Abstract
Adolescents are highly vulnerable to addiction and are four times more likely to become addicted at first exposure than at any other age. The dopamine D1 receptor, which is typically overexpressed in the normal adolescent prefrontal cortex, is involved in drug cue responses and is associated with relapse in animal models. In human drug addicts, imaging methods have detected increased activation in response to drug cues in reward- and habit-associated brain regions. These same methods can be applied more quantitatively to rodent models. Here, changes in neuronal activation in response to cocaine-conditioned cues were observed using functional magnetic resonance imaging in juvenile rats that were made to over-express either D1 receptors or green fluorescent protein by viral-mediated transduction. Reduced activation was observed in the amygdala and dopamine cell body regions in the low cue-preferring/control juvenile rats in response to cocaine cues. In contrast, increased activation was observed in the dorsal striatum, nucleus accumbens, prefrontal cortex, and dopamine cell bodies in high cue-preferring/D1 juveniles. The increase in cue salience that is mediated by increased D1 receptor density, rather than excessive cocaine experience, appears to underlie the transition from aversion to reward in cue-induced neural response and may form the basis for habit-forming vulnerability.
Abbreviations: BLA, basolateral amygdala; BOLD, blood oxygenation level determination; DSTR, dorsal striatum; fMRI, functional magnetic resonance imaging; NAc, nucleus accumbens; PFC, prefrontal cortex; pharmacoMRI, pharmacological magnetic resonance imaging; ROI, region of interest; SNc/r, substantia nigra pars compacta/reticulata; VTA, ventral tegmental area
Keywords: Cocaine, Cue, Development, D1, Odor, Striatum
Highlights
-
•
Increased D1 receptors in prefrontal cortex increase BOLD in addiction regions.
-
•
Cocaine-associated cues activated the amygdala when cocaine was preferred.
-
•
Cocaine cues deactivated the amygdala in the absence of cocaine preference.
-
•
Genetic engineering can be used to isolate functional responses in neural circuitry.
1. Introduction
The neural networks that underlie reward valuation and goal-directed behavior involve prefrontal cortex (PFC) innervation to the nucleus accumbens (NAc) (Griffiths et al., 2014). More specifically, D1 receptors on prelimbic PFC (plPFC) inputs to the NAc core modulate cocaine self-administration and reinstatement associated with relapse (McFarland et al., 2004; McLaughlin and See, 2003; Olsen and Duvauchelle, 2006; Rebec and Sun, 2005). D1 receptor over-expression naturally occurs without drug exposure during adolescent development (Brenhouse et al., 2008). Adolescent rats (postnatal day 44) have 4-fold higher expression of D1 receptors on plPFC glutamate neurons projecting to the NAc relative to juvenile (postnatal day 27) and adult (>90 days) rats (Brenhouse et al., 2008). This over-expression of D1 may render adolescents more vulnerable to drug-associated cues that are linked to addictive processes (Kalivas et al., 2005). Similar over-expression of D1 mRNA has been observed in human PFC during adolescence (Rothmond et al., 2012), suggesting that similar processes exist between rat and human development. Typical adolescent rats both take more cocaine (Wong et al., 2013) and show increased place preferences to cocaine-associated cues than adults (Badanich et al., 2006; Brenhouse et al., 2008); these adolescent preferences are reversible with plPFC microinjections of the D1 antagonist SCH-23390 (Brenhouse et al., 2008). To further confirm the role of D1 in adolescent responses to cocaine, we developed a lentiviral vector that over-expresses D1 receptors on glutamate neurons to approximate adolescent levels in adult rats. Behaviorally, adult rats that over-express D1 on glutamate neurons take more cocaine and show increased place preferences to cocaine-associated cues relative to control rats expressing green fluorescent protein (GFP) (Sonntag et al., 2014).
Magnetic resonance imaging (MRI) in rats provides a translational opportunity to assess brain function because this approach offers similar assessment methods to those used in humans. Blood oxygenation level dependent (BOLD) scans have been used to study activation patterns in response to drug-associated cues in adult humans and in animals. In human cocaine addicts, drug cues consistently increase BOLD responses in the striatum (STR), basolateral amygdala (BLA), ventral tegmental area, anterior and prefrontal cortex (PFC), hippocampus, and nucleus accumbens (NAc) (Garavan et al., 2000; Grusser et al., 2004; Jasinska et al., 2014; Lukas et al., 2013; Maas et al., 1998). With repeated and uncontrollable drug use, dorsal striatal responses to drug cues are elevated (Dalley et al., 2011; Volkow et al., 2006). Functional MRI (fMRI) responses in adult rodents exposed to drug-associated cues following chronic cocaine intake show remarkable anatomical faithfulness to these human fMRI changes (Johnson et al., 2013; Liu et al., 2013a). Because these responses are both reliable and robust, genetically modified animals could provide mechanistic insight into the underlying pharmacological basis for cue responses at the network level.
Imaging paradigms in small animals have been developed to determine structural and functional changes (reviewed by Febo, 2011), with the latter including indirect measures of BOLD (Huang et al., 2011), receptor expression/function with pharmacoMRI (Becerra et al., 2013; Chen et al., 2005), and more recently, resting state activation (Tian et al., 2006). These methodologies can be applied in normal, drug-exposed (Andersen et al., 2008; Reneman et al., 2001; van der Marel et al., 2014) or genetically-modified (e.g., Huang et al., 2011) animals and used to observe network changes in brain activity (discussed by Borsook et al., 2006).
To date, however, the majority of these applications have been restricted primarily to analyses with the region of interest (ROI) approach and not voxel-wise methodologies. Our first goal was to use whole brain, voxel-wise analysis in rats to reveal unexpected BOLD responses to cocaine-associated cues. Furthermore, its application in animals that have a genetic over-expression of D1 receptors in the plPFC produces a behavioral phenotype of animals that show reinstated responses to cocaine (McFarland et al., 2004). Developmental, preclinical imaging studies (Bouet et al., 2012; Chen et al., 2010; van der Marel et al., 2014) have utilized a region-of-interest (ROI) analytical approach, and not the voxel-wise, whole brain analysis that is commonly used in human studies. BOLD responses in juvenile control rats (with a lentiviral vector expressing GFP) that typically have low preferences for cocaine-associated environments and D1 expression on plPFC neurons (Brenhouse et al., 2008), may reveal brain regions involved in reduced addiction vulnerability. In this sense, a secondary goal of this study was to determine how a precocial increase in plPFC D1 receptors in pre-adolescent rats may be related to the increased vulnerability to use drugs of abuse that occurs during adolescence (Stanis and Andersen, 2014).
The current study used fMRI to determine how D1 dopamine receptor over-expression within the plPFC uniquely impacts widespread responses to cocaine-associated cues in a developmental context. Specifically, we compared the cue-based BOLD response in juvenile animals that showed minimum behavioral preferences for cocaine-associated environments and cues to the response in juveniles that over-expressed D1 by viral-mediated transfer. This approach allowed us to determine the responsiveness within neural circuitry that is key in the transition between low and high risk for addiction. The current study is the first to use whole brain voxel-wise BOLD responses to cocaine-conditioned cues in immature animals manipulated for low and high salience with only two exposures to cocaine; previous animal studies used a region of analysis approach (Johnson et al., 2013; Liu et al., 2013a) in animals chronically exposed to cocaine.
