Abstract
Previous studies have reported that peer groups are one of the most important predictors of adolescent and young adult marijuana use, and yet the neural correlates of social processing in marijuana users have not yet been studied. In the current study, marijuana-using young adults (n = 20) and non-using controls (n = 22) participated in a neuroimaging social exclusion task called Cyberball, a computerized ball-tossing game in which the participant is excluded from the game after a pre-determined number of ball tosses. Controls, but not marijuana users, demonstrated significant activation in the insula, a region associated with negative emotion, when being excluded from the game. Both groups demonstrated activation of the ventral anterior cingulate cortex (vACC), a region associated with affective monitoring, during peer exclusion. Only the marijuana group showed a correlation between vACC activation and scores on a self-report measure of peer conformity. This study indicates that marijuana users show atypical neural processing of social exclusion, which may be either caused by, or the result of, regular marijuana use.
Keywords: Social Influence, exclusion, peer groups, marijuana, cannabis, insula, anterior cingulate cortex, ACC
Marijuana use is steadily increasing among young adults (1). As evidence accumulates that adolescent and young adult marijuana use is associated with greater negative cognitive effects (2) and increased addiction rates (3) compared to adult use, it has become increasingly important to understand behavioral and neural processes underlying motivation for use among this population. Motivation for use among young adults (e.g. age 18–25) is particularly important to understand, as this age group has the highest rate of dependence on marijuana (as well as on alcohol)(4). Young adults are at a critical developmental stage in the initiation of marijuana use; while first use of alcohol and tobacco use occurs between 10–16 years, onset of illegal drugs occurs later(5). Furthermore, during young adulthood, particularly among college students, there is a pronounced shift in influence from parents to peers(6) as young adults are often away from home and therefore independent of parental oversight or control (7), and are seeking to establish a peer network for support (8).
One of the strongest determinants of both initiation and maintenance of use of marijuana is peer networks (9–11). Particularly for adolescents and young adults, peer groups are one of the most important predictors of marijuana initiation, progression to regular use, and failure to discontinue use (11). It is therefore possible that the desire to fit in with a peer group, and to avoid social exclusion, is one of the factors that contribute to marijuana use, Young adults tend to use marijuana in social settings among friends (12), and indeed, in a survey of 227 marijuana users (average age = 25.4), over half of participants cited ‘pleasant time with others’ as a primary reason for using (13). However, the relationship between response to social rejection and drug use is complex. Some studies have reported that youth who are isolated from their peers are more likely to use tobacco than those who belong to peer groups (14–16). A study using social network analysis found that even among adolescents who were socially isolated, those peripheral to substance-using peer groups had an increased likelihood of substance use (17).
Group cohesiveness and belonging to a group are fundamental evolutionary strategies for survival, security, reproductive success, and mental health (18). Social exclusion causes significant distress, sadness, and anger, and even sends neural signals of pain (19, 20). It is possible that people who use drugs are more sensitive to social exclusion than non-users, and that their desire to avoid social rejection underlies their desire to use drugs in social settings. It is also possible that social exclusion is a contributing factor that leads individuals to use drugs. Though the directionality is unknown, sensitivity to ostracism may be a fundamental component of drug use.
In the current study, we examined the neural mechanisms underlying social exclusion in young adult light-to-moderate marijuana users (e.g. weekly use or more) (MJ) and in non-using controls (CON). We chose to study lighter users because evidence suggests that peer influence may have more of an impact in recreational users than it would in substance-dependent patients, where drug-taking may become less social and more habitual (21). To elicit feelings of social exclusion, we used Cyberball, an interactive computerized ball-toss game (22) which has been used extensively in functional magnetic resonance imaging (fMRI) studies to examine brain responses to ostracism (e.g. (23–26). A quantitative meta-analysis of the Cyberball paradigm identified three main brain regions that were reliably recruited when a participant was rejected by peers: the anterior insula, the left ventral anterior cingulate cortex (vACC), and the left inferior orbitofrontal cortex (OFC) (27), all of which have been linked to the emotional response to exclusion. In this study, we investigated whether these regions activated differentially in MJ compared to CON. We hypothesized that MJ would show greater activation relative to CON in brain regions associated with peer rejection. As an exploratory analysis, we also investigated whether self-reported peer conformity scores were associated with activation in these regions.
Methods
Study procedures were approved by the Partners Human Research Committee. Participants completed a written, documented informed consent form prior to initiation of study procedures.
Participants
Participants in this study were 42 young adults, ages 18–25 years; 20 who regularly used marijuana (MJ), and 22 non-using controls (CON). Participants were primarily recruited from Boston-area colleges (though current enrollment in college was not a requirement for inclusion). All participants were given a Structured Clinical Interview for the DSM-IV (SCID) (First, 2002). Both groups were medically healthy with no history of head trauma, and did not meet DSM-IV criteria for any current or lifetime Axis I or Axis II psychiatric disorders, with the exception of cannabis use disorders in the MJ group. No participant reported a past history of a psychological disorder, and no participant was taking any medication other than oral contraceptives at the time of the study procedures.
