Abstract
Objective
To investigate the time dependent changes in expression of cytokines that characterizes the spontaneous recovery of reflex voiding after spinal cord injury (SCI). SCI is known to reorganize the neural circuitry of micturition reflex after injury.
Methods
Under isoflurane anesthesia, spinal cord of 18 adult female Sprague-Dawley rats was completely transected at the Th9/10 level. Awake cystometry was performed on controls and 6 SCI animals at each time point of 4, 8 and 12 weeks and bladder was then harvested for analysis of 29 proteins Millipore kit or ELISA. Prophylactic dose of ampicillin 100mg/kg was administered periodically to all SCI animals.
Results
Spontaneous recovery of voiding after SCI at 12 weeks was evident from increased intercontractile interval and voiding efficiency during cystometry. Expression of pro-inflammatory interleukins (IL-1α and IL-1β, IL-2 IL-5, IL-6, IL-18, TNF-α) and CXC chemokines (CXCL1, CXCL2, CXCL10), CX3CL1 and CCL2 showed significant elevation at 4 and 8 weeks with slight decrease at 12 weeks. In contrast, expression of anti-inflammatory interleukin IL-10 and neuroprotective factors, BDNF,CXCL-5 and Leptin was elevated at 8 and 12 weeks (p<0.05). In contrast, expression of CCL3, CCL5 and growth factors VEGF, NGF, EGF, G-CSF, GM-CSF did not show any significant temporal change after SCI.
Conclusions
Spontaneous recovery of reflex voiding at 12 weeks was marked by increased endogenous expression of anti-inflammatory cytokine IL-10 and neuroprotective factors BDNF, CXCL-5 and leptin, which suggests that pharmacological suppression of inflammation, can hasten the emergence of reflex voiding after SCI.
Keywords: Spinal cord injury, IL-10, leptin, BDNF, CXCL-5, reflex voiding
Introduction
The micturition reflex in spinal intact condition is triggered by the small myelinated (Aδ) bladder afferent nerves responding to bladder distension and bladder contraction for voiding is evoked by stimulation of efferent nerves1. Voluntary control over micturition reflex is coordinated at the spinal cord level by the spinobulbospinal reflex pathway passing through the coordination centers (periaqueductal gray and pontine micturition center) located in the rostral brain stem. This spinobulbospinal reflex pathway to the brainstem is therefore directly interrupted by spinal cord injury (SCI), and as a consequence voiding behavior is characterized by bladder areflexia with complete urinary retention.
However, sometime after injury, the bladder function becomes hyperreflexic due to the emergence of a spinal micturition reflex pathway, which is characterized by dyssynergic bladder and sphincter function2. Basic research have demonstrated that SCI induced reorganization of micturition reflex is dependent in part on the plasticity of bladder afferent pathways2 and the unmasking of reflexes triggered by unmyelinated, capsaicin-sensitive, C-fiber bladder afferent neurons3.
Subsequent investigations revealed that plasticity of bladder afferent neurons2 is associated with morphologic, chemical, and electrical changes, which appears to be mediated in part by neurotrophic factors released at the level of bladder and spinal cord. Earlier studies have reported that bladder expression of neurotrophic factor, nerve growth factor (NGF)4 is involved in sprouting of primary afferent neurons5 and in mediating the paracrine signaling responsible for the plasticity of bladder afferent pathways. Neurotrophic action of classic neurotrophic factors like NGF is apparently based, to a significant extent, on their co-operativity with other cytokines/chemokines that participate as a paracrine messengers in bladder6,7. Application of the chemokine, monocyte chemoattractant protein-1 (MCP-1/CCL2) was recently shown to increase the excitability of mechanosensitive unmyelinated peripheral afferents8. Increased expression of chemokine CXCL-5/LIX by the oligodendrocytes was shown to increase the survival of neurons9. In contrast, CX3CL1 (also known as fractalkine) expressed in bladder is considered a key to the neuron-microglia interactions and the increased expression of CX3CL1 and CXCL-10 is associated with apoptosis10 and demyelination of neurons, respectively11. We surmise that complex biological responses involved in SCI induced plasticity of bladder afferent neurons are therefore likely to require additional trans-signaling mechanisms mediated by multiple mediators, which are yet to be investigated.
Here, we investigated the time dependent changes in the expression of neurotrophins, pro-inflammatory and anti-inflammatory cytokines in bladder after SCI in order to identify the molecular phenotype associated with spontaneous recovery of voiding function. The biological role of proteins investigated here has been previously studied in voiding dysfunction and inflammation. Regular antibiotic treatment of experimental animals excluded the confounding effect of infection in the experimental outcomes.
Animal Preparation
Experiments were performed on 24 adult female Sprague-Dawley rats (Hilltop, Pittsburgh, PA). All animal experiments were in accordance with institutional guidelines approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Pittsburgh. Six animals were left spinal intact to serve as controls and rest of the 18 animals received complete spinal cord transection at the level of Th9-10 under isoflurane anesthesia. After Th9-10 laminectomy, the dura and spinal cord were cut with scissors and a sterile Gelform sponge was placed between the cut ends of the spinal cord. The overlying muscle and skin were then sutured back. The bladder of spinalized rats was manually emptied twice daily after spinalization until the experiments. Prophylactic dose of ampicillin 100mg/kg was given daily for first two weeks and then biweekly for remaining 10 weeks to all SCI animals to ward off urinary tract infection.