2. Materials and methods
2.1. Subjects
Sprague-Dawley litters of rats (n = 8 for each group) were obtained from Charles River Laboratories (Boston, MA) at 12 postnatal days of age (P12); only male pups were used, with one pup per litter for each condition. Pups were P16 at the time of surgery. Pups were weaned from the dam at P21 and housed with same-sex littermates. Rats were housed with food and water available ad libitum in constant temperature and humidity conditions on a 12-hr light/dark cycle (light period 0700–1900). The experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (NIH), and were approved by the Institutional Animal Care and Use Committee at McLean Hospital.
2.2. Lentiviral injections
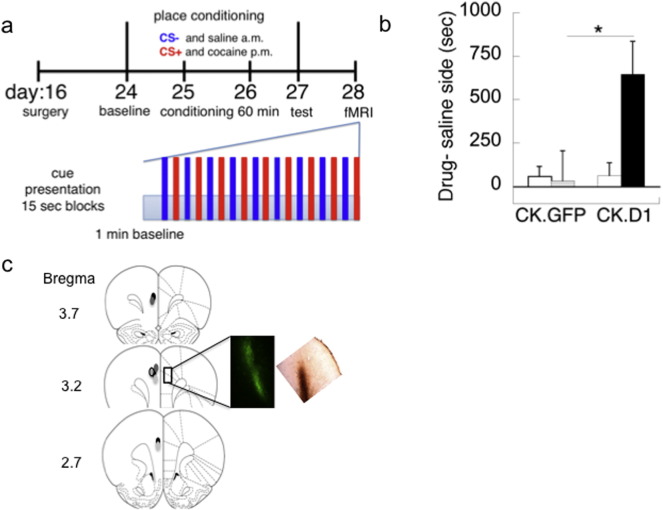
Male rats were anesthetized with a ketamine/xylazine mixture (80/12 mg/kg, respectively). A lentiviral vector (0.6 µL) that targets glutamate neurons by the Calmodulin Kinase II alpha [CK] promoter and expresses either green fluorescent protein (CK.GFP) or D1 dopamine receptors (CK.D1) was produced by the Massachusetts General Hospital Vector Core (Sonntag et al., 2014). Approximately 106 transducing units were injected bilaterally into the prelimbic PFC at stereotaxic coordinates (AP: +2.7, ML: 0.5; DV: −2.7) for P16 rats (Sherwood and Timeras, 1970). Screening for place preferences to cocaine-associated cues began 8 days after surgery to allow for viral expression (Fig. 1a). Expression stability and placement were confirmed by histology (Sonntag et al., 2014) within the plPFC (Fig. 1c shows GFP or D1 expression) or the subjects were excluded from analysis.
Fig. 1.
Determination of behavioral and fMRI effects of conditioned drug cues. a) Timeline of the behavioral and fMRI paradigm. During conditioning the CS− odor was paired with saline, and the CS+ odor was paired with cocaine for 60 min each at 25 and 26 days of age. The assignment of odor cue to CS+ or CS− during conditioning was counter-balanced. During fMRI rose was always presented first, independent of odor cue assignment. BOLD responses to CS+ and CS− were determined after behavioral testing. b) Place preferences to 10 mg/kg cocaine. Relative to pre-conditioning preferences (time on drug side − time on saline side; white bars), CK.GFP show no preference for CS+ environments (gray bar: post-conditioning), whereas CK.D1 subjects show a strong preference (black bar: post-conditioning). Means ± SE presented; *P < 0.05, Bonferroni corrected. c) Histological verification of virus placement in the plPFC (bregma coordinates on far left). GFP immunofluorescence is shown in middle and D1 immunoreactivity with DAB visualization on the right.
2.3. Odorant presentation
Place conditioning chambers had a custom built olfactometer (Lowen and Lukas, 2006) that bubbled filtered room air through two flasks, one containing phenethyl alcohol (“rose” scent), and the other acetophenone (“almond” scent) at a final flow rate of 10 mL/min for each odorant. All tubing throughout was PTFE to minimize stray odors. Each side chamber had an odorant presented into one corner and vented through the opposite corner using the laboratory vacuum system; the side chambers were kept at negative pressure to prevent odor mixing.
2.4. Place conditioning
An unbiased place conditioning protocol was used to establish behavioral preferences or aversions to cocaine cues, as previously published (Andersen et al., 2002). Other operant paradigms, including self-administration, are not suitable for developmental studies in subjects this young because they require extensive training. Briefly, separate groups of juvenile rats (P24) were habituated to the 3-chambered apparatus (Med Associates, St. Albans, VT) for 30 min on Day 1 to establish no baseline preferences for odor-associated environments (defined as spending >18 of 30 min on one side). Initially, these chambers differed by color (black, gray, white), and floor (grid, smooth, and bars). On Days 2 and 3, a 60-minute cue conditioning session was conducted to saline in the morning (where the odor becomes a conditioned stimulus that predicts “no drug” [CS−]) and 10 mg/kg cocaine in the afternoon (a CS odor that predicts drug [CS+]). The 10 mg/kg dose of cocaine was selected because juvenile rats typically do not show place preferences for environments associated with this dose (Brenhouse et al., 2008). The pairing of odor–drug conditions was randomized within group. On Day 4 rats were permitted to freely explore the entire apparatus for 30 min in a drug-free state to determine behavioral preferences to drug-conditioned odor/environments. Place conditioning scores are expressed as time spent on the drug side − time spent on the saline side (in s) on test day.
2.5. Odorant fMRI
These same subjects were anesthetized with isofluorane and imaged with fMRI during odor cue presentation on Day 5. Activation in response to CS+ or CS− odors (Fig. 1a) was observed during a 10-min fMRI experiment. A second, similar olfactometer passed filtered room air at 0.3 L/min through one of three flasks that contained solutions of “rose,” “almond,” or neutral (water) odors. A separate odor pathway exited each flask, and the three odor-bearing tubes were joined 4 in. from the rat to minimize dead space and provide discrete periods of odor exposure. Odors were delivered in the same schedule for all rats. During the 200 vol/10 min scan, odors were turned on and off according to the following schedule: for the first minute, the odorant was set to water, followed by switching to almond for 15 s, return to water for 15 s, then rose, etc.; odorants alternated with water in between each presentation.
2.6. MRI acquisition
The rats were anesthetized at 2% isoflurane and maintained on 1.5% isoflurane and 1.5 L/min breathing quality air during imaging. Physiologic monitoring (Small Animal Instruments, Inc.) included respiration, EKG and temperature. Temperature was maintained using a recirculation water blanket (Gaymar T/Pump). Images were acquired on a 9.4 T Agilent (Palo Alto, CA) MRI system with a 120 mm diameter gradient system. A head-only tight fitting quadrature birdcage RF coil (in house) provided fMRI images with reduced artifacts, and subjects were shimmed to a whole brain line width of <150 Hz. The fMRI experiment followed a clinical protocol model, and used a single-shot Echo-Planar acquisition, TE/TR = 17 ms/3 s; matrix = 64 × 64 on 40 mm FOV with 38 × 0.625 mm slices, 0 mm gap; bandwidth was 357 kHz with an echo spacing of 330 µs. Anatomic images were Fast Spin Echo Multi-Slice (FSEMS) and were acquired with TE/TR = 38.7 ms/3.06 s; matrix 256 × 256 on 40 mm FOV with 38 × 0.625 mm slices with 0 gap; 4 averages, bandwidth 100 kHz (3:16 total).
