Abstract
Folic acid supplementation may meliorate cardiovascular disease risk by improving vascular endothelial structure and function. However, the underlying mechanisms are still lack of a global understanding. To be used, folic acid must be converted to 7,8-dihydrofolate by dihydrofolate reductase to generate one-carbon derivatives serving as important cellular cofactors in the synthesis of nucleotides and amino acids required for cell growth. Therefore, this study explored the effect of dihydrofolate reductase knockdown on endothelial EA.hy926 cell growth and the mechanism involved. We found that down-regulation of dihydrofolate reductase inhibited EA.hy926 cell proliferation, and induced G1 phase arrest. Meanwhile, the expression of regulators necessary for G1/S phase transition, such as cyclin-dependent kinases CDK2, CDK4 and CDK6, were remarkably down-regulated; by contrast, the cell cycle inhibitors p21waf/cip1, p27Kip1 and p53 were significantly up-regulated after dihydrofolate reductase knockdown. Furthermore, supplementation of 5-methyltetrahydrofolate to the dihydrofolate reductase knockdown cells could weaken the inhibitory effect of dihydrofolate reductase knockdown on cell proliferation, simultaneously, inducing the expression of p53 and p21waf/cip1 falling back moderately. Our findings suggest that attenuating dihydrofolate reductase may cause imbalanced expression of cell cycle regulators, especially up-regulation of p53-p21waf/cip1 pathway, leading to G1 cell cycle arrest, thereby inhibiting the growth of endothelial EA.hy926 cells.
Keywords: dihydrofolate reductase, endothelial cell, G1 cell cycle, p21waf/cip1, p53
Introduction
Folic acid (FA) and folate are forms of a water-soluble B vitamin. The former one is a synthetic provitamin, while the latter is found in foods. They are metabolized via the folate cycle in vivo. Dihydrofolate reductase (DHFR, EC 1.5.1.3) is an enzyme that catalyzes the reduction of 7,8-dihydrofolate (7,8-DHF) to 5,6,7,8-tetrahydrofolate (5,6,7,8-THF) using NADPH as a cofactor, and has a pivotal role in folate-based reactions.(1) Since FA has been synthesized, DHFR has taken on another role to initiate FA metabolism by reducing it to 7,8-DHF.(2) The folate cycle generates a series of folate derivatives (FDs), which participate in single-carbon transfer in the biosynthesis of nucleotides and amino acids, as well as in the methylation of DNA, proteins and lipids.(1,3)
One type of FD, 5-methyltetrahydrofolate (5-MTHF), is used for the methylation of homocysteine to methionine.(3) Higher concentration of homocysteine in blood is significantly correlated with cardiovascular disease, potentially causing vascular endothelial dysfunction that promotes the occurrence and development of coronary artery disease.(4,5) Studies have demonstrated that combined FA (0.4–5 mg, daily) and vitamin B6, B12 or E therapy may derease plasma homocysteine concentration and improve vascular endothelial structure and function.(6–9) Short-term FA supplementation (4-week FA, 5 mg/day) was also able to enhance endothelial function and decrease blood pressure in young chronic smokers, suggesting that a simple, nontoxic, and relatively inexpensive vitamin intervention may be useful in primary cardiovascular prevention.(10)
To some extent, the beneficial effects of increasing FA intake in patients with cardiovascular disease may be associated with reduction of homocysteinemia and enhancement of plasma antioxidant capacity. However, there is also evidence that FA appears to exert beneficial effects on endothelial function that are not entirely dependent on its homocysteine-lowering effects.(10) Therefore, the detailed mechanisms involved in FA protection of vascular endothelium still need to be elucidated. Since DHFR is the key enzyme for initiation of FA metabolism to produce the FDs, which act as important cellular cofactors essential for cell growth, we here investigated the effect of knockdown DHFR on endothelial EA.hy926 cell growth and the mechanism involved in this effect.
Materials and Methods
Chemicals and reagents
Primary antibodies for detecting DHFR (15194-1-AP), β actin (60008-1-Ig), CDK2 (10122-1-AP), CDK4 (11026-1-AP), CDK6 (14052-1-AP), Cyclin D1 (60186-1-Ig), Cyclin E (11554-1-AP), and p21waf/cip1 (10355-1-AP) were purchased from Proteintech (Chicago, IL). Antibodies for detection of p27Kip1 (3686), p53 (2527), NF-κB/p65 (6956), phospho-NF-κB/p65 (Ser468) (3039), phospho-NF-κB/p65 (Ser536) (3033), Akt (4691), phospho-Akt (4060), p38 MAPK (9212), phospho-p38 MAPK (4511), p44/42 MAPK (Erk1/2) (4695), phospho-p44/42 MAPK (Erk1/2) (4370), SAPK/JNK (9258), and phospho-SAPK/JNK (4668) were purchased from Cell Signaling Technology (Danvers, MA). Horseradish peroxidase (HRP)-linked secondary antibodies, anti-rabbit IgG (7074P2) and anti-mouse IgG (7076P2), were obtained from Cell Signaling Technology. RIPA lysis buffer (P0013B), phenylmethanesulfonyl fluoride (PMSF) (ST506), and primary antibody dilution buffer (P0023A) were purchased from Beyotime Institute of Biotechnology (Shanghai, China). Protease inhibitor cocktail (BS387) was purchased from Sangon Biotech (Shanghai, China). 5-Methyltetrahydrofolic acid disodium salt (M0132) were obtained from Sigma-Aldrich (St. Louis, MO).
Cell culture
Human endothelial cell line EA.hy926 was purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). Cells were maintained at 37°C under 5% CO2 in high glucose Dulbecco’s modified Eagle’s medium (Thermo Scientific, Waltham, MA) supplemented with 10% fetal bovine serum (Biochrom GmbH, Berlin, Germany).
DHFR knockdown by small RNA interference (siRNA)
DHFR-specific siRNA (DHFR-siRNA) and the negative control siRNA (NC-siRNA) were purchased from Biotend Company (Shanghai, China). The following siRNA sequences were used: DHFR-siRNA1: sense, 5'-GUC UAG AUG AUG CCU UAA ATT-3', and antisense, 5'-UUU AAG GCA UCA UCU AGA CTT-3' (corresponding to bases 770–788 of human DHFR mRNA, GenBank accession number NM_000791.3). DHFR-siRNA2: sense, 5'-UUG GUU CGC UAA ACU GCA UTT-3', and antisense, 5'-AUG CAG UUU AGC GAA CCA ATT-3' (corresponding to bases 497–515 of human DHFR mRNA, GenBank accession number NM_000791.3). NC-siRNA: sense, 5'-CUU ACG CUG AGU ACU UCG ATT-3', and antisense, 5'-UCG AAG UAC UCA GCG UAA GTT-3'.
