Abstract
Myeloperoxidase is an inflammatory enzyme that generates reactive hypochlorous acid in the presence of hydrogen peroxide and chloride ion. However, this enzyme also uses bromide ion or thiocyanate as a substrate to form hypobromous or hypothiocyanous acid, respectively. These species play important roles in host defense against the invasion of microorganisms. In contrast, these enzyme products modify biomolecules in hosts during excess inflammation, indicating that the action of myeloperoxidase is both beneficial and harmful. Myeloperoxidase uses other endogenous compounds, such as serotonin, urate, and l-tyrosine, as substrates. This broad-range specificity may have some biological implications. Target molecules of this enzyme and its products vary, including low-molecular weight thiols, proteins, nucleic acids, and lipids. The modified products represent biomarkers of myeloperoxidase action. Moderate inhibition of this enzyme might be critical for the prevention/modulation of excess, uncontrolled inflammatory events. Some phytochemicals inhibit myeloperoxidase, which might explain the reductive effect caused by the intake of vegetables and fruits on cardiovascular diseases.
Keywords: myeloperoxidase, hypochlorous acid, inflammatory damage, phytochemicals
Introduction
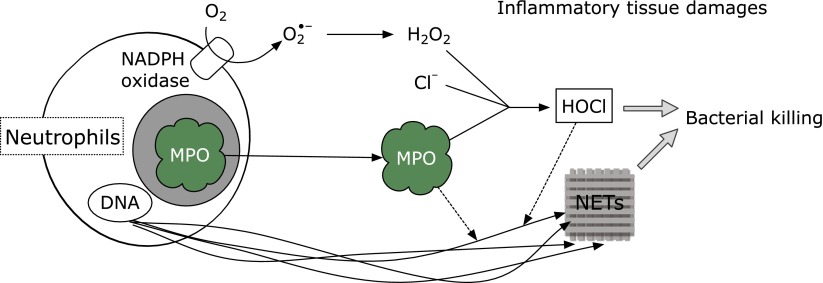
Neutrophil plays an important role in host defense and is equipped with various weapons. In particular, the heme protein myeloperoxidase is most abundantly expressed in azurophilic granules of neutrophils and also known as a key enzyme for the germination of bacteria through the generation of the bactericide hypochlorous acid (HOCl) (Fig. 1).
Fig. 1.
Bacterial killing and inflammatory tissue damage by neutrophils.
H2O2 + Cl− + H+ → HOCl + H2O
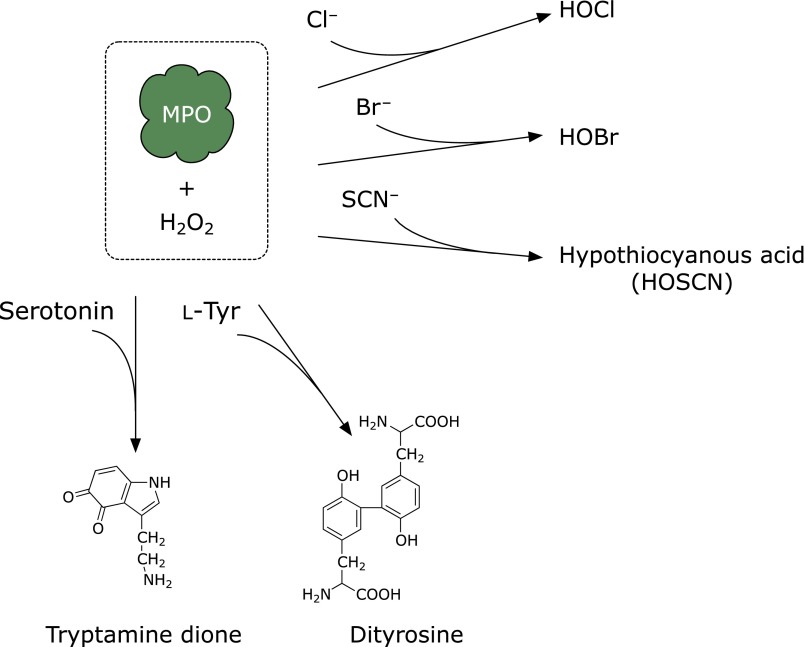
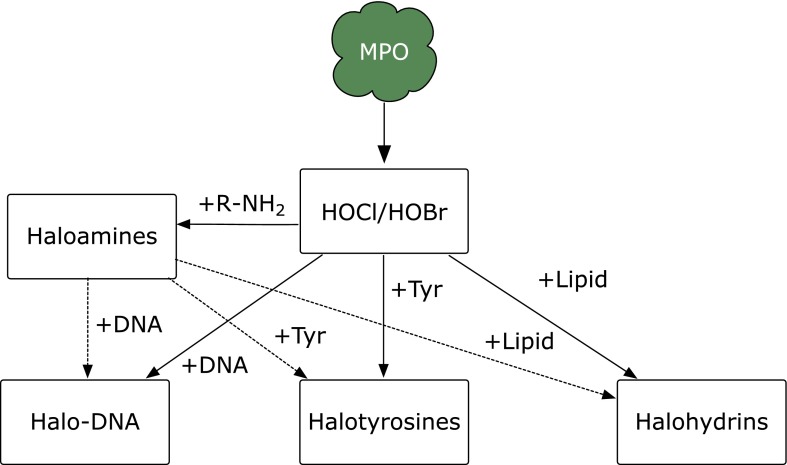
In the presence of physiological concentrations of bromide ion, myeloperoxidase generates hypobromous acid (HOBr) (Fig. 2),(1) and thiocyanate is converted to hypothiocyanous acid by myeloperoxidase. As a minor product, cyanate/isocyanate, which causes carbamylation of proteins,(2) is also generated. In addition, eosinophil peroxidase generates HOBr in the presence of hydrogen peroxide and bromide ion. The hypohalous acids HOCl and HOBr modify amino moieties in proteins, aminophospholipids, or DNA accompanied by the formation of haloamines, chloramine and bromamine (Fig. 3). These halogenating species react with biomolecules in microbes to protect human health. In contrast, these species can also damage host tissues during inflammation, suggesting that the action of these species is a double-edged sword.
