Abstract
This collaborative, community-engaged project developed and tested a Culturally-Tailored Treatment (CTT) for American Indian/Alaska Native (AI/AN) smokers in the Menominee tribal community. One hundred three adult AI/AN smokers were randomized to receive either Standard Treatment (n= 53) or CTT (n = 50) for smoking cessation. Both treatment conditions included 12 weeks of varenicline and four individual counseling sessions but differed in terms of cultural tailoring of the counseling. The primary outcome was 7-day biochemically-confirmed point-prevalence abstinence (PPA) at the 6-month end-of-study visit. Both intention-to-treat (ITT) and responder-only analyses were conducted. There were no statistically significant group differences in 7-day PPA. The overall ITT abstinence rate at 6 months was 20%; the responder-only rate was 42%. The current study represents the first randomized smoking cessation clinical trial testing a culturally-tailored smoking cessation intervention designed for a specific AI/AN tribal community that combined FDA-approved cessation medication (varenicline) and innovative cultural intervention components.
There are well over 500 federally- and state-recognized American Indian/Alaska Native (AI/AN) tribes in North America. In many of these tribes, there is a long history of the use of natural tobacco for ceremonial and spiritual purposes (Winter, 2000). In the last century, however, the addictive abuse of commercially-produced tobacco products such as cigarettes has increased dramatically in many tribal communities (Dennis & Momper, 2012; Samet, 2000). Addictive smoking of tobacco is associated with significant health and other negative consequences (Centers for Disease Control and Prevention, 2003; U.S. Department of Health and Human Services, 2004). Although the prevalence of cigarette smoking in AI/AN communities varies widely (Burgess et al., 2007; Fagan, Moolchan, Lawrence, Fernander, & Ponder, 2007; Nez Henderson, Jacobsen, & Beals, 2005), the rates in many tribal communities are among the highest in the U.S. For example, some Northern Plains tribes in the U.S. have smoking prevalence rates of approximately 50% (Geishirt Cantrell, Hodge, Struthers, & Decora, 2005). The overall prevalence of smoking in AI/AN adults in the U.S. in 2010 was 31.4%, as compared to 21.0% for non-Hispanic Whites and 20.6% for African-Americans (Centers for Disease Control and Prevention, 2011a).
As a consequence of these high rates of addictive tobacco use, AI/ANs suffer disproportionately elevated rates of smoking-related diseases and death which, combined with other factors in tribal communities such as underfunding of healthcare services, result in significant health disparities (Denny, Holtzman, Goins, & Croft, 2005; Hendricks et al., 2014; Watanabe-Galloway et al., 2011). Other factors that contribute to health disparities in AI/AN and other culturally diverse communities include oppression, socioeconomic challenges, and discrimination (Buki, 2007; Buki & Selem, 2012; Herman et al., 2007; Tucker et al., 2007). In particular, AI/AN communities have suffered centuries of oppression through forced relocation of tribes, language and culture suppression, forced assimilation, and removal of AI/AN children from families to send them to government-run boarding schools (Evans-Campbell, 2008; Whitbeck, Adams, Hoyt, & Chen, 2004). The adverse effects of these various kinds of oppression and disempowerment of AI/AN peoples (historical trauma) reverberate through successive generations (multigenerational trauma) in the form of powerlessness and hopelessness which, in turn, contribute to high rates of poverty, alcoholism, substance abuse, depression, suicide, and other health issues (Morgan & Freeman, 2009; Whitbeck, Chen, Hoyt, & Adams, 2004). Thus, the addictive use of commercial tobacco can also be linked to historical trauma and, in addition to harming the physical health of users, it represents an unhealthy relationship with tobacco -- a plant considered by many tribes to be sacred medicine (Winter, 2000).
Smoking cessation interventions are an important secondary prevention approach that can reduce smoking prevalence in AI smokers and, in turn, potentially reduce the health disparities associated with smoking-attributable diseases. However, the extent to which conventional tobacco cessation treatments work in AI/AN smokers is largely unknown due to significant underrepresentation of AI/ANs in cessation clinical trials and a paucity of tribal community-based cessation studies (Carson et al., 2012; Choi et al., 2006; Cox, Okuyemi, Choi, & Ahluwalia, 2011; Johnson, Lando, Schmid, & Solberg, 1997). In fact, in a recent Cochrane review of smoking cessation interventions in indigenous populations (Carson et al., 2012) only one U.S. AI/AN study met criteria as a clinical trial. In this study by Johnson et al. (1997), validated quit rates at 1 year were 6.7% for the intervention group and 6.8% for the control group. The low rates of abstinence success in the Johnson et al. (1997) study and the overall paucity of rigorous cessation clinical trials in AI/AN smokers highlight the urgent need for studies of this kind.
Additionally, there are important cultural and historical aspects of tobacco use by AI/ANs that must be considered (Daley et al., 2006; Oberly & Macedo, 2004; Pego, Hill, Solomon, Chisholm, & Ivey, 1995; Struthers & Hodge, 2004). These considerations include sacred use of tobacco (e.g., for healing) and the importance of tobacco in AI/AN creation stories (Brokenleg & Tornes, 2013). In addition, there are obstacles to conducting research in AI/AN communities (e.g., lack of trust of university-based researchers, inadequate funding) that must be overcome (Burhansstipanov, Christopher, & Schumacher, 2005; Norton & Manson, 1996). Consequently, there is a pressing need to develop and test culturally-appropriate, evidence-based smoking cessation interventions for AI/AN smokers to reduce smoking-related health disparities (Dennis & Momper, 2012; Gohdes et al., 2002).
There is an extensive evidence base for the efficacy of both counseling and pharmacotherapy for increasing cessation success relative to unassisted quit attempts (Fiore et al., 2008; Schlam & Baker, 2013; Stead & Lancaster, 2012). Although unassisted quitting typically yields long-term abstinence rates of only about 3–5% (Hughes, Keely, & Naud, 2004), counseling and pharmacotherapy, individually or combined, yield long-term abstinence rates of between 10% and 30% (Fiore et al., 2008). In 2010, approximately 70% of smokers reported wanting to quit smoking, 52% made a quit attempt, and about 32% had used counseling, medications, or both when they tried to quit (Centers for Disease Control and Prevention, 2011b). However, smokers are much more likely to use pharmacotherapy than behavioral treatments (Shiffman, Brockwell, Pillitteri, & Gitchell, 2008) despite the fact that higher abstinence rates are achieved with a combination of cessation medication and counseling (Fiore et al., 2008). Given the significant contributions of psychosocial factors in the initiation and maintenance of smoking combined with high rates of psychiatric comorbidities in smokers (Mackowick, Lynch, Weinberger, & George, 2012), counseling clearly plays an important role in cessation treatment (Mottillo et al., 2009). Counseling psychologists (CPs) have clinical expertise in a number of relevant areas including behavioral change techniques, understanding the influence of psychosocial/cultural and developmental factors on psychological health, using client strengths to increase effective coping, and the psychotherapeutic treatment of psychiatric conditions -- expertise that enables CPs to be especially effective in assisting smokers to quit the addictive use of tobacco which, in turn, can help to reduce health disparities (Buki, 2007; Leffingwell & Babitzke, 2006; Phillips & Brandon, 2004).
In Wisconsin, the Menominee Indian Tribe of Wisconsin has the highest smoking prevalence rate in the state at 34.0% versus 19.5% statewide (Palmersheim, Voskuil, & Glysch, 2011). As a consequence of these high rates of smoking, the Menominee community suffers from elevated rates of smoking-related diseases, deaths, and health disparities (Ostenso, Remington, & Ahrens, 2001; Wisconsin Tobacco Prevention and Control Program, 2009a). In 2005, the Menominee Tribe expressed interest in collaborating with researchers at the University of Wisconsin-Madison (UW-Madison) on improving the health of tribal community members by increasing the effectiveness of smoking cessation interventions. Researchers from UW-Madison spent the next 3 years (2006–2008) working collaboratively with the Menominee Tribe to develop a culturally-tailored smoking cessation research program. This effort culminated in funding from the Wisconsin Partnership Program (at the University of Wisconsin School of Medicine and Public Health) for a 3-year (2008–2011) Menominee Smoking Cessation Clinical Trial, known also as the START project—Stop Tobacco Abuse Renew Tradition. Also, starting in 2008, the study team expanded to include researchers from the University of Wisconsin-Milwaukee (UW-Milwaukee) and University of New Mexico (UNM).
The purpose of the 3-year START project was to evaluate the efficacy, feasibility, and safety of a culturally-tailored smoking cessation treatment for Menominee and other AI/AN smokers as compared to standard evidence-based cessation treatment (not culturally tailored). This randomized clinical trial (RCT) used a mixed-methods approach in which both quantitative measures and qualitative interview data were collected. As noted by Creswell, Klassen, Plano Clark, & Smith (2011), a mixed-methods approach maximizes the strengths of quantitative and qualitative data by using each to inform the other in an integrated way to increase understanding of complex phenomena viewed from various perspectives. The results reported in this article will focus on the main quantitative outcomes of the study; results for qualitative and mixed-methods analyses will be reported in subsequent articles.
Method
Participants
A total of 103 Menominee and other AI/AN smokers enrolled in the study and initiated treatment. All participants were receiving health care at the Menominee Tribal Clinic (MTC) and were motivated to quit smoking. The majority of participants (80%) were enrolled members of the Menominee Tribe; the remaining participants were enrolled members of 8 other tribes. A higher number of female smokers (n = 64) enrolled in the study than male smokers (n = 39). The mean age of participants was 39.8 years (SD = 13.1, Mdn = 40, range = 19–77). Participants smoked an average of 14.4 cigarettes per day (CPD; SD = 7.9, Mdn = 15, range = 1–40). A total of 85 participants (83%) had made one or more previous quit attempts (Mdn = 3, range = 1–20).
