Abstract
Lack of insight is a well-established phenomenon in schizophrenia, and has been associated with reduced rater-assessed functional performance but increased self-reported well-being in previous studies. The objective of this study was to examine factors that might influence insight (as assessed by the Insight and Treatment Attitudes Questionnaire [ITAQ] or PANSS item G12) and subjective quality-of-life (as assessed by Lehman QoL Interview [LQOLI]), using the large National Institute of Mental Health Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) dataset. Uncooperativeness was assessed by PANSS item G8 (“Uncooperativeness”). In the analysis, we found significant moderating effects for insight on the relationships of subjective life satisfaction assessment to symptom severity (as assessed by CGI-S score), objective everyday functioning (as assessed by rater-administered Heinrichs–Carpenter Quality of Life scale), clinically rated uncooperativeness (as assessed by PANSS G8), and discontinuation of treatment for all causes (all P < 0.05 for statistical interaction between insight and subject QoL). Patients with chronic schizophrenia who reported being "pleased" or "delighted" on LQOLI were found to have significantly lower neurocognitive reasoning performance and poorer insight (ITAQ total score). Our findings underscore the importance of reducing cognitive and insight impairments for both treatment compliance and improved functional outcomes.
Keywords: Insight into illness, schizophrenia, patient-rated quality of life, subjective well-being, neurocognition
1. Introduction
Clinicians, researchers and healthcare authorities have come to recognize personal recovery and reintegration into society as attainable goals in the treatment of chronic, serious mental illness (Farkas, 2007, Frese et al., 2009). Accordingly, the mental health community has shifted its focus from primarily symptom amelioration to the pursuit of improving functioning and quality of life (QoL) outcomes. It is generally understood that QoL is a multidimensional construct, including both objective measures such as rater-assessed independent living, vocational, family and community functioning, and subjective measures such as patient self-reported well-being and life satisfaction (subjective QoL) (Narvacz et al., 2008, Test et al., 2005).
In people with schizophrenia, existing findings suggest that symptoms have only a modest relationship to QoL (Eack and Newhill, 2007), whereas neurocognitive deficits, highly prevalent in schizophrenia, are key factors for poor everyday outcomes (Elvevag and Goldberg, 2000, Green, 1996, Green et al., 2000, Harvey and Keefe, 2001, Harvey et al., 2004, Harvey et al., 2013). While the relationship between neurocognitive performance and objective measures of functional capacity has been well established (Bowie et al., 2006, Bowie et al., 2010, Harvey et al., 2009, Harvey et al., 2011, Harvey et al., 2013), studies investigating the linkage between cognitive performance, objective QoL, and subjective, patient-rated well-being have reported conflicting results (Brekke et al., 2001, Tolman and Kurtz, 2012). Some studies reported positive correlations between neurocognitive performance and objective QoL (as assessed by Heinrichs–Carpenter Quality of Life Scale) (Addington and Addington, 2008, Heinrichs et al., 1984, Lysaker and Davis, 2004, Savilla et al., 2008) or subjective well-being (QoL) (Alpetkin et al., 2005, Herman, 2004). Others, however, reported an inverse relationship between neurocognitive performance and subjective QoL (Corrigan and Buican, 1995, Kurtz and Tolman, 2011, Narvacz et al., 2008), or even no relationships between neurocognitive deficits and either objective or subjective quality of life (Agid et al., 2012, Chino et al., 2009, Fiszdon et al., 2008, Smith et al., 1999).
Lack of insight into illness is a well-established phenomenon in schizophrenia, with the estimated prevalence of poor insight ranging from 50% (Amador et al., 1993) to 81% (Wilson et al., 1986) of individuals with schizophrenia. Although little is known about the underlying mechanism(s), poor insight has been linked to cognitive impairment, increased re-hospitalization rates, worse clinical outcome (Kurtz and Tolman, 2011, Schwartz, 1998, Smith et al., 2004), psychosocial dysfunction (Francis and Penn, 2001, Frank and Gunderson, 1990, Lysaker et al., 1998), and barriers to engagement in treatment (Arango and Amador, 2011, Cuffel et al., 1996, Olfson et al., 2006).
Reduced insight into illness and poorer acceptance of treatment in schizophrenia have been found to be associated with lower depressive symptoms and higher subjective QoL in previous research (Kurtz and Tolman, 2011, Lysaker et al., 2009, Mintz et al., 2003, Morgan and David, 2006, Narvacz et al., 2008, Sellwood et al., 2013, Tolman and Kurtz, 2012), including the Clinical Antipsychotic Trial of Intervention Effectiveness (CATIE) schizophrenia study (Mohamed et al., 2009). This inverse relationship between subjective QoL and insight into illness (Eack and Newhill, 2007, Tolman and Kurtz, 2012) challenges our conventional model on treatment strategies for mental illness, which assumes treatment of symptoms will lead to improved insight, functional outcomes and subjective QoL (i.e. well-being) (Carling, 1995). However, our recent paper (Gould et al., 2015) showed that inability to accurately estimate cognitive and everyday functional skills was a stronger predictor of impairments in everyday functioning than clinical symptoms, cognitive functioning and the ability to perform everyday functional skills. We hereby hypothesized that insight impairment would moderate relationships of patient-rated well-being (subjective QoL) to symptoms, objective functioning, and discontinuation of treatment. Inter-relationships between insight, neurocognitive functioning, and subjective QoL were also evaluated in analyzing data from the Clinical Antipsychotic Trial of Intervention Effectiveness (CATIE) in schizophrenia study (Lieberman et al., 2005).
