintain metabolic homeostasis in mice model. Adipose IL-10+B cells from epididymal and s.c. adipose tissue are CD1dlowCD5-/lowCD11blowCD21/CD35lowCD23-/lowCD25+CD69+CD72highCD185-CD196+IgM+IgD+, which is distinct from any other known IL-10 producing B cells. Of note, the B cell numbers and IL-10 expression in adipose tissues of obese humans were reduced [27].
BREGs in Malignancy
Chronic Lymphocytic Leukemia (CLL) is the most common adult leukemia in North America and Europe. It is characterized by the monoclonal expansion of small, mature, CD5+CD23+CD19+B lymphocytes [28]. DJ DiLillo reported that CLL can be immunosuppressive in humans and mice, and CLL cells share multiple phenotypic markers with regulatory B cells that are competent to produce interleukin (IL)-10 (B10 cells). To identify functional links between CLL cells and regulatory B10 cells, the phenotypes and abilities of leukemia cells from 93 patients with overt CLL to express IL-10 were assessed. CD5- CLL cells purified from 90% of the patients were IL-10-competent and secreted IL-10 following appropriate ex vivo stimulation. Serum IL-10 levels were also significantly elevated in CLL patients. IL-10-competent cell frequencies were higher among CLLs with IgV H mutations, and correlated positively with TCL1 expression. Malignant CLL cells in TCL1-Tg mice also shared immunoregulatory functions with mouse and human B10 cells. Serum IL-10 levels varied in TCL1-Tg mice, but in vivo low-dose lipopolysaccharide treatment induced IL-10 expression in CLL cells and high levels of serum IL-10. Thus, malignant IL-10-competent CLL cells exhibit regulator functions comparable to normal B10 cells that may contribute to the immunosuppression observed in patients and TCL1-Tg mice [24].
BREGs in Sickle cell disease
Transfusion therapy is a life-sustaining treatment for patients with Sickle Cell Disease (SCD), but can cause serious complications including alloimmunization. Phenotypically, no differences in the frequency or numbers of CD24hiCD38hi and CD24hiCD27+ B cell subsets, both previously identified as human BREGs, between alloimmunized and non-alloimmunized SCD patients on regular transfusions. However, CD19+ B cells from alloimmunized SCD patients expressed lower levels of IL-10 following stimulation as compared with non-alloimmunized patients (P < 0.05), and had reduced ability in inhibiting autologous CD141 monocyte TNF-α expression (P<0.05). These findings suggest that BREGs from alloimmunized and non-alloimmunized SCD patients differ in their ability to produce IL-10 and dampen monocyte activation, all consistent with an altered immunoregulatory state in alloimmunized SCD patients [29].
BREG in Helminths
Epidemiological studies showing that in human populations with high rates of parasitic infections, particularly helminth infections, the prevalence of allergic diseases is considerably lower. Chronic helminth infections are often associated with a reduced prevalence of inflammatory disorders, including allergic diseases. Helminths influence the host immune system by down regulating T-cell responses; the cytokine IL-10 appears to play a central role in this process. Helminths appear to be potent inducers of Breg cells [30]. Although it might not necessarily be derived from the B cells alone, it might be of relevance to note that schistosome-infected Gabonese school children have fewer allergic responses and elevated IL-10 levels [31]. There is an emerging trend of immunosuppression during chronic infections with particular parasites despite the relative paucity of data. Via the release of IL-10, and the effect that the humoral immune response and FcR interactions BREGs might play an important role in controlling inflammatory disease induced by CD4+T cells [32].
BREG in Transplantation
Tolerance is the ideal immunological state of transplanted patients, which is defined as a well-functioning transplant in the absence of exogenous immunosuppression [33]. The transplant Recipients who can be rapidly weaned off immunosuppressant's exhibited higher levels of B cell activation compared to those who required continued immunosuppressant therapy [34]. Statistics show that approximately 20% of liver transplant recipients achieve spontaneous operational tolerance [35]. In the peripheral blood of tolerant recipients elevated immunosuppressive BREGs cells and regulatory T cells (Tregs) can be tested [36]. Despite the immunosuppressive effect of BREGs, a recent study demonstrated that human BREGs cells promote Tregs expansion in vivo [37].
