Abstract
Background:
Following radical prostatectomy, success of adjuvant and salvage radiation therapy (RT) is dependent on the absence of micrometastatic disease. However, reliable prognostic/predictive factors for determining this are lacking. Therefore, novel biomarkers are needed to assist with clinical decision-making in this setting. Enumeration of circulating tumor cells (CTCs) using the regulatory-approved CellSearch System (CSS) is prognostic in metastatic prostate cancer. We hypothesize that CTCs may also be prognostic in the post-prostatectomy setting.
Methods:
Patient blood samples (n=55) were processed on the CSS to enumerate CTCs at 0, 6, 12 and 24 months after completion of RT. CTC values were correlated with predictive/prognostic factors and progression-free survival.
Results:
CTC status (presence/absence) correlated significantly with positive margins (increased likelihood of CTCneg disease; P=0.032), and trended toward significance with the presence of seminal vesicle invasion (CTCpos; P=0.113) and extracapsular extension (CTCneg; P=0.116). Although there was a trend toward a decreased time to biochemical failure (BCF) in baseline CTC-positive patients (n=9), this trend was not significant (hazard ratio (HR)=0.3505; P=0.166). However, CTC-positive status at any point (n=16) predicted for time to BCF (HR=0.2868; P=0.0437).
Conclusions:
One caveat of this study is the small sample size utilized (n=55) and the low number of patients with CTC-positive disease (n=16). However, our results suggest that CTCs may be indicative of disseminated disease and assessment of CTCs during RT may be helpful in clinical decision-making to determine, which patients may benefit from RT versus those who may benefit more from systemic treatments.
Introduction
In the United States in 2014, it is estimated that there will be 233 000 new cases of prostate cancer (PCa) diagnosed and 29 480 deaths from this disease.1 Upon diagnosis, one commonly recommended treatment option is surgical resection of the prostate via radical prostatectomy (RP).2 Following surgical intervention, patients are monitored using PSA testing. However, within 10 years of RP, up to 30% of early-stage patients will experience a rise in PSA levels and require additional treatment for residual/recurrent disease.3, 4 Following relapse, evaluation of time to biochemical recurrence, PSA doubling time and pathological features (Gleason score, margin status, seminal vesicle invasion (SVI) and extracapsular extension (ECE)) can assess the risk of PCa-specific mortality. Patients will then be recommended for either surveillance, potentially curative radiation therapy (RT) or palliative hormonal therapy.5 Although these parameters provide a measure of disease aggressiveness, neither they nor available imaging technologies can determine the precise location of recurrent PCa, thereby presenting a unique problem. If recurrent disease is localized to the prostate bed, RT could be curative. However, if the disease has become systemic, local radiation will be insufficient and systemic therapy may be necessary. Therefore, novel biomarkers that could discriminate patients with local recurrence versus those with systemic disease would be of clinical benefit.
The presence of circulating tumor cells (CTCs) in the bloodstream of PCa patients has been correlated with metastatic disease.6 In addition, CTC detection in the metastatic setting is prognostic, correlating with significantly reduced progression-free and overall survival.6 However, given that CTCs are rare and present in a high background of contaminating blood cells, detection and enumeration of CTCs requires highly sensitive and clinically reproducible assays. Currently, the only CTC analysis platform cleared by the United States Food and Drug Administration for prognostic use in metastatic breast, prostate and colorectal cancer is the CellSearch System (CSS) by Janssen Diagnostics,7 thereby making it the current gold standard in CTC technology in the metastatic setting for these disease sites.
Using this platform, the clinical value of CTCs in metastatic PCa has been extensively explored.6, 8, 9 Studies demonstrate that patients with ⩾5 CTCs per 7.5 ml of blood have significantly reduced progression-free and overall survival compared with patients with <5 CTCs.6 In addition, changes in CTC number throughout therapy may be a surrogate end point for treatment efficacy in the metastatic setting.8 However, the clinical value of CTCs in patients with localized PCa is less well described, with the majority of studies focusing on the utility of CTCs in determining biochemical failure (BCF) following RP. However, few have explored the utility of CTCs in determining response to intervention to treat residual disease (that is, RT).10, 11, 12
Based on these initial reports we hypothesized that the presence of CTCs in the blood of PCa patients undergoing adjuvant or salvage radiation may be an indicator of disseminated disease and may ultimately assist with clinical decision-making in this patient cohort. In this study, we specifically assessed whether the presence of CTCs either before or following completion of RT (measured at baseline and 6, 12 and 24 months post-treatment) is indicative of treatment response. To our knowledge, this is the most extensive report in the literature examining the value of CTCs in this uniquely challenging patient population, including serial CTC sampling following treatment completion and the longest period of follow-up to date (up to 3 years in some patients). In addition, we believe we are the first to describe the utility of CTCs in adjuvant patients undergoing RT.