RNA Interference was according to the previous procedure(11) with modifications. After seeding into six-well plates for 24 h, cells were transfected with DHFR-specific siRNA (100 nM) or NC-siRNA (100 nM) using lipofectamine 2000 according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA). Cells were then cultured for 72 h, and gene silencing was detected by western blot analysis of DHFR protein expression.
Cell proliferation assay
In order to determine the effect of DHFR knockdown on EA.hy926 cell growth in vitro, cells were plated in 96-well plates with 100 µl of medium containing 2.5 × 103 cells per well, with six parallel wells for each siRNA. After 24 h, 100 nM of siRNAs were transfected into the cells, respectively. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) dye reduction assay as previously described(11) with some modifications was used to test cell viability on 1, 3, and 5 days post-transfection. 10 µl of MTT (5 mg/ml) was added to each well, and the cells were incubated for 4 h at 37°C. Then, the culture medium was removed, and 100 of µl dimethyl sulfoxide was added to each well. After shaking thoroughly for 10 min, the absorbance of each well was read in a microplate reader (BioTek ELX800, BioTek Instruments Inc., Winooski, VT) at 490 nm. Experiments were performed in triplicate.
Analysis of cell cycle by flow cytometry
Cells were seeded in six-well plates at a density of 3.5 × 105 cells per well, with three parallel wells for each siRNA. After transfection with siRNA, cells were subsequently cultured for 72 h, collected, and centrifuged at 200 × g for 5 min. Cell pellets were re-suspended in 500 µl of ice-cold 70% ethanol and fixed for at least 24 h at –20°C. To detect cellular DNA content, fixed cells were centrifuged at 200 × g for 5 min, then re-suspended in PBS containing ribonuclease A and stained with propidium iodide for 30 min at room temperature. The percentages of cells in G1, S, and G2/M phases of the cell cycle were analyzed with FACSCalibur flow cytometer (BD Co., Franklin Lakes, NJ). Experiments were performed in triplicate.
Western blot analysis
Western blot was performed as previously described(12) with modifications. Briefly, cells were washed with pre-cold PBS, then lysed in RIPA lysis buffer containing phenylmethylsulfonyl fluoride (PMSF) and protease inhibitor cocktail, then centrifuged for 10 min at 14,000 × g under 4°C. Equal amounts of cellular proteins were resolved with SDS-PAGE sample loading buffer. After thermal denaturation for 5 min at 95°C, samples were electrophoresed on 10% SDS-PAGE mini gels, with 20 µg total protein load per lane, and then transferred to polyvinylidene difluoride (PVDF) membranes. Membranes were probed overnight at 4°C with targeted primary antibodies (diluted in primary antibody dilution buffer according to the manufacturer’s instructions), and blotted with the corresponding secondary antibodies (diluted 1:3,000 in blocking buffer with 0.05% Tween-20 and 0.5% bovine serum albumin). Signals were detected by ECL Western Blotting Systems (GE Healthcare, Little Chalfont, Buckinghamshire, UK) according to the manufacturer’s recommendation. The membranes were imaged using the BioRad ChemiDocTM XRS+ System. Densitometry was performed using Image LabTM software (BioRad, Hercules, CA). The value of density ratio (target protein to β-actin in the same sample) represented the relative level of protein expression. Experiments were performed in triplicate.
Supplementation of 5-MTHF to DHFR knockdown cells
Since 5-MTHF, an important biologically active compound of FD, can function as FA within cells.(13) We therefore examined that whether supplementation of 5-MTHF to DHFR knockdown cells could reverse the effect of DHFR knockdown. 5-MTHF was dissolved in PBS at concentration of 2 mM, and added to the cells transfected with DHFR-specific siRNA at final concentrations of 50, 100, and 200 µM, respectively. An equal volume of PBS was given as control (0 µM). Cell proliferation, cell cycle analysis and western blot were performed as described above.
Statistical analysis
Data were expressed as mean ± SD. Student’s t test was used to determine significant differences between compared groups. Values of p<0.05 were considered statistically different.
Results
Down-regulation of DHFR by siRNA transfection inhibited the proliferation of endothelial EA.hy926 cells
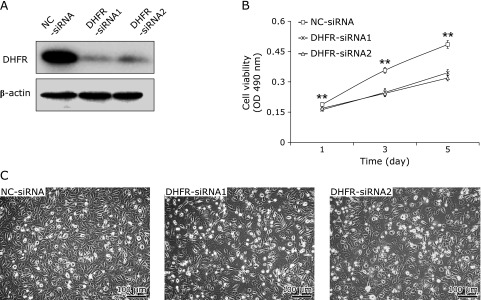
DHFR-siRNAs and NC-siRNA were transfected into EA.hy926 cells. DHFR expression was then evaluated by western blot. At 72 h post-transfection, the protein expression level of DHFR was significantly reduced in EA.hy926 cells transfected with DHFR-siRNAs compared with those transfected with the NC-siRNA (Fig. 1A). To test the effect of DHFR RNAi on EA.hy926 cell proliferation, cell growth was analyzed by the MTT assay. Down-regulation of DHFR had an obvious inhibitory effect on EA.hy926 cell growth as compared to controls (p<0.01, Fig. 1B and C). On days 3 and 5 after DHFR down-regulation, the cell viability was decreased by 30%. The growth inhibitory effect of DHFR knockdown by RNAi is agreed with the observation of previous study. Santiago et al.(14) performed gene knockout of DHFR in a Chinese hamster ovary (CHO) cell line by using engineered zinc-finger nucleases, which resulted in DHFR negative cells unable to grow without thymidine (HT), because lack of DHFR disrupts the reduction of folate during the biosynthesis of purines, thymidine, and glycine.
Fig. 1.
Effect of DHFR knockdown on EA.hy926 cell proliferation. DHFR knockdown in EA.hy926 cells was accomplished by transfection with specific siRNA targeting at DHFR (DHFR-siRNA1/2) or by non-targeting negative control (NC-siRNA). (A) The interference effect was detected with DHFR antibody (dilution 1:800) by western blot for protein expression, at 72 h post-transfection. β-actin served as internal loading control (dilution 1:8,000). (B) Cell viability was measured by MTT assay on days 1, 3, and 5 post-transfection. Experiments were performed in triplicate and data were given as mean ± SD. Representative figure from one out of three experiments is shown. (C) Optical microscopy photos of cells on days 3 post-transfection. Bar scale at low-left = 100 µm. **vs NC-siRNA, p<0.01.