BREGs in Hepatitis
IL-10 is elevated in patients with chronic hepatitis B virus infection (CHB). IL-10 levels were studied longitudinally in patients with CHB undergoing spontaneous disease flares. Results showed that IL-10 producing B cells were enriched in patients, and their frequency correlated temporally with hepatic flares, both after stimulation and directly ex vivo. these data reveal a novel IL-10 producing subset of B cells (CD19+CD24hiCD38hi) able to regulate T cell immunity in CHB [23].
BREGs in Early Human Pregnancy
CD19+CD24hiCD27+B cell were found to be significant higher in normal pregnant
When compared to non-pregnant women. B cells from non-pregnant women cultured with serum from normal pregnant women produced higher IL-10 levels than those cultured with serum from spontaneous abortion patients or autologous serum. CD19+- activated B cells from pregnant women strongly suppressed TNF-α production by CD4+T cells when co-cultured. BREGs play important role in pregnancy which suppress undesired immune responses from maternal T cells and are therefore important for tolerance acquisition [38].
Mechanisms of BREGs function
It remains unknown whether IL-10-producing B cells affect immune responses centrally or depend on regulatory B cells' migration into specific tissues. B-cell infiltration is not observed in the challenged ears of wild type mice during CHS responses [39]. Furthermore, IL-10 transcripts are not significantly increased in B cells from lymph nodes draining Ag challenge sites. Thus, BREGs may predominantly regulate inflammatory responses centrally rather than at the site of inflammation [40].
BREG cells can induce suppression by several effector mechanisms and by targeting different cell subsets [41], as discussed in the following subsections.
(1) IL-10 production
IL-10 is the primary mechanism by which B cells modulate other immune cells with its strongest inhibitory effect on the immune response. It has anti-inflammatory and suppressive effects on most hematopoietic cells. IL-10 was initially described as a cytokine associated with Th2 cells that inhibits Th1 cytokine production and IL-10 can inhibits the differentiation of Th0 cell to Th1 and Th2 cells [42-44]. IL-10 suppresses pro-inflammatory cytokine production by monocytes and macrophages [45]. IL-10 inhibit the Ag-presenting capacity and decreasing costimulatory moleculory molecule expression by professional Ag-presenting cells, including DCs, macrophages, Langerhan's cells, and B cells to suppresses the proliferation of Ag-specific CD4+ T cells [46]. IL-10 may generally play suppressive roles in both inflammatory and autoimmune responses [40]. Impairment of secretion/production of IL-10 by deficiencies of TLR, deficiency of MyD88, and the specificity of the BCR, there appears to be a complex relationship between the innate and adaptive immune systems in the function of Breg [47]. The discovery of cell surface markers associated with IL-10-producing B cells (B10:CD19+CD5+CD1dhi), (T2-MZP: CD23+CD21+CD1dhi) has allowed the enrichment of BREGs and permitted a more rigorous study of B cell-mediated suppression. IL-10–induced suppression of inflammation was found in some autoimmunity models, such as EAE [48, 49], lupus [50], or arthritis [51, 52] by modulating TH cell proliferation and reducing IFN-γ, IL-2, IL-17, or TNF- α levels. Different allergy models [53, 54] clearly indicated that schistosome-induced B cells can also inhibit ovalbumin-specific TH2 cytokine responses in an IL-10– dependent manner, resulting in reduced allergic symptoms. IL-10–independent down-regulation of TH 2 responses has also been reported by B cells from H polygyrus–infected mice [55], suggesting the involvement of cell-cell interaction or other soluble mediators.
(2) Induction of regulatory T cell populations
The concept that B cells can induce Treg cells was first reported by Ashour and Niederkorn in a model of anterior chamber-associated immune deviation [56]. Some other examples can be found in autoimmunity models, such as lupus [57], where IL-10 producing B cells reduced inflammation by the induction of Treg cells.