Study Team and Study Site
The study team consisted of: Menominee clinic staff at the MTC, researchers from UW-Madison with expertise in smoking cessation RCTs, outreach specialists at UW-Madison with expertise in AI/AN community-based outreach supported by Spirit of EAGLES (a national research program funded by the National Cancer Institute to address cancer disparities in AI/AN communities; Kaur, Dignan, Burhansstipanov, Baukol, & Claus, 2006), and researchers from UW-Milwaukee and UNM with expertise in qualitative analysis and AI/AN community-based research. Menominee community members provided vital consultation about the study. The study was conducted at the MTC on the Menominee Reservation in Keshena, WI.
Community-Engaged Research Collaboration
The collaboration between the Menominee Tribe and university-based researchers had its beginnings in January 2005 when a Spirit of EAGLES outreach specialist organized a site visit to the MTC with a team of UW-Madison cancer scientists to discuss tribal concerns about high rates of lung cancer. During this meeting, the MTC Director and the MTC Wellness Director noted the high rates of tobacco abuse (i.e., use of commercial cigarettes rather than traditional tobacco) in the Menominee community as well as significant health disparities stemming from smoking-related illnesses including lung cancer. This discussion also included tribal interest in exploring ways to reduce smoking in their community and, in order to continue the discussions, a subsequent conference call was scheduled that included tribal representatives and UW-Madison researchers (a clinical oncologist, a tobacco policy scientist, a clinician/researcher from the University of Wisconsin Center for Tobacco Research and Intervention, and the co-directors of the Carbone Cancer Center’s newly formed Cancer Health Disparities Initiative). During this conference call and a second call, there was discussion of Menominee community smoking-related data provided by the tribal representatives as well as possible ways to reduce smoking and smoking-related health disparities. Based on these discussions and other considerations, the Menominee Tribe set adult tobacco cessation as a top priority to improve the health of the community. In addition, there was agreement among tribal and UW-Madison representatives to continue working together to develop a research program to improve cessation treatment at the MTC.
Following these conference calls, UW-Madison representatives made several visits to the Menominee community to work together with tribal partners to clarify specific research questions and goals. These meetings were very helpful in building effective working relationships and increasing trust among the research partners. These meetings and conference calls continued throughout 2006 and culminated in a grant application to the National Cancer Institute in late 2006 (via a Spirit of EAGLES pilot study mechanism) requesting funding for a Menominee family-based tobacco cessation pilot project. This grant application was not funded but the research partners continued to meet in 2007 to discuss how to improve the research design and methods. In addition, the Tribe conducted interviews in summer 2007 with 14 Menominee smokers and ex-smokers (see section below on “Cessation Intervention Development” for more details) to help inform the re-design of the cessation study and interventions (Arndt et al., 2013). Meetings and calls among the research partners continued and by early 2008, the Tribe made a decision to focus on developing an RCT to test the efficacy of a culturally appropriate, evidence-based tobacco cessation intervention compared to a standard evidence-based cessation intervention.
Based on the extensive and productive discussions of the research partners from 2005–2008 as well as the knowledge gained from the interviews with smokers and ex-smokers, a grant application was developed and submitted in April 2008 for funding from the Wisconsin Partnership Program at the University of Wisconsin School of Medicine and Public Health. Funding for the project approved in July 2008 with a total budget of $500,000 over 3 years with over 60% of the funding being allocated to the Menominee Tribe.
Tribal and UW-Madison Approval for the Study
The Menominee Tribal Legislature issued Tribal Resolution No. 08–12 on April 7, 2008, that approved the Menominee Smoking Cessation Clinical Trial to be conducted at the MTC. Institutional Review Board (IRB) approval for the study was granted by the UW-Madison Health Sciences IRB. Both the Menominee Tribe and UW-Milwaukee deferred IRB oversight of the study to the UW-Madison Health Sciences IRB. In addition, the study was governed by a formal Memorandum of Agreement (MOA) between the Menominee Tribe and UW-Madison signed by both parties. The MOA set forth covenants and agreements about study-related issues including tribal and UW-Madison sharing of data and materials, right of review by the Menominee Tribe of materials proposed for publication, expectations about human subjects protection to protect the rights and welfare of Menominee and other AI/AN research participants, professional fees for eminent tribal persons who would provide consultation to the project, sovereign immunity of the Menominee Tribe, and agreement that the MOA would be governed by laws of the Menominee Indian Tribe of Wisconsin.
Tribal Advisory Committee
In 2010, seven Menominee Tribe members were invited to be part of a Tribal Advisory Committee that would be an integral part of the community-academic collaboration. Members of the committee represented the interests of the Menominee tribal community and provided guidance about community and cultural issues relevant to the study. In addition, the committee assisted the core study team with interpretation of study findings as well as how the study results potentially can be of benefit to the Menominee Tribe. These individuals included respected Menominee elders as well as a younger community member, all of whom were invited because of their knowledge of both commercial and traditional tobacco use in the Menominee community. Six tribal members (4 females, 2 males) accepted the invitation; half are ex-smokers and half have never smoked. One member is a former Chair of the Menominee Nation and is currently Director of the Menominee Language and Culture Commission; one teaches AI/AN studies at a local university; one is employed at the Menominee Tribal Culture Museum; and the other members are distinguished by their longtime involvement with community and family, promoting and preserving Menominee culture and heritage.
Cessation Intervention Development
Focused developmental work on a Standard Treatment (ST) cessation intervention and a Culturally-Tailored Treatment (CTT) intervention started in 2007 and included regular team meetings and conference calls to discuss possible options for increasing cessation success and expanding cessation treatment at the MTC via cultural tailoring. These discussions incorporated input from Menominee tribal representatives (MTC staff, community members), findings from relevant AI/AN tobacco-related research studies, consultation with outside experts with expertise conducting AI/AN cessation research, and information from a preliminary study conducted by the Menominee Tribe. Early consensus was achieved on a number of treatment details included in both ST and CTT: (a) provision of varenicline (FDA-approved prescription medication for smoking cessation) to all study participants (this decision was made by MTC staff); (b) counseling delivered individually (rather than group-based); and (c) counseling would be provided by an AI/AN counselor (Fu et al., 2014).
With tribal government approval, MTC staff conducted a preliminary study in 2007 that collected detailed interview information from a sample of 14 Menominee smokers and ex-smokers concerning sacred/traditional tobacco use, commercial tobacco use, cultural aspects of tobacco, and cessation of commercial tobacco use (Arndt et al., 2013). An initial, informal analysis of the interview data helped to inform the development of the culturally-tailored treatment in the START study. Important themes that emerged included: smoking-related health concerns, the challenge of being around others who smoke, stress, family and community support for abstinence, use of alcohol, peer pressure, boredom, and easy availability of commercial cigarettes. In addition, sacred use of tobacco was noted as important by some respondents.
The study team worked for about 3 years (2007–2009) on securing funding and developing the ST and CTT interventions along with operational protocols, with final versions completed during the first year of START project funding (October 2008 to September 2009). In September 2009, an application for IRB approval was submitted to the UW-Madison Health Sciences IRB with approval granted in February 2010; the first study participant was enrolled that same month.
Treatment Protocols in the ST and CTT Interventions
All study participants were scheduled to receive four evidence-based cessation counseling sessions with counseling based on recommendations in the 2008 U.S. Public Health Service (PHS) clinical practice guideline Treating Tobacco Use and Dependence: 2008 Update (2008 PHS Guideline; Fiore et al., 2008). In addition, all participants received 12 weeks of varenicline. Of note, the ST and CTT interventions were the same except that the CTT intervention included additional culturally-tailored treatment elements designed to address tobacco-related issues specific to Menominee and other AI/AN smokers. Counseling was provided by the study coordinator who is an enrolled member of the Menominee Tribe and trained as an AODA counselor; she also completed the Mayo Clinic Nicotine Dependence Center Tobacco Treatment Specialist Certification Program (Rochester, MN).
Evidence-based counseling provided to both treatment groups
The 2008 PHS Guideline Panel reviewed hundreds of published studies and found sufficient evidence to recommend two counseling approaches appropriate for smokers who are motivated to make a quit attempt: problem-solving and skills training and supportive treatment (Fiore et al., 2008). This counseling involves discussion of 3 broad topics: (a) provision and discussion of basic information about smoking, nicotine addiction, and the nature and course of withdrawal symptoms, (b) discussion about how to decrease the risk for smoking or relapse including recognition of certain situations (e.g., being around other smokers), activities (e.g., drinking alcohol), and internal states (e.g., urges and craving to smoke, negative affect, stress) that are associated with an increase in the risk for smoking lapses or full relapse, and (c) developing effective coping skills, including how to anticipate and avoid situations that might trigger urges to smoke, how to reduce negative affect without smoking, how to reduce stress, and how to cope with smoking urges (e.g., distraction, changing routines). For supportive treatment, the Guideline Panel recommended that the counselor (a) encourage the smoker in the quit attempt (e.g., by communicating belief in the smoker’s ability to quit, by encouraging the use of effective treatments), (b) communicate caring and concern for the smoker (e.g., by asking about fears and ambivalence about quitting, by offering to help as often as needed); and (c) encourage the smoker to talk about the quitting process (e.g., smokers’ reasons for quitting, prior success in quitting, challenges or difficulties in prior quit attempts).
Smoking cessation medication provided to both treatment groups
All study participants received 12 weeks of varenicline which is an FDA-approved prescription medication for smoking cessation that binds to the same brain receptors to which nicotine (from tobacco) binds. Varenicline does not contain nicotine but, like nicotine, helps reduce craving and withdrawal symptoms (this is the agonist property of varenicline). However, it also blocks the pleasurable effects of nicotine from tobacco because it binds more readily than nicotine to the nicotinic neuronal receptors (this is the antagonist property of varenicline). Across 15 efficacy clinical trials, varenicline has been shown to increase the odds of successful quitting two-to-threefold relative to placebo with an estimated 6-month abstinence rate of approximately 28% compared to 12% for placebo pills (Cahill, Stevens, & Lancaster, 2014). The most common side effect of varenicline is mild to moderate nausea affecting about 30% of smokers using varenicline; about 10% of smokers discontinue varenicline because of adverse effects (Cahill, Stead, & Lancaster, 2011). Varenicline has a good safety profile for carefully screened smokers but the FDA requires a warning on the labelling for varenicline regarding potential risks of neuropsychiatric and cardiovascular events.