2.7. MR image processing
The BOLD fMRI images were processed from raw data files using an in-house program that used standard FFT and filtering. Nyquist ghosting was minimized using a parameterized phase correction in which spatially constant and linear terms were adjusted (Kutter et al., 1994). Both magnitude and phase images were obtained.
2.8. Anatomical scan modification and registration
The voxel size of the anatomic scans were isotropically relabeled by a factor of 10. All brains were then registered to the MNI 1 mm standard (human) brain using FSL software (FMRIB, Oxford University) and averaged together. The brains were then registered to this average and averaged again. The process was repeated two more times; the converged result served as the standard anatomic rat brain for this study.
2.9. BOLD fMRI modification and preprocessing
The voxel size of the fMRI scans was anisotropically relabeled so that the brain size matched the MNI standard and allowed the use of the clinical Brain Extraction Tool (BET/FSL). The voxel dimensions of the extracted brains were then relabeled again to match the anatomical brain size described above. Standard preprocessing followed and comprised motion correction, slice timing correction, spatial smoothing to 10 mm FWHM (1 mm in native space), and high pass filtering with a cutoff of 100 s.
2.10. fMRI subject analysis
Subject-wise BOLD activation was determined using two passes of standard general linear model (GLM) processing (FSL). This was a voxel-wise analysis that was carried out over the entire brain. Subject-wise results were a contrast between the response to the CS+ odors in comparison to the response to the CS− odors, within subject. In the first pass two regressors of interest were used, one for almond and one for rose, that corresponded to the odor delivery times and these were convolved with a standard hemodynamic response function and the same high-pass filter was applied to that data. Standard nuisance regressors were also included and comprised temporal derivatives of each of the two regressors of interest and the six estimated motion time courses. The derivatives were included to model any variation in the timing between odor onset and neural activity, due to unanticipated effects. The image phase change from the first image formed a voxel-wise nuisance regressor. In the second pass the results from the first pass were discarded except for the residuals, which were reduced using a principal component analysis to the first eight components. The component time courses were used as 8 × 1D global nuisance regressors (Madsen and Lund, 2006). The residuals were also averaged over each slice within each volume and then replicated throughout that slice forming an additional, voxel-wise nuisance regressor. A second GLM analysis was performed including all the regressors from the first model, as well as the ten listed above derived from the residuals.
2.11. Registration
Subject-wise images from the second GLM were linearly registered (FSL 5.0.6) to corresponding anatomical scans with 7 degrees of freedom, and then to the standard anatomic rat brain (described above) with 12 degrees of freedom. The resulting registrations were applied to the statistical image output of the second GLM to obtain registered statistical images for group analysis.
2.12. fMRI group analysis
Subject-wise results were then combined in a mixed-effects group analysis for the contrasts Almond–Rose and Rose–Almond only (Chase et al., 2011). This group analysis was performed as a voxel-wise analysis, relying on the registered statistical images. Three regressors were used. The first (“D1”) was +1 for D1 rats trained with almond as the active odor, −1 for D1 rats trained with rose as the active odor, and 0 for GFP rats. The second (“GF”) was +1 for GFP rats trained with almond as the active odor, −1 for GFP rats trained with rose as the active odor, and 0 for D1 rats. The third was the conditioned place preference score (“BH” for behavior), orthogonalized with respect to the other two regressors (see Supplementary Fig. 1). Statistical significance of these voxel-wise results were established as follows. First, results were converted to z scores, and thresholded to z > 2.3 (uncorrected) for each voxel. Then the Gaussian random field theory was used to find clusters of contiguous thresholded voxels that had a cluster significance level of P < 0.05, family-wise error corrected for multiple comparisons over the whole brain.
Supplemental Fig. 1.

Design matrix for the group analysis showing the dopamine odor conditioning interaction (D1), control drug conditioning interaction (GF), and the orthogonalized place preference score (BH). Each row corresponds to a subject. The gray scale to the right represents the regressor values. The +1 or −1 represents white or black color the subject was cocaine-conditioned to almond or rose. The table below shows the statistical contrasts defined as a function of these regressors. This is standard output from the FSL fMRI analysis program.
2.13. Post-hoc regional analysis of the left BLA
A post-hoc regional analysis was performed for the BLA because of its central role in the mediation of cue response. This anatomic region was drawn by hand (FSLView) with the aid of the anatomic atlas used for identification of regions in the voxel-wise results (Fig. 3a). Anatomic landmarks were identified on the composite anatomic image as referenced from the atlas (Sherwood and Timeras, 1970) and hand drawn regions were drawn based on those landmarks.
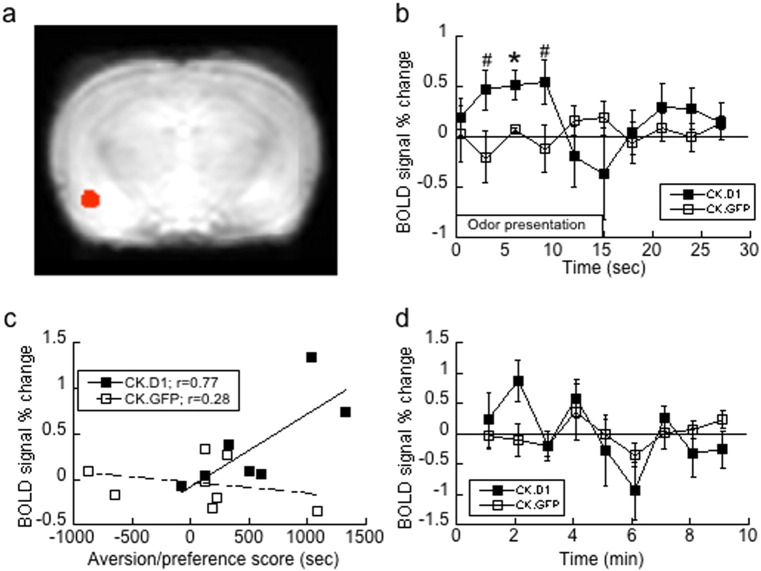
Fig. 3.
Group and individual responses to cocaine cues in the left BLA. Responses are from n = 8 CK.GFP subjects and n = 7 CK.D1 subjects. a) Hand-drawn region of interest of the left BLA based on the atlas of Paxinos and Watson (1986). b) Group time-course differences in the differential BOLD response between CS+ and CS− associated odors (Chapuis et al., 2009) for CK.D1 (filled squares) and CK.GFP (open squares) groups in the left BLA region of interest. Odor responses were averaged within an atlas-based left BLA region, across blocks, yielding event-related group specific responses; * indicates the significant (P < 0.01; #P = 0.06) time point. c) Individual differential responses in the BLA. BOLD activation was averaged across similar blocks, the difference taken between odors, and plotted against place preference scores (time spent on drug side − time spent on saline side). CK.D1, filled squares; CK.GFP, open squares; linear regression (line) yields a significant (P < 0.04) positive correlation for CK.D1, but not CK.GFP. d) Assessment of odor habituation in the BLA ROI. Figure represents demeaned (CS+ − CS−) odor response averaged within each 1 min odor cycle, for minutes 1–9 (after baseline) in the 10 min experiment. There was no significant time effect (Ps > 0.05).