2. Methods
This post-hoc analysis used data from the CATIE study in patients with chronic schizophrenia (Lieberman et al., 2005). Insight and attitudes towards medications were assessed by the Insight and Treatment Attitudes Questionnaire (ITAQ) (McEvoy et al., 1989) and PANSS item G12 ("lack of judgment and insight") (Kay et al., 1987), respectively. The ITAQ is a semi-structured interview scale consisting of 11 items that measure awareness of illness (5 items) and attitude towards treatment (6 items). Total score ranges from 0 (no insight) to 22 ("full insight", no impairment). Insight impairment was defined categorically as PANSS G12 ≥ 4 (moderate to extreme) and ITAQ total score not indicating "full insight" (= 22). Objective functioning was assessed using the rater-administered Heinrichs–Carpenter Quality of Life (HQOL) scale (Heinrichs et al., 1984). The HQOL scale is a 21-item clinician-rated scale rated from 0 (severe impairment) to 6 (normal/unimpaired), assessing 4 domains, namely, interpersonal relations, instrumental role, intrapsychic foundations, and participation in community (common objects and activities). The HQOL total score ranges from 0 to 6. Subjective QoL was assessed by the single item from the Lehman QOL Interview (LQOLI) that asks patients to rate their level of well-being on a 7-point scale: from terrible = 1, unhappy = 2, mostly dissatisfied = 3, mixed = 4, mostly satisfied = 5, pleased = 6, to delighted = 7 (Lehman, 1988, Lehman and Burns, 1990). Neurocognitive functioning was assessed by a battery of neurocognitive tests, reflecting 5 domains: Processing Speed, Verbal Memory, Vigilance, Reasoning, and Working Memory (Keefe et al., 2007). Clinical symptoms were assessed by the Clinical Global Impression of Severity (CGI-S), a single-item clinician assessment of overall illness severity on a 7-point scale, with higher scores associated with greater illness severity (Guy, 1976). Depression was assessed by the Calgary Depression Scale for Schizophrenia (CDSS) (Addington and Addington, 2008, Addington et al., 1990), and uncooperativeness by PANSS item G8 (Kay et al., 1987).
Multiple regression method was applied to evaluate the inter-relationships between the subjective QoL (sQoL as assessed by LQOLI), objective functioning (as assessed by HQOL), cognition, dropouts, and uncooperativeness; as well as the moderating effect that insight impairment had on the inter-relationships of these clinical outcomes. The moderating effects of insight were evaluated by the statistical interaction term (insight-by-sQoL) in these models. The multivariate regression models were also adjusted for age and symptom severity (CGI-S) to reduce the confounding effects of these variables.
3. Results
3.1. Cross-sectional associations between symptoms, insight into illness, neurocognitive function, and patients' self-rated satisfaction with life
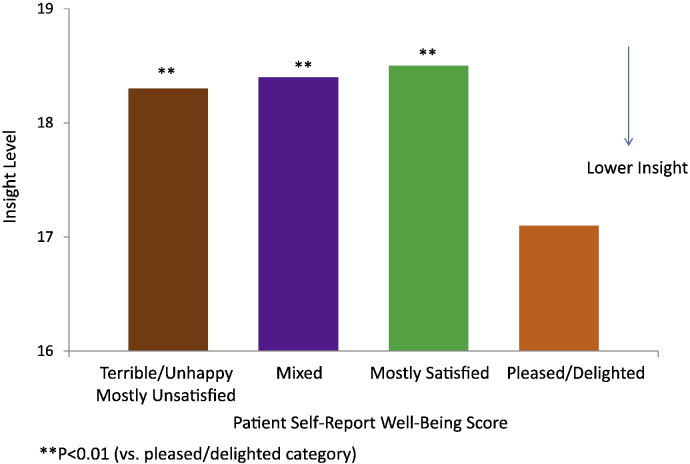
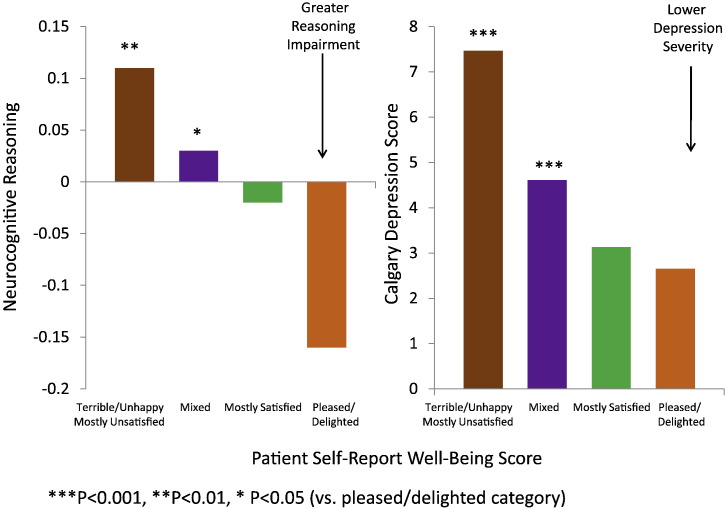
Fig. 1 shows that lack of insight and medication acceptance were inversely related to patients' self-rated, subjective satisfaction with life (LQOLI) in chronic schizophrenia (P = 0.03; P = 0.004 after accounting for age and symptom severity as assessed by CGI-S). Age (related to illness duration) and CGI-S were included as confounding variables in the regression models, because of their significant correlations with insight in univariate analysis (all P < 0.007). When compared to those rated "mostly dissatisfied" or below (≤ 3) on a scale from 1 (= terrible) to 7 (= delighted), patients who rated "delighted' (7) or "pleased" (6) on LQOLI had significantly higher levels of impairment in the neurocognitive composite score (P = 0.011, mean composite score − 0.11 vs. + 0.10 [SD = 1]), and neurocognitive domains that included: reasoning and problem solving (P = 0.001, mean − 0.16 vs. + 0.11, SD = 0.996), and vigilance (P = 0.012, mean − 0.08 vs. + 0.14, SD = 0.997). The inverse relationship between higher subjective life satisfaction score and the lack of insight into illness could be explained, in part, by the high level of impairment in reasoning and problem solving (P < 0.05), as well as the low level of self-reported depressive symptoms (P < 0.001) after adjusting for age and symptom severity (CGI-S) in a multivariate regression model (Fig. 2, Table 1). These two factors, low levels of depression and impaired reasoning and problem solving, together accounted for about 77% of the aged-adjusted inverse relationship between assessed insight into illness and self-reported well-being (LQOLI). The inverse relationship between low insight into illness and high subjective satisfaction of life was non-significant (P = 0.487) after depressive symptoms were entered into the model as a predictor. Depressive symptoms (P < 0.001), neurocognitive reasoning (P = 0.036), and symptom severity (P = 0.031) remained significant predictors of subjective satisfaction with life indicating direct effects, while age and insight into illness were non-significant (Table 1).
