Abstract
Differential scanning fluorimetry (DSF) is used to assess protein stability, transition states, or the Kd’s of various ligands, drug molecules and antibodies. All fluorescent probes published to date are either incompatible with hydrophobic proteins/ligands, which precludes analyses of transmembrane or membrane associated proteins, or have excitation and detection wavelengths outside the range of RT-PCR machines, necessitating the use of dedicated devices. Herein, we describe a thiol-reactive probe BODIPY FL L-cystine (BFC) to overcome both of these shortcomings. The probe supports an inexpensive application of DSF measurements suitable for detection with standard RT-PCR machines in a hydrophilic or hydrophobic environment.
Keywords: Differential scanning fluorimetry, standard RT-PCR device, thiol-reactive fluorescent probe, BODIPY FL L-cystine, membrane proteins, high-throughput assay
Introduction
Differential scanning fluorimetry (DSF) has versatile applications in protein biochemistry, (membrane-) protein crystallography, electron microscopy, as well as ligand, drug and antibody screening (1–12). Thermal denaturation of a protein can be monitored with different fluorescent probes that interact with exposed hydrophobic moieties or Cys residues. The change of fluorescence upon interaction is monitored by a UV/ VIS- detector and is plotted as a function of temperature (4). The response curves then can be used to determine the melting temperature of the protein calculated as the first derivative of the sigmoidal curve, or by fitting the curve to the Boltzmann equation (4, 5, 13, 14).
Herein, we introduce the boron-dipyrromethene (BODIPY) based probe BFC which enabled DSF measurements of various proteins in a hydrophobic environment and detection of the fluorescent signal (λex = 504 and λem = 511 nm) with a standard RT-PCR machine. The functionality of the BFC probe as a reporter dye was assessed with chicken lysozyme C (UNIPROT ID: P00698) and bovine rhodopsin (UNIPROT ID: P02699), as a representative membrane protein.
Materials and Methods
Preparation and storage of the BFC probe
One mg of BFC was purchased from Invitrogen (B-20340). The probe was diluted to a final concentration of 10 mM in DMSO and stored in 1 μl aliquots in the dark at −20 °C. The final working concentration of 2 μM BFC was used throughout the DSF experiments by diluting the DMSO stock with deionized water before adding it to a sample.
Preparation and storage of the Sypro-orange probe
The Sypro-orange 5000x concentrate was purchased from Invitrogen (S-6650), and stored in the dark at 4 °C. A final working concentration of 5x Sypro-orange was used throughout the DSF experiments by adding the DMSO stock solution to the sample (6). If the required volume was less than 1 μl, 5000x Sypro-orange was diluted in deionized water before adding it to a sample.
Preparation of the lysozyme samples
Lysozyme C (UNIPROT ID: P00698) was purchased from Sigma Aldrich (L6876). Lyophilized lysozyme powder was solubilized in PBS buffer (NaCl, 137 mM; KCl 2.7 mM; Na2HPO4, 10 mM; and KH2PO4, 1.8 mM) pH 7.4 to a final concentration of 10 mg/ml. A series of two-fold dilutions in PBS was prepared in a MicroAmp Fast Optical 96-Well Reaction Plate (4346906) from Applied Biosystems.
Purification of bovine rhodopsin (UNIPROT ID: P02699)
All manipulations were carried out in a darkroom under dim red illumination (>650 nm). Rod outer segments (ROS) were prepared as previously described (15, 16). Briefly, crude ROS were isolated from dark adapted bovine retina (W. L. Lawson Co., Lincoln, NE) by a sucrose gradient extraction method. Purified ROS membranes (20 mg/mL rhodopsin) were solubilized by the zinc/alkyl-glucoside extraction method and centrifuged at 100,000g for 40 min to extract rhodopsin (17). The clear supernatant was further purified by gel filtration chromatography on a Superdex-200 column (GE-Healthcare) in 50 mM Tris, pH 7.5, 100 mM NaCl, 1 mM MgCl2 and 0.02% n-dodecyl β-D-maltoside. Purified rhodopsin was concentrated to 2.6 mg/ml and used for DSF experiments.
