Abstract
To elucidate the role of autonomic nervous system in functional dyspepsia patients, we examined 24-h heart rate variability: the basal levels, responses after lunch, cold pressor and mental arithmetic tests, and the efficacy of an autonomic drug (tofisopam). The high-frequency component (HF: 0.15–0.40 Hz) and the ratio of HF to the low-frequency component (LF: 0.04–0.15 Hz; LF/HF ratio) were used as indicators of parasympathetic and sympathetic autonomic nervous system function. The HF component in the 24-h, daytime, and nighttime was low in 86.7%, 97.8%, and 66.7% of patients (n = 45) and the LF/HF ratio was high in 51.1%, 73.3%, and 26.6% of patients. Gastrointestinal symptom tended to be severe in patients with autonomic nervous system disorder (p = 0.085). The abnormal response in HF component after lunch occurred in 38.2% (13/34) of patients who revealed a greater tendency towards in indigestion score (p = 0.061). Delays in recovery to the basal autonomic nervous system level after stimulus of the cold pressor and the mental arithmetic tests occurred in parts of patients. Tofisopam partially improved autonomic nervous system dysfunction and abdominal pain/indigestion. Imbalanced autonomic nervous system function and vulnerability for recovery from external stimuli were observed in functional dyspepsia patients, which was associated with dyspeptic symptoms.
Keywords: functional dyspepsia, autonomic nervous system, brain-gut interaction, neural transmission, heart rate variability
Introduction
Functional dyspepsia (FD) has garnered recent attention in the field of gastroenterology, and was defined in the Rome III criteria published in 2006 as a functional gastroduodenal disorder (FGIDs).(1) The Rome III diagnostic criteria for FD consist of 4 symptoms: (1) bothersome postprandial fullness, (2) early satiation, (3) epigastric pain, and (4) epigastric burning; and also include the required duration of symptoms for diagnosis. However, several uncertainties remain as to the pathophysiology of the disease, and multiple factors: gastric acid secretion, H. pylori infection, gastrointestinal (GI) motility, and neuropsychological factors are putatively involved in the FD pathophysiology.(2,3) The cumulative findings of pathophysiological research have suggested the possibility of a link between gastric acid secretion and GI motility and an occurrence of dyspeptic symptoms. The Japanese guidelines now recommend the use of drugs that inhibit acid secretion and improve GI motility as a first-line therapy for patients with FD.(4–6)
GI tract possesses the enteric nervous system (ENS), which regulates the GI tract functions: motility, endocrine, exocrine, and microcirculation independently from the central nervous system (CNS). On the basis of development standpoint, it is well known that the ENS is linked to the CNS for regulating GI functions mediated by the autonomic nervous system (ANS). Sensory information from the GI tract was transmitted to the CNS through the afferent vagal nerve, while efferent signal from the CNS through the vagal nerve to the ENS upregulates the GI motility and secretion and downregulates the sympathetic nerve activity.(7) As indicated by the term “brain-gut interaction”, these GI functions are the product of a mutual flow of information between the brain and the stomach.(8,9) In an experimental study, neurotransmitters such as nitric oxide, ghrelin, cholecystokinin regulate gastric accommodation or emptying via vagal pathway.(10–12) In fact, vagotomy causes upper abdominal symptoms and gastric distension abnormalities in dysmotility like FD.(13) All these study findings also imply that the pathophysiological cause of FD can be attributed to a general dysfunction that includes pathophysiological mechanisms in GI tract or, in other words, the ANS. To the best of our knowledge, however, there are no published studies describing the association between ANS dysfunction and FD pathology in detail. The present study therefore investigated the relationship between ANS dysfunction and GI symptoms in FD patients, and the vulnerabilities in their homeostatic response to ANS instabilities caused by food and other external stimuli were examined. We also evaluated the efficacy of the autonomic drug tofisopam to treat FD patients in the pilot study.