Fig. 1.
Baseline patient self-report well-being and insight towards illness in chronic schizophrenia.
Fig. 2.
Baseline patient self-report well-being, neurocognitive reasoning, and depression in chronic schizophrenia.
Table 1.
Multivariate model for subjective patient's rated satisfaction of life.
| Variables | Estimate | Standard Error | t Value | Pr > |t| |
|---|---|---|---|---|
| Intercept | 5.48 | 0.256 | 21.42 | < .001 |
| Insight into illness | − 0.006 | 0.007 | − 0.84 | 0.399 |
| Depression score | − 0.124 | 0.008 | − 14.93 | < .001 |
| Neurocognitive reasoning | − 0.080 | 0.038 | − 2.10 | 0.036 |
| CGI-S | − 0.085 | 0.039 | − 2.16 | 0.031 |
| age | − 0.004 | 0.003 | − 1.07 | 0.285 |
3.2. Moderators of the relationship between insight into illness and patients' self-rated satisfaction with life
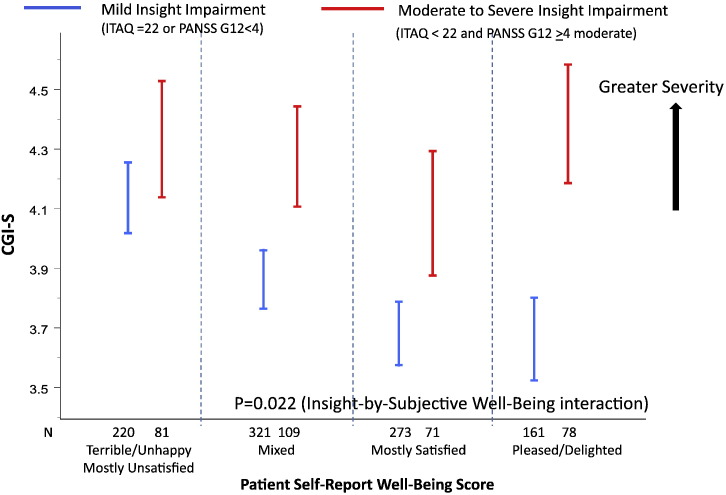
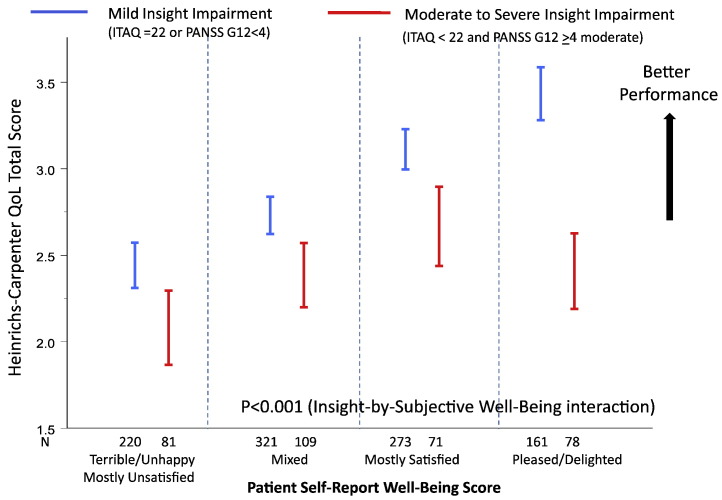
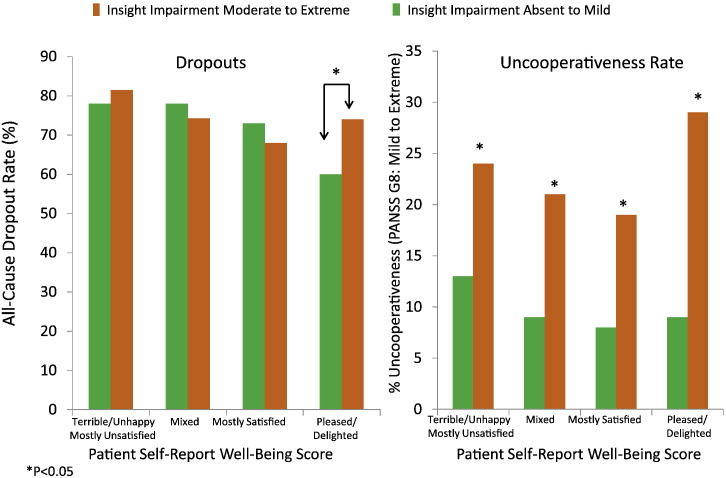
There was a significant correlation between the Insight and Treatment Attitudes Questionnaire (ITAQ) total score and PANSS item G12 ("lack of judgment and insight") (Spearman correlation r = − 0.49, P < 0.001). In the statistical interaction analysis to assess moderating effects, moderate to extreme impairment in insight and judgment was defined as PANSS G12 ≥ 4 (moderate to extreme) and ITAQ total score < 22 where 22 represents full insight (no impairment). Among the 402 (33% of 1237) subjects who had moderate to extreme PANSS G12 scores, 85 had ITAQ score of 22 (full insight) and hence were excluded from the insight impairment class in the analysis. Mild to absence of insight impairment was defined as PANSS G12 < 4 (mild to absent) or ITAQ total score = 22 (full insight, no impairment). Fig. 3, Fig. 4, Fig. 5 show the significant moderating effects that insight into illness had on the relationships of subjective life satisfaction assessment (LQOLI) to symptom severity (as assessed by CGI-S score), everyday functioning (as assessed by rater-administered HQOL scale), clinically rated uncooperativeness (as assessed by PANSS G8), as well as discontinuation of treatment for all causes. Greater satisfaction with life was associated with lower symptom severity, better HQOL functioning score, lower treatment dropout, and lower uncooperativeness symptoms in patients with mild or absence of insight impairment, while the relationship of subjective life satisfaction score to symptom severity was reversed (i.e., greater satisfaction with life was associated with higher symptom severity) in patients with moderate to extreme impairment in insight and high satisfaction with life (P = 0.022 for interaction effects of insight and subjective satisfaction of life scores on CGI-S). Similar inverse trends were observed for the HQOL functioning score and dropouts (all P < 0.05) (Fig. 4, Fig. 5). The PANSS uncooperativeness symptom was significantly higher in patients with lack of insight into illness and high subjective satisfaction with life, compared to patients with mild insight impairment and high subjective satisfaction with life (Fig. 5 right). We found consistent statistical interaction test results using continuous ITAQ total score (ITAQ-by-sQoL interaction effect) for all clinical outcomes in the moderator analysis. In particular, statistical tests for ITAQ total score by sQoL interaction effect were significant for CGI-S (P = 0.0128), uncooperativeness (P = 0.0265), and objective functioning HQOL score (P = 0.002).