Fig. 2.
Substrates of myeloperoxidase (MPO) and corresponding products.
Fig. 3.
Modification of biomolecules by HOX and haloamines.
The peroxidase oxidizes endogenous and exogenous compounds, such as serotonin, l-tyrosine, or phytochemicals, as substrates.(3) Thus, some compounds seem to be genuine substrates (or inhibitors) of myeloperoxidase, indicating that myeloperoxidase may be a multifunctional enzyme. From this point of view, the physiological implications of this enzyme are of great interest.
Novel Aspects of Myeloperoxidase Function
A recent study has shown that myeloperoxidase binds to the surface of fibroblasts and is internalized, an action which induces collagen synthesis by the fibroblasts.(4) Such bioactivity is due to the peroxidase activity of this enzyme and not the generation of hypohalous acid because horseradish peroxidase also possesses the same property. The modulation of extracellular matrix synthesis by myeloperoxidase may play an important role in the reconstitution of injured tissue and also explains why the infiltration of inflammatory cells has often been observed in inflammatory tissue. The same group has serially shown that myeloperoxidase and eosinophil peroxidase were internalized by human umbilical vein endothelial cells and the event had pro-angiogenic activity.(5) 4-Aminobenzohydrazide, an inhibitor of peroxidase, attenuated the effect, suggesting that the peroxidase activity might play an important role for angiogenesisis. Neutrophils kill bacteria by extracellular traps composed of neutrophilic DNA and histones. Myeloperoxidase and/or HOCl might contribute to the bacterial killing process via the extracellular traps.(6,7)
Products of HOCl or HOBr
Specific products derived from HOCl or HOBr (i.e., chlorinated or brominated molecules) are chemical markers for the myeloperoxidase-derived modification of biomolecules. For example, hypohalous acids and haloamines further react with protein tyrosines to generate halotyrosines, such as 3-chlorotyrosine and 3-bromotyrosine. The 3-halotyrosines are further modified to 3,5-dihalotyrosine.(8–10) The increment of dihalotyrosine even within aged, unexposed skin indicates that myeloperoxidase halogenates protein tyrosine not only during acute/intense inflammation, such as sunburn, but also during chronic/long-term mild inflammation.(11) It is noteworthy that bromotyrosine is not a specific marker for eosinophil peroxidase activity because myeloperoxidase also generates bromotyrosine under physiological conditions.(1)
Peroxynitrite, formed from the reaction between nitric oxide and superoxide, generates 3-nitrotyrosine. Myeloperoxidase may also contribute to the formation of nitrotyrosine,(12) although a recent report has shown that myeloperoxidase may scavenge peroxynitrite.(13) 3-Nitrotyrosine is chlorinated by HOCl to form 3-chloro-5-nitrotyrosine.(14) This indicates that 3-nitrotyrosine is not a specific marker for the action of macrophages, which have inducible nitric oxide synthase.
Reduced glutathione (GSH) reacts with HOCl to yield oxidized glutathione (disulfide form, GSSG) as a major product. HOCl further converts GSSG to 5-hydroxybutyrolactam.(15) Glutathione sulfonamide and dehydroglutathione(16) are also generated by HOCl.