Standard Treatment (ST) intervention
ST was modeled on the general cessation treatment approach utilized at the MTC for many years. This approach consisted of FDA-approved cessation medication (if medically appropriate) as well as counseling as recommended in the 2008 PHS Guideline (Fiore et al., 2008). Specific information concerning the details and timing of ST is provided in Table 1.
Table 1.
Treatment Components of the START Study Standard Treatment (ST) and Culturally-Tailored Treatment (CTT) Interventions
| Study Visit/Phone Call | Standard Treatment (ST) | Culturally-Tailored Treatment (CTT) |
|---|---|---|
| Study Visit 1 (Prequit) | Pre-cessation counseling including instructions for using varenicline; participant picks up varenicline (initial 1 month supply) at Menominee Tribal Clinic pharmacy. | Pre-cessation counseling including instructions for using varenicline; participant picks up varenicline (initial 1 month supply) at Menominee Tribal Clinic pharmacy. |
| Study Visit 2 (Prequit) | Cessation counseling topics: Nature of nicotine addiction; effects of nicotine on the brain; steps to prepare for quitting; setting a quit date. Cessation Booklet: “Clearing the Air” (National Cancer Institute). |
ST plus: Discussion of traditional vs. commercial tobacco use; tobacco as medicine; spiritual aspects of tobacco Cessation Resource Booklet: Culturally-Tailored for Menominee/AI Smokers. |
| Phone Call 1 (1 day postquit) | Cessation counseling topics: Nicotine withdrawal, barriers and challenges to staying quit, coping skills, effectively dealing with urges and craving, importance of seeking support, rewarding self for success in staying quit, importance of taking varenicline, positive benefits of quitting smoking. ST only: encourage keeping a journal about quitting experience. | ST plus: Stone Metaphor (gather stone, bring to next visit). |
| Study Visit 3 (1 week postquit) | Cessation counseling topics: Smoking triggers and how to cope with them, positive self-talk, how to effectively deal with high-risk situations. ST only: encourage putting together a “Cessation Tool Kit” (card with coping strategies, chewing gum, hard candy). |
ST plus: Make a traditional tobacco pouch (symbol of long life). |
| Study Visit 4 (3 weeks postquit) | Cessation counseling topics: Relapse prevention, stress management, relaxation techniques, healthy diet, exercise, dealing with weight gain. ST only: encourage listening to conventional relaxation tape (not culturally-tailored). Varenicline refill for second and third months arranged. |
ST plus: Encourage listening to culturally-tailored relaxation/affirmation CD. Varenicline refill for second and third months arranged. |
Culturally-Tailored Treatment (CTT) intervention
To address cultural aspects of the CTT intervention, the study team utilized the Indigenist Stress-Coping model of Walters and Simoni (2002). This model allowed the team to frame nicotine addiction (i.e., abuse of commercial tobacco) in the context of key life stressors experienced by AI/AN people including the effects of historical trauma (Brave Heart, Chase, Elkins, & Altschul, 2011) as well as the beneficial psychological and emotional effects of positive Native cultural factors (e.g., strength of family and community) that can buffer the adverse effects of the stressors. This model explicitly values and incorporates Indigenous knowledge, understanding, and science including traditional teachings about the use of sacred tobacco.
The final version of the CTT intervention for the START study was based on the following: (a) preliminary study findings, (b) advice from external consultants, (c) scientific literature on AI/AN tobacco use and cessation, (d) the 15+ years of experience by the MTC Wellness Director in providing cessation treatment at the MTC, and (e) input from Menominee Tribe members. Specific details concerning the timing and nature of the CTT intervention are provided in Table 1. Specific CTT intervention components are listed below.
Discussion of traditional vs. commercial tobacco use
This discussion focused on the positive aspects of traditional tobacco in AI/AN and Menominee culture (e.g., tobacco being used in prayer, purification, and cleansing and for healings, blessings, as a gift) in contrast to the harmful effects of addictive use of commercial tobacco (cigarettes). Many tribes including the Menominee Tribe have traditional stories about the sacredness of the tobacco plant. The nature and extent of the discussion of traditional vs. commercial tobacco use in the counseling varied depending on the beliefs and preferences of individual participants. Inclusion of this discussion is supported by recent focus group research with AI/AN current and former smokers by Fu et al. (2014).
Cessation booklet culturally tailored for Menominee and Other AI/AN smokers
This 33-page CTT cessation booklet was designed by MTC staff and other Menominee representatives to address Menominee cultural, community, and historical issues related to both the abuse of commercial cigarettes and the traditional or sacred use of tobacco. It was written primarily in English with some information in the Menominee language; illustrations for the booklet were drawn by Menominee artists. The booklet incorporated both evidence-based cessation information as well as Menominee and other AI/AN information, culture, and wisdom. For example, the booklet has a section on “Menominee Tobacco Related Illness” as well as a section on spirituality including how traditional tobacco is used for prayer and healing. This section also includes the Menominee Tobacco Story. Inclusion of culturally-tailored cessation materials such as this booklet is supported by findings reported by Fu et al. (2014).
Stone metaphor
Participants in the CTT group were encouraged to gather a stone from the Wolf River that runs through the Menominee Reservation; the counselor described the stone as having special meaning that could potentially help in healing and in the cessation process or journey. More specifically, the stone was characterized by the counselor as a direct gift from the spirit world through nature and that the stone may have a shape, color, or image that would enhance healing (cessation) through harmony with mother earth. The idea for using the stone metaphor originated with Menominee study team members experienced in counseling AI/AN clients.
Traditional tobacco pouch
Participants in the CTT group were given materials to make their own traditional tobacco pouch, which is a symbol of a long life in Menominee culture. Participants were encouraged to carry the pouch with them to have natural tobacco to use for prayers and other purposes (e.g., as a gift to others) if so desired; smoking of the tobacco was not encouraged unless smoked for ceremonial purposes. The provision of materials for a tobacco pouch was based on findings from our preliminary qualitative study of Menominee smokers and ex-smokers (Arndt et al., 2013).
Culturally-tailored relaxation and affirmation CD, “Smoking Cessation – Meditations for Honoring Our Naeqnemaw”
“Naeqnemaw” means tobacco in the Menominee language. This 16-minute CD was specially commissioned for our study and was created by prominent Menominee musician Wade Fernandez (Native flute and voice; www.wadefernandez.com). The CD has six tracks: (a) Introduction, (b) Preparation, (c) The Body, (d) The Brain, (e) The Heart, and (f) The Spirit. Cover art for the CD was drawn by Cliff W. Wilber, enrolled member of the Menominee Tribe. In Menominee culture, it is believed that sound and music carry certain power and energy. Songs are used in ceremonies, but also for celebrations, games, storytelling, hunting, gathering, traveling, and for a wide variety of everyday dilemmas like illnesses that may arise. The rhythmic pattern of the flute on the CD was intended to help listeners focus their attention and to assist in relaxation. Affirmations were intended to assist in healing. An example of an affirmation is “I pray that all my addictions and abuse of tobacco be released, be released, into the past and that all Menominee people come into their proper relationship with tobacco.” Several studies support the benefit of affirmations in promoting health (see Cohen & Sherman, 2014).
Eligibility Criteria and Recruitment Procedures
Inclusion criteria
Adult smokers who met the following criteria were eligible for enrollment in the study: (a) at least 18 years of age, (b) smoked cigarettes, (c) eligible to receive health care services at the MTC, (d) primary care provider (PCP) was at the MTC, and (e) must be medically able and willing to take varenicline.
Exclusion criteria
Potential participants were excluded from the study for the following reasons: (a) end-stage renal disease with hemodialysis, (b) any prior suicide attempts, (c) current or recent (past 12 months) suicidal ideation, (d) currently pregnant or breastfeeding, (e) unwilling to use appropriate methods of birth control while taking study medication and for 1 month after discontinuing study medication, and (f) PCP determined that the individual should not take varenicline.
Recruitment and enrollment procedures
Potential participants consisted of smokers seeking cessation assistance at the MTC who were self-referred or clinician-referred. The standard MTC cessation program involves self- or clinician-referral to the MTC Wellness Director (the START project director), who then provides or makes arrangements for treatment (usually cessation medication plus counseling). For purposes of START study recruitment, the Wellness Director modified the standard procedures as follows: (a) Prior to meeting with the smoker, the Wellness Director or his designee determined the smoker’s eligibility to receive health care services at the MTC, and (b) adult smokers who were eligible to receive services at the MTC were informed about the START study. Smokers who were interested in the study were then provided a brief description including details about the provision of free cessation medication and counseling. Smokers interested in participating then completed the Enrollment Visit. Smokers not interested in the study received standard cessation treatment consistent with the current clinical procedures at the MTC.
Enrollment Visit (EV)
During the EV, an in-depth description of the study was provided and all participant questions were answered. For those interested in participating in the study, informed consent and HIPAA authorization were obtained. All inclusion and exclusion criteria were then evaluated to ensure study eligibility. For smokers who met study eligibility criteria, an appointment for Study Visit 1 (SV1) was scheduled to occur within 1 week. Participants also received instructions and counseling about the appropriate use of varenicline as well as a take-home information sheet on varenicline that described how to use it, common side effects, and when to contact their doctor. Smokers who were ineligible for the study or were no longer interested in the study were referred back to the Wellness Director who provided the standard MTC smoking cessation treatment.
In addition to self- and clinician-referral to the study, an IRB-approved flyer was posted on community bulletin boards on the Menominee Reservation and published in the Menominee Nation News (tribal newspaper) to generate interest in the study.