Fig. 3.
Relationship between symptom severity and subjective life satisfaction assessment depended on level of insight impairment.
Fig. 4.
Relationship between Heinrichs–Carpenter Quality of Life and subjective life satisfaction depended on level of insight impairment.
Fig. 5.
Relationships of insight into illness and subjective life satisfaction assessment to dropout and uncooperativeness.
Greater satisfaction with life was associated with a lower all-cause dropout rate but only in patients with greater insight and medication acceptance. This trend of decreasing dropout rate with increased satisfaction with life was reversed in the low insight group which exhibited high, self-rated satisfaction with life ("pleased" or "delighted" on LQOLI) (P = 0.039, chi-square = 4.36, Cox model, adjusted for treatment, age, marital status). Patients who had a high, self-rated satisfaction with life ("pleased" or "delighted") tended to be more severely ill (i.e., had a higher CGI-S score) and were more likely to drop out of treatment in the lower insight group, compared to the higher insight group.
4. Discussion
Our findings suggest that the relationships of subjective QoL to clinical symptoms and treatment discontinuation depend on the level of insight impairment into illness and attitude toward medications. These results extend previous findings in the analysis of CATIE data by demonstrating the moderating relationship of insight impairment with subjective QoL and objective functioning HQOL outcomes. Patients with chronic schizophrenia who reported being "pleased" or "delighted" on LQOLI were liable to have lower neurocognitive reasoning performance, plus lower insight into their illness and poorer attitude towards treatment. These patients were more likely to discontinue treatment if they exhibited moderate to extreme symptoms but rated their overall well-being as "pleased" or "delighted".
The association between symptom severity and subjective QoL was moderated by the subject's insight and judgment, such that at every respective level of self-reported subjective QoL subjects with poor insight suffered significantly higher symptoms than their counterparts with better insight (P = 0.022 for insight-by-subjective well-being interaction). This was especially pronounced for the subgroup with the most insight impairment and skewed self-reported life satisfaction (i.e. "pleased" or "delighted" on LQOLI). The same pattern prevailed when it came to the primary outcome of CATIE, all cause discontinuation of treatment during the 18-month trial. Subjects with higher or lower levels of insight generally did not behave significantly differently, except for the subgroup with the most impaired insight which also reported high life satisfaction.
Patients in the CATIE trial who had moderate to severe insight impairment were also significantly more likely to be uncooperative (as assessed by PANSS item G8 "uncooperativeness"), versus those with no or mild insight impairment, irrespective of self-reported subjective QoL. These results suggest that those subjects with poor insight in addition to a distorted sense of personal well-being were most likely to be uncooperative, and, hence, more likely to be non-compliant with treatment.