Materials and methods
Patient population
All studies were carried out under a protocol approved by the Western University's Health Sciences Research Ethics Board. All patients were enrolled following informed consent. Fifty-five PCa patients who had consented to adjuvant or salvage RT following RP were enrolled. Inclusion criteria for adjuvant patients included the presence of any adverse pathological findings such as ECE, positive margins and/or SVI without the presence of a detectable PSA (<0.1 ng ml−1). Patients who were enrolled with the intent of adjuvant radiation but who had detectable PSA levels pre-radiation were categorized as adjuvant/salvage and analyzed separately. Inclusion criteria for salvage patients included PSA value of >0.1 ng ml−1 (http://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=0534) and a minimum of three PSA values taken 1 month apart, in order to calculate doubling time. Pre-RT bone scan and computed tomography scan were performed at the discretion of the physician. Blood was drawn before the initiation of RT to determine baseline PSA and CTC levels. Additional baseline characteristics were noted, including Gleason score, pathologic T (pT) stage, margin status, lymph node status, presence of ECE, presence of SVI, months free from relapse, mean PSA and mean PSA doubling time where appropriate. All patients were treated with radiation to the prostate bed as per Radiation Therapy Oncology Group guidelines with 6600 cGy in 33 fractions using a 5 field intensity modulated RT technique.13 Following completion of RT, PSA levels were determined at 3, 6, 12, 18, 24, 30 and 36 months, whereas CTC levels were determined at 6, 12 and 24 months post-RT.
PSA determination and BCF
Blood samples for PSA determination were analyzed by the London Health Sciences Centre Endocrinology Laboratory on the AutoDelfia automatic immunoassay system (Perkin Elmer, Waltham, MA, USA) using a time-resolved fluoroimmunoassay. BCF following RT was defined as three consecutive rises in PSA during follow-up.
CTC enumeration
All blood samples for CTC analysis were drawn into CellSave tubes (Janssen, Raritan, NJ, USA) and analyzed within 96 h.14 CTCs were identified as being selected by anti-EpCAM (epithelial cell adhesion molecule), positive for cytokeratin (8/18/19) and the DNA stain 4′, 6-diamidino-2-phenylindole (DAPI), >4 μm diameter and with an intact cell membrane. CTC results were analyzed by two independent and blinded observers and enumerated using the criteria described above. A positive CTC result was defined as ⩾1 CTCs per 7.5 ml of blood.
Statistical analysis
Comparisons were made between patients with CTCs at baseline (CTCpos), versus those without CTCs (CTCneg). A two-tailed Fisher's exact test was used to analyze differences between CTCpos and CTCneg groups relative to Gleason score (>7), SVI, margin status, ECE, radiation type (salvage, adjuvant or adjuvant/salvage) and BCF at 2 years. Unpaired t-tests were used to assess age differences between CTCpos and CTCneg groups. Log-rank tests were utilized to examine time to BCF.
Results
Fifty-five PCa patients from the adjuvant or salvage settings were enrolled in the study. Of these, 19 (34.5%) were classified as adjuvant, 33 (60%) as salvage and 3 (5.5%) as adjuvant/salvage. The clinicopathological risk factors (CRFs) of study patients are presented in Table 1. Mean pre-radiation PSA (and range) was observed to be 0.33 (0.11–1.37; salvage setting) and 0.42 (0.11–1.0; adjuvant/salvage setting). Mean PSA doubling time (and range) was 16.4 (2–78) months (only measured in salvage patients). No correlation was observed between PSA levels and CTC status (presence/absence).
Table 1. Comparison of the clinicopathologic factors and CTC status of adjuvant, salvage and adjuvant/salvage patient populations.
| Adjuvant, N (%) | Salvage, N (%) | Adjuvant/salvage, N (%) | Total, N (%) | |
|---|---|---|---|---|
| Total | 19 (34.5) | 33 (60) | 3 (5.5) | 55 (100) |
| T stage | ||||
| T2 | 2 (11) | 15 (45) | 0 (0) | 17 (31) |
| T3a | 15 (79) | 14 (42) | 3 (100) | 32 (58) |
| T3b | 2 (11) | 4 (12) | 0 (0) | 6 (11) |
| Gleason score | ||||
| 6 | 1 (5) | 6 (18) | 0 (0) | 7 (13) |
| 7 | 17 (89) | 25 (76) | 2 (67) | 44 (80) |
| 8–10 | 1 (5) | 2 (6) | 1 (33) | 4 (7) |
| Marginspos | 12 (63) | 17 (52) | 2 (67) | 31 (56) |
| ECEpos | 17 (89) | 18 (55) | 3 (100) | 38 (69) |
| SVIpos | 2 (11) | 5 (15) | 1 (33) | 8 (15) |
| CTCpos (baseline) | 3 (16) | 4 (12) | 2 (67) | 9 (16) |
| CTCpos (any time)a | 5 (26) | 9 (27) | 2 (67) | 16 (29) |
Abbreviations: CTC, circulating tumor cell; ECE, extracapsular extension; SVI, seminal vesicle invasion.