Experimental sample preparation and fluorescence measurements
The dilution series were constructed in duplicate. Each well contained 9 μl of protein sample plus 1 μl of dye. The plate was sealed with ClearSeal film (HR4-521) from Hampton Research. The final probe concentration was 2 μM for BFC and 5x times for Sypro-orange. The plate was incubated for 10 min on ice prior to DSF measurements. The dilution series revealed the optimal working concentration for DSF measurements, which was then verified in quadruplicate.
All DSF measurements were performed with a StepOnePlus System (4376600) from Applied Biosystems. The melting curve experiments were conducted and recorded using StepOne Software v2.3 from Applied Biosystems. The SYBR®, FAM™ and ROX™ channels were used simultaneously to record the change in fluorescence. The run was set to cool down to 4 °C within 10 s, kept at 4 °C for 1 min and then increased 1 °C per min in a step and hold manner up to 99.9 °C. The multicomponent data was exported on a Microsoft EXCEL sheet and analyzed with the XLfit package 5 (5).
Data analysis
Data were analyzed by averaging and normalizing the fluorescence intensities from the multicomponent experiments. Normalized curves were fitted to the Boltzmann equation (i) using the XLfit package 5.
Results
BODIPY FL L-cystine method validation
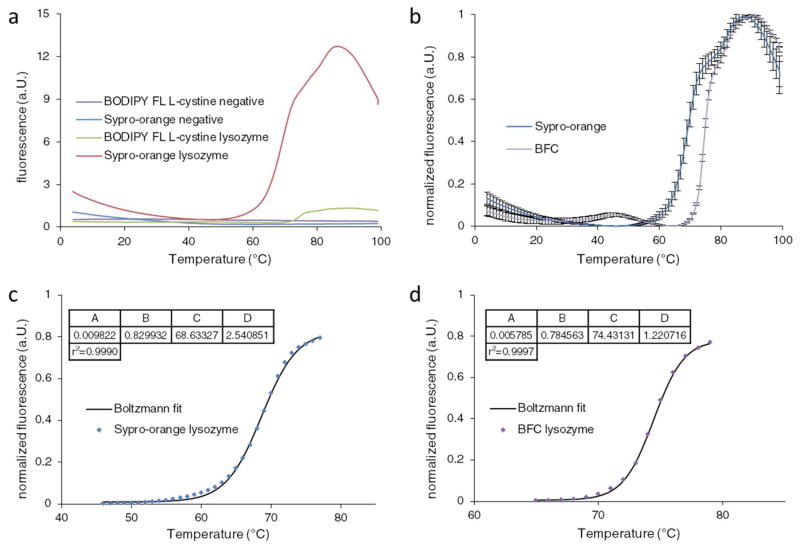
The melting temperature of lysozyme (74 ± 0.5 °C) w as previously determined using different methodology (18–21). A comparison between Sypro-orange and BFC was made with a lysozyme concentration at 2.25 mg/ml (Fig. 1a & b). The melting temperatures were 68.6 °C with Sypro-orange (Fig. 1b) and 74.4 °C with the BF C probe (Fig. 1d). The melting temperature obtained with BFC coincides with label-free measurements as stated above. Conversely, measurements with the Sypro-orange probe differed by 5.8 °C. This difference originates from the pH dependence of the lysozyme DSF data measured with the Sypro-orange probe as reported previously (21). Agreement of the melting temperature between label free measurements and BFC supports the usage of this probe for DSF.
Figure 1.
DSF measurements of lysozyme. a) Fluorescence emitted by Sypro-orange and BFC in the presence and absence of lysozyme. Measurements in the absence of protein are shown in violet for BFC and blue for Sypro-orange. Fluorescence in the presence of lysozyme is presented in green for BFC and red for Sypro-orange. b) Normalized fluorescence of lysozyme measured with BFC in violet and Sypro-orange in blue. Error bars indicate the standard deviation of quadruplicate samples. c) Thermal unfolding of lysozyme measured with Sypro-orange. The first transition of the normalized curve was fitted to the Boltzmann equation and resulted in a melting temperature determined at 68.6 °C. d) Thermal unfolding of lysozyme measured with BFC. The first transition of the normalized curve was fitted to the Boltzmann equation and resulted in a melting temperature of 74.4 °C. Variables A –D correspond to the values described by the Boltzmann equation.