Further, our findings show that neurocognitive reasoning was significantly correlated with self-reported level of subjective well-being. Subjects who scored high on the depression scale at baseline were significantly more likely (P < 0.001) to report lower satisfaction with life, as reflected by low LQOLI scores. These results corroborate the large existing literature suggesting that severity of depression is associated with poor self-reported well-being (subjective QoL), and is responsible for the poor prognosis and long-term outcome that subsequently follow (Amador et al., 1994, Bowie et al., 2007, Ruggeri et al., 2005). Our findings are consistent with recent research suggesting that patients with particularly low depression scores are likely to over-estimate their cognitive abilities and their level of everyday functioning (Durand et al., 2015).
In our analysis, we also identified a challenging subgroup of patients with chronic schizophrenia who manifest marked psychiatric symptoms and poor insight. These individuals are prone to be more uncooperative in treatment and hence predisposed to discontinue their medications early, possibly related to potential "positive bias" in this group of non-depressed patients, who rated "pleased or delighted" on a subjective patients-rated well-being (LQOLI) but had higher CGI-S score and lower objective clinicians-rated quality of life (HQOL). This is in contrast to the "depressive realism" found in mild-to-moderately depressed patients, which reflected greater insight towards their mental illness and more accurate self-assessment of current functioning status (Alloy and Abramson, 1988, Mohamed et al., 2009, Moore and Fresco, 2012, Smith et al., 2004). Both groups present important challenges to healthcare providers, underscoring the need for customized treatment interventions that cater to their specific needs, which might range from focused interventions for depression to adjunctive cognitive behavioral therapy (CBT) for improving insight, neurocognitive performance, functioning (objective QoL), and treatment adherence (Lincoln et al., 2007, Turner et al., 2014).
In the above-mentioned longitudinal analysis of CATIE data (Mohamed et al., 2009), improved insight was associated with reduction in symptoms as assessed by PANSS total and subscales, and improvement in objective functioning as assessed by HQOL total and 4 subscales. These longitudinal results are consistent with findings in this cross-sectional analysis as well as the meta-analysis by Lincoln et al. (2007), which together lend support to the observation that improved insight is associated with greater likelihood for improved functioning and long-term outcomes. Reducing insight impairment in conjunction with neurocognitive and functional deficits may improve long-term outcomes, including both objective and subjective quality of life in patients with schizophrenia.
A limitation of this study involves the use of cross-sectional data to examine the association of subjective QoL with objective functioning, insight and neurocognitive function, which may not be consistent with their causal relationships. The use of a one-item scale for measuring sQoL can be another limitation. The psychometric relationship of this measure of sQoL to various dimensions of life satisfaction in the full Lehman QoL Interview scale (LQOLI) and other subjective QoL scales (e.g. the Lancashire scale or the Satisfaction with Life Scale) (Diener et al., 1985, Lehman, 1988, van Nieuwenhuizen et al., 2001) remains unknown. In addition, the links between sQoL and the Heinrichs HQOL scale may have been inflated in this study, since the Heinrich's scale was developed to assess both the deficit syndrome as well as objective functioning, and the deficit syndrome includes aspects of poor sQoL as part of its definition. There is, however, a consistent relationship between the levels of depressive symptoms and sQoL, thus supporting the external validity of sQoL (Insel, 2010).
To conclude, our findings highlight the complex nature of using subjective QoL measures to assess patient recovery and effectiveness of treatment in chronic schizophrenia, and the importance of reducing cognitive and insight impairments for improving both treatment compliance and long-term functional outcomes.
Ethical Approval
The CATIE study was approved by the institutional review board at each site, and written informed consent was obtained from the patients or their legal guardians.
Role of Funding Source
None. Dr. Harvey is supported by NIMH grant number 1 RO1 MH93432.
Contributors
Dr. Siu and Harvey undertook the analysis. All authors contributed to the critical review and approved the final manuscript.
Conflict of Interest
Dr. Siu serves as a consultant for Sunovion Pharmaceuticals Inc. and Pfizer Inc. Dr. Harvey serves as a consultant/advisory board member for Boeheringer-Ingelheim, Forum Pharma, Genentech, Otsuka-America, Roche Pharma, Sanofi, Sunovion, and Takeda. Dr. Remington has received research support from the Canadian Diabetes Association, the Canadian Institutes of Health Research, Medicure, Neurocrine Biosciences, Novartis, Research Hospital Fund–Canada Foundation for Innovation, and the Schizophrenia Society of Ontario and has served as a consultant or speaker for Novartis, Laboratorios Farmacéuticos Rovi, Synchroneuron, and Roche. Dr. Agid has served on Advisory Board of Janssen-Ortho (Johnson and Johnson); Sepracor, Sunovion, Roche, Novartis, BMS, Otsuka, Lundbeck; Speaking engagements for Janssen-Ortho (Johnson and Johnson); Novartis; SepracorInc.,US.; Sunovion, Lundbeck.; and research Contracts for Pfizer, Inc., Janssen-Ortho (Johnson and Johnson), and Otsuka. Drs. Waye, Brambilla and Choi have declared no conflict of interest.