Similar results were shown in a model of collagen-induced arthritis, where adoptive transfer of IL-10 producing regulatory B cells induced FoxP3+ Treg cells, resulting in reduced TH1 and TH17 frequencies and decreased inflammation [58].
In a model for EAE, B-cell–deficient mice displayed delayed emergence of Foxp3+ and IL-10+T cells in the central nervous system, which was corrected by reconstitution with B cells and resulted in recovery from disease, normalized IL-10 and Foxp3 expression [59]. Of note, BREG and Treg cell numbers appear to peak at different disease stages in EAE, while BREG cell activity enhanced during early EAE initiation and Treg cells providing protection during late-phase EAE [60]. Therefore, BREG cells and Treg cells may have partly independent roles in controlling inflammation even though BREG cells may induce Treg cells [41].
(3) BREG cells can suppress T-cell proliferation and/or T-cell cytokine production
TH1 cells or TH2 cytokine responses can be suppressed by B cells in an IL-10–dependent manner [50, 51, 60]. This suppressive effect was potentiated via CD40 ligation.
(4) BREG cells can inhibit DC antigen processing and presentation and expression of molecules
Myeloid DC function was impaired in Schistosoma heamatobium–infected individuals [61]. it is tempting to speculate that the increased Breg activity during schistosome infection is responsible for the altered DC function [41].
(5) The involvement of CD80 and CD86 is an important feature of suppressive capacity of human regulatory B cells [62]
The interaction between CD80 and CD86 interactions between B cells and CD4+ T cells work synergistically with B cell IL-10 production to suppress CD4+T cell cytokine production. In a TCRα knockout mouse model of intestinal inflammation, CD86 in particular has been reported to mediate the suppressive effects of B cells [59]. Moreover, it has been shown that blockade of CD80 impairs Treg cell suppressive capacity [63]. A requirement for CD80 and CD86 expression has been proven in experimental models of arthritis or lupus, but expression of these molecules by B cells is essential in remission of EAE and in B-cell-mediated protection from experimental colitis [59, 64].
(6) Other mechanisms
Not all BREG cells express IL-10 upon in vitro stimulation, which suggests that production of IL-10 might not be the only mechanism by which BREG cells suppress immune response [65]. The release of other ‘regulatory’ cytokines, such as transforming growth factor β (TGF-β), has also been implicated in mediating the suppressive effect of B REG cells [66]. Recent studies indicate that antibodies may also be involved in the suppression of immune responses. The activation of dendritic cells can be suppressed through the binding of IgG to FcγRIIB, as well as IgG-mediated clearance of potentially pathogenic host apoptotic cells. Both IgG4 and IgA belong to the group of anti-imflammatory antibody isotypes as it is unable to activate complement [41].
Outstanding questions and concluding remarks
The precise phenotype and characteristic markers of BREGs are still the subject of debate. IL-10 cannot be used to select BREG because it requires permeabilization of the cell. So one of the first steps in learning more about the development of BREG is determining the cell surface markers associated with them to allow their selection and expansion [47]. It remains to be established whether BREGs can affect other cells of the immune system and whether, similarly to Tregs, different subpopulations of BREGs can differentially participate in immune modulation [67]. Are BREG cells a developmentally distinct B-cell subset? Do BREG cells display a specific transcriptional signature such as FoxP3 for regulatory T cells? Concerning to BREG cell biology, how many BREG lineages exist? How many BREG subsets are there? Is the phenotype of BREG cells regulated by environmental input? Where do BREG cells exert their suppressive effect? Do BREG cells recirculate to sites of inflammation? Do BREG cells present antigen to T cells? [68].
Mechanisms of human Breg-mediated immune regulation
CD19+CD24hiCD38hi (so-called immature transitional B cells) was capable of suppressing IFN-γ and TNF- α secretion which dependent on IL-10 and CD80/CD86 co-stimulation. This subset was enriched in HIV and CHB patients and could suppress CD8+T cells' function. It was able to suppress the differentiation of Th1 effector cell too while this function was functionally impaired in SLE patients.