3. Results
3.1. Place conditioning
No baseline preferences for either odor were observed for the pre-conditioning values. After conditioning, CK.GFP rats demonstrated only mild preferences or aversions for the CS+ relative to the CS− (Fig. 1b), whereas CK.D1 rats had strong preferences for the CS+ relative to pre-conditioning baseline (mixed ANOVA: Virus × [Conditioning]: F1,15 = 4.30, P = 0.05). These same subjects were then used for imaging the following day in order to determine their BOLD responses to the CS+ and CS− odors.
3.2. Imaging
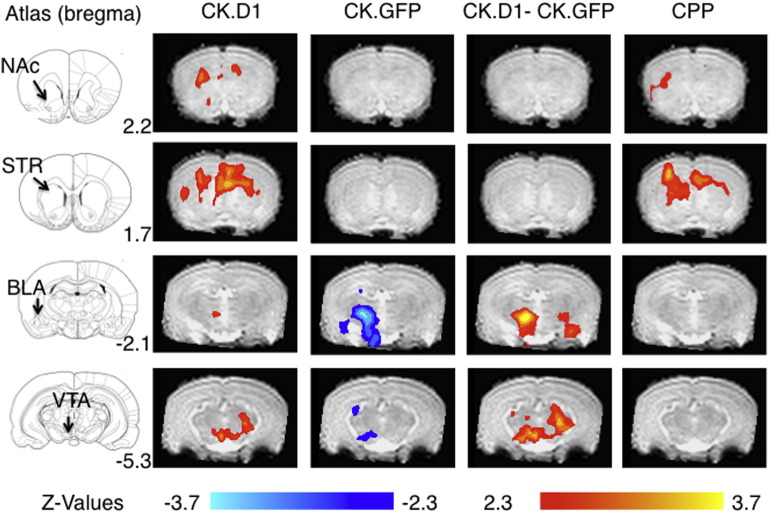
MRI data were obtained from n = 8 CK.GFP subjects and n = 7 CK.D1 subjects. We observed three significant effects concerning the hypotheses in this study and applied a post-hoc regional analysis to further examine one result. First, CK.GFP juveniles, with mild preferences or aversions, exhibited a deactivation BOLD response to the CS+ odors relative to the CS− odors (Fig. 2) that occurred primarily within the left BLA and the VTA. Second, and in contrast to the CK.GFP juveniles, the CK.D1 juveniles with strong place preferences for the CS+ odors, demonstrated significantly elevated BOLD responses to the CS+ odors relative to the CS− odors in the plPFC, left BLA, NAc, dorsal striatum (DSTR), and VTA (Fig. 2). Third, in a comparison between subject groups (CK.D1–CK.GFP), there was an increased differential response to the CS+ vs CS− odor cues in the left BLA and in the VTA. Images of activation as determined by the voxel-wise approach for all other regions are found in Supplementary Fig. 2a–d.
Fig. 2.
BOLD signal responses to CS+ versus CS− across different conditions from a voxel-wise analysis carried out over the whole brain. Scale bar hue indicates group-wise z-score with increases in red/yellow areas, decreases in blue/cyan. Clusters shown are significant (P < 0.05) after correction for multiple comparisons across the whole brain. From left to right, data are from CK.D1, CK.GFP, a differential contrast (CK.D1 − CK.GFP), and the effect of the behavioral conditioned place preference (CPP) response to CS+ vs. CS− independent of virus condition. Responses are from n = 8 CK.GFP subjects and n = 7 CK.D1 subjects. Key figures are identified: NAc: nucleus accumbens; STR: striatum; BLA: basolateral amygdala; VTA: ventral tegmental area. All regions are shown in Supplementary Fig. 2.
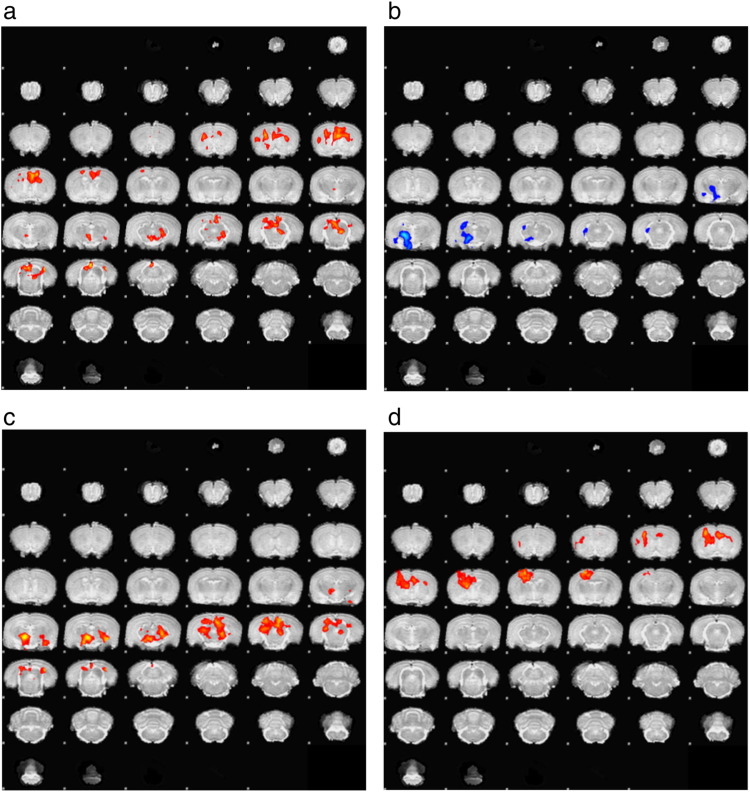
Supplemental Fig. 2.
Whole brain display of the selected slices shown in Fig. 2. BOLD signal responses to CS+ versus CS− across different conditions. The hue indicates z-score with increases in red/yellow areas, decreases in blue/cyan. The scale bar can be found in Fig. 2. Clusters shown are significant (P < 0.05) after correction for multiple comparisons across the whole brain. a) Data are from CK.D1; b) CK.GFP; c) the difference between the two (CK.D1 – CK.GFP); and d) those regions activated by behavioral conditioned place preference (CPP) responses to CS+ vs. CS− independent of virus condition. “R” indicates the anatomical right side of the subject. Responses are from n = 8 CK.GFP subjects and n = 7 CK.D1 subjects.
A post-hoc regional analysis of the time-course of the activity in the left BLA (Fig. 3b) was performed in order to characterize the odor response, and habituation effects, and demonstrate behavioral effects if any. The regional time course of the activation of the left BLA was associated with the CS+ odor in the CK.D1, but not CK.GFP, rats during the 15-second odor presentations (Fig. 3b). Additionally, the relative magnitude of the observed regional BLA activity on the left side was significantly correlated with cue preferences for individual CK.D1 subjects, but not CK.GFP (Fig. 3c; r = 0.77, P = 0.04 and r = 0.28, P = 0.5, respectively). Finally, this regional data was used to test for habituation effects. The (CS+–CS−) response was averaged within the left BLA region within odor blocks for each minute after the initial 1 min baseline, within the CK.D1 and CK.GFP groups. The time-courses were demeaned (Fig. 3d). CK.D1 subjects did not show a significant decrease in activation over the course of the experiment, nor did CK.GFP subjects (Ps > 0.05; repeated measures ANOVA), demonstrating a lack of habituation.