Awake Cystometry
Awake cystometry was conducted at all-time points, as previously described3. Under 0.4% halothane anesthesia, a midline abdominal incision was made and a PE50 catheter with fire-flared ends was passed through the bladder dome to access the bladder. The catheter tip was secured via ligation at the bladder entrance. The abdominal wall and overlying skin was separately closed with suture. Following surgery, animals were immediately positioned and secured in a Ball-man-type restraining device. The bladder catheter was connected to a Statham pressure transducers via a 3-way stopcock. The bladder infusion line connected to a saline filled syringe and mounted upon a Harvard Micron pump was also connected to the 3-way stopcock. The first two hours of CMG were considered as the recovery and acclimatization period. Thereafter, intercontraction interval ( ICI) , maximum voiding pressure and voiding efficiency, were evaluated. Animal was euthanized at the end of cystometry by pentobarbital for harvesting the bladder tissue for cytokine/chemokine analysis.
Measurement of Cytokines/Chemokines/Growth Factors
Bladder tissues harvested from spinal cord intact control animals and SCI animals sacrificed at each time point were homogenized using cold CelLytic™ MT Mammalian Tissue Lysis/Extraction Reagent (sigma) containing 2mM sodium orthovanadate, 1mM PMSF and protein cocktail inhibitor (1X, Sigma). The homogenate was centrifuged at 10,000 rpm for 10 min and the resulting supernatants were stored at −80°C until assayed. 29 proteins including interleukins IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p70, IL-13, IL-17A and IL-18; CXC chemokines( CXCL1, CXCL2, CXCL5 and CXCL10), CC chemokines ( CCL2, CCL3, CCL5); CX3CL1; Growth factors NGF, BDNF, VEGF and G-CSF, GM-CSF and other inflammatory mediators such as eotaxin, leptin, IFN-γ and TNFα levels were determined on a Luminex 100 using a MILLIPLEX MAP Rat Cytokine/Chemokine Panel (Millipore, Billerica, MA). Levels of NGF and BDNF were determined using individual ELISA kits procured from Promega, USA. Protein estimation was done by BCA Protein Assay Kit (Pierce, Rockford, Illinois) to standardize the chemokine concentrations relative to tissue protein levels, which are expressed as pg/mg of total protein12.
Statistical Analysis
Cystometric data was analyzed for significance by one-way ANOVA followed by Tukey’s post-test for multiple comparison. Since the expression of inflammatory mediators followed non-normal distribution, differences between bladder tissues harvested at different time points were analyzed by Kruskal-Wallis followed by Dunn’s test using GraphPad Prism 5.0.
Results
Effect of SCI on Cystometry
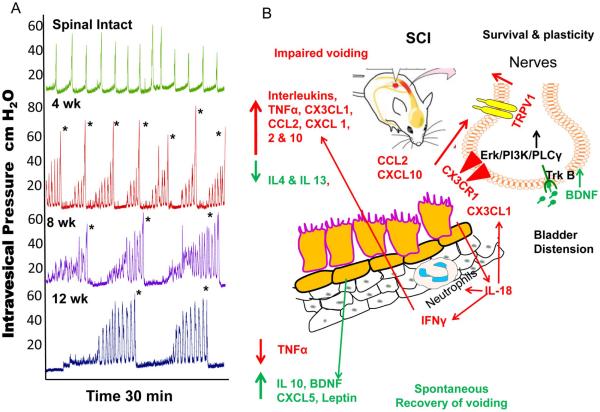
Awake CMG of spinal intact and SCI animals at various time points revealed time dependent changes in the voiding function induced by SCI. Representative CMG tracings from controls and SCI animals each time point is shown in Fig.1A. Relative to spinal intact control animals, the ICI between two voiding events in SCI animals progressively increased from 4 to 12 weeks, with maximum at the time point of 12 weeks. Significantly increased ICI of SCI animals was recorded at 12 weeks relative to ICI recorded at 4 weeks (p<0.05; ANOVA followed by Tukey’s test). ICI of SCI animals is generally higher than spinal intact animals due to urinary retention and increased bladder capacity. Maximum voiding pressure in SCI animals was highest at 4 weeks and then gradually decreased at the 8 and at 12 week time point.
Fig.1A. Effect of SCI on Cystometry.
SCI progressively increased the intercontractile interval from 4 to 12 weeks. ICI in SCI animals was maximum at the time point of 12 weeks. Generally, spinal intact animals have lower ICI than SCI animals due to the urinary retention and increased bladder capacity of SCI animals. Voiding in SCI animals is marked by * in each representative trace. Fig.1B: Schematic illustration of the SCI induced voiding changes and the respective expression of inflammatory mediators. Acute SCI induced bladder distension at 4 and 8 weeks evokes upregulation of IL-18 from injured urothelium which induces expression of chemokines CXCL-1, CXCL-10, CCL2 promoting adhesion and transmigration of neutrophils, infiltrating macrophages, which cause secondary inflammatory damage and neuronal apoptosis through the release of pro-inflammatory mediators such as TNFα, interleukins1 α, β, 2, 5,6, CX3CL1 at 4-8 weeks. Impaired voiding coincides with initial upregulation of the Th1 response along with reduced expression of anti-inflammatory mediators (IL-4, IL-10 and IL-13). Chemokines (CCL2, CX3CL1, CXCL-10) act on receptors expressed on adjacent nociceptor nerve terminals to cause peripheral nociceptor sensitization by activating TRPV1 receptor. By 12 weeks there is spontaneous recovery of voiding, Th1 response is suppressed and the Th2 response is upregulated with time dependent increased expression of IL-10 and neuroprotective factors, leptin, BDNF and CXCL5 to attenuate the inflammation and neuronal apoptosis in SCI bladder.
