Abstract
Epidemiologic studies have demonstrated that HIV-1 discordant couples who share HLA-B alleles were more likely to transmit HIV-1. These data lead us to hypothesize that individuals who match at both HLA-B alleles should have a reduced allogeneic response than those who are not matched. We observed diminished killing of CD4+ target cells only when HLA-B alleles were matched. We propose that for cell-associated HIV-1 transmission the ability of the recipient to eliminate infected cells from a donor partner may be enhanced when HLA-B alleles are different between partners. These findings suggest a novel mechanism for protection against HIV infection.
Keywords: HIV-1 transmission, T-cells, allogeneic responses, HLA-B, mixed lymphocyte reaction, Cytotoxic T-cells
INTRODUCTION
Epidemiologic studies demonstrated that HIV-1 discordant couples sharing HLA-B more likely transmit HIV-1, a finding not seen when sharing HLA-A or HLA-C alleles[1, 2]. Furthermore, mother and child HLA concordance increased risk of vertical transmission[3–6] and children sharing both HLA-B alleles with their mothers more likely acquired HIV-1 via vertical transmission[5]. Additional studies, demonstrated allo-immunization in humans elicited cellular responses that inhibited HIV-replication in vitro[7, 8]. More recently, it was demonstrated that monogamous partners practicing unprotected sex developed allogeneic CD8+ T-cell responses to HLA class I antigens that differed among partners. These responses inhibited HIV-1 infectivity in PBMC ex vivo in the heterosexual partners[9]. In vitro studies demonstrated allo-antigen stimulated anti-HIV activity in alloantigen-activated lymphocytes suppressing both heterologous and autologous HIV-infected cells, where effectors were obtained from seronegatives[10–12]. Further studies demonstrated HIV-1 suppressive CTLs from HIV-infected individuals mismatched at all HLA class I alleles[13]. These findings give merit to the idea that allogeneic responses confer some protection against HIV-infection, but do not explain the epidemiologic evidence that HLA-B sharing is most relevant. Therefore, we sought to determine a biological mechanism for this effect by asking if sharing of HLA-B but not HLA-A or HLA-C dampens the CD8 T-cell-mediated allogeneic response, benefiting cell-to-cell transmission of HIV-1. We propose that HLA-B mismatch between partners may impede cell-associated HIV transmission, as HIV-infected cells bearing mismatched HLA-B are recognized as “foreign” and targeted for elimination.
MATERIALS AND METHODS
Study Population
Cryopreserved PBMC from 36 HIV-seronegatives and 38 HIV-seropositives with HLA class I genotypes were used. HLA class I alleles were typed to four-digit specificity by PCR amplification using sequence-specific primers (Pel-Freez Clinical Systems, Brown Deer, WI) as previously described[14]. Informed consent was obtained under the guidelines of the Institutional Review Board of the University of Alabama at Birmingham.
CFSE based Mixed Lymphocyte Reaction (MLR) and Intracellular Cytokine Staining Assay (ICS)
We combined the ability to measure proliferation and cytokine production by flow cytometry for the detection of allo-stimulation in a mixed lymphocyte assay[15]. Briefly, PBMC stained with CFSE[16] were used as effectors (1 × 106 cells) and incubated with irradiated (3000R) matched or mismatched stimulators (examples Table1) at a 1:1 ratio for 5 days, 37°C, 5% C02. On day 5 Brefeldin was added for 6 hrs. Surface staining with anti-CD3-Alexa780 (ebioscience), anti-CD4-PeCy7 and anti-CD8-PerCPCy5.5 for 20 minutes in the dark at RT followed. Washed cells were permeabilized with Cytofix/cytoperm reagent for 20 minutes at RT in the dark. ICS for the production of IFN-γ-Alexa700, Perforin-PE and Granzyme B-V450 followed. At least 100,000 CD3+ events were acquired using a LSRII cytometer (BD, San Jose, CA). Data was analyzed using FlowJo Version 9.4 (TreeStar, San Carlos, CA). Proliferation was measured off the CD3+CD8+ gate and the media control values were used to set gates. Our analysis measured the cytokine-secreting cells as a proportion of the total proliferating cell population (CFSElo). SEB was the positive control and unstimulated cells the negative control (data not shown). Antibodies were obtained from BD except where noted.
Table 1.