Acknowledgements
Data used in the preparation of this article were obtained from the limited access datasets distributed from the NIH-supported “Clinical Antipsychotic Trials of Intervention Effectiveness in Schizophrenia” (CATIE-Sz). This is a multisite, clinical trial of persons with schizophrenia comparing the effectiveness of randomly assigned medication treatment. The CATIE-Sz study was supported by NIMH Contract #N01MH90001 to the University of North Carolina at Chapel Hill. The ClinicalTrials.gov identifier is NCT00014001.
This manuscript reflects the views of the authors and may not reflect the opinions or views of the CATIE-Sz Study Investigators or the NIH.
References
- Addington J., Addington D. Social and cognitive functioning in psychosis. Schizophr. Res. 2008;99:176–181. doi: 10.1016/j.schres.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Addington D., Addington J., Schissel B. A depression rating scale for schizophrenics. Schizophr. Res. 1990;3:247–251. doi: 10.1016/0920-9964(90)90005-r. [DOI] [PubMed] [Google Scholar]
- Agid O., McDonald K., Siu C. Happiness in first-episode schizophrenia. Schizophr. Res. 2012;141:98–103. doi: 10.1016/j.schres.2012.07.012. [DOI] [PubMed] [Google Scholar]
- Alloy L.B., Abramson L.Y. Depressive realism: four theoretical perspectives. In: Alloy L.B., editor. Cognitive Processes in Depression. Guilford Press; New York: 1988. pp. 223–265. [Google Scholar]
- Alpetkin K.Y., Akvardar K., Akdede B.B. Is quality of life associated with cognitive impairments in schizophrenia? Neuropsychopharmacol. Biol. Psychiatry. 2005;29:239–244. doi: 10.1016/j.pnpbp.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Amador X.F., Strauss D.H., Yale S.A. Assessment of insight in psychosis. Am. J. Psychiatry. 1993;50:873–879. doi: 10.1176/ajp.150.6.873. [DOI] [PubMed] [Google Scholar]
- Amador X.F., Strauss D.H., Yale S.A. Awareness of illness in schizophrenia. Arch. Gen. Psychiatry. 1994;51:826–836. doi: 10.1001/archpsyc.1994.03950100074007. [DOI] [PubMed] [Google Scholar]
- Arango C., Amador X. Lessons learned about insight. Schizophr. Bull. 2011;37:27–28. doi: 10.1093/schbul/sbq143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie C.R., Reichenberg A., Patterson T.L. Determinants of real-world functional performance in schizophrenia subjects, correlations with cognition, functional capacity, and symptoms. Am. J. Psychiatry. 2006;163:418–425. doi: 10.1176/appi.ajp.163.3.418. [DOI] [PubMed] [Google Scholar]
- Bowie C.R., Twamley E.W., Anderson H. Self-assessment of functional status in schizophrenia. J. Psychiatr. Res. 2007;41:1012–1018. doi: 10.1016/j.jpsychires.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie C.R., Depp C., McGrath J.A. Prediction of real-world functional disability in chronic mentaldisorders, a comparison of schizophrenia and bipolar disorder. Am. J. Psychiatry. 2010;167:1116–1124. doi: 10.1176/appi.ajp.2010.09101406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brekke J.S., Kohrt B., Green M.F. Neuropsychological functioning as a moderator of the relationship between psychosocial functioning and the subjective experience of self and life in schizophrenia. Schizophr. Bull. 2001;27:697–708. doi: 10.1093/oxfordjournals.schbul.a006908. [DOI] [PubMed] [Google Scholar]
- Carling P.J. Guilford Press; New York: 1995. Return to Community: Building Support for People with Psychotic Disabilities. [Google Scholar]
- Chino B., Nemoto T., Fujii C., Mizuno M. Subjective assessments of the quality-of-life, well-being and self-efficacy in patients with schizophrenia. Psychiatry Clin. Neurosci. 2009;63:521–528. doi: 10.1111/j.1440-1819.2009.01995.x. [DOI] [PubMed] [Google Scholar]
- Corrigan P.W., Buican B. The construct validity of subjective quality of life for the severely mentally ill. J. Nerv. Ment. Dis. 1995;183:281–285. doi: 10.1097/00005053-199505000-00001. [DOI] [PubMed] [Google Scholar]
- Cuffel B.J., Alford J., Fischer E.P., Owen R.R. Awareness of illness in schizophrenia and out-patient treatment compliance. J. Nerv. Ment. Dis. 1996;184:653–659. doi: 10.1097/00005053-199611000-00001. [DOI] [PubMed] [Google Scholar]
- Diener E., Emmons R.A., Larsen R.J., Griffin S. The Satisfaction with Life Scale. J. Pers. Assess. 1985;49:71–75. doi: 10.1207/s15327752jpa4901_13. [DOI] [PubMed] [Google Scholar]
- Durand D., Strassnig M., Sabbag S. Factors influencing self-assessment of cognition and functioning in schizophrenia, implications for treatment studies. Eur. Neuropsychopharmacol. 2015;25:185–191. doi: 10.1016/j.euroneuro.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eack S.M., Newhill C.E. Psychiatric symptoms and quality of life in schizophrenia, a meta-analysis. Schizophr. Bull. 2007;3:1225–1237. doi: 10.1093/schbul/sbl071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvevag B., Goldberg T.E. Cognitive impairment in schizophrenia is the core of the disorder. Crit. Rev. Neurobiol. 2000;14:1–21. [PubMed] [Google Scholar]