Methods
Subjects
This observational study was conducted in a tertiary care hospital. From January 2002 to December 2006, FD patients were diagnosed based on Rome II criteria.(14) We have retrospectively re-evaluated each medical record of these patients. According to the Rome III criteria including duration, onset, and frequency of dyspeptic symptoms,(15) we have diagnosed respective subtype (epigastric pain syndrome: EPS and postprandial syndrome: PDS) of FD patients. Finally, 45 FD patients [EPS type, n = 24, 53.1 ± 2.1 years of age (mean ± SEM); 10 male and PDS type, n = 21; 52.1 ± 3.3 years of age (mean ± SEM); 10 male] were recruited for this study (Table 1). Nine healthy volunteers (controls) with no GI symptoms or signs (33.5 ± 4.3 years of age; 6 male) were also enrolled. All patients underwent routine peripheral blood tests, upper GI endoscopy, and abdominal ultrasound. Patients with esophagitis or erosive gastritis on endoscopy and heartburn and patients with acid regurgitation as a predominant symptom were excluded. No subjects had any history of abdominal surgery, psychiatric illness, or treatment with non-steroidal anti-inflammatory drugs, steroids, or other drugs. Written informed consent was obtained from all subjects. The investigation was conducted in accordance with the ethical guidelines for clinical studies, including consideration of patients’ human rights and privacy. The protocol was approved by the institutional review board of the Osaka City University Ethics Committee.
Table 1.
Background of patients with FD
| FD type |
||
|---|---|---|
| EPS | PDS | |
| Cases (%) | 24 (58.5%) | 21 (41.5%) |
| Mean age | 53.1 ± 2.1 | 52.1 ± 3.3 |
| Male:Female | 10:14 | 10:11 |
EPS, epigastric pain syndrome; PDS, postprandial syndrome.
Assessment of the autonomic nervous system (ANS)
ANS function was assessed using the Active Tracer AC-301 (Nihon Kohden Corporation, Tokyo, Japan) to monitor 24-h heart rate variability (HRV), analyze 24-h HRV based on the maximum entropy method (MemCalc2000, Tokyo, Japan) and perform time domain and spectral analyses. In spectral analysis of HRV, the low-frequency component (LF: 0.04–0.15 Hz) and high-frequency component (HF: 0.15–0.4 Hz; indicator of parasympathetic nervous system activity) were confirmed as previously reported; the changes that occur in a single day between daytime and nighttime indicate a circadian variation resembling a cosine curve.(16) These indicators were then investigated in the following scenarios: (1) HF component and LF/HF ratio over the entire 24-h period; (2) HF component and LF/HF ratio in the daytime and nighttime; (3) changes and level of recovery in each parameter after eating a usual lunch; (4) changes and level of recovery in each parameter before and after the ANS stimulus tests;(17,18) and (5) changes in ANS function and FD symptoms before and after oral administration of the autonomic drug tofisopam. Daytime and night-time were defined as 6 a.m. to 10 p.m. and 10 p.m. to 6 a.m.
Analysis of the HRV
HRV analysis was carried out based on the guidelines of the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology.(19) However, because HRV is influenced by race, sex, and age,(20,21) we used Japanese HRV parameter value as reference values according to sex and age.(22)
Symptom questionnaires
At an initial visit to our hospital, all subjects completed three questionnaires to assess GI and psychological symptoms: the GI Symptom Rating Scale (GSRS), the Zung Self-rating Depression Scale, and the State-Trait Anxiety Inventory. The GSRS comprises 15 items evaluating 5 domains: abdominal pain, reflux, indigestion, diarrhea, and constipation. The severity of abdominal pain symptoms was evaluated by summing the scores of items 1, 4, and 5. The severity of indigestion symptoms was evaluated by summing the scores of items 6, 7, 8, and 9. The response to each item ranges from 0 (absence of symptom) to 3 (symptom present to maximum degree). All symptoms scores were calculated using a previously reported method.(23,24)
Statistical analysis
Data were expressed as the means ± SEM and analyzed using a t test or analysis of variance with a Bonferroni correction. The significance level was set at p<0.05. The treatment response in each questionnaire score was evaluated before and after treatment using Wilcoxon’s signed rank test.
Results
Mean HF (msec2) and mean LF/HF ratio in the 24-h in FD patients
The mean 24-h HF component for the entire FD group was lower than the mean standard value in 86.7% (39/45) of FD patients.(22) Meanwhile, the LF/HF ratio was higher than the mean in 51.1% (23/45) of FD patients. In other words, ANS dysfunction was observed in FD patients over the entire 24-h period in terms of higher sympathetic nervous system activity and lower parasympathetic nervous system activity. In the PDS-type FD group, the HF component was low in 87.5% (21/24) of patients, and the LF/HF ratio was high in 54.2% (13/24) of patients. In the EPS-type FD group, the HF component was low in 85.7% (18/21) of patients, and the LF/HF ratio was high in 47.6% (10/21) of patients (Table 2).
