Abstract
Although soft-shelled turtle eggs (STE) have been used as a folk medicine for revitalization and the prevention of lifestyle-related diseases, the scientific evidence to support the use of STE in this manner is scarce. To clarify the physiological evidence, STE was administered to diabetic rats and the inhibitory effects on the formation of advanced glycation end-products (AGEs), which are known to increase with the progression of lifestyle-related diseases, were examined. STE and citric acid were administered to diabetic rats for 3 months, and serum Nε-(carboxymethyl)lysine (CML) contents were measured by liquid chromatography-tandem mass spectrometry (LC-MS/MS). Although the administration of STE did not affect the body weight, glycoalbumin or ketone body levels, it significantly reduced the serum level of CML. The accumulation of AGEs, which was measured by fluorescence intensity in the auricle skin and the lower gums, was also reduced by the administration of STE to a similar extent to that observed with citric acid. This report provides the first evidence that the oral administration of STE reduces the formation of AGEs, suggesting that one of the health effects of STE may be the inhibition of AGEs formation.
Keywords: AGEs, soft-shelled turtle eggs, diabetes mellitus, CML, oxidation
Introduction
With the rapidly increasing population of patients with lifestyle-related diseases, disease prevention through daily meals has become more important. Advanced glycation end-products (AGEs) are known to accumulate in our body with age,(1) and to be increased by the pathogenesis of diabetic complications,(2) nephropathy,(3) atherosclerosis and age-related diseases.(4) Thus, the inhibition of AGEs by daily meals is expected to become an effective means of preventing lifestyle-related diseases. Furthermore, high AGEs diets is reported to induce insulin resistance.(5) We previously demonstrated that Nε-(carboxymethyl)lysine (CML), a major antigenic AGE, is generated by the hydroxyl radicals and peroxynitrite,(6,7) which indicates that CML is a marker of oxidation in vivo. The modification of proteins by AGEs causes the denaturation of skeletal proteins such as collagen and the inactivation of enzymes.(8) However, since it remains technically difficult to accurately quantify AGEs in physiological samples, most of the foods that shows inhibitory effects against AGEs have only done so in vitro experiments.
We previously measured serum AGE levels using high performance liquid chromatography (HPLC) and estimated skin AGEs levels using a fluorescence detector. These analysis systems clarified that a hot water extract of mangosteen inhibits the formation of AGEs in vivo.(9) In our search for new natural products that inhibit AGEs formation, we focused on soft-shelled turtle eggs (STE) because they have long been used as a revitalizer and a health-promoting food, especially in Asian countries. Huanling et al.(10) demonstrated that STE inhibits cholesterol absorption in rats, resulting in a reduction of blood cholesterol. Rawendra et al.(11) isolated novel angiotensin-converting enzyme-inhibitory tripeptide from the enzymatic hydrolyzate of turtle egg white. Furthermore, although STE is expected to provide nutritional fortification and to exert preventive effects against atherosclerosis, myocardial infarction and hypertension, the scientific evidence to support these effects is scarce. In the present study, STE was administered to diabetic rats and its inhibitory effect against AGEs formation in serum, skin and gums was evaluated. This study provides the first evidence to support that STE prevents the formation of AGEs in vivo, and may explain the health effects of STE.
Materials and Methods
Chemicals
Streptozotocin (STZ) was purchased from Sigma-Aldrich Japan (Tokyo, Japan). Acetonitrile, H2O and formic acid were purchased from Nacalai Tesque, Inc. (Kyoto, Japan). Ribose and citric acid monohydrate were purchased from Wako (Osaka, Japan). All of the other chemicals were of the best grade that was commercially available.
The preparation of rat chow containing whole extracts of STE
The STE was a gift from DOMO Corporation (Tokyo, Japan). Briefly, the eggs were collected from farmed soft-shelled turtles, Pelodiscus sinesis, which were of more than 3 years of age. Whole STE, including their shells, were vacuum dried until their water content reached less than 3%. The STE powder that was obtained was stored at –20°C until use. Powdered normal rodent chow was kneaded with 20% water in the presence or absence of 1% (v/v) or 5% (v/v) STE, then cut, shaped and baked at 70°C for 3 h.