- Farkas M. The vision of recovery today, what is and what it means for services. World Psychiatry. 2007;6:68–74. [PMC free article] [PubMed] [Google Scholar]
- Fiszdon J.M., Choi J., Goulet J., Bell M.D. Temporal relationship between change in cognition and change in functioningin schizophrenia. Schizophr. Res. 2008;105:105–113. doi: 10.1016/j.schres.2008.06.010. [DOI] [PubMed] [Google Scholar]
- Francis J.L., Penn D.L. The relationship between insight and social skills in persons with severe mental illness. J. Nerv. Ment. Dis. 2001;189:822–829. doi: 10.1097/00005053-200112000-00003. [DOI] [PubMed] [Google Scholar]
- Frank A., Gunderson J.G. The role of the therapeutic alliance in the treatment of schizophrenia. Arch. Gen. Psychiatry. 1990;47:228–236. doi: 10.1001/archpsyc.1990.01810150028006. [DOI] [PubMed] [Google Scholar]
- Frese F.J., III, Knifht L., Saks S. Recovery from schizophrenia, with views from psychiatrists, psychologists and others diagnosed with this disorder. Schizophr. Bull. 2009;35:370–380. doi: 10.1093/schbul/sbn175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould F., McGuire L.S., Durand D. Self-assessment in schizophrenia, Accuracy of assessment of cognition and everyday functioning. Neuropsychology. 2015 doi: 10.1037/neu0000175. 2015 Feb 2. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M.F. What are the functional consequences of neurocognitive deficits in schizophrenia? Am. J. Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Green M.F., Kern R.S., Braff D.L., Mintz J. Neurocognitive deficits and functional outcome in schizophrenia, are we measuring the "right stuff"? Schizophr. Bull. 2000;26:119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Guy W., editor. US Department of Health, Education, and Welfare Public Health Service Alcohol, Drug Abuse, and Mental Health Administration; Rockville, MD: 1976. ECDEU Assessment Manual for Psychopharmacology. [Google Scholar]
- Harvey P.D., Keefe R.S. Studies of cognitive change in patients with schizophrenia following novel antipsychotic treatment. Am. J. Psychiatry. 2001;158:176–184. doi: 10.1176/appi.ajp.158.2.176. [DOI] [PubMed] [Google Scholar]
- Harvey P.D., Green M.F., Keefe R.S., Velligan D.I. Cognitive functioning in schizophrenia, a consensus statement on its role in the definition and evaluation of effective treatment of the illness. J. Clin. Psychiatry. 2004;65:361–372. [PubMed] [Google Scholar]
- Harvey P.D., Helldin L., Bowie C.R. Performance-based measurement of functional disability in schizophrenia, a cross-national study in the United States and Sweden. Am. J. Psychiatry. 2009;166:821–827. doi: 10.1176/appi.ajp.2009.09010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey P.D., Raykov T., Twamley E.W. Validating the measurement of real-world functional outcomes, phase I results of the VALERO study. Am. J. Psychiatry. 2011;168:1195–1201. doi: 10.1176/appi.ajp.2011.10121723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey P.D., Siu C.O., Hsu J. Effect of lurasidone on neurocognitive performance in patients with schizophrenia: a short-term placebo- and active-controlled study followed by a 6-month double-blind extension. Eur. Neuropsychopharmacol. 2013;23:1373–1382. doi: 10.1016/j.euroneuro.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Heinrichs D.W., Hanlon T.E., Carpenter W.T. The Quality of Life Scale, an instrument for rating the schizophrenic deficit syndrome. Schizophr. Bull. 1984;10:388–398. doi: 10.1093/schbul/10.3.388. [DOI] [PubMed] [Google Scholar]
- Herman M. Neurocognitive functioning and quality of life among dually diagnosed and non-substance abusing schizophrenia patients. Int. J. Ment. Health Nurs. 2004;13:282–291. doi: 10.1111/j.1440-0979.2004.00346.x. [DOI] [PubMed] [Google Scholar]
- Insel T.R. Rethinking schizophrenia. Nature. 2010;468:187–193. doi: 10.1038/nature09552. [DOI] [PubMed] [Google Scholar]
- Kay S.R., Fiszbein A., Opler L.A. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Keefe R.S.E., Bilder R.M., Davis S.M. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE trial. Arch. Gen. Psychiatry. 2007;64:633–647. doi: 10.1001/archpsyc.64.6.633. [DOI] [PubMed] [Google Scholar]
- Kurtz M.M., Tolman A. Neurocognition, insight into illness and subjective quality-of-life in schizophrenia, what is their relationship? Schizophr. Res. 2011;127:157–162. doi: 10.1016/j.schres.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman A.F. The quality of life interview for the severe mentally ill. Eval. Program. Plan. 1988;11:51–62. [Google Scholar]
- Lehman A.F., Burns B.J. Severe mental illness in the community. In: Spiker B., editor. Quality of Life Assessment in Clinical Trials. Raven Press; New York: 1990. [Google Scholar]