4. Discussion
In the current study, we used a localized genetic manipulation to increase the expression of dopamine D1 receptors in juvenile animals to probe BOLD responses to cocaine-conditioned odors. Our data were analyzed using a voxel-wise approach over the whole brain, much like those used in humans that will allow a direct translation of these studies of cocaine effects to clinical results. There are two main findings: First, control CK.GFP juvenile subjects show a deactivation response to the CS+ odors relative to the CS− odors in the BLA and VTA regions. Second, CK.D1 juveniles showed elevated responses to the CS+ odors relative to the CS− odors in the NA, BLA, plPFC, DSTR, and VTA. These findings are consistent with an aversive/neutral behavioral response to the CS+ odors relative to the CS− odors for the CK.GFP juveniles, and a preference for CS+ vs. CS− for the CK.D1 juveniles. Also, the increased differential BOLD response to cues in the left BLA suggests stronger associations between stimulus and response in the CK.D1 group that are indicative of significant conditioning to the odor in these animals. It is interesting to note methodologically that place preference scores are associated with increased activation in the DSTR that is evident in the imaging results.
We over-expressed D1 receptors selectively in the plPFC, as we have previously observed a four-fold increase in this receptor population in adolescent rats (Brenhouse et al., 2008). Our lentiviral vector produces approximately a four-fold change in receptor expression, and a 30% activation of c-fos response to a D1 agonist (Sonntag et al., 2014). To what extent the observed changes in CS+ responsiveness are due to D1 over-expression, or to the affiliated decrease in NAc D2 receptors that we have observed when D1 is over-expressed (Sonntag et al., 2014), is not known. Decreases in D2 receptors have been reported in animals that have high trait impulsivity that is associated with compulsive cocaine use (Dalley et al., 2007). Increased D1 dopamine receptor expression in the juvenile CK.D1 group corresponded to an increased BOLD activation pattern to drug cues in the same brain regions that have been previously observed to being activated in adult rats following chronic and habitual drug use.
The development of our fMRI approach could enable us to test drug-associated cues and their underlying mechanisms in immature systems, which is not ethically possible in children. Whether an existing reward anticipation task that has been used in young adolescents (Bjork et al., 2004) assesses a similar phenomenon is unknown, as no drug stimuli were used. In our study, changes in BLA responses in CK.D1 juveniles correlated with increasing preferences for cocaine-associated odors, but this effect was not evident in the CK.GFP juveniles. While this relationship does not show causality, this observation further implicates the BLA in helping to encode the saliency of the cue (Nishijo et al., 1998; Winston et al., 2005) as does the data presented in Fig. 3b. The divergence of the slopes of the two lines between regional BLA BOLD activity and place conditioning behavior between the two groups is not consistent with the role of the immature BLA to encode the value of the reward to its cue (Balleine and Killcross, 2006). Otherwise, cocaine cue preferences would have produced similar BLA activation regardless of D1 status. At the level of group analysis, the deactivation of the BLA and VTA that was observed in the juvenile CK.GFP group is consistent with the average behavioral data that suggests no preference to cocaine in these subjects. The BLA has also been associated with increased aversion to the affective salience of an odor associated with food (Nishijo et al., 1998; Winston et al., 2005) and the aversive representation of that cue (Chapuis et al., 2009), nicotine cues (Vollstadt-Klein et al., 2011), the conditioning of cocaine cues (Buffalari and See, 2010), and the predictability of the cue (Baxter and Murray, 2002). The VTA is involved in the encoding of an action tendency in response to a reward-associated cue (the CS+ (Guitart-Masip et al., 2011)). The lack of motivated response to the cocaine-associated cue may reflect the deactivation of the VTA. Together then, the deactivation of the BLA and/or the VTA in this study is consistent with the negative subjective effects of amphetamine that have been reported in pre-pubertal boys (Rapoport et al., 1980). Additional fMRI animal studies in the future may conclusively show that these BLA responses reflect an aversion to psychostimulants at this age.
The diminished BOLD responses that were observed within brain regions with dopamine cell bodies (including the VTA) in the CK.GFP group are consistent with reduced NAc activity in individuals reporting reduced drug craving (Janse Van Rensburg et al., 2012). Typical juvenile rats do not show preferences to cocaine-associated cues at low doses, suggesting reduced sensitivity compared to older ages (Brenhouse et al., 2008). We have previously demonstrated that neither plPFC D1 activity alone (microinjection) nor low-dose cocaine alone was sufficient to produce a place preference to cocaine-associated environments in juvenile rats, but that low-dose cocaine plus a D1 agonist microinjected into the plPFC did increase place preferences (Brenhouse et al., 2008). Data in this current study expand these previous observations by suggesting that a lack of response in brain activation (based on deactivation in BOLD activity in the dopamine cell body regions (SNc/r and VTA), and no activity in NAc and the plPFC) reduced behavioral preferences. Others have shown that the amygdala, NAc, and ingulate gyrus circuitry are vital for processing drug cues and for reinstatement (Kalivas et al., 2003; Liu et al., 2013a). Together, deactivated BOLD responses in these regions as seen in the control CK.GFP subjects may indicate low addiction vulnerability in children and young teens.
In contrast, the BOLD responses of the CK.D1 juvenile rats resembled those in adult rats with cue-induced increases in BOLD activity in the PFC, NAc, DSTR, and VTA after a history of self-administration for a minimum of 4 weeks (Liu et al., 2013a). Our CK.D1 juveniles had 2 days of passive cocaine exposure. We hypothesize that the CK.D1-elevated striatal activity in juvenile rats maybe sufficient to facilitate a Pavlovian instrumental transfer of stimulus–response associations relevant to habitual and addictive processes (Everitt and Robbins, 2005). Repeated cocaine exposure progressively recruits the activity in regions from the ventral to dorsal striatal regions in a spiral fashion (Haber et al., 2006; Haber and Knutson, 2010). Increased D1 during adolescence may facilitate this process. Adolescent rats, which typically overexpress D1 (Brenhouse et al., 2008), may be highly predisposed to addiction via striatal activity following cue presentation even without significant chronic cocaine exposure. Consistent with this hypothesis, a greater proportion of neurons in the DSTR of adolescents are activated in anticipation of reward relative to adults, suggesting greater potential of learning at this stage (Sturman and Moghaddam, 2012). Together, elevated plPFC D1 and greater DSTR activity in response to reward (or its cues) may transiently increase vulnerability to cocaine addiction.
One methodological point we feel we need to address is anesthesia. Our rats were initially conditioned to the cocaine-associated odors during an awake state, but were anesthetized with 1.5% isoflurane for the fMRI experiment. The robust fMRI BOLD patterns that we observed are consistent with those from awake individuals with addiction in response to cocaine-associated cues (Johnson et al., 2013; Liu et al., 2013a). We believe that the use of anesthesia is needed to reduce the stress that is associated with restraint, as used in other studies in adult animals (for a full discussion of the pros and cons of this approach, see Febo, 2011). Training the animal to remain immobile is also not realistic for rats at this young age as they would be activating brain regions to accomplish this task, rather than the task at hand. Finally, research shows that the effects of anesthesia are type- and dose-dependent (Febo, 2011). Relatively light levels of isoflurane anesthesia seem to have a minimal effect on brain function, although isoflurane does act as a vasodilator (Liu et al., 2013b; Sicard et al., 2003). For example, resting state networks of the sensorimotor system gradually became highly synchronized and less spatially specific when isoflurane anesthesia transitioned from 1.0%, 1.5% to 1.8% (Liu et al., 2013b). The differences between light (1.0%) and moderate (1.5%) anesthesia on resting state BOLD were not significantly different from each other. While a reversal in BOLD response occurs at high anesthesia (3.5% isoflurane), local field potentials remain congruent with BOLD signals at lower isoflurane levels (1.8%) where breathing rates are maintained at ~75 breaths/min (Gong et al., 2014). Our BOLD responses were acquired when both groups of rats were anesthetized at 1.5% isoflurane. The differences between the CK.GFP/D1 groups still produced BOLD responses that are similar to those in studies of awake humans employing cue responses (Garavan et al., 2000; Lukas et al., 2013) and moreover, are temporally linked with odor presentation (Fig. 3b). Together, these data argue against significant interference of isoflurane to influence the detection of the odor or a hemodynamic response to its conditioned associations within the brain.