Examples of matched and mismatched HLA allotypes for testing
| HLA-A1 | HLA-A2 | HLA-B1 | HLA-B2 | HLA-C1 | HLA-C2 | ||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| HLA-A Match | |||||||
| Effectors: | Vol#1 | *03;01 | *11;01 | *07;02 | *55;01 | *03;03 | *07;02 |
| Targets: | Match | *03;01 | *11;01 | *35;04 | *51;01 | *04;01 | *15;02 |
| Mismatch | *01;01 | *24;02 | *40;02 | *40;02 | *02;02 | *02;02 | |
| HLA-B Match | |||||||
| Effectors: | Vol#2 | *11;01 | *31;01 | *40;02 | *44;02 | *02;02 | *05;01 |
| Targets: | Match | *02;01 | *29;02 | *40;01 | *44;03 | *03;04 | *16;01 |
| Mismatch | *24;25 | *69;01 | *35;22 | *55;01 | *01;02 | *04;01 | |
| HLA-C Match | |||||||
| Effectors: | Vol#3 | *30;02 | *30;02 | *07;02 | *53;01 | *07;02 | *08;02 |
| Targets: | Match | *01;01 | *68;02 | *08;01 | *14;02 | *07;01 | *08;02 |
| Mismatch | *03;01 | *11;01 | *35;04 | *51;01 | *04;01 | *15;02 | |
| HLA-B Match | plus one HLA-C match | ||||||
| Effectors: | Vol#4 | *23;02 | *24;02 | *08;01 | *57;01 | *06;02 | *07;02 |
| Targets: | Match | *02;01 | *30;01 | *08;01 | *57;02 | *07;01 | *08;02 |
| Mismatch | *36;01 | *68;02 | *15;03 | *81;01 | *02;01 | *18;01 | |
Killing Assay (p24)
CD8-depleted PBMCs from exclusively HIV-seronegative individuals were activated with PHA for 2 days prior to infection with HIV-1 NL4.3 (MOI=0.1) as previously described[17, 18]. During infection, CD8+ T-cells (effectors) from exclusively HIV-seronegative individuals were purified using CD8 untouched Dynal Beads (per manufacturer’s instructions, Invitrogen). Purified CD8+ T-cells (100,000) were mixed at a 1:1 and/or 10:1 ratio with infected CD4+ T-cells from matched or mismatched individuals for HLA class I alleles (Table1). Cells were co-cultured in duplicate and incubated for 72 hrs at 37° C, 5% CO2. After incubation, cells were stained with surface markers (CD4-Pecy7, CD8-PercypCy5.5, and CD3-Pacific Blue) and permeabilized with Perm A reagent followed by intracellular staining with p24-FITC (Beckman-Coulter) in Perm B buffer (Caltag). The killing of targets was measured by p24 reduction by measuring the %p24. Gates were set using the uninfected targets as previously described[18]. Percent killing is calculated as follows: 100- (%p24 with effectors/%p24 no effectors) × 100.
Cytotoxicity assay using 7-aminoactinomycin D (7-AAD) staining
Targets were CD8-depleted from isolated PBMCs of HLA class I matched HIV-seronegative donors as above. The targets were activated with PHA (5μg/mL) in the presence of IL-2 (100 U/mL) for 2 days. Activated matched or mismatched CD4 targets (5×105 cells) were co-cultured with appropriate HLA CD8 T-cells (Effectors) from HIV-seronegatives for 24 hrs at four effector to target (E/T) ratios. Cells were surface-stained with anti-CD3-Pacific Blue and anti-CD4-Alexa780 (eBioscience) followed by staining with 0.25μg 7-AAD solution (BD Biosciences) for 20 mins to detect dead cells. Dead targets were measured by gating on 7-AAD+CD4+ T-cell populations, and samples with no effectors were used to set the background gates. Target cell death was determined by comparing the net percentage of 7-AAD+CD4+ T-cells in the presence of effectors relative to no effectors. Percent killing is calculated as follows: (% 7-AAD targets with effectors - %7-AAD targets without effectors/100- %7-AAD targets without effectors) × 100[19]. All flow cytometry data was analyzed using FlowJo Version 9.4 software (TreeStar, San Carlos, CA).
Statistics
Paired data used for p24 killing and statistical analyses were performed using the non-parametric Wilcoxon Signed Rank test. Area under the curve data was generated by comparing the % killing from 7-AAD for all E:T ratios. The p-values were calculated using a paired t-test. Differences were considered significant on the basis of 95% confidence intervals. Analyses were done with Graphpad Prism 5.0 for Mac.