CD19+CD25highCD86high IL-10high TGF-βhigh cells were defined as “Breg cells”. This subset can suppress the proliferative capacity of CD4+T cells. Foxp3 and CTLA-4 expression in Treg cells were enhanced by a direct contact with these “Breg cells” on a TGF-β but not IL-10 dependent way.
CD19+-CD25+B cells present antigen more efficiently to allogeneic CD4+T cells by strongly express CD27 and CD80 and secrete high levels of inhibitory cytokine IL-10. CD25+B cells were found to display a high activated and mature phenotype in RA, SLE and remission ANCA-related vasculitis patients.
CD24+CD27+ B cells were characterized as a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells and named it as “human B10 and progenitor B10 cells”. After 5 hours of ex vivo stimulation, this subset can express cytoplasmic IL-10.
CD19+ CD1d+ CD5+ B cell were found in the patients with tuberculosis with stronger suppressive activity than such cells from healthy donors. Furthermore, the frequency of CD19+CD1d+CD5+ B cells in peripheral blood was inversely correlated with that of Th17 cells in patients with tuberculosis.
CD1d+ B cells were found present in the peripheral blood of helminth-infected patients with Multiple Sclerosis(MS), producing high levels of IL-10 in response to CD40 ligation. Helminth-infected patients with MS, were able to suppress T-cell proliferation in an IL-10-dependent manner in vitro while uninfected patients with MS didn't have this suppressive function.
Figure: 1.
References
- 1.Duddy M, Niino M, Adatia F, Hebert S, Freedman M, et al. Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis. J Immunol. 2007;178:6092–6099. doi: 10.4049/jimmunol.178.10.6092. [DOI] [PubMed] [Google Scholar]
- 2.Duddy ME, Alter A, Bar-Or A. Distinct profiles of human B cell effector cytokines: A role in immune regulation? Journal of Immunology. 2004;172:3422–3427. doi: 10.4049/jimmunol.172.6.3422. [DOI] [PubMed] [Google Scholar]
- 3.Gantner F, Hermann P, Nakashima K, Matsukawa S, Sakai K, et al. CD40-dependent and -independent activation of human tonsil B cells by CpG oligodeoxynucleotides. Eur J Immunol. 2003;33:1576–1585. doi: 10.1002/eji.200323444. [DOI] [PubMed] [Google Scholar]
- 4.Tu W, Lau YL, Zheng J, Liu Y, Chan PL, et al. Efficient generation of human alloantigen-specific CD4+ regulatory T cells from naive precursors by CD40-activated B cells. Blood. 2008;112:2554–2562. doi: 10.1182/blood-2008-04-152041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng J, Liu Y, Lau YL, Tu W. CD40-activated B cells are more potent than immature dendritic cells to induce and expand CD4(+) regulatory T cells. Cell Mol Immunol. 2010;7:44–50. doi: 10.1038/cmi.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Llorente L, Richaudpatin Y, Fior R, Alcocervarela J, Wijdenes J, et al. In-Vivo Production of Interleukin-10 By Non-T Cells In Rheumatoid-Arthritis, Sjogrens-Syndrome, And Systemic Lupus-Erythematosus - A Potential Mechanism of B-Lymphocyte Hyperactivity And Autoimmunity. Arthritis and Rheumatism. 1994;37:1647–1655. doi: 10.1002/art.1780371114. [DOI] [PubMed] [Google Scholar]
- 7.Bouaziz JD, Calbo S, Maho-Vaillant M, Saussine A, Bagot M, et al. IL-10 produced by activated human B cells regulates CD4(+) T-cell activation in vitro. Eur J Immunol. 2010;40:2686–2691. doi: 10.1002/eji.201040673. [DOI] [PubMed] [Google Scholar]
- 8.Blair PA, Noreña LY, Flores-Borja F, Rawlings DJ, Isenberg DA, et al. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity. 2010;32:129–140. doi: 10.1016/j.immuni.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 9.Correale J, Farez M, Razzitte G. Helminth infections associated with multiple sclerosis induce regulatory B cells. Ann Neurol. 2008;64:187–199. doi: 10.1002/ana.21438. [DOI] [PubMed] [Google Scholar]