DNA is one of the target molecules of halogenating species that produces halogenated nucleotides; on exposure to HOCl, 5-chlorocytosine is formed as a stable product.(17) N4,5-dichloro-deoxycytidine, which has two chlorines in its base, has been identified in inflammatory tissue.(18) On exposure to HOCl, deoxyguanosine is converted to 8-chlorodeoxyguanosine and other products.(19) Bromination of DNA has also been investigated. For example, 5-bromodeoxycytidine is formed by eosinophil peroxidase and is successively incorporated into DNA as 5-bromouracil,(20) suggesting possible mutagenesis by the oxidative damage of nucleotide precursors. The bromination of deoxyguanosine induces the formation of 8-bromodeoxyguanosine. Compared with other oxidative stress markers, such as 8-hydroxydeoxyguanosine and nitrotyrosine, 8-halodeoxyguanosines are observed along with myeloperoxidase infiltration into tissue, an early event during inflammation, and excreted into the urine within 2–3 days, suggesting that 8-halodeoxyguanosine is an earlier marker for inflammatory tissue damage.(21) These oxidation products may be markers of not only myeloperoxidase (or eosinophil peroxidase)-derived oxidative stress but also DNA mutation.(22,23) As a marker of inflammation-induced tissue damage, 8-bromodeoxyguanosine has been used to estimate the preventive effect of orally administered Coprinus comatus, an edible mushroom, on ultraviolet (UV) light-induced inflammation.(24) A recent study showed that 8-bromoguanosine is transformed into cysteinylguanosine in the presence of l-cysteine.(25) 8-Nitroguanosine 3',5'-cyclic monophosphate acts as a second messenger via protein S-guanylation.(26) This implies that the bromination of guanosine moieties may contribute to some biological phenomena during inflammation. Another recent study has shown that dibrominated taurine (taurine dibromamine) efficiently induces the formation of 8-bromodeoxyguanosine, suggesting that taurine dibromamine might be a plausible mediator of deoxyguanosine bromination in vivo.(27)
Lipids, including cholesterol, that have a double bond in their structures form chlorohydrins on exposure to HOCl.(28–30) The potential of chlorohydrin as a biomarker of HOCl has been discussed.(30) The polar head of phosphatidylethanolamine (PE) is also targeted by HOCl to form N,N-dichlorinated PE. The dichlorinated PE decomposes into an N-centered radical species, which initiates lipid oxidation.(31)
Products Derived from Peroxidase Activity
Myeloperoxidase uses chloride ion, bromide ion, and thiocyanate as substrates to successively generate HOX (Fig. 2). Hence, the enzyme also acts as a peroxidase. Myeloperoxidase catalyzes the formation of dityrosine using l-tyrosine and hydrogen peroxide as substrates.(32) Dityrosine, myeloperoxidase, and macrophages have been found to be colocalized in atherosclerotic lesions.(33) Because dityrosine is also generated by peroxidases, various reactive oxygen species, and UV irradiation, dityrosine is considered to be a universal marker of protein oxidation.
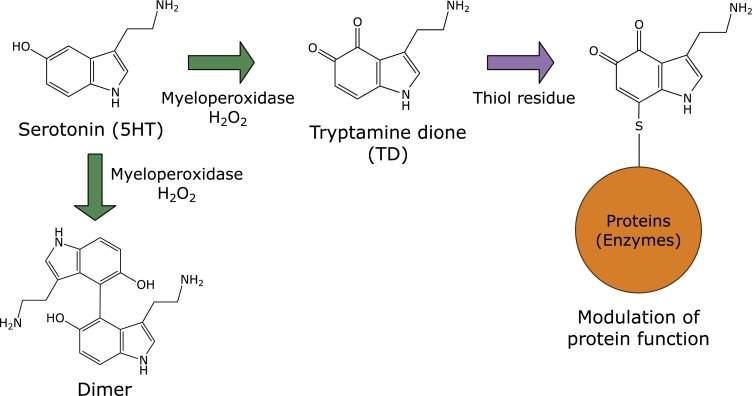
A docking study has shown that serotonin or melatonin binds to the heme pocket of myeloperoxidase.(34) Indeed, serotonin is oxidized by the enzyme in the presence of hydrogen peroxide to form tryptamine-4,5-dione (TD) and serotonin dimers(3,35) (Fig. 4). TD is a putative neurotoxin and covalently reacts with the thiol moieties of proteins, which might inactivate certain enzymes such as α-ketoglutarate dehydrogenase and pyruvate dehydrogenase complexes.(36) Chloride ions at physiological concentrations do not compete with TD-derived modifications, suggesting that serotonin is a promising physiological substrate of myeloperoxidase in vivo.(37) Cytoskeletal proteins, α- and β-tubulins, vimentin, and neurofilament-L, in SH-SY5Y neuroblastoma cells have been identified as target proteins. Self-polymerization of tubulins was modulated by the exposure of TD in vitro. It has also been confirmed that the metabolite of serotonin, 5-hydroxyindoleacetic acid, is a favorable substrate and that the successive formation of quinone gives rise to the covalent conjugation of protein thiols.(37) It has been shown that urate is also a possible physiological substrate for myeloperoxidase.(38) These results suggest that myeloperoxidase may use many endogenous substrates in vivo. However, the physiological significance of its peroxidase activity remains to be clarified.(39)
Fig. 4.