Animal experiments
The experimental protocol was approved by the ethics review committee of Tokai University for animal experimentation (protocol #141067). Wistar rats were purchased from Kyudo (Kumamoto, Japan). These rats were housed in pathogen-free conditions (12 h/12 h light/dark cycle) and were fed a normal rodent chow diet (Clea, Tokyo, Japan). Diabetes was induced in 8-week-old male rats (weight ~285 g) by a single intravenous (tail vein) injection of STZ (70 mg/kg body weight) in 200 µl of 0.05 M saline-citrate buffer (pH 4.5). Three weeks after the induction of diabetes (blood glucose ≥200 mg/dl), the diabetic rats were randomly divided into four groups an untreated control group (n = 8) and three treated groups (n = 8, each), which were fed a normal rodent chow diet containing 1.0 or 5.0% STE (in chow), or citric acid (2 g/L) (in drinking water). Fasting (6 h) blood samples were obtained from the tail vein. The blood glucose concentration was measured by a Life Check sensor (Gunze Limited, Kyoto, Japan). The animals were killed under pentobarbital anesthesia at 21 weeks after the injection of STZ. Tissues were immediately frozen and stored at −80°C until use. Acetoacetic acid levels were measured using a JCA-BM 8000 automatic analyzer (Japan Electron Optics Laboratories, Tokyo) by standard NAD(H)-linked enzymatic methods. To measure glycoalbumin levels, serum was treated with ketoamine oxidase and produced hydrogen peroxide was measured with peroxidase using a JCA-BM 8000.
The measurement of CML in the serum by LC-MS/MS
The CML content in the serum was measured by LC-MS/MS as described previously.(12) Serum (5 µl) was reduced with NaBH4 (2 µl of 1 M NaBH4 in 0.1 N NaOH) in 20 µl of 200 mM sodium borate buffer, pH 9.1, at room temperature for 4 h. Standard [2H2] CML (PolyPeptide Laboratories, Strasbourg, France) and [13C6] Lysine (Cambridge Isotope Laboratories, Inc., Tewksbury, MA) was added to the pellets, which were hydrolyzed with 1 ml of 6 N HCl at 100°C for 18 h. The dried sample was resuspended in 1 ml of distilled water and passed over a Strata-X-C column (Phenomenex, Torrance, CA) which had been pre-washed with 1 ml of methanol and equilibrated with 1 ml of distilled water. The column was then washed with 3 ml of 2% formic acid and eluted with 3 ml of 7% ammonia. The pooled elution fractions were dried and resuspended in 1 ml of 20% acetonitrile containing 0.1% formic acid. The samples were subjected to an LC-MS/MS assay using a TSQ Vantage triple stage quadrupole mass spectrometer (Thermo Fisher Scientific, Waltham, MA). LC was conducted on a ZIC-HILIC column (150 × 2.1 mm, 5 µm) (Merck Millipore, Billerica, MA). The mobile phase was performed using solvent A (distilled water containing 0.1% formic acid) and solvent B (acetonitrile containing 0.1% formic acid). The flow rate was 0.2 ml/min and the column was kept at 40°C. The retention time for CML and lysine were approximately 12 and 13 min, respectively. CML, lysine, and the standard were detected by electrospray positive ionization-mass spectrometric multiple reaction monitoring. The parent ions of CML and [2H2] CML were 205 (m/z) and 207 (m/z) respectively. Fragment ions of 130 (m/z) from each parent ion were measured for the analysis of CML and [2H2] CML in serum samples.
The estimation of AGEs by autofluorescence
The inhibitory effect of STE on AGEs accumulation in the skin was estimated by measuring autofluorescence from the auricle skin and lower gums in each rat. Similar to previous reports,(9) animal skin autofluorescence was recorded with a prototype device that consisted of a light emitting diode (LED) light source, a spectral apparatus system with a 2048-pixel CCD linear image sensor and grating and a biantennary silica-based optical fiber. A Y-shaped flexible optical fiber coated in polyurethane was used to detect fluorescence signals at the same location on the skin. The quartz Y shape fiber consisted of two core fibers (0.6 mm in diameter). One side of the fibers was connected to a spectrometer while the other was connected to an LED light source. The animal skin autofluorescence spectrum showed a stable emission peak at 460 nm. All measurements were performed in a semi-dark, temperature-controlled room.