The number of CTCs detected in patients within each patient population are as follows: 1 (n=4) and 3 (n=1) (adjuvant); 1 (n=6), 2 (n=1), 4 (n=1) and 5 (n=1) (salvage, with one patients having CTCs at baseline (4 CTCs) and 12 months (1 CTC)); 1 (n=1) and 2 (n=1) (adjuvant/salvage).
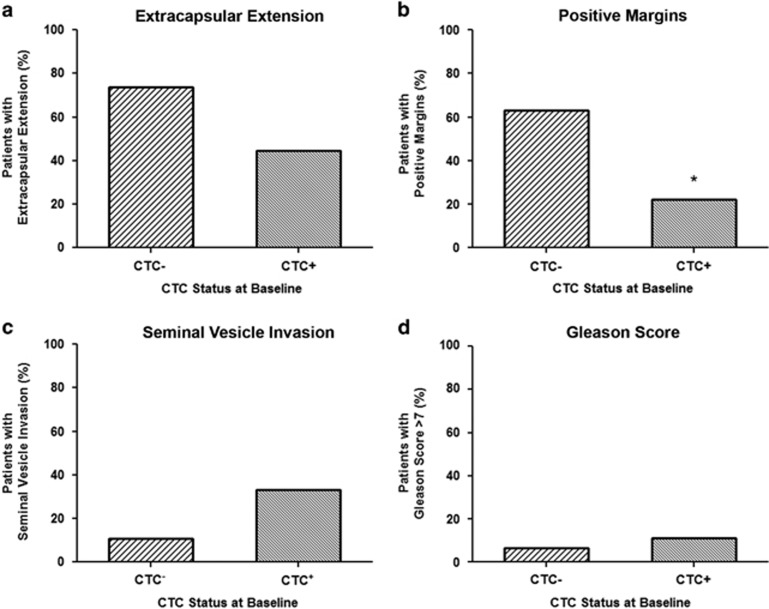
To determine the relationship between CTCs at baseline and CRFs, patients were characterized as either CTCneg (0 CTCs; n=46) or CTCpos (⩾1 CTCs; n=9; Figure 1 and Supplementary Table 1). No significant differences were observed for patients with a Gleason score of >7 and CTCneg (n=3) versus CTCpos (n=1) status at baseline (P>0.05). However, a trend toward statistical significance was observed when considering the presence of ECE (P=0.116) or SVI (P=0.113). For CTCneg patients, there was a trend toward the presence of ECE, with 73.9% presenting with ECE (n=34) versus 44.4% of CTCpos patients (n=4). However, when considering SVI the opposite trend was observed, with 10.9% of CTCneg patients presenting with SVI (n=5) versus 33.3% of CTCpos patients (n=3). A statistically significant difference was observed in relation to margin status (P=0.032), with 63.0% of CTCneg patients presenting with positive margins (n=29) versus 22.2% of CTCpos patients (n=2).
Figure 1.
Circulating tumor cell (CTC) status at baseline correlates with previously recognized patterns of disease recurrence of the clinicopathologic risk factors, extracapsular extension, seminal vesicle invasion and margin status. (a) Percentage of patients with CTCs absent (CTCneg/−; n=34) versus CTCs present (CTCpos/+; n=4) at baseline who presented with extracapsular extension. (b) Percentage of patients with CTCneg (n=29) versus CTCpos (n=2) at baseline who presented with positive margins. (c) Percentage of patients with CTCneg (n=5) versus CTCpos (n=3) at baseline who presented with seminal vesicle invasion. (d) Percentage of patients with CTCneg (n=3) versus CTCpos (n=1) at baseline who presented with a Gleason score of >7.