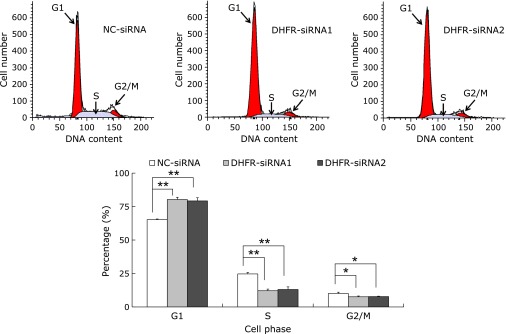
DHFR knockdown induced EA.hy926 cell arresting in G1 phase cell cycle
Down-regulation of DHFR suppressed the growth of EA.hy926 cells. To elaborate the mechanism of this inhibitory effect, we further examined the cell cycle of EA.hy926 by flow cytometry. Knockdown of DHFR by specific siRNA transfection induced G1 phase arrest in EA.hy926 cells (Fig. 2). After 72 h, the cells transfected with DHFR-siRNAs in G1 phase were significantly increased compared with controls (p<0.01); meanwhile cells in S and G2/M phases were significantly decreased (p<0.01 for S phase, p<0.05 for G2/M phase). Cell populations in G1, S and G2/M phases were 65.27 ± 0.49, 24.68 ± 1.08 and 10.05 ± 0.97%, respectively, in cells transfected with NC-siRNA; and 80.29 ± 1.66, 12.11 ± 1.20 and 7.60 ± 0.56%, respectively, in DHFR-siRNA1-transfected cells; and 79.25 ± 2.32, 13.04 ± 1.98 and 7.71 ± 0.36%, respectively, in DHFR-siRNA2-transfected cells. These results indicated that DHFR exhibited important role in regulation of G1/S cell cycle transition.
Fig. 2.
DHFR knockdown affected the cell cycle of EA.hy926. After transfection with siRNA for 72 h, cells were collected and stained with propidium iodide, and the distribution of cell cycle fractions (G1, S and G2/M phase) were then detected by flow cytometry. The number of gated cells in G1, G2/M, or S phase is presented as a percentage of total cells (%). Percentages of populations in each cell cycle phase were averaged from three parallel experiments. *vs NC-siRNA, p<0.05; **vs NC-siRNA, p<0.01.
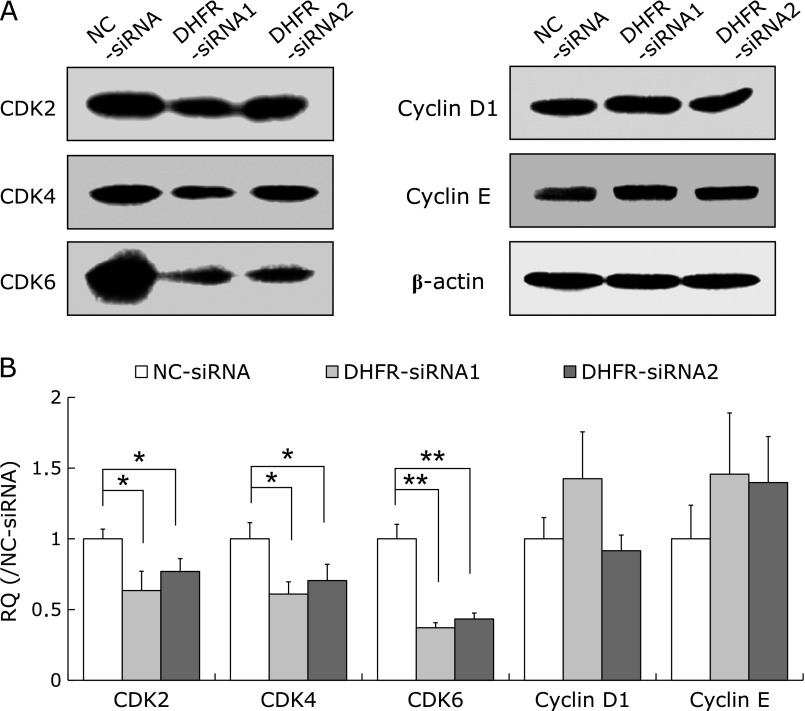
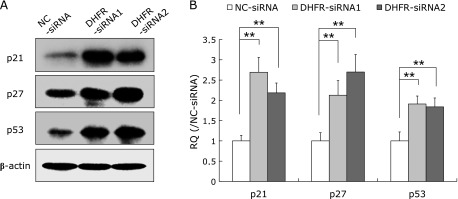
DHFR knockdown down-regulated CDK2, CDK4 and CDK6, while up-regulated p21waf/cip1, p27Kip1 and p53
As DHFR knockdown results in G1 phase arrest, we investigated whether the expression of several key factors regulating G1/S cell cycle transition were affected. Results showed that the expression of cyclin D1 and cyclin E were not observably affected at 72 h post-transfection, while the expression of cyclin-dependent kinases CDK2, CDK4 and CDK6 were remarkably decreased, in particular, CDK6 was down-regulated by approximately 60% (vs control, p<0.01) (Fig. 3); meanwhile, cell cycle inhibitors such as p21waf/cip1, p27Kip1, and p53 were up-regulated by about two-folds after DHFR knockdown (vs control, p<0.01, Fig. 4).
Fig. 3.
Effect of DHFR knockdown on the expression of CDK2, CDK4, CDK6, cyclins D1 and E. At 72 h post-transfection with siRNA, cells were lysed with RIPA lysis buffer, and the lysates were then analyzed by western blot with specific antibodies (dilution 1:800). β-actin served as internal loading control (dilution 1:8,000). (A) Representative gels from 3 experiments are shown as protein expression levels of cyclin-dependent kinases CDK2, CDK4 and CDK6, as well as cyclins D1 and E. (B) Densitometric analysis was performed for each protein band relative to β-actin in the same sample using Image LabTM software. To compare the relative quantity (RQ) of proteins between NC-siRNA and DHFR-siRNA groups, the values of density ratio in NC-siRNA group were normalized to 1.00. *vs NC-siRNA, p<0.05; **vs NC-siRNA, p<0.01.
Fig. 4.
DHFR knockdown up-regulated the expression of p21waf/cip1, p27Kip1 and p53. After DHFR knockdown by siRNA for 72 h, the cell lysates were analyzed by western blot with specific antibodies (dilution 1:500). β-actin served as internal loading control (dilution 1:8,000). (A) Representative gels from 3 experiments are shown as the protein expression levels of p21waf/cip1, p27Kip1 and p53. (B) Densitometric analysis was performed for each protein band relative to β-actin in the same sample using Image LabTM software. To compare the relative quantity (RQ) of proteins between NC-siRNA and DHFR-siRNA groups, the values of density ratio in NC-siRNA group were normalized to 1.00. **vs NC-siRNA, p<0.01.