Formation of serotonin oxidation products by myeloperoxidase.
Detection of Products as Biomarkers
At present, liquid chromatography–tandem mass spectrometry is the most commonly used method for measuring the production of biomarkers because of its accuracy and sensitivity. In particular, brominated or chlorinated compounds have specific molecular weights because they have stable isotopic ions, 78Br and 81Br or 35Cl and 37Cl, which show characteristic mass spectrums.(21,40) Specific antibodies have been established and applied for the quantification of biomarkers. Monoclonal antibodies to HOCl-modified LDL have been prepared and applied to immunohistochemical detection,(41–43) and ELISA using antichlorohydrin monoclonal antibody has been shown to be a more sensitive detection method than thin-layer chromatography.(44) With regard to halotyrosines, specific antibodies against monohalotyrosine/dihalotyrosine have been prepared, and the formation of halogenated proteins in tissue during inflammation has been immunohistochemically confirmed.(45–48)
N4,5-dichloro-deoxycytidine has been detected in the liver and lung of mice treated with lipopolysaccharides (LPS) by a specific antibody.(18) An antibody against 8-halodeoxyguanosines has been established and applied for the staining of LPS-induced inflammatory(21) and UV-B-irradiated tissue.(24) The immunochemical methods (immunohistochemical staining) can clarify the localization of biomarkers, and multiple samples can be measured at once by ELISA.
Inhibitors of Myeloperoxidase
There are at least two ways to prevent inflammatory damage caused by myeloperoxidase, the quenching of HOCl and the inhibition of the enzyme itself. Some biological compounds, such as GSH or methionine, rapidly detoxify HOCl. HOCl reacts with quercetin or galloylated catechins followed by the formation of chlorinated products.(6,49)
Ceruloplasmin is an endogenous inhibitor of myeloperoxidase,(50) and hydrogen peroxide inactivates the enzyme.(51) Myeloperoxidase is also inhibited by some chemicals.(41–43) For example, myeloperoxidase is completely inactivated by azide, a heme poison. Aromatic hydroxamates, 2-thioxanthines, 4-aminobenzohydrazide, and isoniazid are also inhibitors of this enzyme.(52,53) Acetaminophen is a competitive inhibitor of myeloperoxidase;(54) a tripeptide, N-acetyl lysyltyrosylcysteine amide, also inhibits this enzyme.(55)
Some food-derived polyphenols have inhibitory effects on myeloperoxidase(56,57) and/or have direct effect on HOCl quenching.(6,49) This moderate/mild inhibition of myeloperoxidase by ingested foods might be enough to maintain human health. Judging from the inhibitory effect on dityrosine formation, phytochemicals, such as curcumin and quercetin, inhibit the activity of myeloperoxidase(56) The specific immunological extraction followed by enzymatic detection method has been developed and applied to evaluate the inhibitory effect of curcumin and tetrahydrocurcumin.(58) Myricitrin is a substrate and inhibitor of myeloperoxidase.(59) Quercetin and its metabolites inhibit myeloperoxidase by interacting with its active site.(57) A quercetin metabolite has been found to be colocalized with myeloperoxidase in atherosclerotic plaques.(57) The biological meaning of this colocalization is not completely known, but flavonoids from daily meal may reduce the risk of some diseases via the inhibition of myeloperoxidase in part because the ingestion of vegetables and fruits seems to reduce the risk of cardiovascular diseases.
Some previous reports on myeloperoxidase inhibitors contribute to the other fields of research. For instance, the novel glycoside methyl syringate β-gentiobioside, leptosperin, has been isolated from manuka honey and identified(60) as an inhibitor of myeloperoxidase; manuka honey has functional properties, including anti-bacterial and -inflammatory effects.(61,62) Because the glycoside leptosperin is exclusively found in honeys from the flowers of leptospermum species, the contents can be used to authenticate premium manuka honey.(63,64) However, the functionality, such as anti-inflammatory effect, of the compound remains to be elucidated.
Closing Remarks
Neutrophil myeloperoxidase plays multiple roles in the human body. Although there are many reports on this enzyme, the physiological implications of its peroxidase activity remain inadequately understood. The products generated by myeloperoxidase activity often react further with other biomolecules, and the modification of the biomolecule may modulate its function in vivo.
Acknowledgments
This work was supported in part by Strategic International Collaborative Research Program, SICORP from Japan Science and Technology Agency, JST.
Conflicts of Interest
Y. K. is an expert adviser of the Healthcare Systems, Inc. and received scholarship funds from the company. The company is the co-holder of a patent for leptosperin with Y. K.