- 10.Brisslert M, Bokarewa M, Larsson P, Wing K, Collins LV, et al. Phenotypic and functional characterization of human CD25+ B cells. Immunology. 2006;117:548–557. doi: 10.1111/j.1365-2567.2006.02331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amu S, Tarkowski A, Dörner T, Bokarewa M, Brisslert M. The human immunomodulatory CD25+ B cell population belongs to the memory B cell pool. Scand J Immunol. 2007;66:77–86. doi: 10.1111/j.1365-3083.2007.01946.x. [DOI] [PubMed] [Google Scholar]
- 12.Eriksson P, Sandell C, Backteman K, Ernerudh J. B cell abnormalities in Wegener's granulomatosis and microscopic polyangiitis: role of CD25+-expressing B cells. J Rheumatol. 2010;37:2086–2095. doi: 10.3899/jrheum.100074. [DOI] [PubMed] [Google Scholar]
- 13.Kessel A, Haj T, Peri R, Snir A, Melamed D, et al. Human CD19(+)CD25(high) B regulatory cells suppress proliferation of CD4(+) T cells and enhance Foxp3 and CTLA-4 expression in T-regulatory cells. Autoimmunity Reviews. 2012;11:670–677. doi: 10.1016/j.autrev.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 14.Iwata Y, Matsushita T, Horikawa M, DiLillo DJ, Yanaba K, et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood. 2011;117:530–541. doi: 10.1182/blood-2010-07-294249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang M, Zheng X, Zhang J, Zhu Y, Zhu X, et al. CD19(+)CD1d(+)CD5(+) B cell frequencies are increased in patients with tuberculosis and suppress Th17 responses. Cellular Immunology. 2012;274:89–97. doi: 10.1016/j.cellimm.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Díaz-Alderete A, Crispin JC, Vargas-Rojas MI, Alcocer-Varela J. IL-10 production in B cells is confined to CD154+ cells in patients with systemic lupus erythematosus. J Autoimmun. 2004;23:379–383. doi: 10.1016/j.jaut.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Amel Kashipaz MR, Huggins ML, Lanyon P, Robins A, Powell RJ, et al. Assessment of Be1 and Be2 cells in systemic lupus erythematosus indicates elevated interleukin-10 producing CD5+ B cells. Lupus. 2003;12:356–363. doi: 10.1191/0961203303lu338oa. [DOI] [PubMed] [Google Scholar]
- 18.Amu S, Strömberg K, Bokarewa M, Tarkowski A, Brisslert M. CD25-expressing B-lymphocytes in rheumatic diseases. Scand J Immunol. 2007;65:182–191. doi: 10.1111/j.1365-3083.2006.01889.x. [DOI] [PubMed] [Google Scholar]
- 19.Cooper AM. Cell-Mediated Immune Responses in Tuberculosis. Annual Review of Immunology. 2009;27:393–422. doi: 10.1146/annurev.immunol.021908.132703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cowley SC, Elkins KL. CD4(+) T cells mediate IFN-gamma-independent control of Mycobacterium tuberculosis infection both in vitro and in vivo. Journal of Immunology. 2003;171:4689–4699. doi: 10.4049/jimmunol.171.9.4689. [DOI] [PubMed] [Google Scholar]
- 21.Siewe B, Stapleton JT, Martinson J, Keshavarzian A, Kazmi N, et al. Regulatory B cell frequency correlates with markers of HIV disease progression and attenuates anti-HIV CD8(+) T cell function in vitro. Journal of Leukocyte Biology. 2013;93:811–818. doi: 10.1189/jlb.0912436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, McGavern DB, et al. Interleukin-10 determines viral clearance or persistence in vivo. Nature Medicine. 2006;12:1301–1309. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Das A, Ellis G, Pallant C, Lopes AR, Khanna P, Peppa, et al. IL-10-producing regulatory B cells in the pathogenesis of chronic hepatitis B virus infection. J Immunol. 