Effect of DHFR knockdown on the expression and phosphorylation of MAPKs, NF-κB and AKT
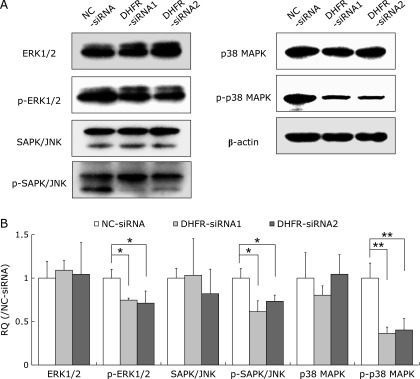
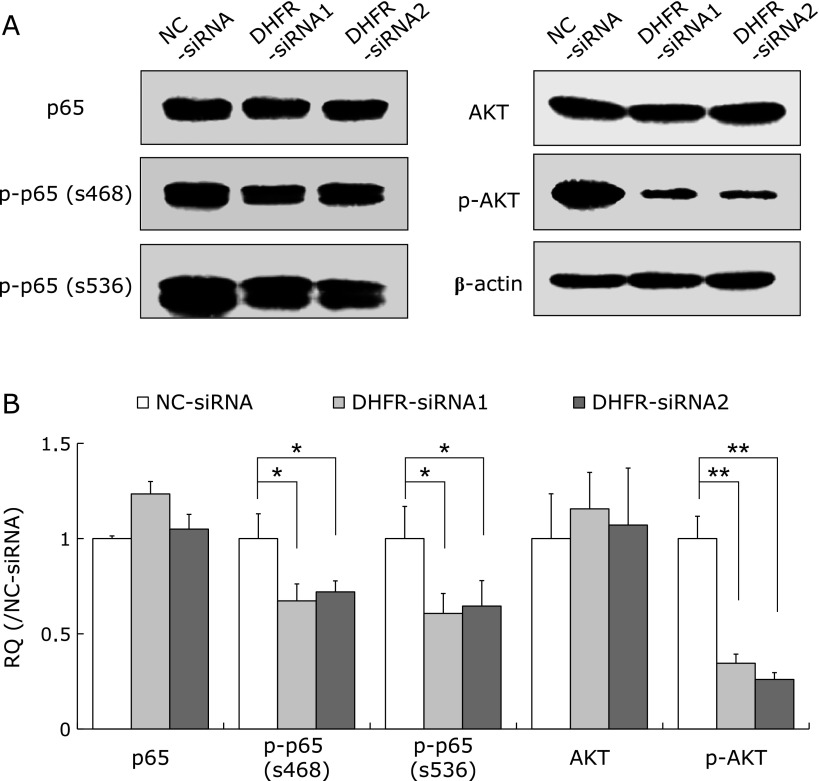
To further investigate the downstream molecules regulating the effects of DHFR knockdown on cell proliferation, we performed western blot to examine the expression and phosphorylation of proteins in several important signaling pathways, e.g., mitogen-activated protein kinases (MAPKs), nuclear factor-κB (NF-κB) and protein kinase B (AKT). Results showed that DHFR knockdown reduced the phosphorylation levels of MAPKs, including ERK1/2, SAPK/JNK and p38 (Fig. 5), as well as NF-κB/p65 and AKT (Fig. 6), at 72 h post-transfection. Especially, the activities of p38 and AKT were reduced to 40% and 30% (vs control, p<0.01), respectively.
Fig. 5.
DHFR knockdown impacts MAPKs activity. The expression and phosphorylation levels of mitogen-activated protein kinases (MAPKs), including ERK1/2, SAPK/JNK and p38, were tested by western blot with specific antibodies (dilution 1:1,000) after DHFR knockdown for 72 h. β-actin served as internal loading control (dilution 1:8,000). (A) Representative gels from 3 experiments are shown. “p-” means phosphorylated state. (B) Densitometric analysis was performed for each protein band relative to β-actin in the same sample using Image LabTM software. To compare the relative quantity (RQ) of proteins between NC-siRNA and DHFR-siRNA groups, the values of density ratio in NC-siRNA group were normalized to 1.00. *vs NC-siRNA, p<0.05; **vs NC-siRNA, p<0.01.
Fig. 6.
Effect of DHFR knockdown on the expression and phosphorylation of nuclear factor-κB/p65 (NF-κB/p65) and AKT. The expression and phosphorylation levels of NF-κB/p65 and protein kinase B (AKT) were tested by western blot with specific antibodies (dilution 1:500) after DHFR knockdown for 72 h. β-actin served as internal loading control (dilution 1:8,000). (A) Representative gels from 3 experiments are shown. “p-” means phosphorylated protein. p-p65 (s468) and p-p65 (s536) represent NF-κB/p65 phosphorylated at serines 468 and 536, respectively. (B) Densitometric analysis was performed for each protein band relative to β-actin in the same sample using Image LabTM software. To compare the relative quantity (RQ) of proteins between NC-siRNA and DHFR-siRNA groups, the values of density ratio in NC-siRNA group were normalized to 1.00. *vs NC-siRNA, p<0.05; **vs NC-siRNA, p<0.01.