Table 2.
ANS components in patients with FD
| total (n = 45) |
EPS (n = 21) |
PDS (n = 24) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| HF | LF/HF | HF | LF/HF | HF | LF/HF | ||||
| 24 h | Increased | 2 (4.4%) | 23 (51.1%) | 0 | 10 (47.6%) | 2 (8.3%) | 13 (54.2%) | ||
| Decreased | 39 (86.7%) | 13 (28.9%) | 18 (85.7%) | 8 (38.1%) | 21 (87.5%) | 5 (20.8%) | |||
| Normal | 4 (8.9%) | 9 (20.0%) | 3 (14.3%) | 3 (14.3%) | 1 (4.2%) | 6 (25.0%) | |||
| Daytime | Increased | 1 (2.2%) | 33 (73.3%) | 0 | 14 (66.7%) | 1 (4.2%) | 19 (79.2%) | ||
| Decreased | 44 (97.8%) | 8 (17.8%) | 21 (100%) | 5 (23.8%) | 23 (95.8%) | 3 (12.5%) | |||
| Normal | 0 | 4 (8.9%) | 0 | 2 (9.5%) | 0 | 2 (8.3%) | |||
| Nighttime | Increased | 6 (13.3%) | 12 (26.6%) | 2 (9.5%) | 4 (19.0%) | 4 (16.7%) | 8 (33.3%) | ||
| Decreased | 30 (66.7%) | 26 (57.8%) | 15 (71.5%) | 14 (66.7%) | 15 (62.5%) | 12 (50.0%) | |||
| Normal | 9 (20.0%) | 7 (15.6%) | 4 (19.0%) | 3 (14.3%) | 5 (20.8%) | 4 (16.7%) | |||
EPS, epigastric pain syndrome; PDS, postprandial syndrome; HF, high-frequency; LF, low-frequency.
Mean HF (msec2) and mean LF/HF ratio in the daytime in FD patients
Next, each of the indicators was tested in the daytime and nighttime, during which ANS balance shows considerable changes. In entire FD group patients, the HF component in the daytime was low in 97.8% (44/45) of patients, and the LF/HF ratio was high in 73.3% (33/45) of patients. In the PDS-type FD group, the daytime HF component was low in 95.8% (23/24) of patients, and the daytime LF/HF ratio was high in 79.2% (19/24) of patients. In the EPS-type FD group, the daytime HF component was low in 100% (21/21) of patients, and the daytime LF/HF ratio was high in 66.7% (14/21) of patients (Table 2).
Mean HF (msec2) and mean LF/HF ratio in the nighttime in FD patients
In entire FD group patients, the HF component in the nighttime was low in 66.7% (30/45) of patients, and the LF/HF ratio in the nighttime was high in 26.6% (12/45) of patients. In the PDS-type FD group, the nighttime HF component was low in 62.5% (15/24) of patients, and the nighttime LF/HF ratio was high in 33.3% (8/24) of patients. In the EPS-type FD group, the nighttime HF component was low in 71.5% (15/21) of patients, and the nighttime LF/HF ratio was high in 19.0% (4/21) of patients (Table 2).
Mean 24-h ANS modulation and symptoms in FD patients
The entire FD group was divided into patients who exhibited ANS modulation (n = 25) and those (n = 9) who did not, and their respective GSRS scores and anxiety scores were compared. The results showed a tendency towards high GSRS total scores (p = 0.085) (Table 3).
Table 3.
Comparison of dyspeptic symptoms and anxiety between imbalanced and balanced HF in patients with FD
| HF component |
p value | ||
|---|---|---|---|
| Modulation (n = 25) | Normal (n = 9) | ||
| GSRS score | |||
| Total | 13.6 ± 6.3 | 9.2 ± 8.2 | 0.085 |
| Reflux | 2.0 ± 1.6 | 2.2 ± 2.3 | 0.88 |
| Abdominal pain | 3.4 ± 1.8 | 2.8 ± 3.4 | 0.31 |
| Indigestion | 4.2 ± 2.9 | 3.5 ± 3.8 | 0.49 |
| State-anxiety | 53.5 ± 14.6 | 48.2 ± 11.4 | 0.42 |
| Trait-anxiety | 52.0 ± 12.5 | 54.4 ± 8.5 | 0.64 |
HF, high-frequency.