Effect of SCI on the expression of Pro-inflammatory Cytokines
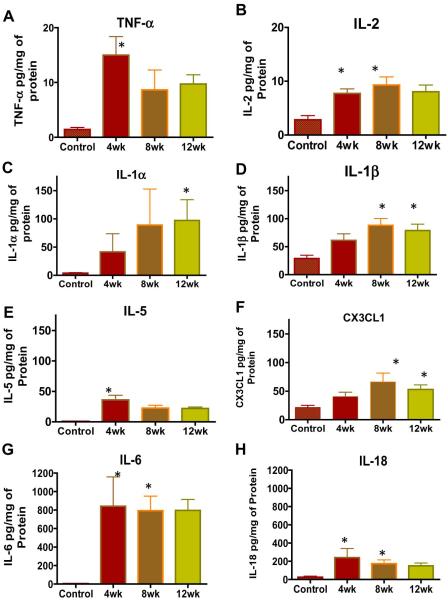
SCI caused significant alterations in the expression of pro-inflammatory interleukins IL-1α, IL-1β IL-2, IL-5, IL-6, and IL-18 (Fig.1B), which showed a gradual elevation from 4 to 8 weeks followed by a slight decrease at 12 week time point. Relative to spinal intact animals, IL-2 (Fig. 2B) was significantly elevated at the time points of 4 and 8 weeks, whereas IL-1α (Fig. 2C), IL-1β (Fig. 2D) and IL-6 (Fig. 2G) reached significance at the time point of 12 weeks (*p<0.05, Kruskal-Wallis followed by Dunn’s test). Tissue levels of IL-1β and IL-6 also reached significance at the 8 week time point and the bladder tissue levels of IL-6 (Fig. 2G) were quantitatively higher than any of other 28 cytokines or chemokines measured at any point after SCI, which indicates the predominant role of IL-6 in mediating the paracrine signaling responsible for voiding dysfunction.
Fig.2. Effect of SCI on the expression of pro-inflammatory cytokines.
Relative to spinal intact animals, SCI caused significant alterations in the expression of pro-inflammatory cytokines in bladder tissue (*p<0.05, Kruskal-Wallis followed by Dunn’s test in Panel A-G). Highest expression of TNF-α (Panel A), IL-18 (Panel H), IL-5(Panel E) and IL-6 (Panel G) was noted at 4 weeks, when subsequently dropped gradually till 12 weeks without significance. In contrast, the expression of pro-inflammatory interleukins IL-1α (Panel C), IL-1β (Panel D),(Panel B) and Fractalkine ( CX3CL1(Panel F) showed a gradual elevation from 4 to 8 weeks followed by a slight decrease at 12 week time point. Regular antibiotic treatment of experimental animals excluded the confounding effect of infection in the results. Bladder tissue levels of cytokine levels measured as pg/ml were normalized to the protein concentration and expressed as pg/mg. Notice the drastic change in the scale of y-axis for IL-6 and IL-18 compared to other cytokines, which indicates their relatively increased expression.
Highest expression of TNF-α (Fig. 2A), IL-5 (Fig. 2E) and IL-18 (Fig. 2H) was detected at 4 weeks point after SCI with significance (*p<0.05, Kruskal-Wallis followed by Dunn’s test), whereas the apparent decrease in tissue levels of all three cytokines at 8 and 12 weeks did not reach significance. IL-18 is considered a downstream mediator of signaling initiated by CX3CL1 (Fig. 2F), and expression of CX3CL1 was found to be significant at the 8 and 12 week time point. The expression of other inflammatory cytokines, IL-12p70 and IL-17 was very low and inconsistent at all-time points after SCI (data not shown).
Effect of SCI on the expression of Anti-inflammatory Cytokines
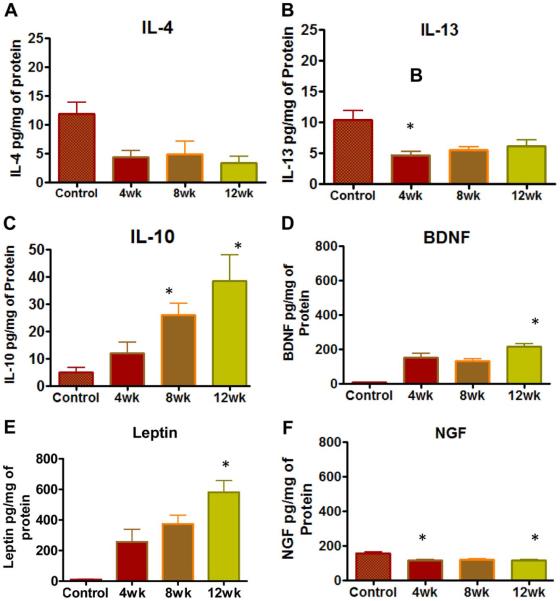
In contrast to the consistent elevation of pro-inflammatory cytokines, there was a divergence in the expression for the old triad of anti-inflammatory cytokines (IL-4, IL-10, and IL-13)13. The expression of IL-4 (Fig.3A) and IL-13(Fig.3B) was down -regulated after SCI and relatively constant levels were detected from 4-12 weeks with statistical significance. Relative to spinal intact controls, the levels of IL-13 were significantly lower in SCI animals at the 4 week time point (*p<0.05, Kruskal-Wallis followed by Dunn’s test; Fig.3). The levels of IL-4 were significantly lowered after SCI, but there was no significance with respect to individual time points on Dunn’s test performed after ANOVA. In contrast, the expression of IL-10(Fig.3C) showed a gradual elevation with maximum expression at 12 weeks (*p<0.05; Kruskal-Wallis followed by Dunn’s test). IL-10 levels were nearly double of the IL-4 or IL-13 tissue levels at all-time points and significance was reached for the time point of 8 and 12 weeks.