5-MTHF supplementation moderately counteracted the inhibitory effect of DHFR knockdown on EA.hy926 cells proliferation
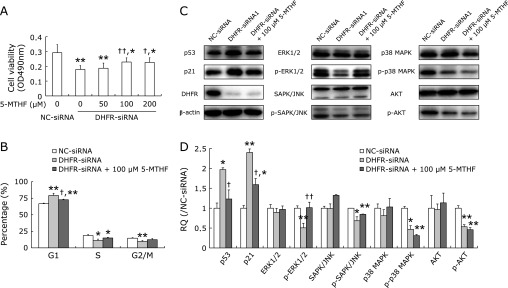
Our above studies demonstrated that DHFR knockdown inhibited EA.hy926 cell proliferation, so we further tested whether FDs supplementation could reverse the growth inhibitory effect of DHFR knockdown. Addition of 100 or 200 µM 5-MTHF to DHFR knockdown cells for 72 h could restore their proliferation ability to some degree, the growth inhibitory rate was decreased from approximately 35% to 20% (Fig. 7A). Then, 5-MTHF at concentration of 100 µM was selected for further test. Treated with 5-MTHF for 72 h, cells were significantly retreated from G1 phase arrest induced by DHFR knockdown, although the cell proportion accumulating in G1 phase was still higher than that of NC-siRNA-transfected cells (p<0.01, Fig. 7B). Cell populations in G1, S and G2/M phases were 66.74 ± 0.77, 18.60 ± 1.37 and 14.65 ± 0.75%, respectively, in NC-siRNA-transfected cells without 5-MTHF treatment; and 79.10 ± 3.3, 11.20 ± 2.04 and 9.69 ± 1.35%, respectively, in DHFR-siRNA-transfected cells without 5-MTHF treatment; and 72.70 ± 0.89, 14.94 ± 0.91 and 12.36 ± 1.68%, respectively, in DHFR-siRNA-transfected cells with 100 µM 5-MTHF treatment. As expected, up-regulation of p53 expression consequent on DHFR knockdown was remarkably fell back by 5-MTHF supplementation, while the expression of p21waf/cip1 was just mild declined (Fig. 7C and D), which indicating p53-independent pathways may be also involved in p21waf/cip1 regulation. At the same time, the phosphorylation level of ERK1/2 reduced by DHFR knockdown was rose again, which was neared to the level of control; however, supplementation of 5-MTHF can not observably re-activate the other MAPKs, as well as AKT, inhibited by DHFR knockdown (Fig. 7C and D). These data confirmed that DHFR and FDs can participate in the control of EA.hy926 cell proliferation.
Fig. 7.
5-MTHF supplementation moderately counteracted the inhibitory effect of DHFR knockdown on EA.hy926 cells. Total of 100 nM DHFR-siRNA (mixture of DHFR-siRNA1 and -siRNA2 with 1:1) or negative control siRNA (NC-siRNA) were transfected into EA.hy926 cells, respectively. The DHFR knockdown cells were additionally treated with 5-MTHF. (A) The cell viability was measured by MTT assay at 72 h post-transfection. Experiments were performed in triplicate and data were given as mean ± SD. Representative figure from one out of three experiments is shown. (B) After DHFR knockdown by siRNA for 72 h, the cell cycle was analyzed by flow cytometry. The number of gated cells in G1, G2/M or S phase was presented as a percentage of total cells (%). Percentages of populations in each cell cycle phase were averaged from three parallel experiments. (C) The cell lysates were analyzed by western blot with specific antibodies (dilution 1:500). β-actin served as internal loading control (dilution 1:8,000). Representative gels from 3 experiments are shown. “p-” means phosphorylated protein. D) Densitometric analysis was performed for each protein band relative to β-actin in the same sample using Image Lab™ software. To compare the relative quantity (RQ) of proteins between NC-siRNA and DHFR-siRNA groups, the values of density ratio in NC-siRNA group were normalized to 1.00. *vs NC-siRNA, p<0.05; **vs NC-siRNA, p<0.01; †vs DHFR-siRNA with 0 µM 5-MTHF, p<0.05, ††vs DHFR-siRNA with 0 µM 5-MTHF, p<0.01.
Discussion
Folate deficiency may lead to the probable microvascular abnormalities, supplementation with FA in folate-deficient subjects can ameliorate cardiovascular disease risk.(15–17) To be effective, FA must first be converted to 7,8-dihydrofolate by DHFR, subsequently enters the folate metabolic cycle to generate a series of FDs as one-carbon donors required for de novo synthesis of nucleotides and amino acids. The eight types of FDs include the following: 7,8-DHF, 5,6,7,8-THF, 5,10-methenyltetrahydrofolate (5,10-CH+-THF), 5,10-methylenetetrahydrofolate (5,10-CH2-THF), 5-methyltetrahydrofolate (5-CH3-THF), 5-formyltetrahydrofolate (5-CHO-THF), 10-formyltetrahydrofolate (10-CHO-THF) and 5-formiminotetrahydrofolate (5-NH=CH-THF). Each FD has a specific role in transferring one-carbon group in six major biosynthetic pathways: (I) 10-CHO-THF and 5,10-CH+-THF in the synthesis of purines; (II) 5,10-CH2-THF in the synthesis of pyrimidine deoxythymidine-5'-monophosphate (dTMP); (III) 10-CHO-THF in the formylation of methionyl-tRNA; (IV) 5,6,7,8-THF and 5,10-CH2-THF in the metabolism of serine and glycine; (V) 5-NH=CH-THF in the metabolism of histidine and glutamate; and (VI) 5-CH3-THF (5-MTHF) in the synthesis of methionine.(18) Lack DHFR or inhibition of its activity can even lead to cell death, as purine and thymidylate biosynthesis and DNA replication are disrupted.(19,20) 5-MTHF is a cofactor used for conversion of homocysteine to methionine. Methionine is the precursor of S-adenosylmethionine, which is the methylation cofactor involved in almost 100 reactions, including the methylation of DNA, proteins and lipids.(18) FA deficiency can induce global DNA hypomethylation and DNA damage in human WIL2-NS cells.(21) In rodent model, maternal protein restriction caused significant changes in DNA methylation and gene expression in the liver of newborn rats, predominately associating with metabolic and cardiovascular diseases. Supplementing the protein-restricted diet with FA largely reversed this epigenetic alteration.(22) Thus, DHFR and FA metabolism have important roles in controlling cell growth.
In this study, knockdown DHFR by siRNA resulted in the growth inhibition of endothelial EA.hy926 cell, accompanied by G1 phase cell cycle arrest, while addition of 5-MTHF to DHFR knockdown cells could restore their proliferation ability and decrease the cells accumulating in G1 phase, to some degree. Prem veer Reddy and Pardee(23) have observed that DHFR could be translocated into cell nuclei, and interacted with other enzymes involved in DNA metabolism to form the complex “replitase”. The authors suggest that the assembly of the replitase complex possibly signals S phase cell cycle initiation. In accord with this suggestion, in our study, knockdown of DHFR may possibly restrain the assembly of “replitase”, consequently retarding the initiation of S phase, thereby leading to G1 phase cell cycle arrest. Interestingly, the expression of DHFR has been found to fluctuate during cell cycle progression. DHFR was not de-repressed in G0/G1, while it was induced dramatically at the G1/S boundary after growth stimulation, its activity even increasing by as much as tenfold.(20,24,25) These findings indicate that DHFR may participate in the regulation of the G1/S cell cycle transition.