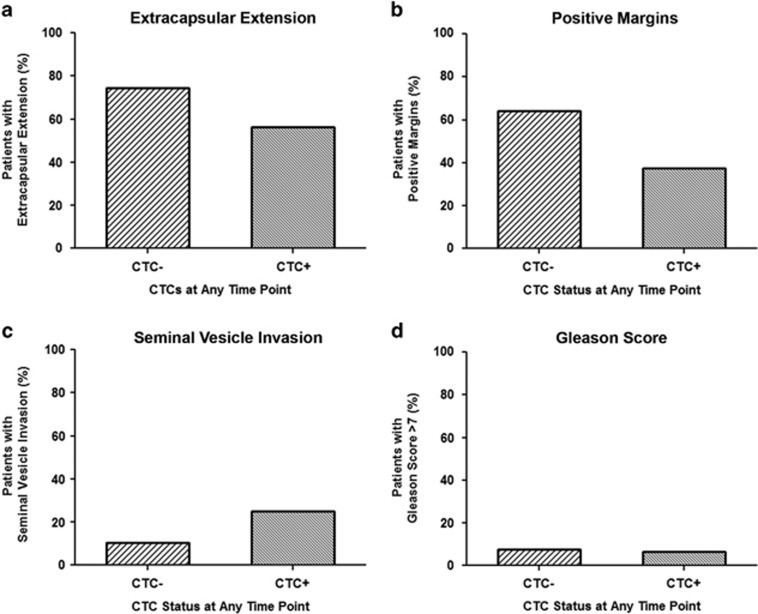
In addition to CTC status at baseline, analysis was also performed to determine the relationship between detectable CTCs at any time point and CRFs. Patients were characterized as either CTCneg (n=39) at all time points or CTCpos (n=16) at any time point, including at baseline (Figure 2 and Supplementary Table 2). No significant differences were observed when considering ECE, SVI or Gleason score of >7 and CTCneg (n=29, 4 and 3, respectively) versus CTCpos (n=9, 4 and 1, respectively) status (P>0.05). However, a trend toward statistical significance was observed when considering margin status (P=0.083). For CTCneg patients, there was a trend toward the presence of positive margins, with 64.1% of CTCneg patients presenting with positive margins (n=25) versus 37.5% of CTCpos patients (n=6), similar to that observed for CTC status at baseline.
Figure 2.
Circulating tumor cell (CTC) status at any time point correlates with previously recognized patterns of disease recurrence of the clinicopathologic risk factor, margin status. (a) Percentage of patients with CTCs absent (CTCneg/-; n=29) at all time points versus CTCs present (CTCpos/+; n=9) at any time point who presented with extracapsular extension. (b) Percentage of patients with CTCneg (n=25) at all time points versus CTCpos (n=6) at any time point who presented with positive margins. (c) Percentage of patients with CTCneg (n=4) at all time points versus CTCpos (n=4) at any time point who presented with seminal vesicle invasion. (d) Percentage of patients with CTCneg (n=3) at all time points versus CTCpos (n=1) at any time point who presented with a Gleason score of >7.
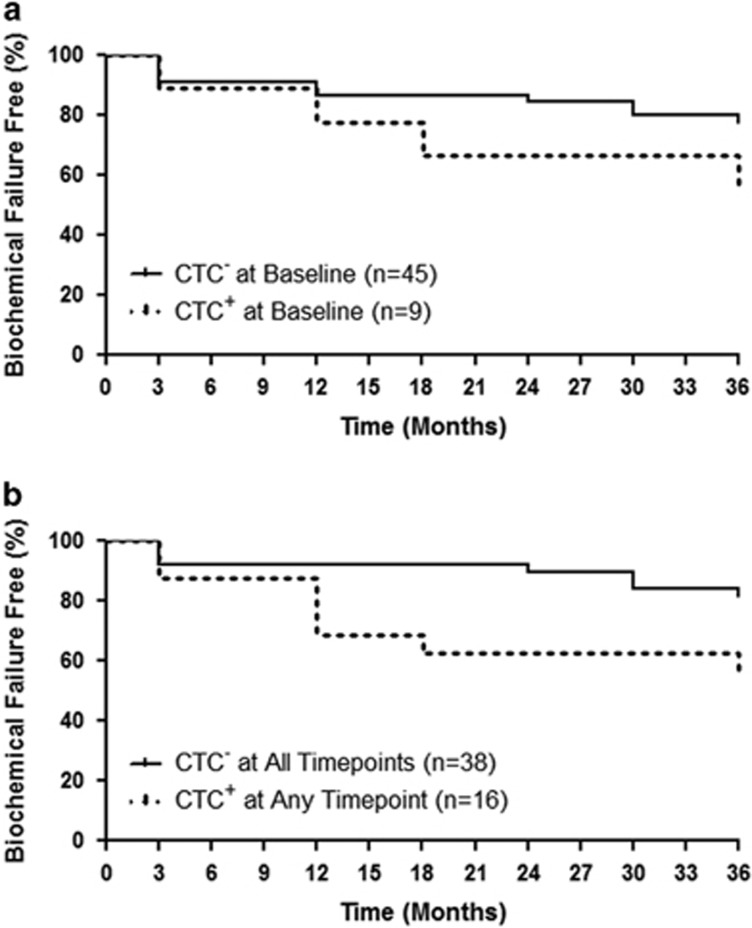
Ultimately, the usefulness of CTCs in this patient cohort depends on their ability to determine who will experience BCF and who will not. Therefore, patients were divided into those with CTCs absent (n=45) or present (n=9) at baseline and log rank analysis was utilized to assess differences in time to BCF in these patient subgroups (Figure 3a). Although there was a trend toward a decreased time to BCF in baseline CTCpos patients, this trend was not significant (P=0.166). Similar analysis was then performed on patients subdivided as CTCneg at all time points (n=38) versus those who were CTCpos at any time point (n=16). The results demonstrated a significantly decreased time to BCF in CTCpos patients (P=0.043; Figure 3b). No significant difference was observed in BCF at 2 years in patients with CTCneg versus CTCpos disease at baseline (Supplementary Table 1). However, a significant difference was observed in BCF at 2 years when considering patients with CTCneg versus CTCpos disease at any time point (P=0.049; Supplementary Table 2). No significant difference was observed in BCF at 2 years or time to BCF between adjuvant and salvage patient groups (Supplementary Table 3).