RESULTS and DISCUSSION
Proliferation and effector molecule production by CD8+ T cells in a modified MLR assay
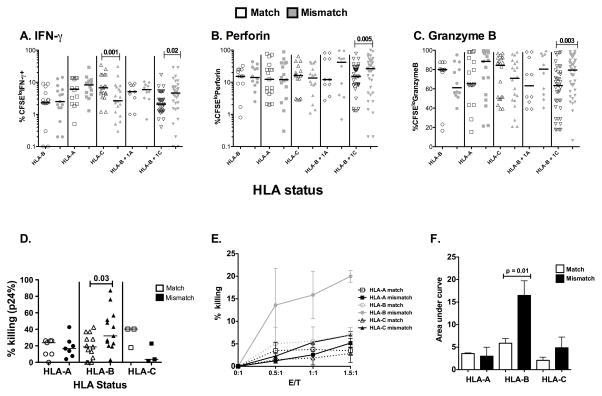
We hypothesized that individuals who match at both HLA-B alleles should have a reduced mixed lymphocyte reaction (MLR) than those who are not matched. To test this hypothesis, we performed a variation of the MLR by incubating pairs of matched (for both copies of any of the three HLA class I genes) and mismatched PBMC at all alleles (Table1). Paring was done to account for the variability between subjects in the MLR. For our analysis we measured the percent effector molecule secretion from the total proliferating population (CFSElo). Allogeneic CD8 T cells, matched at HLA-B plus one HLA-C allele (due to linkage disequilibrium), have significantly decreased percentage of proliferating CD8+ T cells producing IFN-γ, Perforin or Granzyme compared with cells that are mismatched at all class I alleles (Figure1A–C). This effect was not seen when PBMC were mismatched for HLA-A, HLA-B or -C alleles alone (Figure1A–C) or with CD4+ T cells or CD3− cells (non-T cells) (data not shown).
Figure 1. HLA-B mismatching is associated with reduced killing in a MLR assay.
Percent production of effector molecules from proliferating cells: A. IFN-γ, B. Perforin, and C. Granzyme-B production by proliferating (CFSElo) CD8+ T cells in response to PBMC matched at HLA-B, -A, -C, -B + 1 A, or -B + 1 C locus and mismatched at all loci. Matched samples are open symbols, mismatches are shown in grey. D. The diminution of p24 was measured when CD8+ T-cells from HIV seronegatives (HLA-A N=7, HLA-B N=13 and HLA-C N=3) were incubated with CD4 targets infected with HIV-1. Comparisons were made in a paired manner such that responses from the same effectors were compared to matched and mismatched targets. For example, responses from effectors from Vol#1: HLA-A*1101/3101, HLA-B*4002/4402, HLA-C*0202/0501 were co-cultured with targets from Vol#2: HLA-A*0201/2902, HLA-B*4001/4403, HLA-C*0304/1601 as an HLA-B match and from targets from Vol#3: HLA-A*2425/6901, HLA-B*3522/5501, HLA-C*0102/0401 as a mismatch. E. CD8+ T-cells from seronegatives were incubated with CD4+ targets from seronegatives and labeled with 7-AAD and the % killing was measured. In this assay HIV-1 is not present. Two samples were tested for each locus, open symbols are matched, closed symbols mismatched F. Area under the curve was determined and the p values were calculated by comparing % killing from 7-AAD assay using a paired t test. All other statistically significant differences were determined by Wilcoxon Signed rank test. Graphpad Prism 5.0 for Mac was used for all statistical analysis. Differences were considered to be significant on the basis of 95% confidence intervals.
Killing of allogeneic targets is diminished only when HLA-B class I is matched
While proliferation is a traditional tool used to measure allogeneic responses, it is only a surrogate in terms of analyzing elimination of infected CD4 T cells. To experimentally demonstrate whether individuals who matched at HLA-B alleles have a decreased ability to kill allogeneic targets, we tracked the elimination of CD4+ targets by infection with HIV-1 in the p24 killing assay. We co-cultured matched or mismatched HIV-infected CD4+ targets with CD8+ T-cells from the same allogeneic uninfected individual and measured killing by p24+ CD4 T-cell diminution. Effector cell killing was compared among paired samples, such that the responses from the same effectors were measured to either matched or mismatched targets (Table1). Only three pairs of HLA-C matched individuals were tested because it was difficult to find enough uninfected individuals who only matched at HLA-C alleles without additionally matching HLA-A or HLA-B alleles in our cohort. We noted a significant diminution in killing between individuals who matched both HLA-B alleles compared to those who mismatched for all alleles (Figure1D).