5. Conclusion
In conclusion, we genetically increased dopamine D1 receptor expression in juveniles to levels typically found in adolescents to increase motivational salience. Elevated D1 levels increased behavioral preferences for cocaine-associated cues after just two exposures in CK.D1 juvenile rats, whereas control juveniles did not prefer cocaine cues. More importantly, conditioned cues in CK.D1 juveniles produced fMRI activation patterns in the plPFC, BLA, NA, dopamine cell body regions, and DSTR. These patterns are consistent with those found in rats and humans exposed chronically to cocaine. Our fMRI observations are also the first to show cue-induced deactivation of BLA and dopamine regions in control CK.GFP juveniles. These findings suggest that a strong predisposition towards habitual use is mediated by increased PFC D1 receptor expression that is found in adolescents, and possibly, children at high-risk.
The following are the supplementary data related to this article.
Supplemental Fig. 3.
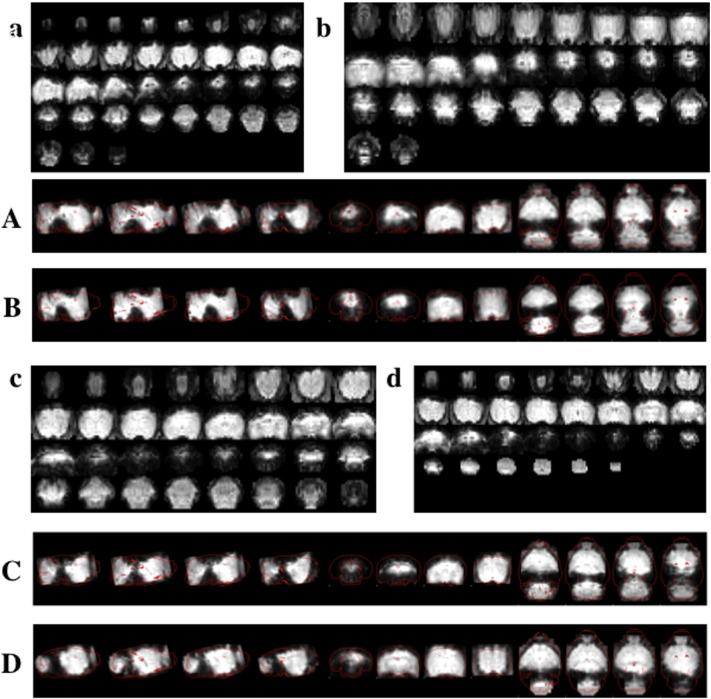
Sample raw EPI and registration displays for five representative rats. a–d) Raw EPI data, taken from the middle volume of the data analyzed. Note that all six edges were reduced to just fit the brain individually for each rat, yielding variable dimensions. A–D) Corresponding registration displays for the rats. The averaged functional data are shown in grayscale, while the outline of the standard space employed appears in red.
Conflict of interest
The authors declare no competing financial interests.
Acknowledgments
The authors acknowledge the support of DA-010543 and DA-026485 (to SLA). This project was also sponsored in part by the Counter-Drug Technology Assessment Center (CTAC), an office within the Office of National Drug Control Policy (ONDCP), via contract Number DBK39-03-C-0075 awarded by the Army Contracting Agency. The content of the information does not necessarily reflect the position or the policy of the Government and no official endorsement should be inferred. This project also was sponsored in part by the NIH grant S10 RR019356.
Contributor Information
Steven B. Lowen, Email: slowen@mclean.harvard.edu.
Michael L. Rohan, Email: mrohan@mclean.harvard.edu.
Timothy E. Gillis, Email: tgillis@mclean.harvard.edu.
Britta S. Thompson, Email: bthompson@mclean.harvard.edu.
Clara B.W. Wellons, Email: cwellons@mclean.harvard.edu.
Susan L. Andersen, Email: sandersen@mclean.harvard.edu.
References
- Andersen S.L., Arvanitogiannis A., Pliakas A.M., LeBlanc C., Carlezon W.A., Jr. Altered responsiveness to cocaine in rats exposed to methylphenidate during development. Nat. Neurosci. 2002;5(1):13–14. doi: 10.1038/nn777. 11731802 [DOI] [PubMed] [Google Scholar]
- Andersen S.L., Napierata L., Brenhouse H.C., Sonntag K.C. Juvenile methylphenidate modulates reward-related behaviors and cerebral blood flow by decreasing cortical D3 receptors. Eur. J. Neurosci. 2008;27(11):2962–2972. doi: 10.1111/j.1460-9568.2008.06254.x. 18588536 [DOI] [PubMed] [Google Scholar]
- Badanich K.A., Adler K.J., Kirstein C.L. Adolescents differ from adults in cocaine conditioned place preference and cocaine-induced dopamine in the nucleus accumbens septi. Eur. J. Pharmacol. 2006;550(1–3):95–106. doi: 10.1016/j.ejphar.2006.08.034. 17011546 [DOI] [PubMed] [Google Scholar]
- Balleine B.W., Killcross S. Parallel incentive processing: an integrated view of amygdala function. Trends Neurosci. 2006;29(5):272–279. doi: 10.1016/j.tins.2006.03.002. 16545468 [DOI] [PubMed] [Google Scholar]
- Baxter M.G., Murray E.A. The amygdala and reward. Nat. Rev. Neurosci. 2002;3(7):563–573. doi: 10.1038/nrn875. 12094212 [DOI] [PubMed] [Google Scholar]
- Becerra L., Upadhyay J., Chang P.C., Bishop J., Anderson J., Baumgartner R., Schwarz A.J., Coimbra A., Wallin D., Nutile L., George E., Maier G., Sunkaraneni S., Iyengar S., Evelhoch J.L., Bleakman D., Hargreaves R., Borsook D. Parallel buprenorphine phMRI responses in conscious rodents and healthy human subjects. J. Pharmacol. Exp. Ther. 2013;345(1):41–51. doi: 10.1124/jpet.112.201145. 23370795 [DOI] [PubMed] [Google Scholar]
- Bjork J.M., Knutson B., Fong G.W., Caggiano D.M., Bennett S.M., Hommer D.W. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. J. Neurosci. 2004;24(8):1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. 14985419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsook D., Becerra L., Hargreaves R. A role for fMRI in optimizing CNS drug development. Nat. Rev. Drug Discov. 2006;5(5):411–424. doi: 10.1038/nrd2027. 16604100 [DOI] [PubMed] [Google Scholar]
- Bouet V., Klomp A., Freret T., Wylezinska-Arridge M., Lopez-Tremoleda J., Dauphin F., Boulouard M., Booij J., Gsell W., Reneman L. Age-dependent effects of chronic fluoxetine treatment on the serotonergic system one week following treatment. Psychopharmacol. Berl. 2012;221(2):329–339. doi: 10.1007/s00213-011-2580-1. 22205158 [DOI] [PubMed] [Google Scholar]
- Brenhouse H.C., Sonntag K.C., Andersen S.L. Transient D1 dopamine receptor expression on prefrontal cortex projection neurons: relationship to enhanced motivational salience of drug cues in adolescence. J. Neurosci. 2008;28(10):2375–2382. doi: 10.1523/JNEUROSCI.5064-07.2008. 18322084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalari D.M., See R.E. Amygdala mechanisms of Pavlovian psychostimulant conditioning and relapse. Curr. Top. Behav. Neurosci. 2010;3:73–99. doi: 10.1007/7854_2009_18. 21161750 [DOI] [PubMed] [Google Scholar]
- Chapuis J., Garcia S., Messaoudi B., Thevenet M., Ferreira G., Gervais R., Ravel N. The way an odor is experienced during aversive conditioning determines the extent of the network recruited during retrieval: a multisite electrophysiological study in rats. J. Neurosci. 2009;29(33):10287–10298. doi: 10.1523/JNEUROSCI.0505-09.2009. 19692603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase H.W., Eickhoff S.B., Laird A.R., Hogarth L. The neural basis of drug stimulus processing and craving: an activation likelihood estimation meta-analysis. Biol. Psychiatry. 2011;70(8):785–793. doi: 10.1016/j.biopsych.2011.05.025. 21757184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.C., Choi J.K., Andersen S.L., Rosen B.R., Jenkins B.G. Mapping dopamine D2/D3 receptor function using pharmacological magnetic resonance imaging. Psychopharmacology (Berl.) 2005;180(4):705–715. doi: 10.1007/s00213-004-2034-0. 15536545 [DOI] [PubMed] [Google Scholar]