Level of recovery in HF and LF/HF values in the FD patients at 120 min after lunch
Meals are one of the typical events that affect ANS balance during the day. The present study therefore investigated changes in ANS balance after lunch, and the level of recovery at 120 min after eating. In the control group, the HF component and the LF/HF ratio recovered to pre-prandial levels in 88.9% (8/9). However, the HF component did not recover to pre-prandial levels in 38.2% (13/34) of FD patients, and the LF/HF ratio failed to recover to pre-prandial levels in 44.1% (15/34) of FD patients (Table 4). A comparison of GI symptoms in the 13 patients whose postprandial HF component recovered and the 21 patients whose HF component did not recover revealed a greater tendency towards indigestion scores in the group with no recovery (p = 0.061) (Table 5).
Table 4.
Ratio of normal response of ANS to meal intake
| HF component | LF/HF ratio | |
|---|---|---|
| Healthy controls (n = 9) | 8 (88.9%) | 8 (88.9%) |
| FD patients (n = 34) | 13 (38.2%) | 15 (44.1%) |
HF, high-frequency; LF, low-frequency.
Table 5.
Comparison of dyspeptic symptoms between modulated and normal HF in patients with FD
| HF component |
p value | ||
|---|---|---|---|
| Modulation (n = 21) | Normal (n = 13) | ||
| GSRS score | |||
| Total | 13.6 ± 6.1 | 11.1 ± 7.9 | 0.36 |
| Reflux | 2.3 ± 1.5 | 1.6 ± 1.9 | 0.17 |
| Abdominal pain | 3.4 ± 2.0 | 3.2 ± 2.6 | 0.69 |
| Indigestion | 4.8 ± 2.6 | 3.0 ± 3.3 | 0.061 |
HF, high-frequency.
Comparison of HF and LF/HF in the FD patients after ANS stimulus tests (cold pressor and mental arithmetic tests)
Two of the 16 patients (12.5%) who underwent the cold pressor test showed a delayed recovery after stimulus. Moreover, 2 of the 13 patients (15.4%) who underwent the mental arithmetic test did not recover to baseline levels (Table 6).
Table 6.
Ratio of ANS response to stimulation in FD patients
| HF component | LF/HF ratio | ||
|---|---|---|---|
| Cold pressure test (n = 16) | Normal | 14 (87.5%) | 14 (87.5%) |
| Modulation | 2 (12.5%) | 2 (12.5%) | |
| Mental arithmetic test (n = 13) | Normal | 11 (84.6%) | 11 (84.6%) |
| Modulation | 2 (12.5%) | 2 (12.5%) | |
HF, high-frequency; LF, low-frequency.
Comparison of HF, LF/HF ratio and GI symptoms in the FD patients after oral administration of the autonomic drug (tofisopam)
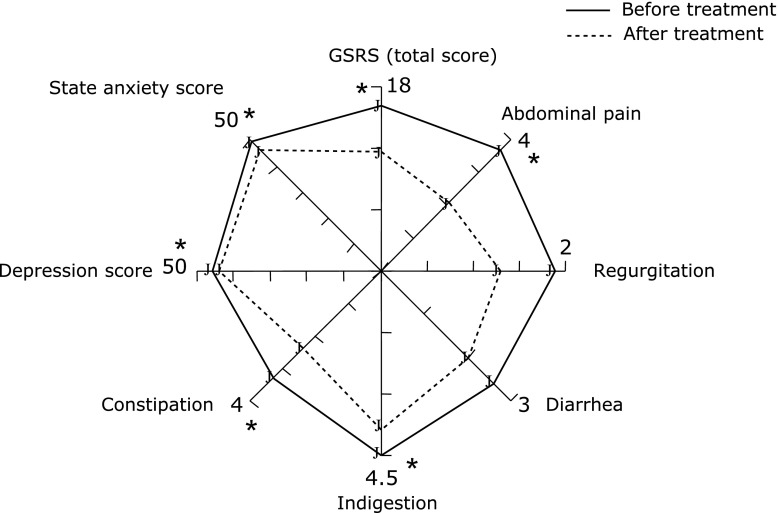
In FD patients treated with the autonomic drug (tofisopam), a change to within reference levels occurred in 4 patients (including those in whom both sympathetic nervous system modulation and parasympathetic nervous system modulation were within reference levels), and a tendency towards recovery was seen in 6 patients. Conversely, only 1 patient showed deterioration (Fig. 1). A comparison of symptoms among all 14 patients indicated a significant improvement in total scores and abdominal pain/indigestion scores (Fig. 2).