While MLR is likely to be independent of HIV infection, especially since the samples used are from uninfected individuals, it is possible that these responses are skewed in the presence of HIV infection. This may especially be the case for the observation that we saw effects at the HLA-B allele but not the others[1, 20]. To ascertain whether HIV-infection itself is responsible for the observed differences, we used a 7-AAD killing assay, were killing is measured by increases in 7-AAD and no viral infection is needed (as in the p24 killing assay). PBMC from two individuals for each class I allele pair were used as targets, one matching and one mismatching all HLA class I alleles from the effector CD8 T-cells. Killing was measured by an increase in 7-AAD (Figure1E). HLA-A and HLA-C alleles did not show differences among match and mismatch combinations. Taking the area under the curve for the 7-AAD data, we observed a significant increased killing when differences between matched and mismatched HLA-B allele experiments were compared. Again, this was not seen with HLA-A or HLA-C (Figure1F). Lastly, when we compared the differences in the fold-increase in 7-AAD between match and mismatched samples, differences where only seen between samples matching at HLA-B and their paired mismatch samples (p=0.0005, data not shown).
In this report killing of CD4+ targets was diminished when HLA-B alleles were matched relative to paired allogeneic cells that are mismatched at all class I alleles. The fact that proliferation data demonstrating differences only if HLA-B plus one HLA-C allele were matched maybe due to the strong linkage disequilibrium between HLA-B and HLA-C alleles. Moreover, the killing assay is more reminiscent of the in vivo biology whereby the allogeneic effect is most likely to function via a killing mechanism than through a proliferative mechanism. Overall, these data support allogeneic responses as a possible mechanism for protection in HIV-discordant couples and mother and infants who do not share HLA-B alleles[1, 5].
While other immune factors such as anti-HLA antibodies[21, 22] have been shown to inhibit HIV-infection, these seem less likely to be playing a role in the increased susceptibility to infection since in mother-infant pairs these antibodies were not found to be associated with protection[23, 24]. Resistance to HIV-1 infection has also been shown in women alloimmunized with their partner’s lymphocytes. In these studies, down regulation of CCR5 on T-cells and an increase in the secretion of antiviral factors as a result of allogeneic responses were demonstrated[7]. Although the down-regulation of CCR5 is clearly not playing a role in our studies due to the fact that we infected target CD4 T-cells prior to exposure to the effector CD8 T-cells, the secretion of anti-viral factors maybe playing a role in the differential killing observed. However, our assays cannot discern this effect. Other non-mutually exclusive mechanisms to explain HLA-mediated effects influencing HIV acquisition include HLA interactions with NK cell receptors that could mediate innate immunity and modulate HIV transmission[25, 26]. The percent NK cells in our cultures was <2.8% by measuring CD3-CD8+ cells (range 0.1–2.8%, mean=1%, median=0.9%) and therefore unlikely to be mediating this effect. Finally, viruses that have escaped T-cell recognition in a donor could be more easily transmitted to a recipient if HLA class I alleles are shared.
In our opinion these data best support the explanation that differences in HLA between partners could induce allogeneic CD8+ T-cell responses that eliminate incoming infected cells reducing transmission. The fact that allogeneic responses mediated by HLA-B are the only responses that significantly affect cell killing and proliferation can partly be explained by the increase in precursor frequency observed against HLA-B alleles relative to HLA-A demonstrated in former studies by others [27, 28]. Why HLA-B alleles have an increase in precursor frequency as shown by others and why this results in diminished killing of matched HLA-B targets is not clear. A further understanding of the unique aspects of these HLA-B-associated effects is warranted as a possible alternative HIV vaccine design based on allogeneic immunity.
Acknowledgments
All flow cytometry experiments were performed by the UAB CFAR Flow Cytometry Core/Joint UAB Flow Cytometry Facility which are funded in part by NIH/NIAID P30 AI27767.
Funding: This work was supported by NIH grant AI064060 awarded to EH and a developmental program award from UAB Center for AIDS Research, P30 AI027767 awarded to SS.
Footnotes
AUTHOR CONTRIBUTIONS:
NS, YW, VYD and SS performed experiments; JT HLA-typed the samples, SS analyzed the data, PAG, EH & SS designed experiments and wrote the manuscript.
Disclosure of conflict of Interest: The authors have no competing financial interests.