The cyclins and their partners, the cyclin-dependent kinases (CDKs), play a well-established role in the regulation of the cell cycle.(26,27) There are two G1 cyclin-CDK complexes, cyclin d-CDK4/6 and cyclin E-CDK2, that are required for S phase entry.(28) In contrast, CDK inhibitors (CKIs) such as the cip/Kip family proteins, p21waf/cip1 and p27Kip1 bind to and inhibit the activity of the above cyclin-CDK complexes, which are involved in the negative control of cell cycle progression.(29,30) Increased levels of the p21waf/cip1 and p27Kip1 proteins typically cause cells to arrest in G1 phase cell cycle. We found that, although the expressions of cyclin D1 and cyclin E in EA.hy926 cells were not observably affected by DHFR knockdown, the expressions of CDK2, CDK4 and CDK6 were remarkably decreased. In particular, CDK6 was down-regulated by approximately 60%; meanwhile p21waf/cip1, p27Kip1, and p53 were up-regulated by about two-fold. The expression of p21waf/cip1 is tightly controlled by the tumor suppressor protein p53, through which this protein mediates the p53-dependent cell cycle G1 phase arrest in response to a variety of stress stimuli.(31–33) DNA damage is a typical stressor that activates p53 expression, leading to p53-dependent G1 cell cycle arrest.(34–37) In human WIL2-NS cells, FA deficiency could induce DNA damage.(21) Folate deficiency caused both massive incorporation of uracil into human DNA as well as chromosome breaks.(38) As expected, p53 was significantly up-regulated by DHFR knockdown in our study, and supplementation with 5-MTHF could reverse this effect. Besides siRNA, down regulation of DHFR has been also performed by micro RNA and inhibitor experiments. Over-expression of miR-215 in osteosarcoma and colon cancer cells down regulates DHFR and reduces cell proliferation rate, triggers cell cycle arrest at G2 phase, and this effect is accompanied by a p53-dependent up regulation of p21, respectively.(39) Although miR-215 down regulates DHFR, it has multiple targets, the authors demonstrated that denticleless protein homolog, one of its critical targets, which produced the above effect in those cancer cells, but not DHFR. In our study, DHFR was knocked down in direct by specific siRNA in the endothelial EA.hy926 cells. Furthermore, Lladó V et al.(40) reported that 2-hydroxyoleic acid, a potent anti-tumor compound, represses the expression of DHFR, and inhibits cell growth, triggers cell cycle arrest at G0/G1 phase, down regulates cdk2 and cyclin B3 expressions in human lung cancer A549 or lymphoblastic leukemia Jurkat cells, although 2-hydroxyoleic acid does not seem to directly inhibit DHFR activity. Therefore, the imbalanced expression of CDKs and CKIs may be one reason that contributes to G1 phase cell cycle arrest induced by DHFR knockdown in the endothelial EA.hy926 cells.
Additionally, constitutive activation of many signal transduction pathways also stimulates cell growth, such as MAPKs, NF-κB and AKT pathway.(41–43) We found that the cell growth inhibition induced by DHFR knockdown was accompanied by the down-regulation of the phosphorylation levels of MAPKs, including ERK1/2, SAPK/JNK and p38, as well as NF-κB/p65 and AKT. Especially, the activity of p38 and AKT were reduced to 40% and 30%, respectively. Recently, Yu et al.(44) found that FA could stimulate neural stem cell proliferation by modifying DNA methylation levels in the PI3K/Akt/CREB pathway. Inhibition of the PI3K/Akt pathway by the PTEN tumor suppressor protein blocks the cell cycle progression beyond G1 phase.(45) In this study, 5-MTHF was supplied to the DHFR knockdown cells, which significantly rising the phosphorylation level of ERK1/2 reduced by DHFR knockdown; while the activity of other MAPKs, as well as AKT, were not found to be re-activated. These suggested that ERK1/2 pathway may be predominantly engaged in 5-MTHF promoting EA.hy926 cell proliferation.
It has been reported that DHFR may also aid in the regeneration of BH4 in endothelial cells needed for coupled nitric oxide synthase (NOS) activity.(46–48) Nitric oxide (NO), produced by eNOS, is a critical regulator of vascular homeostasis.(46) In the absence of tetrahydrobiopterin (BH4), eNOS becomes “uncoupled” and generates superoxide rather than NO.(47) Excessive production of reactive oxygen species (ROS) also contributes to cardiovascular pathogenesis.(48) DHFR knockdown could increase ROS production, while this effect was abolished by supplementing with BH4.(46) Overexpression of DHFR restored NO production and diminished eNOS production of superoxide in angiotensin II-stimulated cells.(48) These findings indicate that DHFR has multiple roles in the regulation of vascular endothelial structure and function.
Taken together, our study has revealed that knockdown of DHFR may down-regulate the expression of CDK2/4/6 required for G1/S phase cell cycle transition, while simultaneously up-regulating CKIs such as p21waf/cip1 and p53, leading to a cell cycle progression block at G1 phase, thereby inhibiting the growth of endothelial EA.hy926 cells. These results provide novel insights into the mechanisms involved in the regulation of endothelial cell growth by DHFR and FDs.
Acknowledgments
This work was supported by the Science and Technology Commission foundation of Shanghai, China (No. 13140903802); the Medicine and Engineering Cross foundation of Shanghai Jiaotong University (No. YG2012MS33).