2012;189:3925–3935. doi: 10.4049/jimmunol.1103139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DiLillo DJ, Weinberg JB, Yoshizaki A, Horikawa M, Bryant JM, et al. Chronic lymphocytic leukemia and regulatory B cells share IL-10 competence and immunosuppressive function. Leukemia. 2013;27:170–182. doi: 10.1038/leu.2012.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 26.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 27.Nishimura S, Manabe I, Takaki S, Nagasaki M, Otsu M, et al. Adipose Natural Regulatory B Cells Negatively Control Adipose Tissue Inflammation. Cell Metab. 2013 doi: 10.1016/j.cmet.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 28.Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med. 2005;352:804–815. doi: 10.1056/NEJMra041720. [DOI] [PubMed] [Google Scholar]
- 29.Bao W, Zhong H, Manwani D, Vasovic L, Uehlinger J, et al. Regulatory B-cell compartment in transfused alloimmunized and non-alloimmunized patients with sickle cell disease. Am J Hematol. 2013;88:736–740. doi: 10.1002/ajh.23488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hussaarts L, van der Vlugt LE, Yazdanbakhsh M, Smits HH. Regulatory B-cell induction by helminths: implications for allergic disease. J Allergy Clin Immunol. 2011;128:733–739. doi: 10.1016/j.jaci.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 31.van den Biggelaar AH, van Ree R, Rodrigues LC, Lell B, Deelder AM, et al. Decreased atopy in children infected with Schistosoma haematobium: a role for parasite-induced interleukin-10. Lancet. 2000;356:1723–1727. doi: 10.1016/S0140-6736(00)03206-2. [DOI] [PubMed] [Google Scholar]
- 32.Mauri C, Ehrenstein MR. The ‘short’ history of regulatory B cells. Trends Immunol. 2008;29:34–40. doi: 10.1016/j.it.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Ashton-Chess J, Giral M, Brouard S, Soulillou JP. Spontaneous operational tolerance after immunosuppressive drug withdrawal in clinical renal allotransplantation. Transplantation. 2007;84:1215–1219. doi: 10.1097/01.tp.0000290683.54937.1b. [DOI] [PubMed] [Google Scholar]
- 34.Newell KA, Asare A, Kirk AD, Gisler TD, Bourcier K, et al. Immune Tolerance Network ST. Identification of a B cell signature associated with renal transplant tolerance in humans. Journal of Clinical Investigation. 2010;120:1836–1847. doi: 10.1172/JCI39933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sánchez-Fueyo A. Identification of tolerant recipients following liver transplantation. Int Immunopharmacol. 2010;10:1501–1504. doi: 10.1016/j.intimp.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 36.Pallier A, Hillion S, Danger R, Giral M, Racapé M, et al. Patients with drug-free long-term graft function display increased numbers of peripheral B cells with a memory and inhibitory phenotype. Kidney Int. 2010;78:503–513. doi: 10.1038/ki.2010.162. [DOI] [PubMed] [Google Scholar]
- 37.Olkhanud PB, Damdinsuren B, Bodogai M, Gress RE, Sen R, et al. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4+ T cells to T-regulatory cells. Cancer Res. 2011;71:3505–3515. doi: 10.1158/0008-5472.CAN-10-4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rolle L, Memarzadeh Tehran M, Morell-García A, Raeva Y, Schumacher A, et al. IL-10-Producing Regulatory B Cells in Early Human Pregnancy. Am J Reprod Immunol. 2013;70:448–453. doi: 10.1111/aji.12157. [DOI] [PubMed] [Google Scholar]