- Chen Y.I., Choi J.K., Xu H., Ren J., Andersen S.L., Jenkins B.G. Pharmacologic neuroimaging of the ontogeny of dopamine receptor function. Dev. Neurosci. 2010;32(2):125–138. doi: 10.1159/000286215. 20523024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley J.W., Everitt B.J., Robbins T.W. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69(4):680–694. doi: 10.1016/j.neuron.2011.01.020. 21338879 [DOI] [PubMed] [Google Scholar]
- Dalley J.W., Fryer T.D., Brichard L., Robinson E.S., Theobald D.E., Lääne K., Peña Y., Murphy E.R., Shah Y., Probst K., Abakumova I., Aigbirhio F.I., Richards H.K., Hong Y., Baron J.C., Everitt B.J., Robbins T.W. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315(5816):1267–1270. doi: 10.1126/science.1137073. 17332411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt B.J., Robbins T.W. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat. Neurosci. 2005;8(11):1481–1489. doi: 10.1038/nn1579. 16251991 [DOI] [PubMed] [Google Scholar]
- Febo M. Technical and conceptual considerations for performing and interpreting functional MRI studies in awake rats. Front. Psychiatry. 2011;2:43. doi: 10.3389/fpsyt.2011.00043. 21808625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H., Pankiewicz J., Bloom A., Cho J.K., Sperry L., Ross T.J., Salmeron B.J., Risinger R., Kelley D., Stein E.A. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am. J. Psychiatry. 2000;157(11):1789–1798. doi: 10.1176/appi.ajp.157.11.1789. 11058476 [DOI] [PubMed] [Google Scholar]
- Gong L., Li B., Wu R., Li A., Xu F. Brain-state dependent uncoupling of BOLD and local field potentials in laminar olfactory bulb. Neurosci. Lett. 2014;580:1–6. doi: 10.1016/j.neulet.2014.07.034. 25079901 [DOI] [PubMed] [Google Scholar]
- Griffiths K.R., Morris R.W., Balleine B.W. Translational studies of goal-directed action as a framework for classifying deficits across psychiatric disorders. Front. Syst. Neurosci. 2014;8:101. doi: 10.3389/fnsys.2014.00101. 24904322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grüsser S.M., Wrase J., Klein S., Hermann D., Smolka M.N., Ruf M., Weber-Fahr W., Flor H., Mann K., Braus D.F., Heinz A. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacol. Berl. 2004;175(3):296–302. doi: 10.1007/s00213-004-1828-4. 15127179 [DOI] [PubMed] [Google Scholar]
- Guitart-Masip M., Fuentemilla L., Bach D.R., Huys Q.J., Dayan P., Dolan R.J., Duzel E. Action dominates valence in anticipatory representations in the human striatum and dopaminergic midbrain. J. Neurosci. 2011;31(21):7867–7875. doi: 10.1523/JNEUROSCI.6376-10.2011. 21613500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber S.N., Kim K.S., Mailly P., Calzavara R. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. J. Neurosci. 2006;26(32):8368–8376. doi: 10.1523/JNEUROSCI.0271-06.2006. 16899732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber S.N., Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35(1):4–26. doi: 10.1038/npp.2009.129. 19812543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Heffernan M.E., Li Z., Zhang N., Overstreet D.H., King J.A. Fear induced neuronal alterations in a genetic model of depression: an fMRI study on awake animals. Neurosci. Lett. 2011;489(2):74–78. doi: 10.1016/j.neulet.2010.11.069. 21134416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janse Van Rensburg K., Taylor A., Benattayallah A., Hodgson T. The effects of exercise on cigarette cravings and brain activation in response to smoking-related images. Psychopharmacol. Berl. 2012;221(4):659–666. doi: 10.1007/s00213-011-2610-z. 22234380 [DOI] [PubMed] [Google Scholar]
- Jasinska A.J., Stein E.A., Kaiser J., Naumer M.J., Yalachkov Y. Factors modulating neural reactivity to drug cues in addiction: a survey of human neuroimaging studies. Neurosci. Biobehav. Rev. 2014;38:1–16. doi: 10.1016/j.neubiorev.2013.10.013. 24211373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson T.R., Smerkers B., Moulder J.K., Stellar J.R., Febo M. Neural processing of a cocaine-associated odor cue revealed by functional MRI in awake rats. Neurosci. Lett. 2013;534:160–165. doi: 10.1016/j.neulet.2012.11.054. 23262077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas P.W., McFarland K., Bowers S., Szumlinski K., Xi Z.X., Baker D. Glutamate transmission and addiction to cocaine. Ann. N. Y. Acad. Sci. 2003;1003:169–175. doi: 10.1196/annals.1300.009. 14684444 [DOI] [PubMed] [Google Scholar]
- Kalivas P.W., Volkow N., Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal–accumbens glutamate transmission. Neuron. 2005;45(5):647–650. doi: 10.1016/j.neuron.2005.02.005. 15748840 [DOI] [PubMed] [Google Scholar]
- Kutter M., Angwin D., Maynard P., Rohan M. Automated ghost tuning of echo-planar images. Proc. SMR, Second Annual Scientific Meeting. 1994;836 [Google Scholar]
- Liu H.S., Chefer S., Lu H., Guillem K., Rea W., Kurup P., Yang Y., Peoples L., Stein E.A. Dorsolateral caudate nucleus differentiates cocaine from natural reward-associated contextual cues. Proc. Natl. Acad. Sci. U. S. A. 2013;110(10):4093–4098. doi: 10.1073/pnas.1207531110. 23431137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Zhu X.H., Zhang Y., Chen W. The change of functional connectivity specificity in rats under various anesthesia levels and its neural origin. Brain Topogr. 2013;26(3):363–377. doi: 10.1007/s10548-012-0267-5. 23208517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowen S.B., Lukas S.E. A low-cost, MR-compatible olfactometer. Behav. Res. Methods. 2006;38(2):307–313. doi: 10.3758/bf03192782. 16956107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas S.E., Lowen S.B., Lindsey K.P., Conn N., Tartarini W., Rodolico J., Mallya G., Palmer C., Penetar D.M. Extended-release naltrexone (XR-NTX) attenuates brain responses to alcohol cues in alcohol-dependent volunteers: a bold FMRI study. Neuroimage. 2013;78:176–185. doi: 10.1016/j.neuroimage.2013.03.055. 23571420 [DOI] [PubMed] [Google Scholar]