Statistical analysis
All of the data were expressed as means ± SD. Differences between the groups were examined using the Mann-Whitney U test and a non-repeated measures ANOVA. P values of <0.05 were considered to indicate statistical significance.
Results
Changes in the body weight and blood glucose concentration of rats
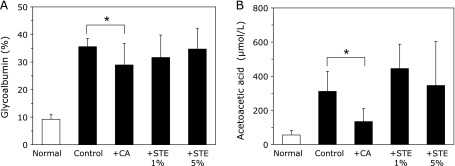
To demonstrate the changes in the fluorophore content that occurred due to diabetes, diabetes was induced in rats by STZ and their blood glucose concentration levels were measured. Although the body weight of normal rats increased in a time-dependent manner, the body weight of diabetic rats showed a slight decrease at 3 months (Fig. 1A), most likely due to the progression of type 1 diabetes. The mean body weight gain was not changed by the administration of STE or citric acid for 90 days. The blood glucose level steeply increased after the induction of diabetes (Fig. 1B), and the mean blood glucose level was not changed by the administration of STE or citric acid for 90 days. The concentrations of glycoalbumin (Fig. 2A) and ketone bodies (Fig. 2B) were increased due to the progression of type 1 diabetes and these concentrations were decreased by the administration of citric acid. In contrast, there were no changes in the glycoalbumin or ketone body concentrations of the 1% and 5% STE groups (Fig. 2A and B).
Fig. 1.
Changes in the body weight and blood glucose levels of rats. Diabetes was induced in rats with streptozotocin and the changes in the body weight (A) and blood glucose levels (B) of normal rats (n = 6) and all of the diabetic (DM) rat groups (n = 8, each) were measured. The data are presented as the means ± SD.
Fig. 2.
The measurement of glycoalbumin and acetoacetic acid in rats. The glycoalbumin (A) and acetoacetic acid (B) levels of normal rats (open bar, n = 8) and all of the diabetic rat groups (closed bar, n = 8, each) were measured. The data are presented as the means ± SD. *p<0.05 vs control rats.
The measurement of serum CML by LC-MS/MS and fluorescence intensity in the auricle skin and lower gums
As shown in Fig. 3A, the parent ions of CML and [2H2] CML were 205 (m/z) and 207 (m/z), respectively, and fragment ions of 130 (m/z) were detected to measure CML and [2H2] CML. Typical fragment ion chromatograms of CML and [2H2] CML (10 pmol) are shown in Fig. 3B. Treatment with citric acid or STE significantly reduced the CML concentration in the diabetic rats by 30% (Fig. 3C). Furthermore, the AGEs contents in hard tissues such as the skin and gums were estimated via the measurement of fluorescence intensity. The increased fluorescence intensity of the auricle skin in diabetic rats was suppressed in the citric acid and STE groups (Fig. 4A). The same tendency was observed in the measurement of fluorescence intensity in the lower gums (Fig. 4B), demonstrating that the increased AGEs formation in those tissues was ameliorated by the administration of STE.
Fig. 3.
The measurement of CML levels in rat sera by LC-MS/MS. The parent ions of CML and [2H2] CML were 205 (m/z) and 207 (m/z) respectively. Deuterium in [2H2] CML was marked by an asterisk. Fragment ions of 130 (m/z) were detected to measure CML and [2H2] CML (A). Peaks of the fragment ions of CML and [2H2] CML (10 pmol) were detected in diabetic rat sera (B). The CML levels in normal rat sera (open bar, n = 8) and the sera of all of the diabetic rat groups (closed bar, n = 8, each) were measured by LC-MS/MS (C), as described in Materials and Methods. CA, citric acid. Data are presented as the means ± SD. *p<0.001 vs control rats.
Fig. 4.
The measurement of fluorescence intensity of the auricle skin and lower gums in rats. The fluorescence intensity of the auricle skin (A) and the lower gums (B) of normal rats (open bar, n = 8) and in all of the diabetic rats groups (closed bar, n = 8, each) was measured as described in Materials and Methods. CA, citric acid. The data are presented as the means ± SD. *p<0.001 vs control rats.