References
- 1.Senthilmohan R, Kettle AJ. Bromination and chlorination reactions of myeloperoxidase at physiological concentrations of bromide and chloride. Arch Biochem Biophys. 2006;445:235–244. doi: 10.1016/j.abb.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Wang Z, Nicholls SJ, Rodriguez ER, et al. Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat Med. 2007;13:1176–1184. doi: 10.1038/nm1637. [DOI] [PubMed] [Google Scholar]
- 3.Ximenes VF, Maghzal GJ, Turner R, Kato Y, Winterbourn CC, Kettle AJ. Serotonin as a physiological substrate for myeloperoxidase and its superoxide-dependent oxidation to cytotoxic tryptamine-4,5-dione. Biochem J. 2009;425:285–293. doi: 10.1042/BJ20090776. [DOI] [PubMed] [Google Scholar]
- 4.DeNichilo MO, Panagopoulos V, Rayner TE, Borowicz RA, Greenwood JE, Evdokiou A. Peroxidase enzymes regulate collagen extracellular matrix biosynthesis. Am J Pathol. 2015;185:1372–1384. doi: 10.1016/j.ajpath.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 5.Panagopoulos V, Zinonos I, Leach DA, et al. Uncovering a new role for peroxidase enzymes as drivers of angiogenesis. Int J Biochem Cell Biol. 2015;68:128–138. doi: 10.1016/j.biocel.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Binsack R, Boersma BJ, Patel RP, et al. Enhanced antioxidant activity after chlorination of quercetin by hypochlorous acid. Alcohol Clin Exp Res. 2001;25:434–443. [PubMed] [Google Scholar]
- 7.Parker H, Winterbourn CC. Reactive oxidants and myeloperoxidase and their involvement in neutrophil extracellular traps. Front Immunol. 2013;3:424. doi: 10.3389/fimmu.2012.00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu W, Chen Y, d'Avignon A, Hazen SL. 3-Bromotyrosine and 3,5-dibromotyrosine are major products of protein oxidation by eosinophil peroxidase: potential markers for eosinophil-dependent tissue injury in vivo. Biochemistry. 1999;38:3538–3548. doi: 10.1021/bi982401l. [DOI] [PubMed] [Google Scholar]
- 9.Fu S, Wang H, Davies M, Dean R. Reactions of hypochlorous acid with tyrosine and peptidyl-tyrosyl residues give dichlorinated and aldehydic products in addition to 3-chlorotyrosine. J Biol Chem. 2000;275:10851–10858. doi: 10.1074/jbc.275.15.10851. [DOI] [PubMed] [Google Scholar]
- 10.Chapman AL, Senthilmohan R, Winterbourn CC, Kettle AJ. Comparison of mono- and dichlorinated tyrosines with carbonyls for detection of hypochlorous acid modified proteins. Arch Biochem Biophys. 2000;377:95–100. doi: 10.1006/abbi.2000.1744. [DOI] [PubMed] [Google Scholar]
- 11.Ishitsuka Y, Maniwa F, Koide C, et al. Increased halogenated tyrosine levels are useful markers of human skin ageing, reflecting proteins denatured by past skin inflammation. Clin Exp Dermatol. 2012;37:252–258. doi: 10.1111/j.1365-2230.2011.04215.x. [DOI] [PubMed] [Google Scholar]
- 12.Sampson JB, Ye Y, Rosen H, Beckman JS. Myeloperoxidase and horseradish peroxidase catalyze tyrosine nitration in proteins from nitrite and hydrogen peroxide. Arch Biochem Biophys. 1998;356:207–213. doi: 10.1006/abbi.1998.0772. [DOI] [PubMed] [Google Scholar]
- 13.Koyani CN, Flemmig J, Malle E, Arnhold J. Myeloperoxidase scavenges peroxynitrite: a novel anti-inflammatory action of the heme enzyme. Arch Biochem Biophys. 2015;571:1–9. doi: 10.1016/j.abb.2015.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curtis MP, Neidigh JW. Kinetics of 3-nitrotyrosine modification on exposure to hypochlorous acid. Free Radic Res. 2014;48:1355–1362. doi: 10.3109/10715762.2014.954110. [DOI] [PubMed] [Google Scholar]
- 15.Yuan W, Wang Y, Heinecke JW, Fu X. Hypochlorous acid converts the gamma-glutamyl group of glutathione disulfide to 5-hydroxybutyrolactam, a potential marker for neutrophil activation. J Biol Chem. 2009;284:26908–26917. doi: 10.1074/jbc.M109.005496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harwood DT, Kettle AJ, Winterbourn CC. Production of glutathione sulfonamide and dehydroglutathione from GSH by myeloperoxidase-derived oxidants and detection using a novel LC-MS/MS method. Biochem J. 2006;399:161–168. doi: 10.1042/BJ20060978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henderson JP, Byun J, Heinecke JW. Molecular chlorine generated by the myeloperoxidase-hydrogen peroxide-chloride system of phagocytes produces 5-chlorocytosine in bacterial RNA. J Biol Chem. 1999;274:33440–33448. doi: 10.1074/jbc.274.47.33440. [DOI] [PubMed] [Google Scholar]