MJ reported using marijuana recreationally at least once a week, and were asked to refrain from using substances on the day of the study. Those who presented with clinical signs of intoxication on the day of the study were rescheduled. CON had used marijuana on less than 5 lifetime occasions, and had not used marijuana in the past 3 months. Participants were excluded if they met abuse or dependence criteria for any drug other than marijuana, including alcohol and nicotine, although they may have tried other drugs in the past. No participants were regular cigarette smokers. Before study procedures were initiated, we performed a qualitative urine drug screen (Medimpex United Inc) that tested for illicit substances, in order to ensure that no MJ participant tested positive for any drug other than marijuana, and that no control participants tested positive for marijuana or any other drug.
Cyberball Task Design
As in previous Cyberball studies, participants were told prior to scanning that we were interested in ‘mental visualization’ ability, so that participants were not aware that we were studying social exclusion. They were told that they would play a game of catch over the Internet with two other players, and were asked to try to imagine the experience as vividly as possible. In reality, the actions of these ‘other players’ were actually preprogrammed to include or exclude the participant in different phases of the task. There were four successive blocks: (a) spectating block, in which participants were told that they would just be observing the other players; (b) inclusion, in which participants played with the other players; (c) exclusion, in which participants were then excluded from the game, and (d) re-inclusion, in which participants were re-included in the game for the remainder of the experiment. In the inclusion conditions, the other players threw the ball to the participant approximately 75% of the time to involve the participant enough to distinguish them from the spectating and exclusion phases. Each block lasted approximately 1.5 to 2 minutes (about 30 throws). Exact block length varied slightly with each block due to a number of factors designed to increase ecological validity (e.g. the participant could choose when to throw the ball). The game was self-paced. After the study, participants were debriefed to ensure they knew that they were not playing with real people.
Acquisition and Analysis of Neuroimaging Data
Participants were scanned using a 3 T Siemens (Erlangen, Germany) Skya scanner with a 32 channel head coil at the Martinos Center for Biomedical Imaging. Whole-brain T1-weighted 1 mm isotropic structural scans were collected using a 3D multiecho MPRAGE sequence (176 sagittal slices, 256 mm FoV, TR 2530 ms, TI 1200 ms, 2x GRAPPA acceleration, TE 1.64/3.5/5.36/7.22 ms, BW 651 Hz/px, Tacq 6:03 mins) (28). Functional scans were collected using a 2D gradient echo EPI sequence (31 slices, 3 mm thick, 0.6 mm gap, 216 mm FoV, 3 mm2 in-plane resolution, TR 2 s, TE 30 ms, BW 2240 Hz/px). All acquisitions were automatically positioned using AutoAlign (29).
FMRI data processing was carried out using FEAT (FMRI Expert Analysis Tool) Version 5.98, part of the FSL fMRI processing stream (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl). Each subject’s functional and structural scans were registered using FSL’s linear registration tool (FLIRT), and then these scans were registered to high resolution structural and standard space images using both FLIRT and FSL’s nonlinear registration tool (FNIRT), (30, 31) so that each subject’s brain was registered to the ICBM152 T1 template (32). In addition, the following pre-processing was applied; non-brain removal using FSL’s brain extraction tool (33); spatial smoothing using a Gaussian kernel of FWHM 5 mm; grand-mean intensity normalization of the entire 4D dataset; highpass temporal filtering (Gaussian-weighted least-squares straight line fitting, with sigma=50.0 s). Each subject’s time series data was fit using a linear signal model with the three experimental blocks; (1) Inclusion Block, (2) Exclusion Block, and (3) Re-inclusion Block, relative to the control block (spectating), as well as 6 movement regressors of no interest. Time-series statistical analysis was carried out using FILM with local autocorrelation correction (34). Z (Gaussianised T/F) statistic images were thresholded using clusters determined by Z>2.3 and a (corrected) cluster significance threshold of p<0.05 (35).
Next, higher-level group analysis was carried out using FLAME (FMRIB’s Local Analysis of Mixed Effects) stage 1 (36–38). As with the individual subject analysis, Z (Gaussianised T/F) statistic images were thresholded using clusters determined by Z>2.3 and a (corrected) cluster significance threshold of p<0.05 (35). The analysis consisted of one primary contrast of interest focused on isolating the neural response to ostracism (Exclusion Block vs Inclusion Block), as well as two secondary contrasts of interest focused on the response to social inclusion (Inclusion Block vs Exclusion Block, and the response to inclusion after being ostracized (Reinclusion Block vs Inclusion Block).
In parallel with whole-brain, voxel-based analyses, region of interest (ROI) analyses were used to explore two regions that have been implicated in the processing of social exclusion, right insula and vACC (23–27). We did not extract data from the OFC because neither group showed significant activation in this region. Individual parameter estimates were extracted using the FSL program featquery (http://fsl.fmrib.ox.ac.uk/fsl/fsl4.0/feat5/featquery.html) from anatomical ROIs using masks parcellated from the ICBM152 T1 brain at the MGH Center for Morphometric Analysis (CMA) (39, 40). Values were regressed against peer influence scores, in order to investigate whether brain activation was associated with trait levels of peer conformity. In addition to hypothesized ROIs, peer influence scores were also entered into whole-brain analysis in an exploratory analysis to investigate whether other brain regions were associated with individual differences in peer influence scores. For whole-brain analyses, Z (Gaussianised T/F) statistic images were thresholded using clusters determined by Z>2.3 and a (corrected) cluster significance threshold of p<0.05 (35).