Fig.3. Effect of SCI on the expression of anti-inflammatory & Neuroprotective Factors.
Relative to spinal intact animals, SCI caused divergence in the expression for old triad of anti-inflammatory cytokines IL-4 (Panel A), IL-13 (Panel B), IL-10 (Panel C), brain derived neurotrophic factor (BDNF) (Panel D), leptin (Panel E) and NGF (Panel F). The expression of IL-10 showed a gradual elevation from 4-12 weeks with highest expression noted at 12 weeks, which was significant relative to spinal intact animals (*p<0.05, Kruskal-Wallis followed by Dunn’s test). In contrast, levels of IL-4 and IL-13 were consistently lower in SCI animals and reached significance only for IL-13 at 4th week time point, but were not significant for IL-4. Relative to spinal intact animals, expression of BDNF and leptin was upregulated in SCI animals and was significant at 12 weeks points. In contrast, expression of NGF was lower at all time points after SCI and reached significance at 4 and 12 week time point. NGF was always expressed at lower levels than BDNF. Notice the change in the scale of y-axis for IL-10, which indicates near doubling of the expression noted for IL-4 and IL-13. Notice that expression of leptin and neurotrophin was higher compared to anti-inflammatory cytokines.
Effect of SCI on the expression of neurotrophins, adipokines and growth factors
The expression of brain derived neurotrophic factor (BDNF) was significantly upregulated and remained high at all points after SCI (Fig.3D). Likewise, the expression of adipokine, leptin which is an adipocyte-derived hormone known to regulate food intake and energy expenditure also remained significantly high after SCI (Fig.3E). The highest tissue levels of leptin were recorded at the 12 week time point. In agreement with previous reports14, we noted that compared to spinal intact control animals, the expression of NGF was significantly lower in SCI animals at the 4 week time point (*p<0.05; Fig.3F) and it remained low also at 8 and 12 week time point. However, the expression of EGF, VEGF, G-CSF and GM-CSF in post-SCI animals showed insignificant changes relative to control spinal intact animals (data not shown).
Effect of SCI on the expression of CXC chemokines
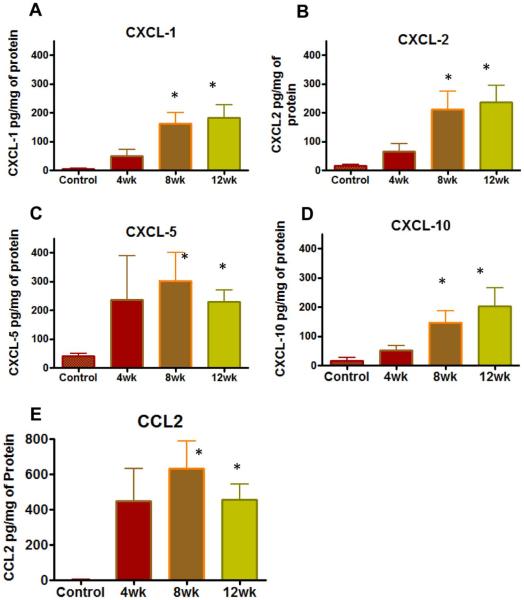
Although the expression of IFN-γ was sustained at slightly higher levels in all SCI animals, it did not reach significance. However, chemokines induced by IFN-γ were measured at significantly higher levels in bladder tissue harvested from SCI animals relative to spinal intact controls. Expression of CXCL-1 (Fig. 4A), CXCL-2(Fig. 4B), CXCL-5(Fig. 4C) and CXCL-10(Fig. 4D) were measured significant at the 8 and 12 week time point in post-SCI animals (*p<0.05; Kruskal-Wallis followed by Dunn’s test; Fig.4). The expression of CXCL-5 was quantitatively higher than other members of CXC chemokine family, which is likely to facilitate sprouting of afferents after SCI.
Fig.4. Effect of SCI on the expression of CXC & CC chemokines.
Relative to spinal intact animals and CC chemokines, the expression of all four CXC chemokines showed gradual elevation, which reached significance at 8 and 12 weeks (*p<0.05, Kruskal-Wallis followed by Dunn’s test). Notice that expression of CXCL-5 (Panel C) was quantitatively higher than other members of CXC chemokine family. Expression of CCL2 (Panel E) was also significant at 8th and 12 week. Notice the y-axis scale of panel E to indicate that that expression of CCL2 was higher than all other members of CXC chemokine family.
Effect of SCI on the expression of CC chemokines
The expression of CCL2(Fig. 4E) was significantly elevated in post-SCI animals, which reached a maximum at 8 week and then dropped slightly at the 12 week time point.CCL2 levels were significantly elevated at both time points of 8 and 12 weeks. In contrast, the slightly elevated levels of CCL3 and CCL5 in post-SCI animals did not reach significance, whereas eotaxin (CCL11) was not detectable in any of the bladder specimens (data not shown). The overall type 1 error during multiple comparisons was not controlled due to exploratory nature of the study.