Rhodopsin stability
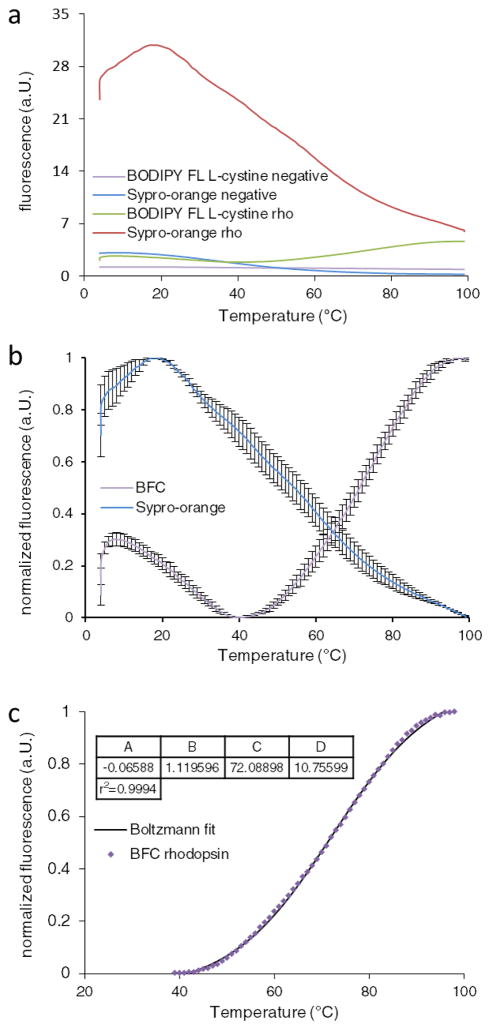
The melting temperatures of bovine rhodopsin in ROS disk membranes are 71.9 °C and 55.9 °C for the dark and bleached states, respectiv ely (22, 23). The measurements with Sypro-orange could not be analyzed because of its high initial fluorescence and the absence of a transition (Fig. 2a & b). Figure 2b reveals the averaged quadruplicate DSF measurements at 9.14 μg/ml sample concentration. Analysis of this sample revealed a melting temperature of 72.1 °C, which is consistent with the published value (Fig. 2c). Overall, the data show that BFC is an effective probe to obtain DSF measurements of hydrophobic proteins.
Figure 2.
DSF measurements of bovine rhodopsin. a) Fluorescence emitted by Sypro-orange and BFC in the presence and absence of rhodopsin. Measurements in the absence of protein are shown in violet for BFC and blue for Sypro-orange. Fluorescence in the presence of rhodopsin is presented in green for BFC and red for Sypro-orange. b) Normalized fluorescence of rhodopsin measured with BFC in violet and Sypro-orange in blue. Error bars indicate the standard deviations of quadruplicate samples. The blue curve shows a high initial fluorescence and no transition curve upon unfolding. The violet curve shows an initial fluorescence which is reduced and subsequently increases upon unfolding of the protein. c) The normalized fluorescence of rhodopsin is fitted to the Boltzmann equation and resulted in a melting temperature of 72.1 °C. Variables A –D co rrespond to the values described by the Boltzmann equation.
Discussion
Comparing DSF measurements between Sypro-orange and BFC demonstrated the superior data obtained with BFC. Moreover, the melting temperature of lysozyme measured with BFC is identical with the results obtained with label-free methods. The concentration-dependent melting temperatures observed with both lysozyme and rhodopsin indicated that DSF measurements have to be conducted at a fixed protein concentration to allow accurate comparison.