- 18.Kawai Y, Morinaga H, Kondo H, et al. Endogenous formation of novel halogenated 2'-deoxycytidine. Hypohalous acid-mediated DNA modification at the site of inflammation. J Biol Chem. 2004;279:51241–51249. doi: 10.1074/jbc.M408210200. [DOI] [PubMed] [Google Scholar]
- 19.Masuda M, Suzuki T, Friesen MD, et al. Chlorination of guanosine and other nucleosides by hypochlorous acid and myeloperoxidase of activated human neutrophils. Catalysis by nicotine and trimethylamine. J Biol Chem. 2001;276:40486–40496. doi: 10.1074/jbc.M102700200. [DOI] [PubMed] [Google Scholar]
- 20.Henderson JP, Byun J, Williams MV, et al. Bromination of deoxycytidine by eosinophil peroxidase: a mechanism for mutagenesis by oxidative damage of nucleotide precursors. Proc Natl Acad Sci U S A. 2001;98:1631–1636. doi: 10.1073/pnas.041146998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asahi T, Kondo H, Masuda M, et al. Chemical and immunochemical detection of 8-halogenated deoxyguanosines at early stage inflammation. J Biol Chem. 2010;285:9282–9291. doi: 10.1074/jbc.M109.054213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sassa A, Kamoshita N, Matsuda T, et al. Miscoding properties of 8-chloro-2'-deoxyguanosine, a hypochlorous acid-induced DNA adduct, catalysed by human DNA polymerases. Mutagenesis. 2013;28:81–88. doi: 10.1093/mutage/ges056. [DOI] [PubMed] [Google Scholar]
- 23.Sassa A, Ohta T, Nohmi T, Honma M, Yasui M. Mutational specificities of brominated DNA adducts catalyzed by human DNA polymerases. J Mol Biol. 2011;406:679–686. doi: 10.1016/j.jmb.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Asahi T, Wu X, Shimoda H, et al. A mushroom-derived amino acid, ergothioneine, is a potential inhibitor of inflammation-related DNA halogenation Biosci Biotechnol Biochem 2015. DOI: 10.1080/09168451.2015.1083396 [DOI] [PubMed] [Google Scholar]
- 25.Suzuki T, Kosaka A, Inukai M. Formation of 8-S-L-cysteinylguanosine from 8-bromoguanosine and cysteine. Bioorg Med Chem Lett. 2013;23:3864–3867. doi: 10.1016/j.bmcl.2013.04.084. [DOI] [PubMed] [Google Scholar]
- 26.Sawa T, Zaki MH, Okamoto T, et al. Protein S-guanylation by the biological signal 8-nitroguanosine 3',5'-cyclic monophosphate. Nat Chem Biol. 2007;3:727–735. doi: 10.1038/nchembio.2007.33. [DOI] [PubMed] [Google Scholar]
- 27.Asahi T, Nakamura Y, Kato Y, Osawa T. Specific role of taurine in the 8-brominated-2'-deoxyguanosine formation. Arch Biochem Biophys. 2015;586:45–50. doi: 10.1016/j.abb.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Heinecke JW, Li W, Mueller DM, Bohrer A, Turk J. Cholesterol chlorohydrin synthesis by the myeloperoxidase-hydrogen peroxide-chloride system: potential markers for lipoproteins oxidatively damaged by phagocytes. Biochemistry. 1994;33:10127–10136. doi: 10.1021/bi00199a041. [DOI] [PubMed] [Google Scholar]
- 29.van den Berg JJ, Winterbourn CC, Kuypers FA. Hypochlorous acid-mediated modification of cholesterol and phospholipid: analysis of reaction products by gas chromatography-mass spectrometry. J Lipid Res. 1993;34:2005–2012. [PubMed] [Google Scholar]
- 30.Winterbourn CC, Kettle AJ. Biomarkers of myeloperoxidase-derived hypochlorous acid. Free Radic Biol Med. 2000;29:403–409. doi: 10.1016/s0891-5849(00)00204-5. [DOI] [PubMed] [Google Scholar]
- 31.Kawai Y, Kiyokawa H, Kimura Y, Kato Y, Tsuchiya K, Terao J. Hypochlorous acid-derived modification of phospholipids: characterization of aminophospholipids as regulatory molecules for lipid peroxidation. Biochemistry. 2006;45:14201–14211. doi: 10.1021/bi0610909. [DOI] [PubMed] [Google Scholar]
- 32.Jacob JS, Cistola DP, Hsu FF, et al. Human phagocytes employ the myeloperoxidase-hydrogen peroxide system to synthesize dityrosine, trityrosine, pulcherosine, and isodityrosine by a tyrosyl radical-dependent pathway. J Biol Chem. 1996;271:19950–19956. doi: 10.1074/jbc.271.33.19950. [DOI] [PubMed] [Google Scholar]