Figure 3.
CTCpos status at baseline and at any time point correlates with a decrease in time to biochemical failure following adjuvant or salvage radiotherapy. (a) Percentage of patients with circulating tumor cells (CTCs) absent (CTCneg/-; n=45) versus CTCs present (CTCpos/+; n=9) at baseline who are biochemical failure free over a 36-month period. (b) Percentage of patients with CTCneg (n=38) at all time points versus CTCpos (n=16) at any time point who are biochemical failure free over a 36-month period.
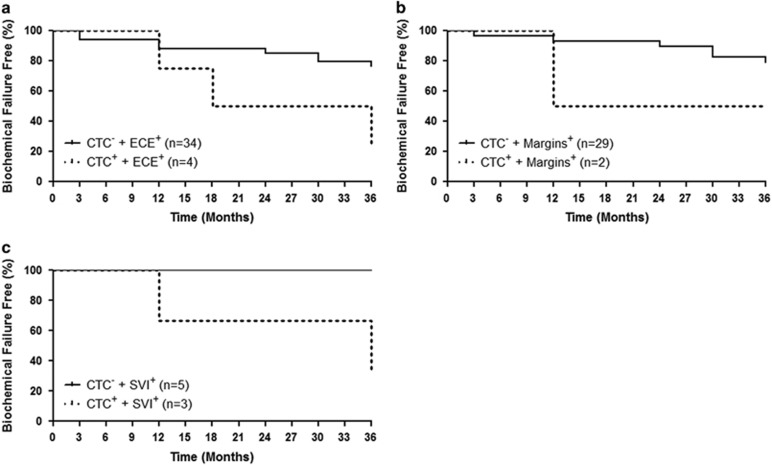
Although CTCs alone at baseline were not an independent predictor of time to BCF, we investigated if combination with one or more of the known CRFs would enhance this ability. This approach demonstrated that patients with the presence of ECE (Figure 4a) or SVI (Figure 4c) in combination with a CTCpos status at baseline had a decreased time to BCF (P=0.027 and P=0.043, respectively) versus those with the presence of ECE or SVI alone. However, a significant difference was not observed when comparing patients with the presence of positive margins and a CTCpos status versus those with the presence of positive margins alone (P=0.250; Figure 4b). As the presence of negative margins in this patient population suggests that patient's disease may no longer be confined to the prostate bed, time to BCF analysis was performed to determine whether a relationship existed between CTC status at baseline and negative margins. Based on this analysis, no significant difference was observed between patients with CTCneg marginneg disease and those with CTCpos marginneg disease (data not shown).
Figure 4.
Combination of circulating tumor cell (CTC) status at baseline and known clinicopathologic risk factors, extracapsular extension (ECE) or seminal vesicle invasion (SVI), can predict for time to biochemical failure following adjuvant or salvage radiotherapy. (a) Percentage of patients who are biochemical failure free over a 36-month period and who presented with ECE, but without CTCs (CTCneg/-; n=34), versus patients with ECE, but with CTCs (CTCpos/+; n=4), at baseline (P=0.027). (b) Percentage of patients who are biochemical failure free over a 36-month period and who presented with positive margins (margins), but CTCneg (n=29), versus patients with positive margins, but CTCpos (n=2), at baseline (P>0.05). (c) Percentage of patients who are biochemical failure free over a 36-month period and who presented with SVI, but CTCneg (n=5), versus patients with SVI, but CTCpos (n=3), at baseline (P=0.043).