BFC belongs to the class of thiol-reactive probes, and therefore it suffers from similar shortcomings as CPM (9). The main limitations are the pH dependence of thiol formation, the interaction with reducing agents and the interference of the probe with a ligand binding site (4, 9, 14, 24). Nevertheless, we have shown these shortcomings can be overcome by careful sample preparation, initial evaluation of the protein, probe concentrations, and careful data analyses.
Conclusions
We demonstrate here that BFC is a versatile thiol-reactive compound that can be used for high-throughput DSF measurements of membrane proteins, proteins with hydrophobic binding sites or hydrophobic ligands. The absorption and fluorescent emission of BFC allows signal detection with a standard RT-PCR device. Moreover, the initial dimeric state of BFC enhances the intensity of the fluorescent signal, producing greater assay sensitivity and permitting lower probe concentrations, if desired. These properties render BFC a superior probe for high-throughput DSF measurements with standard RT-PCR equipment.
Highlights.
BODIPY FL L-cystine as thiol reactive probe for differential scanning fluorimetry
Differential scanning fluorimetry of membrane proteins or in presence of hydrophobic ligands
High-throughput differential scanning fluorimetry measurements with a standard RT- PCR device
Acknowledgments
We thank Drs Yaroslav Tsybovsky, Nathan Alexander, Tivadar Orban, Marcin Golczak, and Leslie T. Webster, Jr. for helpful comments on this manuscript. This work was supported by funding from the National Institutes of Health EY009339 (KP) and the Arnold and Mabel Beckman Foundation, LH is supported by the Swiss National Science Foundation Doc.Mobility fellowship (P1SKP3_158634). KP is John Hord Professor of Pharmacology.
Abbreviations
- BFC
BODIPY FL L-cystine
- CPM
N-[4-(7-diethylamino-4-methyl-3-coumarinyl)phenyl] maleimide
- DSF
differential scanning fluorimetry
- ROS
rod outer segments
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.He F, et al. Screening of monoclonal antibody formulations based on high-throughput thermostability and viscosity measurements: design of experiment and statistical analysis. J Pharm Sci. 2011;100:1330–1340. doi: 10.1002/jps.22384. [DOI] [PubMed] [Google Scholar]
- 2.Senisterra G, Chau I, Vedadi M. Thermal denaturation assays in chemical biology. Assay Drug Dev Technol. 2012;10:128–136. doi: 10.1089/adt.2011.0390. [DOI] [PubMed] [Google Scholar]
- 3.Vedadi M, et al. Chemical screening methods to identify ligands that promote protein stability, protein crystallization, and structure determination. Proc Natl Acad Sci U S A. 2006;103:15835–15840. doi: 10.1073/pnas.0605224103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pantoliano MW, et al. High-density miniaturized thermal shift assays as a general strategy for drug discovery. J Biomol Screen. 2001;6:429–440. doi: 10.1177/108705710100600609. [DOI] [PubMed] [Google Scholar]
- 5.Ericsson UB, Hallberg BM, Detitta GT, Dekker N, Nordlund P. Thermofluor-based high-throughput stability optimization of proteins for structural studies. Anal Biochem. 2006;357:289–298. doi: 10.1016/j.ab.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 6.Niesen FH, Berglund H, Vedadi M. The use of differential scanning fluorimetry to detect ligand interactions that promote protein stability. Nat Protoc. 2007;2:2212–2221. doi: 10.1038/nprot.2007.321. [DOI] [PubMed] [Google Scholar]