Discussion
Inflammatory mediators have been shown to play an important role in the pathological and physiological consequences secondary to SCI15-17. The main findings from this study are that inflammation plays a key role in the time dependent changes in voiding function after SCI. Upregulation of anti-inflammatory mediators and neuroprotective molecules is likely to play an important role in the plasticity of bladder afferent pathways as well as reorganization of synaptic connections in the spinal cord. Poor voiding efficiency at the time point of 4 and 8 weeks after SCI was coincident with upregulation of pro-inflammatory cytokines (IL-1α, IL-1β, IL-2 IL-5, IL-6, IL-18 and TNF-α), chemokines (CX3CL-1, CCL2, CXCL-1, CXCL2, CXCL-10) and downregulation of anti-inflammatory cytokines IL-4 and IL-13. In contrast, spontaneous recovery of voiding function at the 12 week time point was associated with maximum expression of anti-inflammatory cytokine IL-1018, neurotrophin BDNF and CXCL-5 as well as the neuroprotective leptin19 in bladder.
SCI at cervical and high thoracic levels is known to trigger a severe systemic inflammatory response20, but the role of inflammation in complete spinal transection at the mid-thoracic-level and the secondary voiding dysfunction was not investigated so far. An earlier study on SCI patients18 identified IL-6, CXCL-1, and CCL2 as potential serum biomarkers for SCI. Here, we found that, the expression of IL-6 and CCL2 in bladder tissue remained quantitatively higher than other mediators after SCI and the expression decreased slightly at 12 weeks, which coincided with plasticity-dependent recovery of voiding. We have previously published time dependent changes in expression of these and other chemokines in acute rat model of overactive bladder induced by cyclophosphamide12. Therefore, we focused on proteins, which have been previously studied in voiding dysfunction or inflammation.
SCI is known to elicit an inflammatory response that recruits macrophages to the injured spinal cord and bladder, as indicated by reports of increased spinal cord levels of CCL2 chemokine that is chemotactic for macrophages, at 6 hours and also at 4 weeks21 after SCI . Here, we report that pro-inflammatory CCL2 was also upregulated in bladder of SCI animals together with CX3CL1, CXCL-1, CXCL2 and CXCL-10 at 4, 8 and 12 weeks. Upregulation of CCL2 in bladder at 4 and 8 weeks was associated with significantly reduced ICI between two voiding events recorded during awake (conscious) cystometry. Studies from other groups have reported that pharmacological down regulation of CCL2 leads to reduced inflammation and increased neuronal survival after SCI22 . Interestingly, slight lowering of bladder tissue CCL2 levels at 12 weeks accompanied the recovery of voiding function, which led us to suggest that blockade of inflammation locally in bladder can be a potential approach for the future treatment of SCI induced voiding dysfunction.
Time dependent changes in the expression of pro and anti-inflammatory cytokines following SCI suggests that a right mix of paracrine signaling constituted by IL-10, CXCL-5, leptin and BDNF creates an appropriate micro-environment in the bladder for the sprouting of afferents that contribute to the emergence of bladder function after SCI. IL-10 is known to block four different inflammatory cells implicated in SCI, including neutrophils, infiltrating macrophages, and resident microglia. Anti-inflammatory cytokine, IL-10 and IL-4 can both reduce macrophage activation by reducing the NO production23. A previous study found increased levels of IL-4 within 24 after SCI, but levels were decreased at time points thereafter 24 as observed here at 4, 8 and 12 weeks.
The apoptotic action of CX3CL1 and CXCL10 on bladder afferent neurons after SCI appears to be countered by the upregulation of CXCL-5 and BDNF as a homeostatic response9 for ensuring the survival of bladder innervation after SCI. CXCL-59 promotes sprouting of afferents and its levels were higher than other members of CXC chemokine family (CXCL10) and CX3CL1, which promote neuronal apoptosis10,11.
Poor voiding outcomes at 4 and 8 weeks in SCI animals were associated with upregulation of CX3CL1 (also known as fractalkine) in bladder. Levels of CX3CL1 measured here are biologically relevant and much higher than levels reported after chemically induced bladder inflammation25. CX3CL1 is expressed in neurons and astrocytes and is known to promote production of reactive oxygen species (ROS) which induce hyperexcitability of C-fibers by activating TRPA1 receptor. IL-18 expressed in epithelium and astrocytes is considered a potential downstream factor of CX3CL1/CX3CR1 signaling10. The IL-18 was earlier known as IFN-γ -inducing factor and is known to induce gene expression of TNF-α, IL-1(α and β), and several chemokines, which may explain the coincident maximum expression of IL-18 with TNF-α, IL-5 and IL-6 at the 4 week time point after SCI.
The concept of reduced inflammation being conducive to the recovery of voiding after SCI is supported by the highest temporal increase in the endogenous expression of anti-inflammatory cytokine IL-10 at 12 weeks. The homeostatic elevation of IL-10 and slightly diminished bladder tissue levels of IL-18 and IL-2 at 12 week time point indicates a suppression of type 1 T-helper cell (Th1) immune response and enhancement of the Th2 response at the 12 week time point after SCI. Taken together, time dependent changes in cytokine expression shown in Fig. 2-4, Supplementary Fig. 1 suggest SCI causes initial upregulation of the Th1 response, which is subsequently suppressed at 12 weeks and the Th2 response gets upregulated. The suppression of Th1 immune response is also supported by inconsistent measurement of IFN-γ, and IL-12 levels in bladder tissue after SCI.
The anti-inflammatory effects of IL-10 are well established and exogenous delivery of IL-10 by replication deficient virus was shown to help in the functional recovery of SCI animals18. IL-10 appears to be a more potent inhibitor of pro-inflammatory IL-1β, interferons, TNF-α and ROS than IL-4 26. IL-10 receptor is present on astrocytes, oligodendrocytes, microglia, and neurons and the indirect STAT3 pathway is involved in the decreased pro-inflammatory cytokine release and the direct STAT3 pathway is neuroprotective against glutamate excitotoxicity and also initiates an anti-apoptotic cascade and prevents the release of cytochrome c and activation of caspase18.