Discussion
Although STE is used as a revitalizer and health-promoting food, the scientific evidence to support its use is rare. In the present study, we evaluated the inhibitory effect of STE on AGEs formation in vivo. The formation of AGEs progresses in vivo as long as an organism remains alive and results in the denaturation of proteins such as skeletal proteins and enzymes.(8) However, since multiple preparation steps and time-consuming processes are required for the measurement of AGEs in physiological samples, the measurement of AGEs (unlike HbA1c) has not been applied in the clinical setting. Thus, in most cases the only evidence for the AGEs inhibitory effects of certain foods comes from in vitro experiments. In the present study, we focused on the inhibitory effects of STE on CML formation since CML levels increase in inflammatory diseases such as atherosclerosis and acute renal disease.(13,14) The oral administration of STE to diabetic rats did not affect their body weight (Fig. 1A) or their fasting blood glucose levels (Fig. 1B). Furthermore, although STE did not ameliorate the diabetic-induced increase in glycoalbumin (Fig. 2A) and ketone body levels (Fig. 2B), it significantly inhibited the formation of serum CML (Fig. 3) and fluorescent AGEs in the auricle skin (Fig. 4A) and lower gums (Fig. 4B). We previously demonstrated that the oral administration of citric acid ameliorates the ketone bodies in diabetic rats and inhibits the formation of AGEs.(15) Citric acid was used therefore used as a positive control and in the present study, it was found to significantly reduce the formation of AGEs in serum (Fig. 3), fluorescence intensity in the auricle skin (Fig. 4A) and the lower gum (Fig. 4B) of diabetic rats. However, since citric acid reduced AGEs formation in diabetic rats by improving the ketone body metabolism in vivo, citric acid did not inhibit the formation of AGEs in vitro.(15) In contrast, STE apparently inhibits AGEs formation in vitro (data not shown), since it contains proteins and free amino acids and decreases the formation of AGEs that are generated on each protein molecule. Although the oral administration of STE does not lead to an increase in the continuous blood protein concentration, the inhibitory effect of STE on AGEs formation in diabetic rats is considered to involve components other than proteins and amino acids. Unlike citric acid, STE did not suppress the level of ketone bodies (Fig. 2B). Thus, the inhibitory effect of STE on AGEs formation is thought to occur through a different mechanism to citric acid.
The AGEs content in rat organs was also estimated by the measurement of fluorescence intensity.(9) In the present study, the lower gum and auricle skin were chosen as sites for AGEs measurement since inflammation of the gums and xeroderma are likely to occur as a result of diabetes. Similarly to the results that were observed with citric acid, the accumulation of AGEs in the auricle skin (Fig. 4A) and lower gums (Fig. 4B) were reduced by the administration of STE. The fact that the AGEs profile in the gums (Fig. 4B) was similar to that in auricle skin (Fig. 4A), suggests the possibility that AGEs contents in the gums can be evaluated by a simpler skin measurement.
In conclusion, this study provides the first evidence to support that the oral administration of STE inhibits the formation of AGEs in diabetic rats, and demonstrates that the suppressed formation of AGEs in vivo is likely to be one of the mechanisms underlying the health benefits of STE. Aside from the protein and amino acid components, STE contain a variety of ingredients such as minerals, lipids and vitamins, which may play a role in the inhibition of AGEs formation in an additive or synergistic manner.
Acknowledgments
This work was supported in part by Grants-in-Aid for Scientific Research (No. 15H02902 and 15K12364 to Ryoji Nagai) from the Ministry of Education, Science, Sports and Culture of Japan. This work was also supported in part by grants from the Research Institute of Agriculture of Tokai University.