Questionnaires
MJ completed a 90-day time-line follow-back (41) detailing their marijuana use. All participants completed a time-line follow-back for alcohol use (41), as well as the Ten-Item Personality Inventory (TIPI) (42) to assess personality characteristics. Participants also completed the Multidimensional Iowa Suggestibility Scale (MISS) (43), which measured peer conformity as well as other indices of suggestibility including consumer suggestibility (e.g., suggestibility to commercials, products), persuadability (e.g., changing one’s mind based on other peoples’ arguments), physiological suggestibility (e.g., feeling cold when someone else is shivering), physiological reactivity (e.g., feeling jumpy after watching a scary movie). Scores across subscales were summed with higher total values indicating greater suggestibility. Finally, participants also completed a questionnaire administered immediately after the Cyberball task to assess exclusion-related distress (23), using a scale of 10 (no distress) to 50 (extreme distress).
Results
CON and MJ did not differ in age, gender, personality, or alcohol drinking patterns (all p’s > 0.10; Table 1). Six out of 20 MJ reported use of other illicit drugs; none reported using any substance more than 2 times lifetime. The post-game questionnaire indicated that both groups felt mild distress following the experiences of social exclusion (CON = 17.4, MJ = 17.5) with no difference between groups (p = 0.95).
Table 1.
Participant Demographics
| CON (n=22) | MJ (n=20) | |
|---|---|---|
| Gender | 11M/11F | 9M/11F |
| Age | ||
| Yrs | 21.5 (1.9) | 20.6 (2.5) |
| Ethnicity (n) | ||
| Asian | 3 | 4 |
| African American | 3 | 2 |
| White | 14 | 11 |
| Biracial | 1 | 0 |
| Not reported | 1 | 1 |
| TIPI | ||
| Extroversion | 7.5 (2.8) | 9.2 (3.2) |
| Agreeableness | 10.1 (1.9) | 10.2 (2.1) |
| Conscientiousness | 11.5 (2.0) | 10.2 (2.7) |
| Emotional Stability | 11.5 (1.8) | 10.9 (2.4) |
| Openness | 11.1 (2.5) | 11.9 (1.8) |
| Substance Use | ||
| Alcohol Marijuana | ||
| # Drinks/Week | 2.2 (2.5) | 3.0 (2.1) |
| # MJ Use Days/week | 0 | 2.8 (1.5) |
| # MJ Joints per week | 0 | 5.4 (4.5) |
| Age of Onset (years) | N/A | 16.3 (1.7) |
| Duration of Use (years) | N/A | 4.3 (1.7) |
| Other illicit drugs* | ||
| Number reporting any use | 0 | 6 |
| Suggestibility | ||
| Consumer Suggestibility | 18.0 (4.8) | 20.4 (6.2) |
| Persuadability | 41.5 (6.6) | 43.4 (8.4) |
| Physiological Suggestibility | 18.5 (3.3) | 18.9 (4.6) |
| Physiological Reactivity | 41.2 (6.5) | 43.9 (6.3) |
| Peer Conformity | 20.0 (6.6) | 24.4 (6.8)a |
| Mental Control | 38.2 (7.8) | 39.0 (10.0) |
| Unpersuadability | 53.3 (6.6) | 53.5 (8.7) |
| Total Suggestibility | 139.2 (15.8) | 150.9 (22.4)b* |
All values are expressed in means and standard deviations. There were no significant differences between groups on any measure other than peer conformity.
Other illicit drugs include cocaine (n=1), mushrooms (n=5), Lysergic acid diethylamide (LSD) (n=3), N-Dimethyltryptamine (DMT) (n=1), and 3,4-methylenedioxy-methamphetamine (MDMA) (n=2). None were used more than 2 times lifetime.
p = 0.044,
p = 0.057. Suggestibility differences did not meet significance when corrected for multiple comparisons.
Neuroimaging results indicated that during exclusion, compared to fair play (inclusion), CON activated the right anterior insula and vACC, regions reported as regularly activated by this task in a meta-analysis (27), as well as the lingual gyrus. In the MJ group, in contrast, activation was significant only in the vACC in response to social exclusion. A direct comparison between groups indicated that CON had greater activation than MJ in two clusters in the right anterior insula, one of which also encompassed the OFC, but the groups did not differ in vACC activation (Fig 1A). A region-of-interest analysis confirmed the voxelwise results, showing a significant difference between MJ and CON in the right insula, but not in the vACC (Fig 1B).
Fig. 1.
A. Activation to Social Exclusion vs Fair Play (Inclusion) in Each Group. Top images show right anterior insula activation in CON only; group contrasts showed significantly greater activation in CON than in MJ. Bottom images show bilateral vACC activation in both groups, with no significant group differences. Images are thresholded with a cluster significance threshold of p<0.05 after whole-brain correction.
B. Region-of-Interest values. There was a significant difference between groups in insula activation (top), but not in vACC activation (bottom). Longer bars represent the mean of each group; shorter bars represent the 95% confidence interval.