Fig. 1.
Changes in HF component and LF/HF ratio by treatment of the autonomic drug tofisopam. Fourteen FD patients were treated with the autonomic drug tofisopam. Change to within reference levels (including those in whom both sympathetic nervous system modulation and parasympathetic nervous system modulation were within reference levels) occurred in 4 patients, and a tendency towards recovery was seen in 6 patients. Conversely, only 1 patient showed deterioration.
Fig. 2.
Rader chart of the GSRS, SDS, and anxiety scores by treatment of the autonomic drug tofisopam. Among 14 patients, there were significant differences in total GSRS scores, abdominal pain/indigestion scores, and psychological scores. *p<0.05 vs before treatment.
Discussion
Analysis of HRV at the very least enables observation of circadian variations in cardiac parasympathetic nerve function, and the existence of inherent circadian rhythms has also been reported.(16) Physiological phenomena in humans (including physiological functions unique to the GI tract, such as GI motility or gastric acid secretion) show these kinds of rhythmic changes, and the ANS is a mechanism that is known to be involved in controlling these rhythms. Therefore, when considering the regularity of these rhythms, or when considering daytime and nighttime rhythms as separate entities, any investigation of nerve balance in sympathetic and parasympathetic nervous system activities with their reciprocal actions within the ANS must incorporate all ANS function rhythms. Therefore, investigating the association of abdominal symptoms with ANS function is very significant when considering the pathophysiology of FD.
In the present study, the majority of FD patients had diminished parasympathetic nervous system activity, and approximately half of the FD patients had enhanced sympathetic nervous system activity. These findings were more pronounced during daytime activity than during nighttime. In contrast, the parasympathetic nervous system indicator that should have been increased during nighttime was actually inhibited, and the sympathetic nervous system activity during nighttime was also increased in approximately one-third of FD patients. In other words, the sympathetic nervous system constituted the predominant form of ANS function activity in FD patients throughout the entire 24-h period, whereas the parasympathetic nervous system tended to be subdued. When considering this finding from the perspective of the stomach (as the representative organ of the upper GI tract), this pattern of nervous activity would cause reductions in gastric motility and gastric acid secretion. In the lower GI tract, it could trigger symptoms such as reduced peristalsis, upper abdominal bloating and discomfort, anorexia, and constipation. In addition, while GI function during nighttime should be active due to food absorption and other physiological functions, in the present study it was diminished, implying impaired excretory function as a result of diminished food digestion or GI motility. Previous studies have reported that delayed gastric emptying is involved in 30–60% of FD cases,(25–27) and the findings of the present study appear to support this assertion. As an event capable of altering ANS function, meals typically trigger an increase in parasympathetic nervous system activity. However, this response did not occur in approximately 40% of the FD patients. Furthermore, postprandial sympathetic nerve activity during 120 min was also not seen in approximately 44% of FD patients (data not shown). These findings could signify a decline in responsiveness to external ANS activity stimuli. Put simply, in PDS-type FD, which is purportedly closely linked to diet, these declines in ANS responsiveness may be involved in the appearance of symptoms such as postprandial fullness. This association is strongly suggested by the fact that a part of FD patients who experienced a meal-induced decline in ANS function responsiveness also reported potent GI symptoms. Although ANS balance is modified by meals, it must recover to pre-prandial levels within a certain period in order for homeostasis to be maintained. This decline in the ability to recover to pre-prandial levels could imply a decline in the ability of ANS balance to recover in response to meals, which are very common and routine stimuli affecting ANS function. It is intriguing that this recuperative vulnerability was also observed after the two ANS stimulus tests. FGIDs such as FD and irritable bowel syndrome are response abnormalities and functional alterations to external stimuli such as meals, stress, and temperature variation.(28,29) In this context, the findings of the present study appear to imply a decline in ANS adaptability. Thus, the findings may support the putative association between degree of dyspeptic symptoms and the ANS.