Similar analysis was then performed on the ability of CTC status at any time point in combination with one or more of the known CRFs to determine time to BCF. Patients who presented with one or more CRFs were subdivided into those who were either CTCpos versus CTCneg at any time point. This further demonstrated the relationship between the ability of CTCs to determine time to BCF in patients in combination with the presence of ECE (P=0.025; Figure 5a), although the same was not observed in combination with the presence of SVI (P=0.128; Figure 4c). However, a significant relationship was observed when comparing patients with the presence of positive margins and CTCpos status at any time point versus those with the presence of positive margins alone (P=0.001; Figure 5b). In addition, a very strong relationship was observed with the combination of presence of positive margins and ECE and CTCpos status at any time point versus those with the presence of positive margins and ECE alone (P<0.0001; Figure 5d).
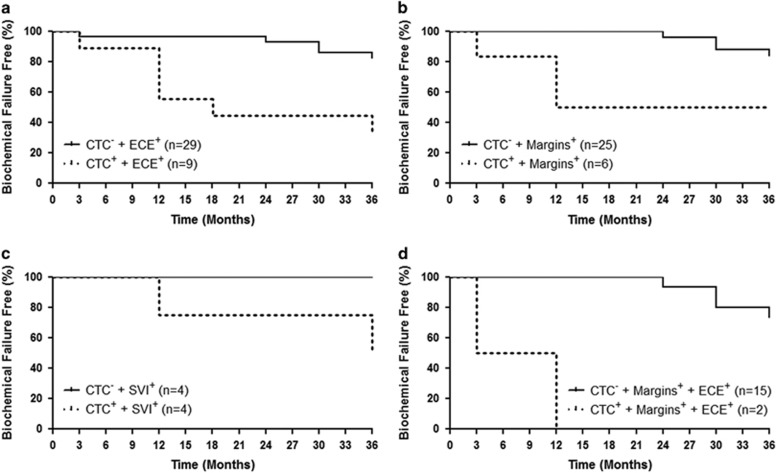
Figure 5.
Combination of circulating tumor cell (CTC) status at any time point and known clinicopathologic risk factors, extracapsular extension (ECE) and/or margin status, can predict for time to biochemical failure following adjuvant or salvage radiotherapy. (a) Percentage of patients who are biochemical failure free over a 36-month period and who presented with ECE, but without CTCs (CTCneg/-; n=29), at all time points versus patients with ECE, but with CTCs (CTCpos/+; n=9), at any time point (P=0.025). (b) Percentage of patients who are biochemical failure free over a 36-month period and who presented with positive margins (margins), but CTCneg (n=25), at all time points versus patients with positive margins, but CTCpos (n=6), at any time point (P=0.001). (c) Percentage of patients who are biochemical failure free over a 36-month period and who presented with seminal vesicle invasion (SVI), but CTCneg (n=4), at all time points versus patients with SVI, but CTCpos (n=4), at any time point (P=0.128). (d) The percentage of patients who are biochemical failure free over a 36-month period and who presented with positive margins and ECE, but CTCneg (n=15), at all time points versus patients with positive margins and ECE, but CTCpos (n=2), at any time point (P<0.0001).
Discussion
Current imaging technologies cannot differentiate local from systemic failure following RP. Regardless, RT is a common treatment option, as biomarkers that can distinguish those who will benefit from RT versus those who will not are unavailable. Unfortunately, for patients whose cancer has become systemic, RT will not provide benefit, resulting in up to 30% of patients experiencing disease recurrence. Therefore, novel biomarkers that could distinguish these patient groups before initiation of RT is essential. To the best of our knowledge, only two published studies have explored CTCs in this patient population. The first was performed in a small number of patients (n=15) using a non-standardized reverse-transcription PCR approach examining the detection of PSA mRNA in the blood.12 This study suggested that the presence of PSA mRNA following RP was indicative of micrometastatic disease and may predict poor response to salvage RT. The second study, published by our group, was the first to explore the detection and enumeration of CTCs using the Food and Drug Administration-cleared CSS.14 This study demonstrated that CTCs were detectable in salvage patients (n=26) using the CSS, and that, similar to metastatic disease, changes in CTC number following RT may be indicative of treatment response. In the current study, we sought to determine whether detection of CTCs before initiation of RT could be utilized as a surrogate biomarker for disseminated disease and therefore an indicator of treatment failure in this patient cohort.