During the inclusion block (compared to exclusion), CON activated the left dorsolateral prefrontal cortex (DLPFC), as well as regions in the parietal and occipital lobes. MJ users demonstrated significantly greater activation to inclusion compared to exclusion in the right frontal pole, right insula, and right thalamus, as well as in parietal and occipital regions. It should be noted that some of these activations may have been the result of higher engagement in the inclusion compared to the exclusion blocks (i.e. the participant was actively pressing buttons to toss the ball, and making decisions about which player to toss the ball to), and therefore, not all activations can be definitively interpreted as responses to social inclusion. No between-group differences were detected in this contrast (Table S1).
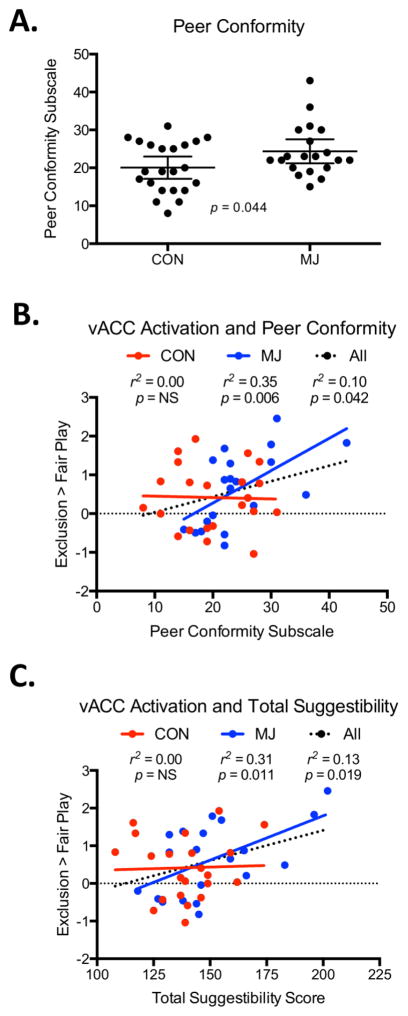
Neither group showed significant differences in activation to reinclusion after ostracism compared to inclusion before ostracism. MJ scored greater on peer conformity on the MISS than CON (t (1,40) = 2.08, p = 0.044; Fig 2A), and showed a trend toward higher ratings on overall suggestibility, (t (1,40) = 1.96, p = 0.057), though it should be noted that these differences were not significant when corrected for multiple comparisons, ans should be followed up with larger samples for confirmation. Across groups, peer conformity scores were positively associated with vACC activation (r2 = 0.10, p = 0.042; Fig 2B). The correlation across groups was driven by the MJ group (r2 = 0.35, p = 0.006); no correlation was detected in CON for peer conformity self-ratings and vACC activation to social exclusion. We found a similar effect with total suggestibility scores, where a significant correlation was found across groups between scores and vACC activation (r2 = 0.13, p = 0.019) (Fig 2C). This correlation was also driven by the MJ group (r2 = 0.31, p = 0.011), with no correlation found in controls. Neither group showed correlations between self-reported suggestibility scores and right insula activation. Other brain regions that were associated with peer influence scores (with whole-brain correction) included the left insula, and two regions in the left temporal cortex; see Table S2.
Fig. 2.
A. MJ reported higher levels in peer conformity than CON (p = 0.044). Longer bars represent the mean of each group; shorter bars represent the 95% confidence interval. B. Association between vACC activation and self-reported peer conformity. Across groups, peer conformity scores were positively associated with vACC activation. The correlation was driven by the MJ group; no correlation was detected in CON. C. Association between vACC activation and self-reported total suggestibility. A significant association was found across groups between total suggestibility scores and vACC activation. This correlation was also driven by the MJ group, with no correlation found in controls.
Discussion
This study is the first to examine neural signals of social exclusion in young adult marijuana users. This study has several novel findings. First, though both groups reported similar levels of distress following social exclusion, the MJ group did not demonstrate significant activation in the right anterior insula during peer rejection, though they showed normative responses in the vACC. Second, though the groups rated similar levels of task-related distress, MJ self-reported higher levels of peer conformity than CON. Finally, vACC activation correlated with levels of self-reported peer conformity and total suggestibility across groups, and these associations were driven by the MJ group.
Though we hypothesized that MJ would show greater activation than CON in regions associated with exclusion such as the insula, we instead found that the MJ group did not show significant activation in the anterior insula. The insula is involved in many brain functions such as subjective awareness (44) and cognitive control (44–47), and has been linked to subjective experiences of physical pain (27, 44, 48) and processing of negative emotions. (49–51). Insula activation to social exclusion was the most highly replicated finding in a meta-analysis of Cyberball paradigms (27). Though the finding that MJ showed less insula activation to social exclusion than CON is counterintuitive, it may reflect impaired processing of social information. A study of adolescents using Cyberball showed that more socially competent adolescents showed heightened activity in the insula, suggesting that heightened interpersonal skills may be associated with increased neural sensitivity to peer rejection (24).
Reduced neural sensitivity in the insula to peer rejection could also indicate that MJ users are less conscious of social norms. Behavioral studies provide additional evidence that individuals with high interpersonal competence are more conscious of peer norms, more advanced cognitively, more sensitive to others’ emotions (52), and more sensitive to relational problems with peers (53). Insula activation may underlie an ability to reflect on social situations, and this ability may produce greater sensitivity to relational problems with peers (53). Furthermore, marijuana use has been associated with anhedonia, (e.g. (54)), indicating that emotional response may be dulled in the MJ group.