- 7.Crowther GJ, et al. Buffer optimization of thermal melt assays of Plasmodium proteins for detection of small-molecule ligands. J Biomol Screen. 2009;14:700–707. doi: 10.1177/1087057109335749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu W, Hanson MA, Stevens RC, Cherezov V. LCP-Tm: an assay to measure and understand stability of membrane proteins in a membrane environment. Biophysical journal. 2010;98:1539–1548. doi: 10.1016/j.bpj.2009.12.4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alexandrov AI, Mileni M, Chien EY, Hanson MA, Stevens RC. Microscale fluorescent thermal stability assay for membrane proteins. Structure. 2008;16:351–359. doi: 10.1016/j.str.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Tol MB, et al. Thermal unfolding of a mammalian pentameric ligand-gated ion channel proceeds at consecutive, distinct steps. The Journal of biological chemistry. 2013;288:5756–5769. doi: 10.1074/jbc.M112.422287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Epps DE, Sarver RW, Rogers JM, Herberg JT, Tomich PK. The ligand affinity of proteins measured by isothermal denaturation kinetics. Anal Biochem. 2001;292:40–50. doi: 10.1006/abio.2001.5047. [DOI] [PubMed] [Google Scholar]
- 12.Chari A, et al. ProteoPlex: stability optimization of macromolecular complexes by sparse-matrix screening of chemical space. Nature methods. 2015;12:859–865. doi: 10.1038/nmeth.3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mezzasalma TM, et al. Enhancing recombinant protein quality and yield by protein stability profiling. J Biomol Screen. 2007;12:418–428. doi: 10.1177/1087057106297984. [DOI] [PubMed] [Google Scholar]
- 14.Layton CJ, Hellinga HW. Thermodynamic analysis of ligand-induced changes in protein thermal unfolding applied to high-throughput determination of ligand affinities with extrinsic fluorescent dyes. Biochemistry. 2010;49:10831–10841. doi: 10.1021/bi101414z. [DOI] [PubMed] [Google Scholar]
- 15.Okada T, et al. Circular dichroism of metaiodopsin II and its binding to transducin: a comparative study between meta II intermediates of iodopsin and rhodopsin. Biochemistry. 1994;33:4940–4946. doi: 10.1021/bi00182a024. [DOI] [PubMed] [Google Scholar]
- 16.Baker BY, et al. Crystallization of proteins from crude bovine rod outer segments. Methods in enzymology. 2015;557:439–458. doi: 10.1016/bs.mie.2014.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okada T, Takeda K, Kouyama T. Highly selective separation of rhodopsin from bovine rod outer segment membranes using combination of divalent cation and alkyl(thio)glucoside. Photochemistry and photobiology. 1998;67:495–499. [PubMed] [Google Scholar]
- 18.Shih P, Holland DR, Kirsch JF. Thermal stability determinants of chicken egg-white lysozyme core mutants: hydrophobicity, packing volume, and conserved buried water molecules. Protein Sci. 1995;4:2050–2062. doi: 10.1002/pro.5560041010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ichimura Y. In: Application Brief. Hifh-Tech H, editor. Vol. 54. Hitachi High-Tech Science Corporation; Tokyo: 1991. p. 2. chap. 1. [Google Scholar]
- 20.JASCO.INC; JASCO.INC, editor. CD application note 01–03. 2015;chap 2 [Google Scholar]
- 21.Yeh AP, McMillan A, Stowell MH. Rapid and simple protein-stability screens: application to membrane proteins. Acta crystallographica Section D, Biological crystallography. 2006;62:451–457. doi: 10.1107/S0907444906005233. [DOI] [PubMed] [Google Scholar]
- 22.Khan SM, Bolen W, Hargrave PA, Santoro MM, McDowell JH. Differential scanning calorimetry of bovine rhodopsin in rod-outer-segment disk membranes. Eur J Biochem. 1991;200:53–59. doi: 10.1111/j.1432-1033.1991.tb21047.x. [DOI] [PubMed] [Google Scholar]
- 23.Landin JS, Katragadda M, Albert AD. Thermal destabilization of rhodopsin and opsin by proteolytic cleavage in bovine rod outer segment disk membranes. Biochemistry. 2001;40:11176–11183. doi: 10.1021/bi0100539. [DOI] [PubMed] [Google Scholar]
- 24.Tyagarajan K, Pretzer E, Wiktorowicz JE. Thiol-reactive dyes for fluorescence labeling of proteomic samples. Electrophoresis. 2003;24:2348–2358. doi: 10.1002/elps.200305478. [DOI] [PubMed] [Google Scholar]