- Lieberman J.A., Stroup T.S., McEvoy J.P. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N. Engl. J. Med. 2005;353:1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- Lincoln T.M., Lullman E., Rief W. Correlates and long-term consequences of poor insight in patients with schizophrenia, a systematic review. Schizophr. Bull. 2007;31:1324–1342. doi: 10.1093/schbul/sbm002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysaker P., Davis L. Social function in schizophrenia and schizoaffective disorder: associations with personality, symptoms and neurocognition. Health Qual. Life Outcomes. 2004;2:15. doi: 10.1186/1477-7525-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysaker P.H., Bell M.D., Bryson G.J., Kaplan E.E. Psychosocial function and insight in schizophrenia. J. Nerv. Ment. Dis. 1998;186:432–436. doi: 10.1097/00005053-199807000-00008. [DOI] [PubMed] [Google Scholar]
- Lysaker P.H., Buck K.D., Salvatore G. Lack of awareness of illness in schizophrenia, conceptualizations, correlates and treatment approaches. Expert. Rev. Neurother. 2009;9:1035–1043. doi: 10.1586/ern.09.55. [DOI] [PubMed] [Google Scholar]
- McEvoy J.P., Apperson L.J., Applebaum P.S. Insight in schizophrenia, its relationship to acute psychopathology. J. Nerv. Ment. Dis. 1989;177:43–47. doi: 10.1097/00005053-198901000-00007. [DOI] [PubMed] [Google Scholar]
- Mintz A.R., Dobson K.S., Romney D.M. Insight in schizophrenia, a meta-analysis. Schizophr. Res. 2003;61:75–88. doi: 10.1016/s0920-9964(02)00316-x. [DOI] [PubMed] [Google Scholar]
- Mohamed S., Rosenheck R., McEvoy J. Cross-sectional and longitudinal relationships between insight and attitudes toward medication and clinical outcomes. Schizophr. Bull. 2009;35:336–346. doi: 10.1093/schbul/sbn067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M.T., Fresco D. Depressive realism: a meta-analytic review. Clin. Psychol. Rev. 2012;32:496–509. doi: 10.1016/j.cpr.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Morgan K.D., David A.S. Insight in psychosis and neuropsychological functions, meta-analysis. Br. J. Psychiatry. 2006;189:204–212. doi: 10.1192/bjp.189.3.204. [DOI] [PubMed] [Google Scholar]
- Narvacz J.M., Twamley E.W., McKibbon C.L. Subjective and objective quality of life in schizophrenia. Schizophr. Res. 2008;91:201–208. doi: 10.1016/j.schres.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olfson M., Marcus S.C., Wilk J., West J.C. Awareness of illness and non-adherence to antipsychotic medications among persons with schizophrenia. Psychiatr. Serv. 2006;57:205–211. doi: 10.1176/appi.ps.57.2.205. [DOI] [PubMed] [Google Scholar]
- Ruggeri M., Nose M., Bonetto C. Changes and predictors of change in objective and subjective quality-of-life, multiwave follow-up study in community psychiatric practice. Br. J. Psychiatry. 2005;187:121–130. doi: 10.1192/bjp.187.2.121. [DOI] [PubMed] [Google Scholar]
- Savilla K., Kettler L., Galletly C. Relationships between cognitive deficits, symptoms and quality of life in schizophrenia. Aust. N. Z. J. Psychiatr. 2008;42:496–504. doi: 10.1080/00048670802050512. [DOI] [PubMed] [Google Scholar]
- Schwartz R.C. Insight and illness in chronic schizophrenia. Compr. Psychiatry. 1998;39:249–254. doi: 10.1016/s0010-440x(98)90031-1. [DOI] [PubMed] [Google Scholar]
- Sellwood W., Morrison A.P., Beck R. Subjective cognitive complaints in schizophrenia, relation to antipsychotic medication dose, actual cognitive performance, insight and symptoms. PLoS One. 2013;8(12):e83774. doi: 10.1371/journal.pone.0083774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T.E., Hull J.W., Goodman M. The relative influence of symptoms, insight and neurocognition in social adjustment in schizophrenia and schizoaffective disorder. J. Nerv. Ment. Dis. 1999;187:102–108. doi: 10.1097/00005053-199902000-00006. [DOI] [PubMed] [Google Scholar]
- Smith T.E., Hull J.W., Huppert J.D. Insight and recovery from psychosis inchronic schizophrenia and schizoaffective disorder patients. J. Psychiatr. Res. 2004;38:169–176. doi: 10.1016/s0022-3956(03)00091-8. [DOI] [PubMed] [Google Scholar]
- Test M.A., Greenberg J.S., Long T.D. Construct validity of a measure of subjective satisfaction with life in adults with severe mental illness. Psychiatr. Serv. 2005;56:292–300. doi: 10.1176/appi.ps.56.3.292. [DOI] [PubMed] [Google Scholar]
- Tolman A., Kurtz M.M. Neurocognitive predictors of objective and subjective quality of life in individuals with schizophrenia, a meta-analytic investigation. Schizophr. Bull. 2012;38:304–315. doi: 10.1093/schbul/sbq077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner D.T., van der Gaag N., Karyotaki E., Cuijpers P. Psychological interventions for psychosis, a meta-analysis of comparative outcome studies. Am. J. Psychiatry. 2014;171:523–538. doi: 10.1176/appi.ajp.2013.13081159. [DOI] [PubMed] [Google Scholar]
- van Nieuwenhuizen C., Schene A.H., Koeter M.W. The Lancashire Quality of Life Profile: modification and psychometric evaluation. Soc. Psychiatry Psychiatr. Epidemiol. 2001;36:36–44. doi: 10.1007/s001270050288. [DOI] [PubMed] [Google Scholar]
- Wilson W.H., Ban T.A., Guy W. Flexible system criteria in chronic schizophrenia. Compr. Psychiatry J. 1986;27:259–265. doi: 10.1016/0010-440x(86)90052-0. [DOI] [PubMed] [Google Scholar]