Though MJ showed an absence of activation in the insula, they showed normative processing in the vACC. Like the insula, the vACC has been implicated in studies of emotion, especially sadness (55–58). However, unlike the insula, the vACC, including the rostral ACC (rACC) and subgenual or subcallosal ACC (sACC), has been more specifically associated with affective conflict and detection of emotional distractors during cognitive tasks (56, 59). Activity in the vACC during social exclusion likely reflects not only negative emotion induced by social exclusion, but also affective monitoring, which may be intact in the MJ group. A recent study reported increased connectivity between vACC and midline default mode network (DMN) regions during social exclusion (23), suggesting an engagement of reflective processing during exclusion blocks, when the participant is no longer engaged in playing the game and perhaps ruminating on the situation. DMN functional connectivity to vACC is stronger in people with depression compared to controls, suggesting a relationship between vACC-DMN connectivity, negative emotion, and rumination (23, 60).
Only the MJ group, but not CON, demonstrated a relationship between vACC activity and self-rated peer conformity on the MISS. In MJ, greater vACC activation was associated with greater peer conformity as well as overall suggestibility. Another study using the Cyberball paradigm showed a negative correlation between activation of the medial prefrontal cortex (part of the vACC) to social exclusion and resistance to peer influence (e.g. more activation, more susceptible to peer influence) in adolescents but not in adults (26). The authors posit that because the ability to resist peer influence develops in a linear fashion in adolescence and plateaus thereafter (61), adolescents, more so than adults, may rely on others’ appraisals of them (62). Though speculative, in the current study, the MJ group may be exhibiting a more “immature” pattern of brain development compared to CON, though it should be noted that studies of adolescents with Cyberball tend to use younger populations than the one used in the current study.
It is important to note that the marijuana users in this study were, in general, non-daily users, averaging about 2–4 times per week. Less than half had a diagnosis of cannabis abuse (8 of 20, or 40%), and a minority had cannabis dependence (2 of 20, or 20%). A meta-analysis of 13 neuroimaging studies demonstrated that among regular cannabis users, those with heavier patterns of use (dose, quantity, frequency, and duration of use) were more subjected to the adverse impacts of cannabis on brain structure (Lorenzetti). It is likely, therefore, that effects seen in this sample of MJ users may be subtler than effects we may observe in heavier users. Furthermore, though we assessed current patterns of MJ use through a detailed 90-day timeline follow-back method, participants may have used more heavily at other times in their lives, and previous heavy use may have had residual effects on brain function. Future studies could compare heavy (e.g. daily) to non-heavy (e.g. weekly) users, as well as current heavy users to past heavy users, in order to investigate whether neural response to social exclusion varies as a function of marijuana exposure over time.
There are several limitations to this study. First, because of the need for ecological validity, the study design only included one block of exclusion. Most neuroimaging studies utilize block designs that alternate between conditions; however, Cyberball studies cannot cycle though blocks of exclusion and inclusion because this would compromise the believability of the task. Furthermore, previous Cyberball studies have shown that one exclusion block is enough to generate a strong brain response to social exclusion (e.g. (63)), and a meta-analysis of 120 Cyberball studies demonstrated that the average ostracism effect is large, with d > |1.4|, and generalizes across structural aspects (number of players, ostracism duration, number of tosses, presentation of block order, etc.)(64). Another limitation of the Cyberball task is that self-reported emotional responses and/or reflections on the experience are measured after the completion of the task rather than in real time; future studies that incorporate collection of responses after each block of scanning could further delineate how emotional responses correlate with brain activity. Second, many participants reported that they suspected that the other players were not real people. However, most participants reported at least some distress in the post-scan questionnaire. Third, although none of the MJ users were acutely high on the day of the study, metabolites of cannabis remain in the body for days to weeks after last use of marijuana; it is therefore possible that there were subtle effects of THC on blood oxygenation measures in the fMRI scan. Finally, as with any cross-sectional study, we cannot determine whether group differences in neural processing of social exclusion were caused by, a result of, or developed along with marijuana use. Future studies using a longitudinal design may be able to disentangle the developmental trajectory of altered social processing and marijuana use.
In conclusion, this study replicated previous findings of insula, vACC, and prefrontal activation in response to social exclusion in CON, but found that interestingly, vACC activation was normal but insula activation was absent in the MJ group. This dissociation between vACC and insula suggests that while the MJ group was cognitively aware of being excluded (a function of the vACC), they were not emotionally responsive to the exclusion (a function of the insula). vACC activation in the MJ group was also proportional to social conformity scores, indicating that those who were more likely to report social conformity experienced greater monitoring of affective states during the task. In conclusion, this study suggests a neural correlate of social exclusion that functions differently in individuals with regular marijuana use. This differential activation in response to social exclusion may be cause or a result of marijuana use.
Supplementary Material
Table 2.