- 39.Watanabe R, Fujimoto M, Ishiura N, Kuwano Y, Nakashima H, et al. CD19 expression in B cells is important for suppression of contact hypersensitivity. Am J Pathol. 2007;171:560–570. doi: 10.2353/ajpath.2007.061279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bouaziz JD, Yanaba K, Tedder TF. Regulatory B cells as inhibitors of immune responses and inflammation. Immunol Rev. 2008;224:201–214. doi: 10.1111/j.1600-065X.2008.00661.x. [DOI] [PubMed] [Google Scholar]
- 41.Hussaarts L, van der Vlugt LEPM, Yazdanbakhsh M, Smits HH. Regulatory B-cell induction by helminths: Implications for allergic disease. Journal of Allergy and Clinical Immunology. 2011;128:733–739. doi: 10.1016/j.jaci.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 42.Ron Y, Sprent J. T-Cell Priming Invivo - A Major Role For B-Cells In Presenting Antigen To T-Cells In Lymph-Nodes. Journal of Immunology. 1987;138:2848–2856. [PubMed] [Google Scholar]
- 43.Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, et al. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunological Reviews. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 44.Ronchese F, Hausmann B. Lymphocytes-B Invivo Fail To Prime Naive T-Cells But Can Stimulate Antigen-Experienced Lymphocytes-T. Journal of Experimental Medicine. 1993;177:679–690. doi: 10.1084/jem.177.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rudolph U, Finegold MJ, Rich SS, Harriman GR, Srinivasan Y, et al. Ulcerative-Colitis and Adenocarcinoma of the Colon in G-Alpha(I2)-Deficient Mice. Nature Genetics. 1995;10:143–150. doi: 10.1038/ng0695-143. [DOI] [PubMed] [Google Scholar]
- 46.Sakaguchi S. Naturally arising Foxp3-expressing CD25(+) CD4(+) regulatory T cells in immunological tolerance to self and non-self. Nature Immunology. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 47.Goode I, Xu H, Ildstad ST. Regulatory B Cells: The New “It” Cell. Transplant Proc. 2013 doi: 10.1016/j.transproceed.2013.08.075. [DOI] [PubMed] [Google Scholar]
- 48.Matsushita T, Yanaba K, Bouaziz JD, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest. 2008;118:3420–3430. doi: 10.1172/JCI36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matsushita T. Regulatory B cell and autoimmune disease. Nihon Rinsho Meneki Gakkai Kaishi. 2010;33:234–241. doi: 10.2177/jsci.33.234. [DOI] [PubMed] [Google Scholar]
- 50.Blair PA, Chavez-Rueda KA, Evans JG, Shlomchik MJ, Eddaoudi A, et al. Selective targeting of B cells with agonistic anti-CD40 is an efficacious strategy for the generation of induced regulatory T2-like B cells and for the suppression of lupus in MRL/lpr mice. J Immunol. 2009;182:3492–3502. doi: 10.4049/jimmunol.0803052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Evans JG, Chavez-Rueda KA, Eddaoudi A, Meyer-Bahlburg A, Rawlings DJ, et al. Novel suppressive function of transitional 2 B cells in experimental arthritis. J Immunol. 2007;178:7868–78. doi: 10.4049/jimmunol.178.12.7868. [DOI] [PubMed] [Google Scholar]
- 52.Lemoine S, Morva A, Youinou P, Jamin C. Regulatory B cells in autoimmune diseases: how do they work? Ann N Y Acad Sci. 2009;1173:260–267. doi: 10.1111/j.1749-6632.2009.04651.x. [DOI] [PubMed] [Google Scholar]
- 53.Amu S, Saunders SP, Kronenberg M, Mangan NE, Atzberger A, et al. Regulatory B cells prevent and reverse allergic airway inflammation via FoxP3-positive T regulatory cells in a murine model. J Allergy Clin Immunol. 2010;125:1114–1124.e8. doi: 10.1016/j.jaci.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 54.Smits HH, Hammad H, van Nimwegen M, Soullie T, Willart MA, et al. Protective effect of Schistosoma mansoni infection on allergic airway inflammation depends on the intensity and chronicity of infection. J Allergy Clin Immunol. 2007;120:932–940. doi: 10.1016/j.jaci.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 55.Wilson MS, Taylor MD, O'Gorman MT, Balic A, Barr TA, et al. Helminth-induced CD19+CD23hi B cells modulate experimental allergic and autoimmune inflammation. Eur J Immunol. 2010;40:1682–1696. doi: 10.1002/eji.200939721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ashour HM, Niederkorn JY. Expansion of B cells is necessary for the induction of T-cell tolerance elicited through the anterior chamber of the eye. Int Arch Allergy Immunol. 2007;144:343–346. doi: 10.1159/000106461. [DOI] [PubMed] [Google Scholar]