- 33.Leeuwenburgh C, Rasmussen JE, Hsu FF, Mueller DM, Pennathur S, Heinecke JW. Mass spectrometric quantification of markers for protein oxidation by tyrosyl radical, copper, and hydroxyl radical in low density lipoprotein isolated from human atherosclerotic plaques. J Biol Chem. 1997;272:3520–3526. doi: 10.1074/jbc.272.6.3520. [DOI] [PubMed] [Google Scholar]
- 34.Hallingback HR, Gabdoulline RR, Wade RC. Comparison of the binding and reactivity of plant and mammalian peroxidases to indole derivatives by computational docking. Biochemistry. 2006;45:2940–2950. doi: 10.1021/bi051510e. [DOI] [PubMed] [Google Scholar]
- 35.Wrona MZ, Dryhurst G. Interactions of 5-hydroxytryptamine with oxidative enzymes. Biochem Pharmacol. 1991;41:1145–1162. doi: 10.1016/0006-2952(91)90653-m. [DOI] [PubMed] [Google Scholar]
- 36.Jiang XR, Dryhurst G. Inhibition of the alpha-ketoglutarate dehydrogenase and pyruvate dehydrogenase complexes by a putative aberrant metabolite of serotonin, tryptamine-4,5-dione. Chem Res Toxicol. 2002;15:1242–1247. doi: 10.1021/tx020029b. [DOI] [PubMed] [Google Scholar]
- 37.Kato Y, Ono S, Kitamoto N, Kettle AJ. Covalent modification of cytoskeletal proteins in neuronal cells by tryptamine-4,5-dione. Redox Biol. 2014;2C:983–990. doi: 10.1016/j.redox.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meotti FC, Jameson GN, Turner R, et al. Urate as a physiological substrate for myeloperoxidase: implications for hyperuricemia and inflammation. J Biol Chem. 2011;286:12901–12911. doi: 10.1074/jbc.M110.172460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davies MJ. Myeloperoxidase-derived oxidation: mechanisms of biological damage and its prevention. J Clin Biochem Nutr. 2011;48:8–19. doi: 10.3164/jcbn.11-006FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kato Y, Dozaki N, Nakamura T, et al. Quantification of modified tyrosines in healthy and diabetic human urine using liquid chromatography/tandem mass spectrometry. J Clin Biochem Nutr. 2009;44:67–78. doi: 10.3164/jcbn.08-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malle E, Waeg G, Schreiber R, Grone EF, Sattler W, Gröne HJ. Immunohistochemical evidence for the myeloperoxidase/H2O2/halide system in human atherosclerotic lesions: colocalization of myeloperoxidase and hypochlorite-modified proteins. Eur J Biochem. 2000;267:4495–4503. doi: 10.1046/j.1432-1327.2000.01498.x. [DOI] [PubMed] [Google Scholar]
- 42.Malle E, Hazell L, Stocker R, Sattler W, Esterbauer H, Waeg G. Immunologic detection and measurement of hypochlorite-modified LDL with specific monoclonal antibodies. Arterioscler Thromb Vasc Biol. 1995;15:982–989. doi: 10.1161/01.atv.15.7.982. [DOI] [PubMed] [Google Scholar]
- 43.Gröne HJ, Gröne EF, Malle E. Immunohistochemical detection of hypochlorite-modified proteins in glomeruli of human membranous glomerulonephritis. Lab Invest. 2002;82:5–14. doi: 10.1038/labinvest.3780390. [DOI] [PubMed] [Google Scholar]
- 44.Carr AC, Vissers MC, Domigan NM, Winterbourn CC. Modification of red cell membrane lipids by hypochlorous acid and haemolysis by preformed lipid chlorohydrins. Redox Rep. 1997;3:263–271. doi: 10.1080/13510002.1997.11747122. [DOI] [PubMed] [Google Scholar]
- 45.Gujral JS, Hinson JA, Jaeschke H. Chlorotyrosine protein adducts are reliable biomarkers of neutrophil-induced cytotoxicity in vivo. Comp Hepatol. 2004;3 (Suppl 1):S48. doi: 10.1186/1476-5926-2-S1-S48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kambayashi Y, Ogino K, Takemoto K, et al. Preparation and characterization of a polyclonal antibody against brominated protein. J Clin Biochem Nutr. 2009;44:95–103. doi: 10.3164/jcbn.08-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kato Y, Kawai Y, Morinaga H, et al. Immunogenicity of a brominated protein and successive establishment of a monoclonal antibody to dihalogenated tyrosine. Free Radic Biol Med. 2005;38:24–31. doi: 10.1016/j.freeradbiomed.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 48.Robaszkiewicz A, Bartosz G, Soszynski M. Detection of 3-chlorinated tyrosine residues in human cells by flow cytometry. J Immunol Methods. 2011;369:141–145. doi: 10.1016/j.jim.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 49.Kawai Y, Matsui Y, Kondo H, et al. Galloylated catechins as potent inhibitors of hypochlorous acid-induced DNA damage. Chem Res Toxicol. 2008;21:1407–1414. doi: 10.1021/tx800069e. [DOI] [PubMed] [Google Scholar]