Activation to Exclusion > Fair Play
| Area | HEM | Region | x | y | z | z-stat | Voxels |
|---|---|---|---|---|---|---|---|
| Activation in CONTROL GROUP | |||||||
| Frontal | L/R | vACC | 0 | 18 | −4 | 4.40 | 273 |
| Occipital | R | Lingual gyrus | 12 | −54 | 4 | 3.85 | 179 |
| L | Lingual gyrus | −8 | −80 | −4 | 3.87 | 683 | |
| Subcortical | R | Insula | 40 | 8 | −14 | 3.75 | 27 |
| Activation in MJ GROUP | |||||||
| Frontal | L/R | vACC | −2 | 20 | −8 | 4.40 | 87 |
| Activation in CONTROL GROUP > MJ GROUP | |||||||
| Subcortical | R | Insula | 40 | 4 | −6 | 3.47 | 57 |
| R | OFC/insula | 42 | 18 | −14 | 3.07 | 32 | |
Whole-brain corrected significant clusters were thresholded using Z>2.3 and a (corrected) cluster significance threshold of p<0.05. vACC = ventral anterior cingulate cortex. OFC = orbitofrontal cortex. HEM represents hemisphere. Coordinates are in MNI space. VOL = volume, in number of voxels (2 × 2 × 2 mm3).
Acknowledgments
This work was supported by NIDA K01 DA034093 (JMG) and NIDA K24 DA030443 (AEE).
Footnotes
Financial Disclosures: A. Eden Evins received grant support from Pfizer and Forum Pharmaceuticals in the past year and consulting fees from Reckitt Benckiser Pharmaceuticals. J. Gilman reports having received lecture fees from AAAS. All other authors report no biomedical financial interests or potential conflicts of interest.
These funding sources had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the manuscript for publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Henry JA, Oldfield WL, Kon OM. Comparing cannabis with tobacco. Bmj. 2003;326:942–943. doi: 10.1136/bmj.326.7396.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jager G, Ramsey NF. Long-term consequences of adolescent cannabis exposure on the development of cognition, brain structure and function: an overview of animal and human research. Curr Drug Abuse Rev. 2008;1:114–123. doi: 10.2174/1874473710801020114. [DOI] [PubMed] [Google Scholar]
- 3.Hurd YL, Michaelides M, Miller ML, Jutras-Aswad D. Trajectory of adolescent cannabis use on addiction vulnerability. Neuropharmacology. 2014;76(Pt B):416–424. doi: 10.1016/j.neuropharm.2013.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.SAMHSA releases Behavioral Health, United States, 2012. Psychiatr Serv. 2013;64:1281. doi: 10.1176/appi.ps.6412news1. [DOI] [PubMed] [Google Scholar]
- 5.Wittchen HUBS, Höfler M, Perkonigg A, Lieb R, Bühringer G, Beesdo K. What are the high risk periods for incident substance use and transitions to abuse and dependence? Implications for early intervention and prevention. Int J Methods Psychiatr Res. 2008 Jun;17(Suppl 1):S16–29. doi: 10.1002/mpr.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larson R, Csikszentmihalyi M, Freeman M. Alcohol and marijuana use in adolescents’ daily lives: a random sample of experiences. Int J Addict. 1984;19:367–381. doi: 10.3109/10826088409057188. [DOI] [PubMed] [Google Scholar]
- 7.Brown BB, Dolcini MM, Leventhal A. Transformations in peer relationships at adolescence: implications for health-related behavior. New York: Cambridge University Press; 1997. [Google Scholar]
- 8.Paul EL, Kelleher M. Precollege concerns about losing and making friends in college. Journal of College Student Development. 1995;36:513–521. [Google Scholar]
- 9.Bahr SJ, Hoffmann JP, Yang X. Parental and peer influences on the risk of adolescent drug use. The journal of primary prevention. 2005;26:529–551. doi: 10.1007/s10935-005-0014-8. [DOI] [PubMed] [Google Scholar]
- 10.Galea S, Nandi A, Vlahov D. The social epidemiology of substance use. Epidemiologic reviews. 2004;26:36–52. doi: 10.1093/epirev/mxh007. [DOI] [PubMed] [Google Scholar]
- 11.van den Bree MB, Pickworth WB. Risk factors predicting changes in marijuana involvement in teenagers. Archives of general psychiatry. 2005;62:311–319. doi: 10.1001/archpsyc.62.3.311. [DOI] [PubMed] [Google Scholar]
- 12.Terry-McElrath YM, O’Malley PM, Johnston LD. Reasons for Drug Use among American Youth by Consumption Level, Gender, and Race/Ethnicity: 1976–2005. J Drug Issues. 2009;39:677–714. doi: 10.1177/002204260903900310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartwell KJ, Back SE, McRae-Clark AL, Shaftman SR, Brady KT. Motives for using: a comparison of prescription opioid, marijuana and cocaine dependent individuals. Addictive behaviors. 2012;37:373–378. doi: 10.1016/j.addbeh.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bailey SL, Ennett ST, Ringwalt CL. Potential mediators, moderators, or independent effects in the relationship between parents’ former and current cigarette use and their children’s cigarette use. Addict Behav. 1993;18:601–621. doi: 10.1016/0306-4603(93)90015-2. [DOI] [PubMed] [Google Scholar]
- 15.Ennett ST, Bauman KE. Peer group structure and adolescent cigarette smoking: a social network analysis. Journal of health and social behavior. 1993;34:226–236. [PubMed] [Google Scholar]
- 16.Fang X, Li X, Stanton B, Dong Q. Social network positions and smoking experimentation among Chinese adolescents. American journal of health behavior. 2003;27:257–267. doi: 10.5993/ajhb.27.3.7. [DOI] [PubMed] [Google Scholar]