The neuroprotective action of IL-10 appears to act in concert with upregulated levels of leptin, BDNF and CXCL-5 in mediating the voiding changes seen on 12 weeks. In a recent study, exogenous overexpression of leptin was associated with increased expression of neuroprotective genes, reduced caspase-3 activity and decreased the expression of pro-inflammatory molecules in SCI animal19. Leptin is known to reduce microglial reactivity and increase the myelin preservation19 and significant elevation of leptin at 12 week time point in our study was associated with upregulation of CXCL-5 and reduced bladder tissue levels of CCL2, IL-5 and IL-18. Earlier studies from our group showed that NGF is increased initially at the 2 week time point after SCI27, but report of Vizzard et al showed that bladder NGF levels at 4 week time point after SCI were lower than in controls14. Our results obtained at 4-12 weeks after SCI corroborate with the NGF expression of previous reports14. BDNF expression was maximum at 12 weeks, and studies from other groups demonstrated that upregulation of BDNF and IL-4 mediates the axonal regeneration and functional recovery following administration of fibroblast growth factor28 in SCI animals. In a recent study, Spinal BDNF expression increased in a time-dependent manner together with emergence of reflex voiding29.
A recent study was unable to quantitate the levels of CXCL2 ( MIP2), CXCL-5 ( LIX), TNF-α, CCL5( RANTES), G-CSF and GM-CSF at 6h time point after SCI in spinal cord tissue and plasma30. On the contrary, the quantitative measurement of the bladder tissue levels of CXCL2 ( MIP2), CXCL-5 ( LIX), TNF-α, CCL5( RANTES), G-CSF and GM-CSF in bladder tissue at the time point of 4, 8 and 12 weeks after SCI indicates a role temporal differences in paracrine signaling from spinal cord to bladder. Only the tissue levels of eotaxin were undetectable in control and SCI animals at all-time points.
Overall, these findings identify the paracrine messengers involved in voiding dysfunction after SCI and the corresponding time dependent homeostatic changes in cytokine expression mediating the recovery of voiding. Regular antibiotic treatment of experimental animals excluded the confounding effect of infection in these results.
The inflammatory mediators identified here may have potential utility as biomarkers and surrogate outcome measures for evaluating biological response to therapeutic interventions. Several existing pharmacological agents can be repositioned to enhance plasticity for improved voiding function after SCI.
Conclusions
SCI induced voiding dysfunction and the spontaneous recovery at 12 weeks is associated with time dependent changes in expression of pro-inflammatory and anti-inflammatory mediators. Investigations at the molecular level are crucial to advance the understanding of SCI induced voiding dysfunction and develop new treatments.
Supplementary Material
Supplementary Fig.1: Schematic illustration of the SCI induced voiding changes and the respective expression of signaling mediators. Acute SCI induced bladder distension evokes upregulation of IL-18 from injured urothelium at 4 and 8 weeks, which induces the expression of CX3CL1 and IFN-γ ( a downstream inducer of CXC chemokines, CXCL-1, CXCL-2, CXCL-5, CXCL-10), CCL2 and pro-inflammatory cytokines TNF-α, IL-5 and IL-6 released from monocytes, macrophages and dendritic cells. CX3CL1 and CXCL-10 are known inducer of neuronal apoptosis ( ) in bladder and the survival of neurons (
) in bladder and the survival of neurons ( ) is promoted by BDNF Leptin, CXCL-5 and IL-10. Impaired voiding at 4 and 8 weeks coincided with lower expression of anti-inflammatory cytokines, IL-4, IL-10 and IL-13. Plasticity-dependent recovery of voiding at 12 weeks coincided with the time dependent increased expression from monocytes and lymphocytes of anti-inflammatory IL-10 to attenuate the expression of TNFα, IL-1β, and ROS from neutrophils and infiltrating macrophages (
) is promoted by BDNF Leptin, CXCL-5 and IL-10. Impaired voiding at 4 and 8 weeks coincided with lower expression of anti-inflammatory cytokines, IL-4, IL-10 and IL-13. Plasticity-dependent recovery of voiding at 12 weeks coincided with the time dependent increased expression from monocytes and lymphocytes of anti-inflammatory IL-10 to attenuate the expression of TNFα, IL-1β, and ROS from neutrophils and infiltrating macrophages ( ). There was slight decrease of pro-inflammatory cytokines and chemokines at 12 weeks. CXCL-1 promotes the infiltration of neutrophils, CXCL-10 promotes infiltration of lymphocytes and CCL2 drive the chemoattraction of monocytes and sensitization of afferent neurons via TRPV1.
). There was slight decrease of pro-inflammatory cytokines and chemokines at 12 weeks. CXCL-1 promotes the infiltration of neutrophils, CXCL-10 promotes infiltration of lymphocytes and CCL2 drive the chemoattraction of monocytes and sensitization of afferent neurons via TRPV1.