- 57.Watanabe R, Ishiura N, Nakashima H, Kuwano Y, Okochi H, et al. Regulatory B cells (B10 cells) have a suppressive role in murine lupus: CD19 and B10 cell deficiency exacerbates systemic autoimmunity. J Immunol. 2010;184:4801–4819. doi: 10.4049/jimmunol.0902385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carter NA, Vasconcellos R, Rosser EC, Tulone C, Muñoz-Suano A, et al. Mice lacking endogenous IL-10-producing regulatory B cells develop exacerbated disease and present with an increased frequency of Th1/Th17 but a decrease in regulatory T cells. J Immunol. 2011;186:5569–5579. doi: 10.4049/jimmunol.1100284. [DOI] [PubMed] [Google Scholar]
- 59.Mann MK, Maresz K, Shriver LP, Tan Y, Dittel BN. B cell regulation of CD4+CD25+ T regulatory cells and IL-10 via B7 is essential for recovery from experimental autoimmune encephalomyelitis. J Immunol. 2007;178:3447–3456. doi: 10.4049/jimmunol.178.6.3447. [DOI] [PubMed] [Google Scholar]
- 60.Matsushita T, Horikawa M, Iwata Y, Tedder TF. Regulatory B cells (B10 cells) and regulatory T cells have independent roles in controlling experimental autoimmune encephalomyelitis initiation and late-phase immunopathogenesis. J Immunol. 2010;185:2240–2252. doi: 10.4049/jimmunol.1001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Everts B, Adegnika AA, Kruize YC, Smits HH, Kremsner PG, et al. Functional impairment of human myeloid dendritic cells during Schistosoma haematobium infection. PLoS Negl Trop Dis. 2010;4:e667. doi: 10.1371/journal.pntd.0000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mauri C. Regulation of immunity and autoimmunity by B cells. Current Opinion in Immunology. 2010;22:761–767. doi: 10.1016/j.coi.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 63.Hajoui O, Janani R, Tulic M, Joubert P, Ronis T, et al. Synthesis of IL-13 by human B lymphocytes: regulation and role in IgE production. J Allergy Clin Immunol. 2004;114:657–663. doi: 10.1016/j.jaci.2004.05.034. [DOI] [PubMed] [Google Scholar]
- 64.Mizoguchi E, Mizoguchi A, Preffer FI, Bhan AK. Regulatory role of mature B cells in a murine model of inflammatory bowel disease. Int Immunol. 2000;12:597–605. doi: 10.1093/intimm/12.5.597. [DOI] [PubMed] [Google Scholar]
- 65.Yanaba K, Bouaziz JD, Matsushita T, Tsubata T, Tedder TF. The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J Immunol. 2009;182:7459–7472. doi: 10.4049/jimmunol.0900270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Parekh VV, Prasad DV, Banerjee PP, Joshi BN, Kumar A, et al. B cells activated by lipopolysaccharide, but not by anti-Ig and anti-CD40 antibody, induce anergy in CD8+ T cells: role of TGF-beta 1. J Immunol. 2003;170:5897–5911. doi: 10.4049/jimmunol.170.12.5897. [DOI] [PubMed] [Google Scholar]
- 67.Balkwill F, Montfort A, Capasso M. B regulatory cells in cancer. Trends in Immunology. 2013;34:169–173. doi: 10.1016/j.it.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 68.Mauri C, Blair PA. Regulatory B cells in autoimmunity: developments and controversies. Nat Rev Rheumatol. 2010;6:636–643. doi: 10.1038/nrrheum.2010.140. [DOI] [PubMed] [Google Scholar]