- 17.Pearson M, Michell L. Smoke Rings: social network analysis of friendship groups, smoking and drug-taking. Drugs: Education, Prevention and Policy. 2000;7:21–37. [Google Scholar]
- 18.Baumeister RF, Leary MR. The need to belong: desire for interpersonal attachments as a fundamental human motivation. Psychological bulletin. 1995;117:497–529. [PubMed] [Google Scholar]
- 19.Bernstein MJ, Claypool HM. Social exclusion and pain sensitivity: why exclusion sometimes hurts and sometimes numbs. Pers Soc Psychol Bull. 2012;38:185–196. doi: 10.1177/0146167211422449. [DOI] [PubMed] [Google Scholar]
- 20.Macdonald G, Leary MR. Why does social exclusion hurt? The relationship between social and physical pain. Psychological bulletin. 2005;131:202–223. doi: 10.1037/0033-2909.131.2.202. [DOI] [PubMed] [Google Scholar]
- 21.Wise RA. Neurobiology of addiction. Curr Opin Neurobiol. 1996;6:243–251. doi: 10.1016/s0959-4388(96)80079-1. [DOI] [PubMed] [Google Scholar]
- 22.Williams KD, Cheung CK, Choi W. Cyberostracism: effects of being ignored over the Internet. Journal of personality and social psychology. 2000;79:748–762. doi: 10.1037//0022-3514.79.5.748. [DOI] [PubMed] [Google Scholar]
- 23.Bolling DZ, Pitskel NB, Deen B, Crowley MJ, McPartland JC, Mayes LC, et al. Dissociable brain mechanisms for processing social exclusion and rule violation. Neuro Image. 2011;54:2462–2471. doi: 10.1016/j.neuroimage.2010.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masten CL, Eisenberger NI, Borofsky LA, Pfeifer JH, McNealy K, Mazziotta JC, et al. Neural correlates of social exclusion during adolescence: understanding the distress of peer rejection. Social cognitive and affective neuroscience. 2009;4:143–157. doi: 10.1093/scan/nsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Onoda K, Okamoto Y, Nakashima K, Nittono H, Ura M, Yamawaki S. Decreased ventral anterior cingulate cortex activity is associated with reduced social pain during emotional support. Social neuroscience. 2009;4:443–454. doi: 10.1080/17470910902955884. [DOI] [PubMed] [Google Scholar]
- 26.Sebastian CL, Tan GC, Roiser JP, Viding E, Dumontheil I, Blakemore SJ. Developmental influences on the neural bases of responses to social rejection: implications of social neuroscience for education. Neuro Image. 2011;57:686–694. doi: 10.1016/j.neuroimage.2010.09.063. [DOI] [PubMed] [Google Scholar]
- 27.Cacioppo S, Frum C, Asp E, Weiss RM, Lewis JW, Cacioppo JT. A quantitative meta-analysis of functional imaging studies of social rejection. Sci Rep. 2013;3:2027. doi: 10.1038/srep02027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Kouwe AJ, Benner T, Salat DH, Fischl B. Brain morphometry with multiecho MPRAGE. Neuroimage. 2008;40:559–569. doi: 10.1016/j.neuroimage.2007.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Kouwe AJ, Benner T, Fischl B, Schmitt F, Salat DH, Harder M, et al. On-line automatic slice positioning for brain MR imaging. Neuroimage. 2005;27:222–230. doi: 10.1016/j.neuroimage.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 30.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- 31.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 32.Chau W, McIntosh AR. The Talairach coordinate of a point in the MNI space: how to interpret it. Neuroimage. 2005;25:408–416. doi: 10.1016/j.neuroimage.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 33.Smith SM. Fast robust automated brain extraction. Human brain mapping. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- 35.Worsley KJ. Statistical analysis of activation images. OUP; 2001. [Google Scholar]
- 36.Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. Neuroimage. 2003;20:1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- 37.Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage. 2004;21:1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 38.Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, et al. Bayesian analysis of neuroimaging data in FSL. Neuroimage. 2009;45:S173–186. doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]
- 39.Gasic GP, Smoller JW, Perlis RH, Sun M, Lee S, Kim BW, et al. BDNF, relative preference, and reward circuitry responses to emotional communication. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:762–781. doi: 10.1002/ajmg.b.30944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perlis RH, Holt DJ, Smoller JW, Blood AJ, Lee S, Kim BW, et al. Association of a polymorphism near CREB1 with differential aversion processing in the insula of healthy participants. Arch Gen Psychiatry. 2008;65:882–892. doi: 10.1001/archgenpsychiatry.2008.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sobell MB, Sobell LC, Klajner F, Pavan D, Basian E. The reliability of a timeline method for assessing normal drinker college students’ recent drinking history: utility for alcohol research. Addict Behav. 1986;11:149–161. doi: 10.1016/0306-4603(86)90040-7. [DOI] [PubMed] [Google Scholar]
- 42.Gosling SD, Rentfrow PJ, Swann WB., Jr A Very Brief Measure of the Big Five Personality Domains. Journal of Research in Personality. 2003;37:504–528. [Google Scholar]
- 43.Kotov R, Bellman SB, Watson DB. Multidimensional Iowa Suggestibility Scale (MISS) Brief Manual. [Accessed 2014 Jun 2010];Stoneybrook Medicine website. 2004 Available: http://medicine.stonybrookmedicine.edu/system/files/MISSBriefManual.pdf.