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Dunn JA, McCance DR, Thorpe SR, Lyons TJ, Baynes JW. Age-dependent accumulation of Nε-(carboxymethyl)lysine and Nε-(carboxymethyl)hydroxylysine in human skin collagen. Biochemistry. 1991;30:1205–1210. doi: 10.1021/bi00219a007. [DOI] [PubMed] [Google Scholar]
- 2.Buongiorno AM, Morelli S, Sagratella E, et al. Immunogenicity of advanced glycation end products in diabetic patients and in nephropathic non-diabetic patients on hemodialysis or after renal transplantation. J Endocrinol Invest. 2008;31:558–562. doi: 10.1007/BF03346408. [DOI] [PubMed] [Google Scholar]
- 3.Beisswenger PJ, Moore LL, Brinck-Johnsen T, Curphey TJ. Increased collagen-linked pentosidine levels and advanced glycosylation end products in early diabetic nephropathy. J Clin Invest. 1993;92:212–217. doi: 10.1172/JCI116552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakata N, Imanaga Y, Meng J, et al. Increased advanced glycation end products in atherosclerotic lesions of patients with end-stage renal disease. Atherosclerosis. 1999;142:67–77. doi: 10.1016/s0021-9150(98)00192-0. [DOI] [PubMed] [Google Scholar]
- 5.Ottum MS, Mistry AM. Advanced glycation end-products: modifiable environmental factors profoundly mediate insulin resistance. J Clin Biochem Nutr. 2015;57:1–12. doi: 10.3164/jcbn.15-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagai R, Ikeda K, Higashi T, et al. Hydroxyl radical mediates Nε-(carboxymethyl)lysine formation from Amadori product. Biochem Biophys Res Commun. 1997;234:167–172. doi: 10.1006/bbrc.1997.6608. [DOI] [PubMed] [Google Scholar]
- 7.Nagai R, Unno Y, Hayashi MC, et al. Peroxynitrite induces formation of Nε-(carboxymethyl)lysine by the cleavage of Amadori product and generation of glucosone and glyoxal from glucose: novel pathways for protein modification by peroxynitrite. Diabetes. 2002;51:2833–2839. doi: 10.2337/diabetes.51.9.2833. [DOI] [PubMed] [Google Scholar]
- 8.Nagai R, Shirakawa J, Fujiwara Y, et al. Detection of AGEs as markers for carbohydrate metabolism and protein denaturation. J Clin Biochem Nutr. 2014;55:1–6. doi: 10.3164/jcbn.13-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohno R, Moroishi N, Sugawa H, et al. Mangosteen pericarp extract inhibits the formation of pentosidine and ameliorates skin elasticity. J Clin Biochem Nutr. 2015;57:27–32. doi: 10.3164/jcbn.15-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huanling Y, Yong L, Junbo W, Liping Z, Weixing Y. Chinese soft-shelled turtle egg powder lowers serum cholesterol, increases faecal neutral steroids and bile acid excretion, and up-regulates liver cytochrome P450 mRNA level in rats. Br J Nutr. 2005;94:315–320. doi: 10.1079/bjn20051485. [DOI] [PubMed] [Google Scholar]
- 11.Rawendra RD, Aisha, Chen SH, et al. Isolation and characterization of a novel angiotensin-converting enzyme-inhibitory tripeptide from enzymatic hydrolysis of soft-shelled turtle (Pelodiscus sinensis) egg white: in vitro, in vivo, and in silico study. J Agric Food Chem. 2014;62:12178–12185. doi: 10.1021/jf504734g. [DOI] [PubMed] [Google Scholar]
- 12.Miyazawa T, Nakagawa K, Shimasaki S, Nagai R. Lipid glycation and protein glycation in diabetes and atherosclerosis. Amino Acids. 2012;42:1163–1170. doi: 10.1007/s00726-010-0772-3. [DOI] [PubMed] [Google Scholar]
- 13.Wang Z, Jiang Y, Liu N, et al. Advanced glycation end-product Nε-carboxymethyl-Lysine accelerates progression of atherosclerotic calcification in diabetes. Atherosclerosis. 2012;221:387–396. doi: 10.1016/j.atherosclerosis.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 14.Coughlan MT, Forbes JM. Temporal increases in urinary carboxymethyllysine correlate with albuminuria development in diabetes. Am J Nephrol. 2011;34:9–17. doi: 10.1159/000328581. [DOI] [PubMed] [Google Scholar]
- 15.Nagai R, Nagai M, Shimasaki S, Baynes JW, Fujiwara Y. Citric acid inhibits development of cataracts, proteinuria and ketosis in streptozotocin (type 1) diabetic rats. Biochem Biophys Res Commun. 2010;393:118–122. doi: 10.1016/j.bbrc.2010.01.095. [DOI] [PMC free article] [PubMed] [Google Scholar]