Despite having only a small number of CTCpos patients before RT, strong correlations were observed with regards to CTC status at baseline and known CRFs. Interestingly, CTCneg disease was most highly correlated with CRFs associated with local recurrence, including ECE and positive margins.15 However, when considering SVI, a CRF associated with systemic relapse,15 a correlation with CTCpos disease was observed. These correlations, although in opposite directions, are consistent with the clinical observations of local versus systemic relapse associated with these CRFs. Based on the propensity for local relapse in ECE+ and margin+ patients, we would anticipate that these patients would exhibit a tendency toward non-disseminated and therefore CTCneg disease versus SVI+ patients, known to have a propensity for systemic relapse, whom we would anticipate would exhibit a tendency toward disseminated and therefore CTCpos disease. Therefore, CTC status appears to be in agreement with existing CRFs, suggesting that CTCs may relate to disease localization in these patients.
Although these associations suggest a relationship between CTCs and disease spread, the value of CTCs in this patient cohort will depend on their ability to predict RT success. Upon examination of BCF at 2 years and time to BCF, we noted a strong trend toward reduced time to BCF in CTCpos versus CTCneg patients at baseline. However, this trend was statistical significant when considering patients with CTCs at any time point for both measures (BCF at 2 years and time to BCF). These promising results suggest that the detection of CTCs at any time may be a surrogate biomarker of metastatic disease, and support a recommendation for early initiation of systemic treatment in this patient cohort.
However, this study also aimed to determine whether CTCs could predict the outcome of RT before treatment initiation, thereby reducing radiation-induced morbidity in patients for which benefit would not be achieved. Therefore, CTC status at baseline was examined in combination with known CRFs to determine whether this approach could improve our ability to discriminate these patient subsets. We have demonstrated that the presence of ECE or SVI in association with CTCpos status at baseline is predictive of poorer response to RT. However, as this study was not powered appropriately to determine definitive associations between combinations of CRFs and CTCs, not all significant associations observed at baseline were significant when considering CTC status at any time point and vice versa. In addition, not all CRF combinations, especially multiple CRFs (for example, ECE+SVI+CTC), could be effectively examined. However, the results presented here, specifically with regards to ECE and CTC status, demonstrate consistently poorer outcomes following RT, further strengthening the existence of a relationship between the presence of CTCs and disease spread. This suggests that the addition of CTCs to a patient's clinicopathologic ‘risk profile' (ECE, SVI and margin status) may further enhance our ability to discriminate patients with localized versus systemic recurrence. Further studies that could elucidate such risk profiles are justified.
The sample size for this study was chosen based on our pilot studies, which demonstrated that over 70% of salvage patients presented with CTCs at baseline using the CSS.14 Unfortunately, in the current study, only 16% of salvage patients and 12% of adjuvant patients presented with CTCs. Interestingly, for patients in the adjuvant/salvage group, 67% had detectable CTCs at baseline and all demonstrated treatment failure within 18 months, suggesting that CTCs may be more readily detectable and particularly valuable in these rapidly progressing patients. However, these results would require confirmation in a larger follow-up study. In agreement with our pilot analysis,14 the majority of patients with detectable CTCs at baseline presented with <2 CTCs (67%), and no patient had >5 CTCs at any point. Although the CSS can detect as few as 1 CTC per 7.5 ml of blood, variability increases significantly as the number of CTCs approaches 0, and with such low rates of detection the potential for false negative/positive results cannot be discounted as a confounding factor of this study. Therefore, proper enumeration of CTCs in these patients may be difficult and likely contributed to the high number of CTCneg patients at baseline that failed RT (66%). The low number of CTCs observed throughout the course of this study presents a statistical challenge that can be overcome by either increasing sample size, increasing CTC capture by collecting additional blood for analysis (>7.5 ml) or utilizing new emerging CTC technologies with increased sensitivity.16 In doing so, analysis of changes in CTC number at baseline compared with a subsequent post-treatment time points may be possible. This measure may also be valuable in determining the origin of disseminating disease (that is, CTCs). For example, if CTC numbers decrease following RT, then this may indicate that the residual disease was localized to the prostate bed and effectively treated using RT. However, should CTC numbers remain unchanged or increase following RT this may be an indicator of metastatic disease.
In summary, we believe the results presented here are the first to demonstrate that CTC enumeration using the clinical gold standard CTC analysis CSS platform may be valuable in clinical decision-making to determine which patients should receive RT versus those who would benefit more from systemic therapy. Validation studies using larger patient cohorts to examine the clinicopathologic 'risk profiles' outlined in this manuscript are necessary and justified based on these novel results.