- 44.Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 45.Critchley HD, Mathias CJ, Dolan RJ. Neural activity in the human brain relating to uncertainty and arousal during anticipation. Neuron. 2001;29:537–545. doi: 10.1016/s0896-6273(01)00225-2. [DOI] [PubMed] [Google Scholar]
- 46.Grinband J, Hirsch J, Ferrera VP. A neural representation of categorization uncertainty in the human brain. Neuron. 2006;49:757–763. doi: 10.1016/j.neuron.2006.01.032. [DOI] [PubMed] [Google Scholar]
- 47.Thielscher A, Pessoa L. Neural correlates of perceptual choice and decision making during fear-disgust discrimination. J Neurosci. 2007;27:2908–2917. doi: 10.1523/JNEUROSCI.3024-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Casey KL, Minoshima S, Morrow TJ, Koeppe RA. Comparison of human cerebral activation pattern during cutaneous warmth, heat pain, and deep cold pain. J Neurophysiol. 1996;76:571–581. doi: 10.1152/jn.1996.76.1.571. [DOI] [PubMed] [Google Scholar]
- 49.Critchley HD, Rotshtein P, Nagai Y, O’Doherty J, Mathias CJ, Dolan RJ. Activity in the human brain predicting differential heart rate responses to emotional facial expressions. Neuro Image. 2005;24:751–762. doi: 10.1016/j.neuroimage.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 50.Hennenlotter A, Schroeder U, Erhard P, Castrop F, Haslinger B, Stoecker D, et al. A common neural basis for receptive and expressive communication of pleasant facial affect. Neuro Image. 2005;26:581–591. doi: 10.1016/j.neuroimage.2005.01.057. [DOI] [PubMed] [Google Scholar]
- 51.Jabbi M, Swart M, Keysers C. Empathy for positive and negative emotions in the gustatory cortex. Neuro Image. 2007;34:1744–1753. doi: 10.1016/j.neuroimage.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 52.Dekovic M, Gerris JRM. Developmental analysis of social cognitive and behavioral differences between popular and rejected children. Journal of Applied Developmental Psychology. 1994;15:367–386. [Google Scholar]
- 53.Hoglund WLG, Lalonde CE, Leadbeater BJ. Social-cognitive competence, peer rejection and neglect, and behavioral and emotional problems in middle childhood. Social Development. 2008;17:528–553. [Google Scholar]
- 54.Bovasso GB. Cannabis abuse as a risk factor for depressive symptoms. Am J Psychiatry. 2001;158:2033–2037. doi: 10.1176/appi.ajp.158.12.2033. [DOI] [PubMed] [Google Scholar]
- 55.Allman JM, Hakeem A, Erwin JM, Nimchinsky E, Hof P. The anterior cingulate cortex. The evolution of an interface between emotion and cognition. Ann N Y Acad Sci. 2001;935:107–117. [PubMed] [Google Scholar]
- 56.Ichikawa N, Siegle GJ, Jones NP, Kamishima K, Thompson WK, Gross JJ, et al. Feeling bad about screwing up: emotion regulation and action monitoring in the anterior cingulate cortex. Cogn Affect Behav Neurosci. 2011;11:354–371. doi: 10.3758/s13415-011-0028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kanske P, Kotz SA. Emotion speeds up conflict resolution: a new role for the ventral anterior cingulate cortex? Cereb Cortex. 2011;21:911–919. doi: 10.1093/cercor/bhq157. [DOI] [PubMed] [Google Scholar]
- 58.Lane RD, Reiman EM, Axelrod B, Yun LS, Holmes A, Schwartz GE. Neural correlates of levels of emotional awareness. Evidence of an interaction between emotion and attention in the anterior cingulate cortex. J Cogn Neurosci. 1998;10:525–535. doi: 10.1162/089892998562924. [DOI] [PubMed] [Google Scholar]
- 59.Mohanty A, Engels AS, Herrington JD, Heller W, Ho MH, Banich MT, et al. Differential engagement of anterior cingulate cortex subdivisions for cognitive and emotional function. Psychophysiology. 2007;44:343–351. doi: 10.1111/j.1469-8986.2007.00515.x. [DOI] [PubMed] [Google Scholar]
- 60.Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Steinberg L, Monahan KC. Age differences in resistance to peer influence. Dev Psychol. 2007;43:1531–1543. doi: 10.1037/0012-1649.43.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pfeifer JH, Masten CL, Borofsky LA, Dapretto M, Fuligni AJ, Lieberman MD. Neural correlates of direct and reflected self-appraisals in adolescents and adults: when social perspective-taking informs self-perception. Child Dev. 2009;80:1016–1038. doi: 10.1111/j.1467-8624.2009.01314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maurage P, Joassin F, Philippot P, Heeren A, Vermeulen N, Mahau P, et al. Disrupted regulation of social exclusion in alcohol-dependence: an fMRI study. Neuropsychopharmacology. 2012;37:2067–2075. doi: 10.1038/npp.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hartgerink CH, van Beest I, Wicherts JM, Williams KD. The ordinal effects of ostracism: a meta-analysis of 120 Cyberball studies. PLoS One. 2015;10:e0127002. doi: 10.1371/journal.pone.0127002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.