Acknowledgement
This work was partly supported by funding from NIH (DK088836 and DK093424) and Department of Defense (W81XWH-11-1-0763)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yoshizawa T, Kadekawa K, Tyagi P, Yoshikawa S, Takahashi R, Takahashi S, Yoshimura N. Mechanisms inducing autonomic dysreflexia during urinary bladder distention in rats with spinal cord injury. Spinal Cord. 2014;53:190–194. doi: 10.1038/sc.2014.233. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi R, Yoshizawa T, Yunoki T, Tyagi P, Naito S, de Groat WC, Yoshimura N. Hyperexcitability of bladder afferent neurons associated with reduction of Kv1.4 alpha-subunit in rats with spinal cord injury. J Urol. 2013;190:2296–304. doi: 10.1016/j.juro.2013.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas C, Kim JH, Torimoto K, Kwon DD, Kim YT, Tyagi P, Yoshimura N, Chancellor MB. Early capsaicin intervention for neurogenic bladder in a rat model of spinal cord injury. Biomed Res. 2007;28:255–9. doi: 10.2220/biomedres.28.255. [DOI] [PubMed] [Google Scholar]
- 4.Ochodnicky P, Cruz CD, Yoshimura N, Michel MC. Nerve growth factor in bladder dysfunction: contributing factor, biomarker, and therapeutic target. Neurourol Urodyn. 2011;30:1227–41. doi: 10.1002/nau.21022. [DOI] [PubMed] [Google Scholar]
- 5.Girard BM, Malley SE, Vizzard MA. Neurotrophin/receptor expression in urinary bladder of mice with overexpression of NGF in urothelium. Am J Physiol Renal Physiol. 2011;300:F345–55. doi: 10.1152/ajprenal.00515.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lutz M, Krieglstein K, Schmitt S, ten Dijke P, Sebald W, Wizenmann A, Knaus P. Nerve growth factor mediates activation of the Smad pathway in PC12 cells. Eur J Biochem. 2004;271:920–31. doi: 10.1111/j.1432-1033.2004.03994.x. [DOI] [PubMed] [Google Scholar]
- 7.Unsicker K, Krieglstein K. Co-activation of TGF-ss and cytokine signaling pathways are required for neurotrophic functions. Cytokine Growth Factor Rev. 2000;11:97–102. doi: 10.1016/s1359-6101(99)00033-7. [DOI] [PubMed] [Google Scholar]
- 8.Kubo A, Taguchi T, Mizumura K. Monocyte chemoattractant protein-1-induced excitation and sensitization to mechanical stimulation of mechanosensitive C-fiber afferents in rat skin. Neurosci Res. 2015;91:13–8. doi: 10.1016/j.neures.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 9.Merabova N, Kaminski R, Krynska B, Amini S, Khalili K, Darbinyan A. JCV agnoprotein-induced reduction in CXCL5/LIX secretion by oligodendrocytes is associated with activation of apoptotic signaling in neurons. J Cell Physiol. 2012;227:3119–27. doi: 10.1002/jcp.23065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bian C, Zhao ZQ, Zhang YQ, Lu N. Involvement of CX3CL1/CX3CR1 signaling in spinal long term potentiation. PLoS One. 2015;10:e0118842. doi: 10.1371/journal.pone.0118842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu MT, Keirstead HS, Lane TE. Neutralization of the chemokine CXCL10 reduces inflammatory cell invasion and demyelination and improves neurological function in a viral model of multiple sclerosis. J Immunol. 2001;167:4091–7. doi: 10.4049/jimmunol.167.7.4091. [DOI] [PubMed] [Google Scholar]
- 12.Smaldone MC, Vodovotz Y, Tyagi V, Barclay D, Philips BJ, Yoshimura N, Chancellor MB, Tyagi P. Multiplex analysis of urinary cytokine levels in rat model of cyclophosphamide-induced cystitis. Urology. 2009;73:421–6. doi: 10.1016/j.urology.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao HQ, Li WM, Lu ZQ, Sheng ZY, Yao YM. The growing spectrum of anti-inflammatory interleukins and their potential roles in the development of sepsis. J Interferon Cytokine Res. 2015;35:242–51. doi: 10.1089/jir.2014.0119. [DOI] [PubMed] [Google Scholar]
- 14.Vizzard MA. Changes in urinary bladder neurotrophic factor mRNA and NGF protein following urinary bladder dysfunction. Exp Neurol. 2000;161:273–84. doi: 10.1006/exnr.1999.7254. [DOI] [PubMed] [Google Scholar]
- 15.Kjell J, Finn A, Hao J, Wellfet K, Josephson A, Svensson CI, Wiesenfeld-Hallin Z, Eriksson U, Abrams MB, et al. Delayed imatinib treatment for acute spinal cord injury; functional recovery and serum biomarkers. J Neurotrauma. 2015 doi: 10.1089/neu.2014.3863. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stammers AT, Liu J, Kwon BK. Expression of inflammatory cytokines following acute spinal cord injury in a rodent model. J Neurosci Res. 2012;90:782–90. doi: 10.1002/jnr.22820. [DOI] [PubMed] [Google Scholar]
- 17.Kwon BK, Stammers AM, Belanger LM, Bernardo A, Chan D, Bishop CM, Slobogean GP, Zhang H, Umedaly H, et al. Cerebrospinal fluid inflammatory cytokines and biomarkers of injury severity in acute human spinal cord injury. J Neurotrauma. 2010;27:669–82. doi: 10.1089/neu.2009.1080. [DOI] [PubMed] [Google Scholar]