Study Procedures Following the EV
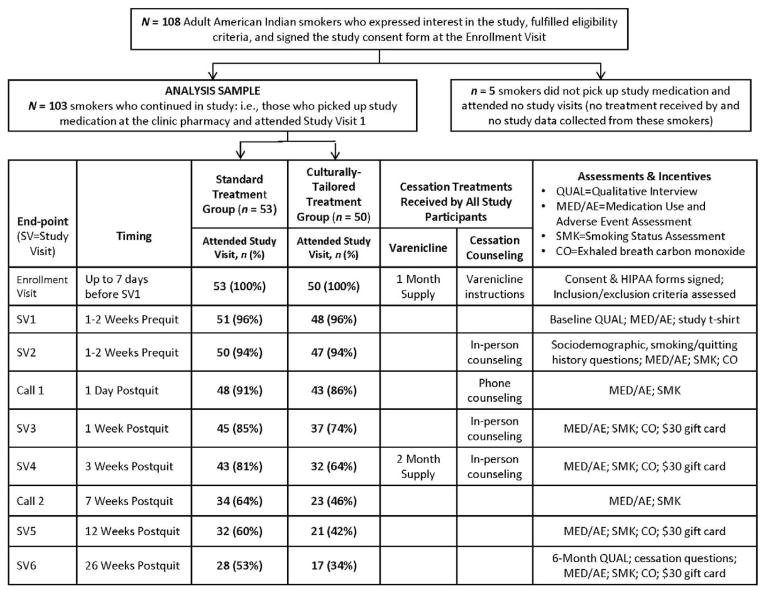
Figure 1 provides an overview of the timing and nature of study participation from the EV through completion of the study at 6 months postquit (SV6). The study coordinator conducted all counseling sessions and study visits.
Figure 1.
CONSORT diagram and study visit schedule.
Prescription for varenicline
As soon as the EV was completed, the study coordinator notified the PCP via the clinic electronic medical record (EMR) that his or her patient was interested in the study and that medical clearance was needed. The study coordinator received notification from the PCP via the EMR usually within 24 hours and, if the participant was medically cleared, the PCP issued a prescription for a 1-month supply of standard open-label varenicline to start prior to SV1. Participants who were not medically cleared to take varenicline were encouraged go through the standard MTC cessation treatment. At SV4, the participant’s PCP issued a prescription for two additional months of varenicline. Participants who reduced or discontinued varenicline after treatment started were still able to continue participating in the study.
Safety monitoring
Participants were carefully monitored by study staff for changes in health or behavior including agitation, depressed mood, suicidal ideation, and suicidal behavior during study participation. In accordance with current safety practices in clinical trials (Friedman, Furburg, & DeMets, 2010), monitoring for these and other symptoms or conditions was accomplished though assessment of adverse events (AEs) and serious adverse events (SAEs) at each study visit and phone contact. Non-urgent AEs were reported in a timely manner to study clinical staff (MDs and RNs). SAEs or AEs that raised concerns (e.g., allergic reaction, significant change in mood or suicidality) were to be immediately reported to the study physician. Individuals who reported any significant mood change or suicidal ideation were contacted immediately by a physician or licensed psychologist who assessed the level of risk and provided referrals as needed. Quarterly reports about the safety and experience of study participants were provided to the Menominee Health and Family Committee as specified in the MOA.
Study visit 1
SV1 was scheduled to take place within 1 week of the EV and consisted of the baseline qualitative interview, questions about medication use, and assessment of AEs. At the conclusion of the visit, the participant was randomly assigned to receive either ST or CTT and received a T-shirt with the START study logo. The T-shirt was intended to be a tangible symbol and reminder of the participants’ commitment to a healthier life.
Study visit 2
Most participants completed SV2 right after SV1. SV2 included: (a) baseline assessment, AE assessment, and exhaled breath carbon monoxide (CO) measurement; (b) prequit cessation counseling including setting a quit date; and (c) scheduling of subsequent study visits and phone calls.
Phone calls 1and 2
The first call occurred 1 day after the quit day and the second call occurred 7 weeks later. The study coordinator proactively contacted study participants by phone to assess smoking status, varenicline use, and AEs, and to provide postquit cessation counseling in support of the quit attempt.
Study visits 3, 4, and 5
SV3 occurred 1 week after the quit day, SV4 3 weeks post-quit, and SV5 3 months post-quit. Assessments at these visits included questions about smoking status, varenicline use, AEs, and CO; cessation counseling was provided as well. Participants also received a $30 gift card at each of these visits as compensation for their participation.
Study visit 6
SV6 occurred 6 months after the quit day and included assessments of smoking status, AEs, CO, and questions about the participant’s experiences in the study. In addition, participants completed the 6-month Qualitative Interview and received a $30 gift card.
Measures and Instrumentation
Baseline self-report measures
Participants completed a questionnaire that included standardized questions on demographics, smoking history, smoking motives and dependence, general health, and other smoking- and cessation-related questions as well as assessment of varenicline use and AEs. Participants also completed the Fagerström Test of Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerström, 1991). The FTND is a widely-used 6-item measure of nicotine dependence with fair internal consistency (α = .61); scores range from 0 to 10 with higher scores indicating greater dependence. The FTND has been found in several studies to be predictive of smoking heaviness and cessation success (e.g., Baker et al., 2007; Bolt et al., 2009).
Post-baseline self-report measures
Smoking status questions, varenicline use, and AEs were collected during all study visits and scheduled phone calls. At SV6, participants completed questions about their experiences in the study including open-ended questions about aspects of the study that they found helpful or not helpful in their cessation attempt and a question eliciting suggestions for improving cessation treatment for AIs. Participants who had relapsed were asked questions at SV6 about the reasons they resumed smoking.
Exhaled breath carbon monoxide (CO) measurement
Exhaled breath CO was measured with the Micro 4 Smokerlyzer® Monitor using a standardized procedure. CO was measured at baseline and at all postquit in-person study visits (to biochemically confirm postquit self-reports of abstinence). CO is a widely used measure in smoking cessation clinical trials and is recommended by the Society for Research on Nicotine and Tobacco (SRNT Subcommittee on Biochemical Verification, 2002). Due to budget constraints, CO was utilized for biochemical confirmation of self-reported abstinence rather than more costly laboratory assays of nicotine or cotinine (a metabolite of nicotine) in saliva, urine, or blood plasma. In the current study, a CO value of <10 parts per million was considered confirmatory of self-reported abstinence.
Primary and secondary outcome measures
The primary outcome measure was self-reported 7-day point-prevalence abstinence (7-day PPA) at 6 months post-quit, defined as abstinence from smoking, even a single puff, for the 7 days prior to the 6-month assessment endpoint (Hughes et al., 2003). Additional postquit assessment end-points included the day after quitting; 1, 3, and 7 weeks; and 3 months after quitting. Self-reported 7-day PPA was biochemically confirmed by CO at SV3, SV4, SV5, and SV6. Secondary outcomes included medication use (1, 3, and 12 weeks post-quit) and reasons for relapse reported at the 6-month follow-up.
Statistical Analyses
All statistical tests were two-tailed; alpha was set at p < .05. χ2 tests (for categorical variables) and t-tests (for continuous variables) were used to test for group differences in baseline characteristics (Table 2). For the primary 7-day PPA outcome, we conducted both intention-to-treat (ITT) analyses and responder-only analyses (Hall et al., 2001; Hughes et al., 2003) at each of the postquit study end-points (1 day; 1, 3, and 7 weeks; 3 and 6 months). ITT analysis includes all 103 participants who were randomized to treatment irrespective of protocol noncompliance, protocol deviations, or withdrawal from the study. ITT analysis provides a conservative estimate of (or lower bound for) 7-day PPA rates because participants with missing smoking status data were assumed to be smoking (resulting in a lower abstinence rate). In contrast, responder-only analysis (also known as complete-case analysis) includes only the participants who provided smoking status data at a given study endpoint. Responder-only analyses are potentially subject to bias because participants who are willing to provide data may differ from participants with missing data in terms of various characteristics (e.g., age, education, socioeconomic status) as well as outcomes. However, responder-only analyses can provide an upper bound for PPA rates and may be more sensitive to treatment effects. We conducted both ITT and responder-only analyses to assess consistency of results across the two approaches and to determine lower and upper bounds for estimated PPA rates.
Table 2.