However, the present study also leaves several unanswered questions. The first is whether HRV was measured on a day that typified homeostasis among the patients; the measurements need to be performed several times on the same patients to confirm the repeatability of the results. The second is that this study was conducted from the perspective of investigating cardiac parasympathetic nerve function, so the results are not necessarily consistent with the level of ANS activity in the GI tract. The lack of currently-available test methods capable of addressing both of these functions means that tests enabling measurement of HRV and ANS function of the GI tract need to be conducted at the same time. More detailed research on the ANS that improves on or compensates for these study limitations is essential if not indispensable for analyzing and elucidating the pathophysiology of FD and for establishing viable therapeutic strategies. A more drug intervention studies of FD patients evaluating the ANS before and after administration of autonomic drugs as well as tofisopam would also contribute to the development of future treatment strategies.
Conclusions
Imbalance of ANS function and vulnerability for recovery from external stimuli were observed in FD patients, which may underlie the pathogenesis of FD causing dyspeptic symptoms. Autonomic drugs such as tofisopam may be effective for FD treatment.
Conflict of Interest
Tetsuo Arakawa has participated in advisory committees for Otsuka Pharmaceutical Co., Ltd. and Eisai Co., Ltd. Yasuhiro Fujiwara has participated in advisory committees for Eisai Co., Ltd. All other authors declare that they have no conflicts of interest.
References
- 1.Drossman DA. The functional GI disorders and the Rome III process. Gastroenterology. 2006;130:1377–1390. doi: 10.1053/j.gastro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Tominaga K, Arakawa T. Kampo medicines for gastrointestinal tract disorders: a review of basic science and clinical evidence and their future application. J Gastroenterol. 2013;48:452–462. doi: 10.1007/s00535-013-0788-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tahara T, Shibata T, Wang F, Yamashita H, Hirata I, Arisawa T. Genetic polymorphisms of molecules associated with innate immune responses, TRL2 and MBL2 genes in Japanese subjects with functional dyspepsia. J Clinl Biochem Nutr. 2010;47:217–223. doi: 10.3164/jcbn.10-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miwa H, Kusano M, Arisawa T, et al. Evidence-based clinical practice guidelines for functional dyspepsia. J Gastroenterol. 2015;50:125–139. doi: 10.1007/s00535-014-1022-3. [DOI] [PubMed] [Google Scholar]
- 5.Iwakiri R, Tominaga K, Furuta K, et al. Randomised clinical trial: rabeprazole improves symptoms in patients with functional dyspepsia in Japan. Aliment Pharmacol Ther. 2013;38:729–740. doi: 10.1111/apt.12444. [DOI] [PubMed] [Google Scholar]
- 6.Matsueda K, Hongo M, Tack J, Saito Y, Kato H. A placebo-controlled trial of acotiamide for meal-related symptoms of functional dyspepsia. Gut. 2012;61:821–828. doi: 10.1136/gutjnl-2011-301454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goyal RK, Hirano I. The enteric nervous system. N Engl J Med. 1996;334:1106–1115. doi: 10.1056/NEJM199604253341707. [DOI] [PubMed] [Google Scholar]
- 8.Tominaga K, Tsumoto C, Ataka S, et al. Regional brain disorders of serotonin neurotransmission are associated with functional dyspepsia. Life Sci. 2015;137:150–157. doi: 10.1016/j.lfs.2015.07.023. [DOI] [PubMed] [Google Scholar]
- 9.Mayer EA, Aziz Q, Coen S, et al. Brain imaging approaches to the study of functional GI disorders: a Rome working team report. Neurogastroenterol Motil. 2009;21:579–596. doi: 10.1111/j.1365-2982.2009.01304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takahashi T, Owyang C. Characterization of vagal pathways mediating gastric accommodation reflex in rats. J Physiol. 1997;504 (Pt 2):479–488. doi: 10.1111/j.1469-7793.1997.479be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asakawa A, Inui A, Kaga T, et al. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology. 2001;120:337–345. doi: 10.1053/gast.2001.22158. [DOI] [PubMed] [Google Scholar]