- Maas L.C., Lukas S.E., Kaufman M.J., Weiss R.D., Daniels S.L., Rogers V.W., Kukes T.J., Renshaw P.F. Functional magnetic resonance imaging of human brain activation during cue-induced cocaine craving. Am. J. Psychiatry. 1998;155(1):124–126. doi: 10.1176/ajp.155.1.124. 9433350 [DOI] [PubMed] [Google Scholar]
- Madsen K.H., Lund T.E. Filtering fMRI Data by Unsupervised Modelling of Physiological Noise Artifacts. The Organization for Human Brain Mapping; Minneapolis: 2006. [Google Scholar]
- McFarland K., Davidge S.B., Lapish C.C., Kalivas P.W. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J. Neurosci. 2004;24(7):1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. 14973230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J., See R.E. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacol. Berl. 2003;168(1–2):57–65. doi: 10.1007/s00213-002-1196-x. 12845418 [DOI] [PubMed] [Google Scholar]
- Nishijo H., Uwano T., Tamura R., Ono T. Gustatory and multimodal neuronal responses in the amygdala during licking and discrimination of sensory stimuli in awake rats. J. Neurophysiol. 1998;79(1):21–36. doi: 10.1152/jn.1998.79.1.21. 9425173 [DOI] [PubMed] [Google Scholar]
- Olsen C.M., Duvauchelle C.L. Prefrontal cortex D1 modulation of the reinforcing properties of cocaine. Brain Res. 2006;1075(1):229–235. doi: 10.1016/j.brainres.2006.01.003. 16460710 [DOI] [PubMed] [Google Scholar]
- Paxinos G., Watson C. Second Edition. Academic Press; San Diego: 1986. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
- Rapoport J.L., Buchsbaum M.S., Weingartner H., Zahn T.P., Ludlow C., Mikkelsen E.J. Dextroamphetamine. its cognitive and behavioral effects in normal and hyperactive boys and normal men. Arch. Gen. Psychiatry. 1980;37(8):933–943. doi: 10.1001/archpsyc.1980.01780210091010. 7406657 [DOI] [PubMed] [Google Scholar]
- Rebec G.V., Sun W. Neuronal substrates of relapse to cocaine-seeking behavior: role of prefrontal cortex. J. Exp. Anal. Behav. 2005;84(3):653–666. doi: 10.1901/jeab.2005.105-04. 16596984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reneman L., De Bruin K., Lavalaye J., Gunning W.B., Booij J. Addition of a 5-HT receptor agonist to methylphenidate potentiates the reduction of [123I]FP-CIT binding to dopamine transporters in rat frontal cortex and hippocampus. Synapse. 2001;39(3):193–200. doi: 10.1002/1098-2396(20010301)39:3<193::AID-SYN1000>3.0.CO;2-F. 11169768 [DOI] [PubMed] [Google Scholar]
- Rothmond D.A., Weickert C.S., Webster M.J. Developmental changes in human dopamine neurotransmission: cortical receptors and terminators. B.M.C. Neurosci. 2012;13:18. doi: 10.1186/1471-2202-13-18. 22336227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood N., Timeras P. A. Stereotaxic Atlas of the Developing Rat Brain. University of California Press; Los Angeles: 1970. [Google Scholar]
- Sicard K., Shen Q., Brevard M.E., Sullivan R., Ferris C.F., King J.A., Duong T.Q. Regional cerebral blood flow and BOLD responses in conscious and anesthetized rats under basal and hypercapnic conditions: implications for functional MRI studies. J. Cereb. Blood Flow Metab. 2003;23(4):472–481. doi: 10.1097/01.WCB.0000054755.93668.20. 12679724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag K.C., Brenhouse H.C., Freund N., Thompson B.S., Puhl M., Andersen S.L. Viral over-expression of D1 dopamine receptors in the prefrontal cortex increase high-risk behaviors in adults: comparison with adolescents. Psychopharmacol. Berl. 2014;231(8):1615–1626. doi: 10.1007/s00213-013-3399-8. 24408208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanis J.J., Andersen S.L. Reducing substance use during adolescence: a translational framework for prevention. Psychopharmacol. Berl. 2014;231(8):1437–1453. doi: 10.1007/s00213-013-3393-1. 24464527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman D.A., Moghaddam B. Striatum processes reward differently in adolescents versus adults. Proc. Natl. Acad. Sci. U. S. A. 2012;109(5):1719–1724. doi: 10.1073/pnas.1114137109. 22307637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L., Jiang T., Wang Y., Zang Y., He Y., Liang M., Sui M., Cao Q., Hu S., Peng M., Zhuo Y. Altered resting-state functional connectivity patterns of anterior cingulate cortex in adolescents with attention deficit hyperactivity disorder. Neurosci. Lett. 2006;400(1–2):39–43. doi: 10.1016/j.neulet.2006.02.022. 16510242 [DOI] [PubMed] [Google Scholar]
- van der Marel K., Klomp A., Meerhoff G.F., Schipper P., Lucassen P.J., Homberg J.R., Dijkhuizen R.M., Reneman L. Long-term oral methylphenidate treatment in adolescent and adult rats: differential effects on brain morphology and function. Neuropsychopharmacology. 2014;39(2):263–273. doi: 10.1038/npp.2013.169. 23851400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N.D., Wang G.J., Telang F., Fowler J.S., Logan J., Childress A.R., Jayne M., Ma Y., Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J. Neurosci. 2006;26(24):6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. 16775146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollstädt-Klein S., Kobiella A., Bühler M., Graf C., Fehr C., Mann K., Smolka M.N. Severity of dependence modulates smokers' neuronal cue reactivity and cigarette craving elicited by tobacco advertisement. Addict Biol. 2011;16(1):166–175. doi: 10.1111/j.1369-1600.2010.00207.x. 20331560 [DOI] [PubMed] [Google Scholar]
- Winston J.S., Gottfried J.A., Kilner J.M., Dolan R.J. Integrated neural representations of odor intensity and affective valence in human amygdala. J. Neurosci. 2005;25(39):8903–8907. doi: 10.1523/JNEUROSCI.1569-05.2005. 16192380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong W.C., Ford K.A., Pagels N.E., McCutcheon J.E., Marinelli M. Adolescents are more vulnerable to cocaine addiction: behavioral and electrophysiological evidence. J. Neurosci. 2013;33(11):4913–4922. doi: 10.1523/JNEUROSCI.1371-12.2013. 23486962 [DOI] [PMC free article] [PubMed] [Google Scholar]