Descriptive Statistics for Baseline Sociodemographic Variables, Smoking History, and Previous Cessation History by Treatment Group
| Baseline Characteristics/Measures | Standard Treatment (ST) (n = 53) | Culturally-Tailored Treatment (CTT) (n = 50) | p-value |
|---|---|---|---|
| Female, % | 64.2% | 60.0% | .664 |
| Age, Mean (SD) | 39.4 (13.5) | 40.1 (12.7) | .778 |
| Education: | .744 | ||
| < High School Degree | 12.0% | 14.9% | |
| High School Degree/GED | 48.0% | 40.4% | |
| > High School Degree | 40.0% | 44.7% | |
| Marital Status: | .694 | ||
| Married/Living with Partner | 38.0% | 29.8% | |
| Divorced/Widowed/Separated | 24.0% | 27.7% | |
| Never Married | 38.0% | 42.6% | |
| Age (years) When First Smoked Cigarettes, Mean (SD) | 13.4 (2.9) | 12.3 (3.7) | .118 |
| Age (years) When First Smoked Regularly, Mean (SD) | 16.8 (3.6) | 15.2 (3.5) | .028 |
| Number of Cigarettes Smoked Per Day, Mean (SD) | 13.9 (7.2) | 14.8 (8.7) | .593 |
| Years of Smoking, Mean (SD) | 22.6 (12.8) | 24.3 (10.1) | .492 |
| Fagerström Test of Nicotine Dependence (FTND) | 3.1 (2.4) | 4.1 (2.5) | .039 |
| Exhaled Breath Carbon Monoxide (in parts per million), Mean (SD) | 12.4 (9.2)) | 12.3 (9.5) | .964 |
| % With Spouse or Partner Who Smoked | 72.2% | 64.3% | .631 |
| Smoking Rules in the Home | .186 | ||
| Smoking Not Allowed in Home | 46.0% | 55.3% | |
| Smoking Allowed in Some Places | 16.0% | 23.4% | |
| Smoking Allowed Anywhere in Home | 38.0% | 21.3% | |
| Smoking By Immediate and Extended Family Members | .959 | ||
| None to a Few, % | 30.0% | 27.7% | |
| About Half, % | 30.0% | 29.8% | |
| Most or All, % | 40.0% | 42.6% | |
| Smoking By Friends: | .235 | ||
| None to a Few, % | 37.5% | 23.9% | |
| About Half, % | 16.7% | 13.0% | |
| Most or All, % | 45.8% | 63.0% | |
| Number of Previous Quit Attempts | .704 | ||
| No Prior Quit Attempts, % | 10.0% | 13.0% | |
| 1–3 Quit Attempts, % | 54.0% | 45.7% | |
| 4 or More Quit Attempts, % | 36.0% | 41.3% | |
| Number of Months Since Last Quit Attempt, Mean (SD) | 35.3 (49.5) | 36.1 (53.6) | .943 |
| Longest Previous Period of Abstinence | .995 | ||
| <1 Month, % | 22.3% | 22.0% | |
| 1–6 Months, % | 42.2% | 41.5% | |
| >6 Months, % | 35.6% | 36.6% | |
| Previously Used Cessation Medications | .906 | ||
| Never Used Cessation Medications, % | 42.0% | 44.7% | |
| Used 1 Type Previously, % | 24.0% | 25.5% | |
| Used 2 or More Types Previously, % | 34.0% | 29.8% | |
| Previously Used Individual or Group Counseling for Smoking Cessation, % | 6.0% | 10.6% | .407 |
Missing data for study outcomes occurred primarily because participants elected to not return for study visits despite concerted efforts by the study coordinator to contact and encourage participants to come to study visits. For the ITT analyses, missing data were handled as follows: for 7-day point-prevalence abstinence, missing was treated as smoking (i.e., participants who did not complete a follow-up assessment were considered to be smoking). For both ITT and responder-only analyses, ST versus CTT group differences were tested using logistic regression (Hosmer, Lemeshow, & Sturdivant, 2013) which yielded odds ratios (ORs) and 95% confidence intervals (CIs) for the ORs (Table 3).
Table 3.
Responder-Only and Intention-To-Treat Abstinence by Treatment Condition
| Study End-Point | Standard Treatment
|
Culturally-Tailored Treatment
|
Odds Ratio (95% CI) | p-value | ||
|---|---|---|---|---|---|---|
| n | % Abstinent | n | % Abstinent | |||
| 1 Day Postquit | ||||||
| Responder Only | 48 | 45.8% | 43 | 51.2% | 1.24 (0.54–2.82) | .612 |
| Intention-to-Treat | 53 | 41.5% | 50 | 44.0% | 1.12 (0.51–2.42) | .798 |
| 1 Week Postquit | ||||||
| Responder Only | 45 | 28.9% | 37 | 40.5% | 1.68 (0.67–4.21) | .270 |
| Intention-to-Treat | 53 | 24.5% | 50 | 30.0% | 1.32 (0.55–3.15) | .533 |
| 3 Weeks Postquit | ||||||
| Responder Only | 43 | 44.2% | 32 | 43.8% | 0.97 (0.39–2.47) | .970 |
| Intention-to-Treat | 53 | 35.8% | 50 | 28.0% | 0.70 (0.30–1.60) | .394 |
| 7 Weeks Postquit | ||||||
| Responder Only | 34 | 52.9% | 23 | 56.5% | 1.16 (0.40–3.35) | .790 |
| Intention-to-Treat | 53 | 34.0% | 50 | 26.0% | 0.68 (0.29–1.60) | .380 |
| 3 Month Postquit | ||||||
| Responder Only | 32 | 40.6% | 21 | 57.1% | 1.95 (0.64–5.95) | .241 |
| Intention-to-Treat | 53 | 24.5% | 50 | 24.0% | 0.97 (0.39–2.39) | .950 |
| 6 Months Postquit | ||||||
| Responder Only | 28 | 42.9% | 17 | 41.2% | 0.93 (0.28–3.17) | .912 |
| Intention-to-Treat | 53 | 22.6% | 50 | 14.0% | 0.56 (0.20–1.55) | .262 |
Note. Abstinence rates at 1 week, 3 weeks, 3 months, and 6 months postquit were biochemically-confirmed via exhaled breath carbon monoxide testing (a value less than 10 parts per million was considered confirmatory of self-reported abstinence). CI = Confidence Interval.
We conducted a set of secondary analyses using χ2 tests that tested treatment group differences in medication use at 1, 3, and 12 weeks post-quit. We also conducted χ2 tests to test for the association between abstinence at the 6-month follow-up and selected participant characteristics (Table 4).
Table 4.
Abstinence Status at the 6 Month Follow-Up by Selected Participant Characteristics
| Participant Characteristic | Abstinence at 6-Month Follow-Up
|
Test Value and p-Value | |
|---|---|---|---|
| n | % Abstinent | ||
| Smoking Status at: 1 Week Postquit | |||
| Abstinent | 28 | 57.1% | χ2(1, N = 103) = 38.28, p < .001 |
| Smoking | 75 | 4.0% | |
| Gender: | |||
| Female | 64 | 23.4% | χ2(1, N = 103) = 2.80, p = .094 |
| Male | 39 | 10.3% | |
| Age Group: | |||
| 19–40 Years | 51 | 9.8% | χ2(1, N = 101) = 5.47, p = .019 |
| 41–77 Years | 50 | 28.0% | |
| Marital Status: | |||
| Married/With Partner | 33 | 30.3% | χ2(2, N = 97) = 3.74, p = .154 |
| Divorced/Widowed/Separated | 25 | 16.0% | |
| Never Married | 39 | 12.8% | |
| Education: | |||
| < High School Degree | 13 | 7.7% | χ2(2, N = 97) = 1.54, p = .464 |
| High School Degree/GED | 43 | 23.3% | |
| > High School Degree | 41 | 19.5% | |
| Does Spouse/Partner Smoke? | |||
| No | 10 | 60.0% | χ2(1, N = 32) = 5.60, p = .018 |
| Yes | 22 | 18.2% | |
| Cigarettes Per Day (Median Split): | |||
| 1–12 Cigarettes Per Day | 47 | 29.8% | χ2(1, N = 97) = 6.02, p = .014 |
| 13–40 Cigarettes Per Day | 50 | 10.0% | |
| Smoking Rules in the Home: | |||
| Smoking Not Allowed in Home | 49 | 18.4% | χ2(2, N = 97) = 0.69, p =.707 |
| Smoking Allowed in Some Places | 19 | 26.3% | |
| Smoking Allowed Anywhere | 29 | 17.2% | |
| Smoking By Immediate and Extended Family Members: | |||
| None to a Few | 28 | 35.7% | χ2(2, N = 97) = 8.36, p = .015 |
| About Half | 29 | 20.7% | |
| Most or All | 40 | 7.5% | |
| Smoking By Friends: | |||
| None to a Few | 29 | 24.1% | χ2(2, N = 94) = 0.75, p = .686 |
| About Half | 14 | 14.3% | |
| Most or All | 51 | 17.6% | |
Note. Abstinence rates at 6 months postquit were biochemically-confirmed via exhaled breath carbon monoxide testing (a value less than 10 parts per million was considered confirmatory of self-reported abstinence). P-values less than .05 are considered statistically significant.
Sample Size and Power to Detect Predicted Effects
In the original study protocol, the study sample size was set at 150 to have sufficient power (80%; two-tailed test; α = .05) to detect a statistically significant group difference in predicted 7-day PPA rates at 6-months of 20% in the ST group (based on prior experience at the MTC) versus 41% in the CTT group. The enrollment goal for the study was subsequently reduced to 120 due to an unanticipated 25% reduction in funding for years 2 and 3 of the study. We were only able to achieve a final sample size of 103 because the start of the study was delayed due to challenges in obtaining IRB approval for the study and because we were not able to continue enrollment past the end of the 3-year funding period of the study. The reduced sample size reduced statistical power to detect predicted effects.
Results
Treatment Group Comparisons for Baseline Measures
Table 2 provides descriptive statistics for baseline sociodemographic, smoking history, cessation history variables, and other smoking-related variables by treatment group. There were no group differences on any of the baseline measures except for two smoking history variables. Specifically, the CTT group had a significantly higher FTND score than the ST group. In addition, participants in the CTT group were significantly younger than ST group participants in terms of when they first regularly smoked cigarettes.
Table 2 also shows some notable results. A high percentage (>64%) of married or partnered participants had a spouse or partner who smoked. Also, nearly half of all participants reported that smoking was allowed inside their homes and >70% of participants reported that at least half of their immediate and extended family members smoked. All of these represent risk factors for relapse. In addition, the average time since the last quit attempt was approximately 3 years and only about one-third of participants were ever able to stay quit for longer than 6 months. Lastly, nearly 60% reported previously using cessation medications whereas only a small percentage (6–10%) reported previously using cessation counseling of any kind.
Study Participation and Follow-up
As shown in Figure 1, a total of 103 participants were randomized to the ST condition (n = 53) or the CTT condition (n = 50). Rates of study visit attendance were similar for the two groups until about SV4 (3 weeks post-quit) when attendance rates started to differ by treatment group. For the CTT group, 64% attended SV4 compared to 81% of the ST group (χ2 (1, N = 103) = 3.82, p = .05). These group differences in study attendance rates continued through to the 6-month study visit (34% for CTT vs. 53% for ST; χ2 (1, N = 103) = 3.71, p = .05). Although the magnitude of the differences appeared to be sizable, no difference was statistically significant at p < .05 but all of the p-values from SV4 to the 6-month visit were quite close to .05 and, thus, were suggestive of a consistent effect over time. The reasons for this differential non-attendance at study visits are unclear and will be explored in future qualitative analyses of participant interviews.