- 12.Raybould HE, Taché Y. Cholecystokinin inhibits gastric motility and emptying via a capsaicin-sensitive vagal pathway in rats. Am J Physiol. 1988;255 (2 Pt 1):G242–G246. doi: 10.1152/ajpgi.1988.255.2.G242. [DOI] [PubMed] [Google Scholar]
- 13.Troncon LE, Thompson DG, Ahluwalia NK, Barlow J, Heggie L. Relations between upper abdominal symptoms and gastric distension abnormalities in dysmotility like functional dyspepsia and after vagotomy. Gut. 1995;37:17–22. doi: 10.1136/gut.37.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drossman DA. The functional gastrointestinal disorders and the Rome II process. Gut. 1999;45 (Suppl 2):1–5. doi: 10.1136/gut.45.2008.ii1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tack J, Talley NJ, Camilleri M, et al. Functional gastroduodenal disorders. Gastroenterology. 2006;130:1466–1479. doi: 10.1053/j.gastro.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 16.Buysschaert M, Donckier J, Dive A, Ketelslegers JM, Lambert AE. Gastric acid and pancreatic polypeptide responses to sham feeding are impaired in diabetic subjects with autonomic neuropathy. Diabetes. 1985;34:1181–1185. doi: 10.2337/diab.34.11.1181. [DOI] [PubMed] [Google Scholar]
- 17.Mourot L, Bouhaddi M, Regnard J. Effects of the cold pressor test on cardiac autonomic control in normal subjects. Physiol Res. 2009;58:83–91. doi: 10.33549/physiolres.931360. [DOI] [PubMed] [Google Scholar]
- 18.Yoshino K, Matsuoka K. Causal coherence analysis of heart rate variability and systolic blood pressure variability under mental arithmetic task load. Biol Psychol. 2005;69:217–227. doi: 10.1016/j.biopsycho.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 20.Choi JB, Hong S, Nelesen R, et al. Age and ethnicity differences in short-term heart-rate variability. Psychosom Med. 2006;68:421–426. doi: 10.1097/01.psy.0000221378.09239.6a. [DOI] [PubMed] [Google Scholar]
- 21.Otsuka K, Nishimun Y, Kubo Y, Cornelissen G, Halberg F. Chronomes (rhythms, chaos, and age trends) of human heart rate variability in both genders. Comput Cardiol. 1997;24:49–52. [Google Scholar]
- 22.Otsuka K. Chronome & Janus-medicine: Heart Rate Variability (HRV) and BP Variavility (BPV) from a Viewpoint of Chronobiology and Ecology. Tokyo: Medical Review; 1998. pp. 70–77. (In Japanese) [Google Scholar]
- 23.Tominaga K, Higuchi K, Iketani T, et al. Comparison of gastrointestinal symptoms and psychological factors of functional dyspepsia to peptic ulcer or panic disorders patients. Inflammopharmacology. 2007;15:84–89. doi: 10.1007/s10787-006-0011-4. [DOI] [PubMed] [Google Scholar]
- 24.Tominaga K, Higuchi K, Ochi M, et al. Concurrent assessment of reservoir and emptying of the stomach for dyspepsia patients. Hepatogastroenterology. 2008;55:744–749. [PubMed] [Google Scholar]
- 25.Ochi M, Tominaga K, Tanaka F, et al. Clinical classification of subgroups according to the Rome III criteria cannot be used to distinguish the associated respective pathophysiology in Japanese patients with functional dyspepsia. Intern Med. 2013;52:1289–1293. doi: 10.2169/internalmedicine.52.9205. [DOI] [PubMed] [Google Scholar]
- 26.Urbain JL, Vekemans MC, Parkman H, et al. Dynamic antral scintigraphy to characterize gastric antral motility in functional dyspepsia. J Nucl Med. 1995;36:1579–1586. [PubMed] [Google Scholar]
- 27.Kamino D, Manabe N, Hata J, Haruma K, Tanaka S, Chayama K. Long-term ultrasonographic follow-up study of gastric motility in patients with functional dyspepsia. J Clin Biochem Nutr. 2008;42:144–149. doi: 10.3164/jcbn.2008021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carbone F, Holvoet L, Tack J. Rome III functional dyspepsia subdivision in PDS and EPS: recognizing postprandial symptoms reduces overlap. Neurogastroenterol Motil. 2015;27:1069–1074. doi: 10.1111/nmo.12585. [DOI] [PubMed] [Google Scholar]
- 29.Fujikawa Y, Tominaga K, Tanaka F, et al. Enteric glial cells are associated with stress-induced colonic hyper-contraction in maternally separated rats. Neurogastroenterol Motil. 2015;27:1010–1023. doi: 10.1111/nmo.12577. [DOI] [PubMed] [Google Scholar]