Acknowledgments
We thank Larry Stitt for his assistance with statistical analysis. Funding was provided by grants from the London Regional Cancer Program Small Grants, a Canadian Association of Radiation Oncology (CARO) Uro-Oncologic Radiation Award (ACURA), and Drs Joseph Chin and Eric Winquist. LEL is supported by a graduate scholarship from the Canadian Institutes for Health Research (CIHR). ALA is supported by a CIHR New Investigator Award and an Early Researcher Award from the Ontario Ministry of Research and Innovation.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Prostate Cancer and Prostatic Diseases website (http://www.nature.com/pcan)
Supplementary Material
References
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 2014; 64: 9–29. [DOI] [PubMed] [Google Scholar]
- Thompson I, Thrasher JB, Aus G, Burnett AL, Canby-Hagino ED, Cookson MS et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol 2007; 177: 2106–2131. [DOI] [PubMed] [Google Scholar]
- Ohori M, Goad JR, Wheeler TM, Eastham JA, Thompson TC, Scardino PT. Can radical prostatectomy alter the progression of poorly differentiated prostate cancer? J Urol 1994; 152: 1843–1849. [DOI] [PubMed] [Google Scholar]
- Pound CR, Partin AW, Epstein JI, Walsh PC. Prostate-specific antigen after anatomic radical retropubic prostatectomy. Patterns of recurrence and cancer control. Urol Clin North Am 1997; 24: 395–406. [DOI] [PubMed] [Google Scholar]
- Freedland SJ, Humphreys EB, Mangold LA, Eisenberger M, Dorey FJ, Walsh PC et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA 2005; 294: 433–439. [DOI] [PubMed] [Google Scholar]
- De Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res 2008; 14: 6302–6309. [DOI] [PubMed] [Google Scholar]
- Lianidou ES, Markou A. Circulating tumor cells in breast cancer: detection systems, molecular characterization, and future challenges. Clin Chem 2011; 57: 1242–1255. [DOI] [PubMed] [Google Scholar]
- Olmos D, Arkenau H-T, Ang JE, Ledaki I, Attard G, Carden CP et al. Circulating tumour cell (CTC) counts as intermediate end points in castration-resistant prostate cancer (CRPC): a single-centre experience. Ann Oncol 2009; 20: 27–33. [DOI] [PubMed] [Google Scholar]
- Danila DC, Heller G, Gignac G a, Gonzalez-Espinoza R, Anand A, Tanaka E et al. Circulating tumor cell number and prognosis in progressive castration-resistant prostate cancer. Clin Cancer Res 2007; 13: 7053–7058. [DOI] [PubMed] [Google Scholar]
- Murray NP, Reyes E, Tapia P, Badinez L, Orellana N, Fuentealba C et al. Redefining micrometastasis in prostate cancer - a comparison of circulating prostate cells, bone marrow disseminated tumor cells and micrometastasis: implications in determining local or systemic treatment for biochemical failure after radical prostatecto. Int J Mol Med 2012; 30: 896–904. [DOI] [PubMed] [Google Scholar]
- Murray NP, Reyes E, Orellana N, Fuentealba C, Bádinez L, Olivares R et al. Secondary circulating prostate cells predict biochemical failure in prostate cancer patients after radical prostatectomy and without evidence of disease. ScientificWorldJournal 2013; 2013: 762064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombal B, Van Cangh PJ, Loric S, Gala J-L. Prognostic value of circulating prostate cells in patients with a rising PSA after radical prostatectomy. Prostate 2003; 56: 163–170. [DOI] [PubMed] [Google Scholar]
- Thompson IM, Valicenti RK, Albertsen P, Davis BJ, Goldenberg SL, Hahn C et al. Adjuvant and salvage radiotherapy after prostatectomy: AUA/ASTRO Guideline. J Urol 2013; 190: 441–449. [DOI] [PubMed] [Google Scholar]
- Lowes LE, Lock M, Rodrigues G, D'Souza D, Bauman G, Ahmad B et al. Circulating tumour cells in prostate cancer patients receiving salvage radiotherapy. Clin Transl Oncol 2012; 14: 150–156. [DOI] [PubMed] [Google Scholar]
- Katz MS, Zelefsky MJ, Venkatraman ES, Fuks Z, Hummer A, Leibel SA. Predictors of biochemical outcome with salvage conformal radiotherapy after radical prostatectomy for prostate cancer. J Clin Oncol 2003; 21: 483–489. [DOI] [PubMed] [Google Scholar]
- Alix-Panabières C, Pantel K. Technologies for detection of circulating tumor cells: facts and vision. Lab Chip 2014; 14: 57–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.