Primary 7-Day Point-Prevalence Abstinence (PPA) Outcome
As shown in Table 3, there were no statistically significant group differences in 7-day PPA in either the ITT or the responder-only analyses. The overall end-of-treatment (3 months) ITT abstinence rate for all 103 participants was 24% and the responder-only rate (based on N = 53 who attended SV5) was 47%; the overall ITT abstinence rate at 6 months was 20% and the responder-only rate was 42% (based on n = 45 who attended SV6). The number of participants who attended a given study visit or completed a given phone call is provided in Figure 1 by group and time-point. We conducted additional analyses in which we added two covariates on which the groups differed at baseline (FTND score and age when first smoked regularly) to the logistic regression models and the treatment group effect remained statistically nonsignificant at all time-points.
Association between 6-Month Abstinence and Participant Characteristics
Because there were no group differences in abstinence by treatment group at any study end-point, we elected to examine selected participant characteristics that may be associated with long-term (6-month) biochemically-confirmed ITT abstinence success (Table 4). Several variables were significantly associated with abstinence. The most robust finding was that abstinence status at 1 week postquit was strongly associated with 6-month abstinence. In particular, 57.1% of participants who were abstinent at 1 week postquit also were abstinent at 6 months compared to only 4.0% of participants who were smoking at 1 week postquit. Other significant results showed that older participants were more likely than younger participants to be abstinent (28.0% vs. 9.8%, respectively). In addition, having a spouse or partner who smokes was associated with a lower likelihood of being abstinent at 6 months. Lighter smokers (1–12 CPD) were 3 times more likely to be quit at 6 months compared to heavier smokers (29.8% vs. 10.0%, respectively). Lastly, participants who reported that most or all of their immediate or extended family members smoked had a very low PPA rate at 6 months (7.5%) compared to participants with only about half of family members smoking (20.7%) or none to a few (35.7%).
Secondary Outcomes: Medication Use and Reasons for Relapse
Overall, 90.2% of study participants reported taking varenicline at 1 week post-quit, 84.0% at 3 weeks post-quit, and 32.1% at 12 weeks post-quit. There were no treatment group differences in rates of medication use. Also, there were no significant associations between medication use and 6-month PPA rates.
A total of 45 study participants attended the 6-month study visit and completed end-of-study assessments including reasons for relapse among 26 participants who had returned to smoking. The most commonly cited reason for relapse was “too much stress” (reported by 46.2% of relapsed participants). Two other frequently-cited reasons for relapse were “too many situations where others were smoking” (30.8%), and “too addicted to nicotine” (19.2%).
Safety
No SAEs occurred during the study. A total of 6 participants discontinued varenicline early; however, all six continued their participation in the study. Three participants discontinued varenicline because they returned to smoking; among the three other participants who stopped taking varenicline, one cited stomach pain, one reported changes in moods, and one reported nausea.
Discussion
To our knowledge, the current study represents the first smoking cessation comparative RCT testing a culturally-tailored smoking cessation intervention designed for a specific AI/AN tribal community (i.e., Menominee) that combined FDA-approved cessation medication (varenicline) and innovative cultural intervention components. The CTT intervention resulted from extensive engagement with community representatives over several years and benefited from the shared experiences of tribal members, MTC staff, and clinical researchers from UW-Madison and UW-Milwaukee. Contrary to prediction, cultural tailoring of the cessation intervention did not result in a significantly higher abstinence rate at any study end-point as compared to the standard non-tailored treatment. However, it is worth noting that responder-only abstinence rates at two study end-points (1 week and 3 months post-quit) were higher in the CTT group than in the ST group but the differences were not statistically significant possibly because of limited sample size. Thus, an important question for future study concerns how to increase and sustain the benefit of cultural tailoring. Another factor that may have negatively affected CTT abstinence rates at post-treatment study endpoints (e.g., 3 months and 6 months) is the greater attrition in the CTT group compared to the ST group. It is unclear whether or not the differential attrition is related to the CTT treatment itself (i.e., an unanticipated iatrogenic effect of cultural tailoring). We plan to explore this and other possible explanations in qualitative analyses that will be reported separately.
Interestingly, the overall ITT abstinence rate at 6 months of 20%, and the overall responder-only rate of 42%, are quite similar to rates reported by D’Silva and colleagues who reported 90-day abstinence rates of 21.8% and 47%, respectively, in a single-group study of a culturally-tailored cessation program conducted on the Fond du Lac reservation in Minnesota (D’Silva, Schillo, Sandman, Leonard, & Boyle, 2011). However, the long-term ITT rate in the current study is substantially higher than the long-term rates reported in most previous AI/AN comparative studies (e.g., Hodge & Casken, 1999; Johnson et al., 1997), yet somewhat lower than the estimated 6-month ITT rate of 27.6% for majority-White smokers reported in the 2008 PHS Guideline meta-analysis for medication plus counseling (Fiore et al., 2008). Taken together, these findings speak to the challenge of sustaining higher rates of abstinence success regardless of race or ethnicity (Cox, Okuyemi, Choi, & Ahluwalia, 2011).
Although the study did not find clear evidence that the cultural tailoring, as initially developed, improved long-term cessation success, it is important to note that this first effort has set the stage for further refinement of the CTT intervention that could lead to more effective cessation treatment. There were a number of study findings that increase understanding of the challenges and promise of cessation treatment for AI/AN smokers. One important finding was that disengagement from treatment was higher than expected and, as such, the addition of interventions or procedures to support continued treatment engagement likely will be an important refinement of interventions. Also, we found that our participants share similarities to treatment-seeking smokers in other racial and ethnic groups but there were notable differences as well. Like other groups of smokers, study participants started smoking at a relatively young age and had been smoking, on average, for over 20 years. Also, on average, participants smoked 14–15 CPD, a rate that is similar to the general population CPD rate in Wisconsin (Ahrens, Bandi, Ullsvik, & Moberg, 2005). Interestingly, the percentage of participants reporting that smoking was not allowed anywhere in the home (50.5%) is very similar to recent Wisconsin and national estimates (Wisconsin Tobacco Prevention and Control Program, 2009b; Zhang, Martinez-Donate, Kuo, Jones, & Palmersheim, 2011).
However, there are some striking findings in the current study. For example, 69% of the participants who were married or with a partner reported that their spouse or partner smoked and this factor was associated with lower likelihood of cessation success at 6 months postquit. Contrary to expectation, 6-month cessation success was not associated with rules about smoking in the home. Another important finding was that more than 70% of the study participants reported having a majority of their immediate and extended family members as smokers and this was associated with a low likelihood of cessation at 6 months. Thus, spouse or partner smoking and having many family members who smoke may be key social risk factors for relapse for AI/AN smokers trying to quit.
A limitation to the study is the sample size. The original study plan specified a target sample size of 150 adult AI/AN smokers but this total was later reduced to 120 due to an unanticipated reduction in funding. The final enrollment total of 103 represents about 69% of the original target sample size and about 86% of the revised enrollment goal. It is unclear whether or not meeting the original or revised enrollment goals would yield stronger or clearer findings for the primary abstinence outcome. Another limitation is the higher-than-expected attrition in the study. In addition, attrition in was higher in the CTT group and this likely lowered the cessation success rate in that group. It is also possible that provision and wearing of the study T-shirt with the START logo on it could have alerted others also participating in the study and information could possibly have been shared among participants about the two different treatments. This may have influenced or biased participants in unknown ways.
We encountered a number of challenges and barriers to collaboration that affected timely progress in developing and fielding our study. As noted previously, the unanticipated reduction in funding (25% reduction in years 2 and 3) required lowering the target sample size; this smaller sample size reduced power to detect effects. In addition, the reduction in funding was perceived by the tribal research partner as inconsistent with the trust placed in the university-based partners. This issue was resolved by way of honest, forthright discussions about the reasons for, and impact of, the funding reduction as well as confirmation that there would be no change in the original agreement that 60% of the funding total would go directly to the Menominee Tribe. The research team also encountered challenges related to matching mainstream research orientations to the needs of the Menominee people. These challenges were resolved by adjusting research materials and procedures to be respectful of Menominee and AI/AN language and worldview issues regarding commercial and ceremonial tobacco. A barrier to starting the study according to the planned timeline was the significant delay in obtaining IRB approval for the study. After we completed the development process for all study procedures, we sought IRB approval as required but there was a significant delay in getting approval because of limited IRB experience with community-based clinical research, especially with tribal communities. Although we attempted to address this by educating IRB staff about community-based research, approval was delayed by at least 3 months and this delay adversely affected the start of study enrollment and contributed to our inability to achieve the revised target enrollment of 120.
There are a number of successes in the current study that are worth noting. The START study represents a successful collaboration between a tribal community and academic partners working together for the benefit of the community as well as the advancement of scientific understanding. The study also utilized rigorous clinical trials methodology and collection of both quantitative and qualitative data, a combination which is rare in AI/AN cessation research. Another success is the participation of 103 AI/AN participants who generously volunteered their time and shared their experiences for the benefit of the tribe, their own well-being, and the advancement of science. The START study also provided a rich training experience for a number of students, both Native and non-Native, who gained invaluable experience in conducting community-based research in “Indian country” and who can potentially contribute to the sustainability of this important work. It is also worth noting that the current results represent the beginning of a series of analyses of study data, both quantitative and qualitative as well as a mixed-methods analysis. These analyses have great potential to advance understanding of a number of tobacco- and cessation-related issues in the Menominee community with possible implications for other tribal communities as well. Preliminary qualitative results point to the potential importance of a family-based approach to cessation and discussions are ongoing with the Menominee Tribe about developing and testing such an intervention.
For counseling psychologists, the current study supports the use of evidence-based counseling in combination with FDA-approved cessation medication such as varenicline. In particular, the overall biochemically-confirmed 6-month ITT abstinence rate for the total sample of 103 participants was 20%. This abstinence success rate is well above the rate for unassisted quitting which is about 5% at 6 months (Hughes et al., 2004). Thus, despite the fact that we did not find predicted group differences, we demonstrated that our AI/AN participants benefitted from more intensive intervention irrespective of cultural tailoring. An important part of the intensive intervention is evidence-based cessation counseling delivered with cultural sensitivity, a treatment approach that counseling psychologists can learn to deliver in an effective and culturally-aware manner.