- 18.Jackson CA, Messinger J, Peduzzi JD, Ansardi DC, Morrow CD. Enhanced functional recovery from spinal cord injury following intrathecal or intramuscular administration of poliovirus replicons encoding IL-10. Virology. 2005;336:173–83. doi: 10.1016/j.virol.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez-Martos CM, Gonzalez P, Rodriguez FJ. Acute leptin treatment enhances functional recovery after spinal cord injury. PLoS One. 2012;7:e35594. doi: 10.1371/journal.pone.0035594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleming JC, Bailey CS, Hundt H, Gurr KR, Bailey SI, Cepinskas G, Lawendy AR, Badhwar A. Remote inflammatory response in liver is dependent on the segmental level of spinal cord injury. J Trauma Acute Care Surg. 2012;72:1194–201. doi: 10.1097/TA.0b013e31824d68bd. discussion 1202. [DOI] [PubMed] [Google Scholar]
- 21.Sandhir R, Gregory E, He YY, Berman NE. Upregulation of inflammatory mediators in a model of chronic pain after spinal cord injury. Neurochem Res. 2011;36:856–62. doi: 10.1007/s11064-011-0414-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang X, Chen C, Ma S, Wang Y, Zhang X, Su X. Inhibition of monocyte chemoattractant peptide-1 decreases secondary spinal cord injury. Mol Med Rep. 2015;11:4262–6. doi: 10.3892/mmr.2015.3330. [DOI] [PubMed] [Google Scholar]
- 23.Bogdan C, Vodovotz Y, Paik J, Xie QW, Nathan C. Mechanism of suppression of nitric oxide synthase expression by interleukin-4 in primary mouse macrophages. J Leukoc Biol. 1994;55:227–33. doi: 10.1002/jlb.55.2.227. [DOI] [PubMed] [Google Scholar]
- 24.Lee SI, Jeong SR, Kang YM, Han DH, Jin BK, Namgung U, Kim BG. Endogenous expression of interleukin-4 regulates macrophage activation and confines cavity formation after traumatic spinal cord injury. J Neurosci Res. 2010;88:2409–19. doi: 10.1002/jnr.22411. [DOI] [PubMed] [Google Scholar]
- 25.Yuridullah R, Corrow KA, Malley SE, Vizzard MA. Expression of fractalkine and fractalkine receptor in urinary bladder after cyclophosphamide (CYP)- induced cystitis. Auton Neurosci. 2006;126-127:380–9. doi: 10.1016/j.autneu.2006.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–22. [PubMed] [Google Scholar]
- 27.Seki S, Sasaki K, Fraser MO, Igawa Y, Nishizawa O, Chancellor MB, de Groat WC, Yoshimura N. Immunoneutralization of nerve growth factor in lumbosacral spinal cord reduces bladder hyperreflexia in spinal cord injured rats. J Urol. 2002;168:2269–74. doi: 10.1016/S0022-5347(05)64369-8. [DOI] [PubMed] [Google Scholar]
- 28.Kuo HS, Tsai MJ, Huang MC, Chiu CW, Tsai CY, Lee MJ, Huang WC, Lin YL, Kuo WC, et al. Acid fibroblast growth factor and peripheral nerve grafts regulate Th2 cytokine expression, macrophage activation, polyamine synthesis, and neurotrophin expression in transected rat spinal cords. J Neurosci. 2011;31:4137–47. doi: 10.1523/JNEUROSCI.2592-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frias B, Santos J, Morgado M, Sousa MM, Gray SM, McCloskey KD, Allen S, Cruz F, Cruz CD. The role of brain-derived neurotrophic factor (BDNF) in the development of neurogenic detrusor overactivity (NDO) J Neurosci. 2015;35:2146–60. doi: 10.1523/JNEUROSCI.0373-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cox AA, Varma A, Vertegel A, Barry J, Banik N. Nanoparticle Estrogen in Rat Spinal Cord Injury Elicits Rapid Anti-inflammatory Effects in Plasma, CSF and Tissue. J Neurotrauma. 2015;32:1413–21. doi: 10.1089/neu.2014.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig.1: Schematic illustration of the SCI induced voiding changes and the respective expression of signaling mediators. Acute SCI induced bladder distension evokes upregulation of IL-18 from injured urothelium at 4 and 8 weeks, which induces the expression of CX3CL1 and IFN-γ ( a downstream inducer of CXC chemokines, CXCL-1, CXCL-2, CXCL-5, CXCL-10), CCL2 and pro-inflammatory cytokines TNF-α, IL-5 and IL-6 released from monocytes, macrophages and dendritic cells. CX3CL1 and CXCL-10 are known inducer of neuronal apoptosis ( ) in bladder and the survival of neurons (
) in bladder and the survival of neurons ( ) is promoted by BDNF Leptin, CXCL-5 and IL-10. Impaired voiding at 4 and 8 weeks coincided with lower expression of anti-inflammatory cytokines, IL-4, IL-10 and IL-13. Plasticity-dependent recovery of voiding at 12 weeks coincided with the time dependent increased expression from monocytes and lymphocytes of anti-inflammatory IL-10 to attenuate the expression of TNFα, IL-1β, and ROS from neutrophils and infiltrating macrophages (
) is promoted by BDNF Leptin, CXCL-5 and IL-10. Impaired voiding at 4 and 8 weeks coincided with lower expression of anti-inflammatory cytokines, IL-4, IL-10 and IL-13. Plasticity-dependent recovery of voiding at 12 weeks coincided with the time dependent increased expression from monocytes and lymphocytes of anti-inflammatory IL-10 to attenuate the expression of TNFα, IL-1β, and ROS from neutrophils and infiltrating macrophages ( ). There was slight decrease of pro-inflammatory cytokines and chemokines at 12 weeks. CXCL-1 promotes the infiltration of neutrophils, CXCL-10 promotes infiltration of lymphocytes and CCL2 drive the chemoattraction of monocytes and sensitization of afferent neurons via TRPV1.
). There was slight decrease of pro-inflammatory cytokines and chemokines at 12 weeks. CXCL-1 promotes the infiltration of neutrophils, CXCL-10 promotes infiltration of lymphocytes and CCL2 drive the chemoattraction of monocytes and sensitization of afferent neurons via TRPV1.