Abbreviations
- AKT
protein kinase B
- CDK
cyclin dependent kinase
- CKI
CDK inhibitor
- DHFR
dihydrofolate reductase
- FA
folic acid
- MAPK
mitogen-activated protein kinase
- 5-MTHF
5-methyltetrahydrofolate
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NF-κB
nuclear factor-κB
- PMSF
phenylmethanesulfonyl fluoride
- siRNA
short interfering RNA
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Nzila A, Ward SA, Marsh K, Sims PF, Hyde JE. Comparative folate metabolism in humans and malaria parasites (part I): pointers for malaria treatment from cancer chemotherapy. Trends Parasitol. 2005;21:292–298. doi: 10.1016/j.pt.2005.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey SW, Ayling JE. The extremely slow and variable activity of dihydrofolate reductase in human liver and its implications for high folic acid intake. Proc Natl Acad Sci U S A. 2009;106:15424–15429. doi: 10.1073/pnas.0902072106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun DF, Weng YR, Chen YX, Lu R, Wang X, Fang JY. Knock-down of methylenetetrahydrofolate reductase reduces gastric cancer cell survival: an in vitro study. Cell Biol Int. 2008;32:879–887. doi: 10.1016/j.cellbi.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 4.Yi X, Zhou Y, Jiang D, Li X, Guo Y, Jiang X. Efficacy of folic acid supplementation on endothelial function and plasma homocysteine concentration in coronary artery disease: a meta-analysis of randomized controlled trials. Exp Ther Med. 2014;7:1100–1110. doi: 10.3892/etm.2014.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baszczuk A, Kopczyński Z. Hyperhomocysteinemia in patients with cardiovascular disease. Postepy Hig Med Dosw (Online) 2014;68:579–589. doi: 10.5604/17322693.1102340. (in Polish) [DOI] [PubMed] [Google Scholar]
- 6.Bleie Ø, Strand E, Ueland PM, et al. Coronary blood flow in patients with stable coronary artery disease treated long term with folic acid and vitamin B12. Coron Artery Dis. 2011;22:270–278. doi: 10.1097/MCA.0b013e328344fff4. [DOI] [PubMed] [Google Scholar]
- 7.MacKenzie KE, Wiltshire EJ, Gent R, Hirte C, Piotto L, Couper JJ. Folate and vitamin B6 rapidly normalize endothelial dysfunction in children with type 1 diabetes mellitus. Pediatrics. 2006;118:242–253. doi: 10.1542/peds.2005-2143. [DOI] [PubMed] [Google Scholar]
- 8.Till U, Röhl P, Jentsch A, et al. Decrease of carotid intima-media thickness in patients at risk to cerebral ischemia after supplementation with folic acid, vitamins B6 and B12. Atherosclerosis. 2005;181:131–135. doi: 10.1016/j.atherosclerosis.2004.12.043. [DOI] [PubMed] [Google Scholar]
- 9.Assanelli D, Bonanome A, Pezzini A, et al. Folic acid and vitamin E supplementation effects on homocysteinemia, endothelial function and plasma antioxidant capacity in young myocardial-infarction patients. Pharmacol Res. 2004;49:79–84. doi: 10.1016/j.phrs.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Mangoni AA, Sherwood RA, Swift CG, Jackson SH. Folic acid enhances endothelial function and reduces blood pressure in smokers: a randomized controlled trial. J Intern Med. 2002;252:497–503. doi: 10.1046/j.1365-2796.2002.01059.x. [DOI] [PubMed] [Google Scholar]
- 11.Spanheimer PM, Cyr AR, Gillum MP, Woodfield GW, Askeland RW, Weigel RJ. Distinct pathways regulated by RET and estrogen receptor in luminal breast cancer demonstrate the biological basis for combination therapy. Ann Surg. 2014;259:793–799. doi: 10.1097/SLA.0b013e3182a6f552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Z, Lin CZ, Peng LJ, et al. High mobility group box 1 activates Toll like receptor 4 signaling in hepatic stellate cells. Life Sci. 2012;91:207–212. doi: 10.1016/j.lfs.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 13.Li W, Yu M, Luo S, et al. DNA methyltransferase mediates dose-dependent stimulation of neural stem cell proliferation by folate. J Nutr Biochem. 2013;24:1295–1301. doi: 10.1016/j.jnutbio.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Santiago Y, Chan E, Liu PQ, et al. Targeted gene knockout in mammalian cells by using engineered zinc-finger nucleases. Proc Natl Acad Sci U S A. 2008;105:5809–5814. doi: 10.1073/pnas.0800940105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schiano C, Vietri MT, Grimaldi V, Picascia A, De Pascale MR, Napoli C. Epigenetic-related therapeutic challenges in cardiovascular disease. Trends Pharmacol Sci. 2015;36:226–235. doi: 10.1016/j.tips.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Kayadibi H, Sertoglu E, Uyanik M. Biochemical view on: precocious markers of cardiovascular risk and vascular damage in apparently healthy women with previous gestational diabetes. Diabetol Metab Syndr. 2015;7:22. doi: 10.1186/s13098-015-0023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L, Li H, Zhou Y, Jin L, Liu J. Low-dose B vitamins supplementation ameliorates cardiovascular risk: a double-blind randomized controlled trial in healthy Chinese elderly. Eur J Nutr. 2015;54:455–464. doi: 10.1007/s00394-014-0729-5. [DOI] [PubMed] [Google Scholar]
- 18.Priest DG, Bunni MA. Folate and folate antagonists in cancer chemotherapy. In: Bailey LB, editor. Folate in Health and Disease. Marcel Dekker; 1995. pp. 379–403. [Google Scholar]
- 19.Antosiewicz A, Senkara E, Cieśla J. Quartz crystal microbalance with dissipation and microscale thermophoresis as tools for investigation of protein complex formation between thymidylate synthesis cycle enzymes. Biosens Bioelectron. 2015;64:36–42. doi: 10.1016/j.bios.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 20.Abali EE, Skacel NE, Celikkaya H, Hsieh YC. Regulation of human dihydrofolate reductase activity and expression. Vitam Horm. 2008;79:267–292. doi: 10.1016/S0083-6729(08)00409-3. [DOI] [PubMed] [Google Scholar]
- 21.Bull CF, Mayrhofer G, O'Callaghan NJ, et al. Folate deficiency induces dysfunctional long and short telomeres; both states are associated with hypomethylation and DNA damage in human WIL2-NS cells. Cancer Prev Res (Phila) 2014;7:128–138. doi: 10.1158/1940-6207.CAPR-13-0264. [DOI] [PubMed] [Google Scholar]
- 22.Altobelli G, Bogdarina IG, Stupka E, Clark AJ, Langley-Evans S. Genome-wide methylation and gene expression changes in newborn rats following maternal protein restriction and reversal by folic acid. PLoS One. 2013;8:e82989. doi: 10.1371/journal.pone.0082989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prem veer Reddy G, Pardee AB. Multienzyme complex for metabolic channeling in mammalian DNA replication. Proc Natl Acad Sci U S A. 1980;77:3312–3316. doi: 10.1073/pnas.77.6.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slansky JE, Farnham PJ. Transcriptional regulation of the dihydrofolate reductase gene. Bioessays. 1996;18:55–62. doi: 10.1002/bies.950180111. [DOI] [PubMed] [Google Scholar]