Although our study did not find clear evidence that cultural tailoring yields higher abstinence rates than a standard treatment, a recent focus group study by Fu and colleagues (Fu et al., 2014) that assessed AI/AN opinions about cessation treatments offers a number of recommendations that may be beneficial to AI/AN smokers. In particular, study participants recommended that cessation programs be led by AI/AN community members with expertise in cessation, that peer support be an integral part of cessation programs, that there be a focus on healthy smoke-free living, that cultural components should be a part of the cessation program (e.g., use of AI/AN images, discussion of traditional tobacco, story-telling) and that this would promote supportive connections with other community members, that children and youth should be included in prevention programs, and that free cessation medication should be provided. We incorporated several of these recommendations in the current study, but additional research is needed to identify which combination of components are most helpful to AI/AN smokers wishing to quit the addictive use of commercial tobacco.
Acknowledgments
This research was funded by the Wisconsin Partnership Program of the University of Wisconsin School of Medicine and Public Health with additional support from the “Spirit of Eagles Community Network Program” (U54 CA153605; PI: Judith Kaur, MD, Mayo College of Medicine), the UW Carbone Cancer Center, and the UW Center for Tobacco Research and Intervention.
Contributor Information
Stevens S. Smith, Department of Medicine and Center for Tobacco Research and Intervention (CTRI), University of Wisconsin School of Medicine and Public Health (UWSMPH), Madison, WI
Leah M. Rouse, Department of Educational Psychology, University of Wisconsin-Milwaukee
Mark Caskey, Menominee Tribal Clinic, Menominee Indian Tribe of Wisconsin, Keshena, WI
Jodi Fossum, Menominee Tribal Clinic, Menominee Indian Tribe of Wisconsin, Keshena, WI
Rick Strickland, UW Spirit of EAGLES, Carbone Cancer Center, UWSMPH, Madison, WI
J. Kevin Culhane, Menominee Tribal Clinic, Menominee Indian Tribe of Wisconsin, Keshena, WI
Jerry Waukau, Menominee Tribal Clinic, Menominee Indian Tribe of Wisconsin, Keshena, WI
References
- Ahrens D, Bandi P, Ullsvik J, Moberg DP. Who smokes? A demographic analysis of Wisconsin smokers. Wisconsin Medical Journal. 2005;104:18–22. [PubMed] [Google Scholar]
- Arndt L, Caskey M, Fossum J, Schmidt N, Davis A, Smith SS, Waukau J. Menominee perspectives on commercial and sacred tobacco use. American Indian and Alaska Native Mental Health Research. 2013;20:1–22. doi: 10.5820/aian.2003.2013.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Bolt DM, Smith SS, Kim SY, Toll BA. Time to first cigarette in the morning as an index of ability to quit smoking: Implications for nicotine dependence. Nicotine & Tobacco Research. 2007;9:S555–S570. doi: 10.1080/14622200701673480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolt DM, Piper ME, McCarthy DE, Japuntich SJ, Fiore MC, Smith SS, Baker TB. The Wisconsin Predicting Patients’ Relapse Questionnaire. Nicotine & Tobacco Research. 2009;11:481–492. doi: 10.1093/ntr/ntp030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brave Heart M, Chase J, Elkins J, Altschul DB. Historical trauma among indigenous peoples of the Americas: Concepts, research, and clinical considerations. Journal of Psychoactive Drugs. 2011;43(4):282–290. doi: 10.1080/02791072.2011.628913. [DOI] [PubMed] [Google Scholar]
- Brokenleg I, Tornes E. Walking toward the sacred: Our Great Lakes tobacco story. Lac du Flambeau, WI: Great Lakes Inter-Tribal Council, Inc; 2013. Retrieved from http://www.glitc.org/forms/Tabacco/tabacco-booklet-web-.pdf. [Google Scholar]
- Buki LP. Reducing health disparities: The perfect fit for counseling psychology. The Counseling Psychologist. 2007;35(5):706–715. doi: 10.1177/0011000007303632. [DOI] [Google Scholar]
- Buki LP, Selem M. Health disparities: Issues and opportunities for counseling psychologists. In: Fouad N, Carter J, Subich L, editors. APA handbook of counseling psychology: Vol. 2. Practice, interventions, and applications. Washington, DC: American Psychological Association; 2012. pp. 235–249. [DOI] [Google Scholar]
- Burawoy M. The extended case method. Sociological Theory. 1998;16:4–33. doi: 10.1111/0735-2751.00040. [DOI] [Google Scholar]
- Burgess D, Fu SS, Joseph AM, Hatsukami DK, Soloman J, van Ryn M. Beliefs and experiences regarding smoking cessation among American Indians. Nicotine & Tobacco Research. 2007;9(1):19–28. doi: 10.1080/14622200601083426. [DOI] [PubMed] [Google Scholar]
- Burhansstipanov L, Christopher S, Schumacher SA. Lessons learned from community-based participatory research in Indian country. Cancer Control. 2005;12:70–76. doi: 10.1177/1073274805012004s10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database of Systematic Reviews (Online) 2011;(2):CD006103. doi: 10.1002/14651858.CD006103.pub5. [DOI] [PubMed] [Google Scholar]
- Cahill K, Stevens S, Lancaster T. Pharmacological treatments for smoking cessation. JAMA. 2014;311:193–194. doi: 10.1001/jama.2013.283787. [DOI] [PubMed] [Google Scholar]
- Carson KV, Brinn MP, Peters M, Veale A, Esterman AJ, Smith BJ. Interventions for smoking cessation in Indigenous populations. Cochrane Database of Systematic Reviews (Online) 2012;(1):CD009046. doi: 10.1002/14651858.CD009046.pub2. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Cigarette smoking-attributable morbidity -- United States, 2000. Morbidity and Mortality Weekly Report. 2003;52:842–844. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Vital signs: Current cigarette smoking among adults aged ≥ 18 years - United States, 2005–2010. Morbidity and Mortality Weekly Report. 2011a;60:1207–1212. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Quitting smoking among adults—United States, 2001–2010. Morbidity and Mortality Weekly Report. 2011b;60:1513–1519. [PubMed] [Google Scholar]
- Choi WS, Daley CM, James A, Thomas J, Schupbach R, Segraves M, Barnoskie R, Ahluwalia JS. Beliefs and attitudes regarding smoking cessation among American Indians: A pilot study. Ethnicity and Disease. 2006;16:35–40. [PubMed] [Google Scholar]
- Cohen GL, Sherman DK. The psychology of change: Self-affirmation and social psychological intervention. Annual Review of Psychology. 2014;65:333–371. doi: 10.1146/annurev-psych-010213-115137. [DOI] [PubMed] [Google Scholar]
- Cox LS, Okuyemi K, Choi WS, Ahluwalia JS. A review of tobacco use treatments in U.S. ethnic minority populations. American Journal of Health Promotion. 2011;25(Suppl):S11–30. doi: 10.4278/ajhp.100610-LIT-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creswell JW, Klassen AC, Plano Clark VL, Smith KC for the Office of Behavioral and Social Sciences Research. Best practices for mixed methods research in the health sciences. Washington, DC: National Institutes of Health; 2011. Retrieved from http://obssr.od.nih.gov/mixed_methods_research. [Google Scholar]
- Daley CM, James AS, Barnoskie RS, Segraves M, Schupbach R, Choi WS. “Tobacco has a purpose, not just a past”: Feasibility of developing a culturally appropriate smoking cessation program for a Pan-Tribal Native population. Medical Anthropology Quarterly. 2006;20:421–440. doi: 10.1525/maq.2006.20.4.421. [DOI] [PubMed] [Google Scholar]
- Dennis MK, Momper SL. “It’s bad around here now”: Tobacco, alcohol and other drug use among American Indians living on a rural reservation. Journal of Ethnicity in Substance Abuse. 2012;11:130148. doi: 10.1080/15332640.2012.675244. [DOI] [PubMed] [Google Scholar]
- Denny CH, Holtzman D, Goins RT, Croft JB. Disparities in chronic disease risk factors and health status between American Indian/Alaska Native and White elders: findings from a telephone survey, 2001 and 2002. American Journal of Public Health. 2005;95:825–827. doi: 10.2105/AJPH.2004.043489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Silva J, Schillo BA, Sandman NR, Leonard TL, Boyle RG. Evaluation of a tailored approach for tobacco dependence treatment for American Indians. American Journal of Health Promotion. 2011;25:S66–S69. doi: 10.4278/ajhp.100611-QUAN-180. [DOI] [PubMed] [Google Scholar]
- Evans-Campbell T. Historical trauma in American Indian/Native Alaska communities: A multilevel framework for exploring impacts on individuals, families, and communities. Journal of Interpersonal Violence. 2008;23:316–338. doi: 10.1177/0886260507312290. [DOI] [PubMed] [Google Scholar]
- Fagan P, Moolchan ET, Lawrence D, Fernander A, Ponder PK. Identifying health disparities across the tobacco continuum. Addiction. 2007;102(suppl 2):5–29. doi: 10.1111/j.1360-0443.2007.01952.x. [DOI] [PubMed] [Google Scholar]
- Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz N, Curry SJ, Wewers ME. Treating tobacco use and dependence:2008 update. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service; 2008. Retrieved from http://www.ahrq.gov/path/tobacco.htm. [Google Scholar]
- Friedman LM, Furberg CD, DeMets DL. Fundamentals of clinical trials. 4. New York: Springer-Verlag; 2010. [Google Scholar]