- 50.Chapman AL, Mocatta TJ, Shiva S, et al. Ceruloplasmin is an endogenous inhibitor of myeloperoxidase. J Biol Chem. 2013;288:6465–6477. doi: 10.1074/jbc.M112.418970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paumann-Page M, Furtmüller PG, Hofbauer S, Paton LN, Obinger C, Kettle AJ. Inactivation of human myeloperoxidase by hydrogen peroxide. Arch Biochem Biophys. 2013;539:51–62. doi: 10.1016/j.abb.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Forbes LV, Furtmüller PG, Khalilova I, Turner R, Obinger C, Kettle AJ. Isoniazid as a substrate and inhibitor of myeloperoxidase: identification of amine adducts and the influence of superoxide dismutase on their formation. Biochem Pharmacol. 2012;84:949–960. doi: 10.1016/j.bcp.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 53.Forbes LV, Sjogren T, Auchère F, et al. Potent reversible inhibition of myeloperoxidase by aromatic hydroxamates. J Biol Chem. 2013;288:36636–36647. doi: 10.1074/jbc.M113.507756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koelsch M, Mallak R, Graham GG, et al. Acetaminophen (paracetamol) inhibits myeloperoxidase-catalyzed oxidant production and biological damage at therapeutically achievable concentrations. Biochem Pharmacol. 2010;79:1156–1164. doi: 10.1016/j.bcp.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 55.Zhang H, Jing X, Shi Y, et al. N-acetyl lysyltyrosylcysteine amide inhibits myeloperoxidase, a novel tripeptide inhibitor. J Lipid Res. 2013;54:3016–3029. doi: 10.1194/jlr.M038273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kato Y, Nagao A, Terao J, Osawa T. Inhibition of myeloperoxidase-catalyzed tyrosylation by phenolic antioxidants in vitro. Biosci Biotechnol Biochem. 2003;67:1136–1139. doi: 10.1271/bbb.67.1136. [DOI] [PubMed] [Google Scholar]
- 57.Shiba Y, Kinoshita T, Chuman H, et al. Flavonoids as substrates and inhibitors of myeloperoxidase: molecular actions of aglycone and metabolites. Chem Res Toxicol. 2008;21:1600–1609. doi: 10.1021/tx8000835. [DOI] [PubMed] [Google Scholar]
- 58.Franck T, Kohnen S, Grulke S, et al. Inhibitory effect of curcuminoids and tetrahydrocurcuminoids on equine activated neutrophils and myeloperoxidase activity. Physiol Res. 2008;57:577–587. doi: 10.33549/physiolres.931086. [DOI] [PubMed] [Google Scholar]
- 59.Meotti FC, Senthilmohan R, Harwood DT, Missau FC, Pizzolatti MG, Kettle AJ. Myricitrin as a substrate and inhibitor of myeloperoxidase: implications for the pharmacological effects of flavonoids. Free Radic Biol Med. 2008;44:109–120. doi: 10.1016/j.freeradbiomed.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 60.Kato Y, Umeda N, Maeda A, Matsumoto D, Kitamoto N, Kikuzaki H. Identification of a novel glycoside, leptosin, as a chemical marker of manuka honey. J Agric Food Chem. 2012;60:3418–3423. doi: 10.1021/jf300068w. [DOI] [PubMed] [Google Scholar]
- 61.Molan P, Rhodes T. Honey: a biologic wound dressing. Wounds. 2015;27:141–151. [PubMed] [Google Scholar]
- 62.Prakash A, Medhi B, Avti PK, Saikia UN, Pandhi P, Khanduja KL. Effect of different doses of Manuka honey in experimentally induced inflammatory bowel disease in rats. Phytother Res. 2008;22:1511–1519. doi: 10.1002/ptr.2523. [DOI] [PubMed] [Google Scholar]
- 63.Kato Y, Fujinaka R, Ishisaka A, Nitta Y, Kitamoto N, Takimoto Y. Plausible authentication of manuka honey and related products by measuring leptosperin with methyl syringate. J Agric Food Chem. 2014;62:6400–6407. doi: 10.1021/jf501475h. [DOI] [PubMed] [Google Scholar]
- 64.Kato Y, Araki Y, Juri M, et al. Immunochemical authentication of manuka honey using a monoclonal antibody specific to a glycoside of methyl syringate. J Agric Food Chem. 2014;62:10672–10678. doi: 10.1021/jf503464a. [DOI] [PubMed] [Google Scholar]