- 25.Kubbies M, Stockinger H. Cell cycle-dependent DHFR and t-PA production in cotransfected, MTX-amplified CHO cells revealed by dual-laser flow cytometry. Exp Cell Res. 1990;188:267–271. doi: 10.1016/0014-4827(90)90169-b. [DOI] [PubMed] [Google Scholar]
- 26.Morgan DO. Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu Rev Cell Dev Biol. 1997;13:261–291. doi: 10.1146/annurev.cellbio.13.1.261. [DOI] [PubMed] [Google Scholar]
- 27.Murray AW, Marks D. Can sequencing shed light on cell cycling? Nature. 2001;409:844–846. doi: 10.1038/35057033. [DOI] [PubMed] [Google Scholar]
- 28.Connell-Crowley L, Elledge SJ, Harper JW. G1 cyclin-dependent kinases are sufficient to initiate DNA synthesis in quiescent human fibroblasts. Curr Biol. 1998;8:65–68. doi: 10.1016/s0960-9822(98)70021-1. [DOI] [PubMed] [Google Scholar]
- 29.Elledge SJ, Winston J, Harper JW. A question of balance: the role of cyclin-kinase inhibitors in development and tumorigenesis. Trends Cell Biol. 1996;6:388–392. doi: 10.1016/0962-8924(96)10030-1. [DOI] [PubMed] [Google Scholar]
- 30.Harper JW, Elledge SJ. Cdk inhibitors in development and cancer. Curr Opin Genet Dev. 1996;6:56–64. doi: 10.1016/s0959-437x(96)90011-8. [DOI] [PubMed] [Google Scholar]
- 31.Nakatsuka A, Wada J, Hida K, et al. RXR antagonism induces G0/G1 cell cycle arrest and ameliorates obesity by up-regulating the p53-p21(Cip1) pathway in adipocytes. J Pathol. 2012;226:784–795. doi: 10.1002/path.3001. [DOI] [PubMed] [Google Scholar]
- 32.Liu W, Dai Q, Lu N, et al. LYG-202 inhibits the proliferation of human colorectal carcinoma HCT-116 cells through induction of G1/S cell cycle arrest and apoptosis via p53 and p21(WAF1/Cip1) expression. Biochem Cell Biol. 2011;89:287–298. doi: 10.1139/o10-162. [DOI] [PubMed] [Google Scholar]
- 33.Kim SY, Kim JE, Lee KW, Lee HJ. Lactococcus lactis ssp. lactis inhibits the proliferation of SNU-1 human stomach cancer cells through induction of G0/G1 cell cycle arrest and apoptosis via p53 and p21 expression. Ann N Y Acad Sci. 2009;1171:270–275. doi: 10.1111/j.1749-6632.2009.04721.x. [DOI] [PubMed] [Google Scholar]
- 34.Panchanathan R, Liu H, Choubey D. Activation of p53 in human and murine cells by DNA-damaging agents differentially regulates aryl hydrocarbon receptor levels. Int J Toxicol. 2015;34:242–249. doi: 10.1177/1091581815578013. [DOI] [PubMed] [Google Scholar]
- 35.Kang KB, Zhu C, Yong SK, Gao Q, Wong MC. Enhanced sensitivity of celecoxib in human glioblastoma cells: Induction of DNA damage leading to p53-dependent G1 cell cycle arrest and autophagy. Mol Cancer. 2009;8:66. doi: 10.1186/1476-4598-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arima Y, Hirota T, Bronner C, et al. Down-regulation of nuclear protein ICBP90 by p53/p21Cip1/WAF1-dependent DNA-damage checkpoint signals contributes to cell cycle arrest at G1/S transition. Genes Cells. 2004;9:131–142. doi: 10.1111/j.1356-9597.2004.00710.x. [DOI] [PubMed] [Google Scholar]
- 37.Yoon HS, Chen X, Yang VW. Krüppel-like factor 4 mediates p53-dependent G1/S cell cycle arrest in response to DNA damage. J Biol Chem. 2003;278:2101–2105. doi: 10.1074/jbc.M211027200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blount BC, Mack MM, Wehr CM, et al. Folate deficiency causes uracil misincorporation into human DNA and chromosome breakage: implications for cancer and neuronal damage. Proc Natl Acad Sci U S A. 1997;94:3290–3295. doi: 10.1073/pnas.94.7.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song B, Wang Y, Titmus MA, et al. Molecular mechanism of chemoresistance by miR-215 in osteosarcoma and colon cancer cells. Mol Cancer. 2010;9:96. doi: 10.1186/1476-4598-9-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lladó V, Terés S, Higuera M, et al. Pivotal role of dihydrofolate reductase knockdown in the anticancer activity of 2-hydroxyoleic acid. Proc Natl Acad Sci U S A. 2009;106:13754–13758. doi: 10.1073/pnas.0907300106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang YQ, Jaganath IB, Manikam R, Sekaran SD. Inhibition of MAPKs, Myc/Max, NFκB, and hypoxia pathways by Phyllanthus prevents proliferation, metastasis and angiogenesis in human melanoma (MeWo) cancer cell line. Int J Med Sci. 2014;11:564–577. doi: 10.7150/ijms.7704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feitelson MA, Arzumanyan A, Kulathinal RJ, et al. Sustained proliferation in cancer: mechanisms and novel therapeutic targets Semin Cancer Biol 2015. DOI: 10.1016/j.semcancer.2015.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Safdari Y, Khalili M, Ebrahimzadeh MA, Yazdani Y, Farajnia S. Natural inhibitors of PI3K/AKT signaling in breast cancer: emphasis on newly-discovered molecular mechanisms of action. Pharmacol Res. 2015;93:1–10. doi: 10.1016/j.phrs.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 44.Yu M, Li W, Luo S, et al. Folic acid stimulation of neural stem cell proliferation is associated with altered methylation profile of PI3K/Akt/CREB. J Nutr Biochem. 2014;25:496–502. doi: 10.1016/j.jnutbio.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 45.Ramaswamy S, Nakamura N, Vazquez F, et al. Regulation of G1 progression by the PTEN tumor suppressor protein is linked to inhibition of the phosphatidylinositol 3-kinase/Akt pathway. Proc Natl Acad Sci U S A. 1999;96:2110–2115. doi: 10.1073/pnas.96.5.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sugiyama T, Levy BD, Michel T. Tetrahydrobiopterin recycling, a key determinant of endothelial nitric-oxide synthase-dependent signaling pathways in cultured vascular endothelial cells. J Biol Chem. 2009;284:12691–12700. doi: 10.1074/jbc.M809295200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crabtree MJ, Tatham AL, Al-Wakeel Y, et al. Quantitative regulation of intracellular endothelial nitric-oxide synthase (eNOS) coupling by both tetrahydrobiopterin-eNOS stoichiometry and biopterin redox status: insights from cells with tet-regulated GTP cyclohydrolase I expression. Biol Chem. 2009;284:1136–1144. doi: 10.1074/jbc.M805403200. [DOI] [PubMed] [Google Scholar]
- 48.Chalupsky K, Cai H. Endothelial dihydrofolate reductase: critical for nitric oxide bioavailability and role in angiotensin II uncoupling of endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2005;102:9056–9061. doi: 10.1073/pnas.0409594102. [DOI] [PMC free article] [PubMed] [Google Scholar]