- Fu SS, Rhodes KL, Robert C, Widome R, Forster JL, Joseph AM. Designing and evaluating culturally specific smoking cessation interventions for American Indian Communities. Nicotine & Tobacco Research. 2014;16:42–49. doi: 10.1093/ntr/ntt111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geishirt Cantrell BA, Hodge FS, Struthers R, Decora LH. The high incidence of cigarette smoking among American Indians of the Northern Plains. Journal of Cancer Education. 2005;20(Suppl):97–100. doi: 10.1207/s15430154jce2001s_19. [DOI] [PubMed] [Google Scholar]
- Gohdes D, Harwell TS, Cummings S, Moore KR, Smilie JG, Helgerson SD. Smoking cessation and prevention: An urgent public health priority for American Indians in the Northern Plains. Public Health Reports. 2002;117:281–290. doi: 10.1016/S0033-3549(04)50162-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall S, Delucchi K, Velicer W, Kahler C, Ranger-Moore J, Hedeker D, Tsoh J, Niaura R. Statistical analysis of randomized trials in tobacco treatment: Longitudinal designs with dichotomous outcome. Nicotine & Tobacco Research. 2001;3:193–202. doi: 10.1080/14622200110050411. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KA. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addictions. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hendricks PS, Westmaas JL, Ta Park VM, Thorne CB, Wood SB, Baker MR, Lawler RM, Webb Hooper M, Delucchi KL, Hall SM. Smoking abstinence-related expectancies among American Indians, African Americans, and women: Potential mechanisms of tobacco-related disparities. Psychology of Addictive Behaviors. 2014;28(1):193–205. doi: 10.1037/a0031938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman KC, Tucker CM, Ferdinand LA, Mirsu-Paun A, Hasan NT, Beato C. Culturally sensitive health care and counseling psychology: An overview. The Counseling Psychologist. 2007;35:633–649. doi: 10.1177/0011000007301672. [DOI] [Google Scholar]
- Hodge FS, Casken J. Characteristics of American Indian women cigarette smokers: Prevalence and cessation status. Health Care for Women International. 1999;20:455–469. doi: 10.1080/073993399245557. [DOI] [PubMed] [Google Scholar]
- Hosmer DW, Lemeshow S, Sturdivant RX. Applied logistic regression. 3. Hoboken, NJ: John Wiley & Sons, Inc; 2013. [Google Scholar]
- Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: Issues and recommendations. Nicotine & Tobacco Research. 2003;5:13–25. doi: 10.1093/ntr/5.1.13. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004;99(1):29–38. doi: 10.1111/j.1360-0443.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- Johnson KM, Lando HA, Schmid LS, Solberg LI. The GAINS project: outcome of smoking cessation strategies in four urban Native American clinics. Giving American Indians No-smoking Strategies. Addictive Behaviors. 1997;22:207–218. doi: 10.1016/S0306-4603(96)00015-9. [DOI] [PubMed] [Google Scholar]
- Kaur JS, Dignan M, Burhansstipanov L, Baukol P, Claus C. The “Spirit of Eagles” legacy. Cancer. 2006;107(Suppl 8):1987–1994. doi: 10.1002/cncr.22152. [DOI] [PubMed] [Google Scholar]
- Leffingwell TR, Babitzke AC. Tobacco intervention practices of licensed psychologists. Journal of Clinical Psychology. 2006;62:313–323. doi: 10.1002/jclp.20234. [DOI] [PubMed] [Google Scholar]
- Mackowick KM, Lynch MJ, Weinberger AH, George TP. Treatment of tobacco dependence in people with mental health and addictive disorders. Current Psychiatry Reports. 2012;14(5):478–485. doi: 10.1007/s11920-012-0299-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottillo S, Filion KB, Bélisle P, Joseph L, Gervais A, O’Loughlin J, Eisenberg MJ. Behavioural interventions for smoking cessation: A meta-analysis of randomized controlled trials. European Heart Journal. 2009;30:718–730. doi: 10.1093/eurheartj/ehn552. [DOI] [PubMed] [Google Scholar]
- Morgan R, Freeman L. The healing of our people: Substance abuse and historical trauma. Substance Use & Misuse. 2009;44:84–98. doi: 10.1080/10826080802525678. [DOI] [PubMed] [Google Scholar]
- Nez Henderson P, Jacobsen C, Beals J. Correlates of cigarette smoking among selected Southwest and Northern Plains tribal groups: The AI-SUPERPFP Study. American Journal of Public Health. 2005;95:867–872. doi: 10.2105/AJPH.2004.050096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton IM, Manson SM. Research in American Indian and Alaska Native communities: Navigating the cultural universe of values and process. Journal of Consulting and Clinical Psychology. 1996;64:856–860. doi: 10.1037/0022-006X.64.5.856. [DOI] [PubMed] [Google Scholar]
- Oberly J, Macedo J. The R word in Indian Country: Culturally appropriate commercial tobacco-use research strategies. Health Promotion Practice. 2004;5:355–361. doi: 10.1177/1524839904267391. [DOI] [PubMed] [Google Scholar]
- Ostenso A, Remington P, Ahrens D. Lung cancer mortality report card: Measuring progress in Wisconsin’s counties, 1979–1998. Wisconsin Medical Journal. 2001;100:70–74. [PubMed] [Google Scholar]
- Palmersheim KA, Voskuil KR, Glysch RL. The Prevalence of Smoking in Wisconsin: Variability at the County Level. Research Brief, Center for Urban Initiative & Research, University of Wisconsin-Milwaukee; 2011. Sep, Available from http://www4.uwm.edu/cuir/news/upload/Wisconsin-County-Smoking-Prevalence.pdf. [Google Scholar]
- Pego CM, Hill RF, Solomon GW, Chisholm RM, Ivey SE. Tobacco, culture, and health among American Indians: A historical review. American Indian Culture and Research Journal. 1995;19:143–164. [Google Scholar]
- Phillips KM, Brandon TH. Do psychologists adhere to the clinical practice guidelines for tobacco cessation? A survey of practitioners. Professional Psychology: Research and Practice. 2004;35(3):281–285. doi: 10.1037/0735-7028.35.3.281. [DOI] [Google Scholar]
- Samet JM. Health effects of tobacco use by Native Americans. In: Winter JC, editor. Tobacco Use by Native North Americans: Sacred Smoke and Silent Killer. University of Oklahoma Press; Norman, OK: 2000. pp. 331–341. [Google Scholar]
- Schlam TR, Baker TB. Interventions for tobacco smoking. Annual Review of Clinical Psychology. 2013;9:675–702. doi: 10.1146/annurev-clinpsy-050212-185602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Brockwell SE, Pillitteri JL, Gitchell JG. Use of smoking-cessation treatments in the United States. American Journal of Preventive Medicine. 2008;34(2):102–111. doi: 10.1016/j.amepre.2007.09.033. [DOI] [PubMed] [Google Scholar]
- SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Stead LF, Lancaster T. Combined pharmacotherapy and behavioural interventions for smoking cessation. Cochrane Database of Systematic Reviews (Online) 2012;(10):CD008286. doi: 10.1002/14651858.CD008286.pub2. [DOI] [PubMed] [Google Scholar]
- Struthers R, Hodge FS. Sacred tobacco use in Ojibwe communities. Journal of Holistic Nursing. 2004;22:209–225. doi: 10.1177/0898010104266735. [DOI] [PubMed] [Google Scholar]
- Tucker CM, Ferdinand LA, Mirsu-Paun A, Herman KC, Delgado-Romero E, van den Berg JJ, Jones JD. The roles of counseling psychologists in reducing health disparities. The Counseling Psychologist. 2007;35:650–678. doi: 10.1177/0011000007301687. [DOI] [Google Scholar]
- U.S. Department of Health and Human Services, Office of the Surgeon General. The health consequences of smoking: A report of the Surgeon General. Washington, DC: U.S. Department of Health and Human Services; 2004. [Google Scholar]
- Walters KL, Simoni JM. Reconceptualizing Native women’s health: An “Indigenist” stress-coping model. American Journal of Public Health. 2002;92(4):520–524. doi: 10.2105/AJPH.92.4.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe-Galloway S, Flom N, Xu L, Duran T, Frerichs L, Kennedy F, Smith CB, Jaiyeola AO. Cancer-related disparities and opportunities for intervention in Northern Plains American Indian communities. Public Health Reports. 2011;126:318–329. doi: 10.1177/003335491112600304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitbeck LB, Adams GW, Hoyt DR, Chen X. Conceptualizing and measuring historical trauma among American Indian people. American Journal of Community Psychology. 2004;33(3/4):119–130. doi: 10.1023/B:AJCP.0000027000.77357.31. [DOI] [PubMed] [Google Scholar]
- Whitbeck LB, Chen X, Hoyt DR, Adams GW. Discrimination, historical loss and enculturation: Culturally specific risk and resiliency factors for alcohol abuse among American Indians. Journal of Studies on Alcohol. 2004;33:409–418. doi: 10.15288/jsa.2004.65.409. [DOI] [PubMed] [Google Scholar]
- Winter JC. Traditional uses of tobacco by Native Americans. In: Winter JC, editor. Tobacco Use by Native North Americans: Sacred Smoke and Silent Killer. University of Oklahoma Press; Norman, OK: 2000. pp. 9–58. [Google Scholar]
- Wisconsin Tobacco Prevention and Control Program. Tobacco Related Disparities in Wisconsin: Selected Tobacco Prevention and Control Data (Updated Report P-00142) Division of Public Health, Wisconsin Department of Health Services; 2009a. Dec, Available from http://www.dhs.wisconsin.gov/publications/P0/P00142.pdf. [Google Scholar]
- Wisconsin Tobacco Prevention and Control Program. Wisconsin Tobacco Facts 2009. Division of Public Health, Wisconsin Department of Health Services; 2009b. Dec, Report P-43021B. Available from http://www.dhs.wisconsin.gov/tobacco/Tobacco%20Facts2009A1.pdf. [Google Scholar]
- Zhang X, Martinez-Donate AP, Kuo D, Jones NR, Palmersheim KA. Trends in home smoking bans in the USA, 1995–2007: prevalence, discrepancies and disparities. Tobacco Control. 2011;21:330–336. doi: 10.1136/tc.2011.043802. [DOI] [PMC free article] [PubMed] [Google Scholar]