References
- 1.Dorak MT, Tang J, Penman-Aguilar A, Westfall AO, Zulu I, Lobashevsky ES, et al. Transmission of HIV-1 and HLA-B allele-sharing within serodiscordant heterosexual Zambian couples. Lancet. 2004;363:2137–2139. doi: 10.1016/S0140-6736(04)16505-7. [DOI] [PubMed] [Google Scholar]
- 2.Lockett SF, Robertson JR, Brettle RP, Yap PL, Middleton D, Leigh Brown AJ. Mismatched human leukocyte antigen alleles protect against heterosexual HIV transmission. J Acquir Immune Defic Syndr. 2001;27:277–280. doi: 10.1097/00126334-200107010-00010. [DOI] [PubMed] [Google Scholar]
- 3.Kuhn L, Abrams EJ, Palumbo P, Bulterys M, Aga R, Louie L, et al. Maternal versus paternal inheritance of HLA class I alleles among HIV-infected children: consequences for clinical disease progression. AIDS. 2004;18:1281–1289. doi: 10.1097/00002030-200406180-00006. [DOI] [PubMed] [Google Scholar]
- 4.MacDonald KS, Embree J, Njenga S, Nagelkerke NJ, Ngatia I, Mohammed Z, et al. Mother-child class I HLA concordance increases perinatal human immunodeficiency virus type 1 transmission. J Infect Dis. 1998;177:551–556. doi: 10.1086/514243. [DOI] [PubMed] [Google Scholar]
- 5.Mackelprang RD, John-Stewart G, Carrington M, Richardson B, Rowland-Jones S, Gao X, et al. Maternal HLA homozygosity and mother-child HLA concordance increase the risk of vertical transmission of HIV-1. J Infect Dis. 2008;197:1156–1161. doi: 10.1086/529528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polycarpou A, Ntais C, Korber BT, Elrich HA, Winchester R, Krogstad P, et al. Association between maternal and infant class I and II HLA alleles and of their concordance with the risk of perinatal HIV type 1 transmission. AIDS Res Hum Retroviruses. 2002;18:741–746. doi: 10.1089/08892220260139477. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Tao L, Mitchell E, Bravery C, Berlingieri P, Armstrong P, et al. Allo-immunization elicits CD8+ T cell-derived chemokines, HIV suppressor factors and resistance to HIV infection in women. Nat Med. 1999;5:1004–1009. doi: 10.1038/12440. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Underwood J, Vaughan R, Harmer A, Doyle C, Lehner T. Allo-immunization elicits CCR5 antibodies, SDF-1 chemokines, and CD8-suppressor factors that inhibit transmission of R5 and X4 HIV-1 in women. Clin Exp Immunol. 2002;129:493–501. doi: 10.1046/j.1365-2249.2002.01936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kingsley C, Peters B, Babaahmady K, Pomeroy L, Rahman D, Vaughan R, et al. Heterosexual and homosexual partners practising unprotected sex may develop allogeneic immunity and to a lesser extent tolerance. PLoS One. 2009;4:e7938. doi: 10.1371/journal.pone.0007938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruhl P, Kerschbaum A, Zimmermann K, Eibl MM, Mannhalter JW. Allostimulated lymphocytes inhibit replication of HIV type 1. AIDS Res Hum Retroviruses. 1996;12:31–37. doi: 10.1089/aid.1996.12.31. [DOI] [PubMed] [Google Scholar]
- 11.Liu H, Ohashi T, Masuda T, Zhou X, Kubo M, Kannagi M. Suppression of HIV-1 replication by HIV-1-irrelevant CD8+ cytotoxic T lymphocytes resulting in preservation of persistently HIV-1-infected cells in vitro. Viral Immunol. 2003;16:381–393. doi: 10.1089/088282403322396172. [DOI] [PubMed] [Google Scholar]
- 12.Pinto LA, Sharpe S, Cohen DI, Shearer GM. Alloantigen-stimulated anti-HIV activity. Blood. 1998;92:3346–3354. [PubMed] [Google Scholar]
- 13.Ohashi T, Kubo M, Kato H, Iwamoto A, Takahashi H, Fujii M, et al. Role of class I major histocompatibility complex-restricted and -unrestricted suppression of human immunodeficiency virus type 1 replication by CD8+ T lymphocytes. J Gen Virol. 1999;80(Pt 1):209–216. doi: 10.1099/0022-1317-80-1-209. [DOI] [PubMed] [Google Scholar]
- 14.Tang J, Wilson CM, Meleth S, Myracle A, Lobashevsky E, Mulligan MJ, et al. Host genetic profiles predict virological and immunological control of HIV-1 infection in adolescents. AIDS. 2002;16:2275–2284. doi: 10.1097/00002030-200211220-00007. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka Y, Ohdan H, Onoe T, Asahara T. Multiparameter flow cytometric approach for simultaneous evaluation of proliferation and cytokine-secreting activity in T cells responding to allo-stimulation. Immunol Invest. 2004;33:309–324. doi: 10.1081/imm-120038079. [DOI] [PubMed] [Google Scholar]
- 16.Sabbaj S, Pass RF, Goepfert PA, Pichon S. Glycoprotein B vaccine is capable of boosting both antibody and CD4 T-cell responses to cytomegalovirus in chronically infected women. J Infect Dis. 2011;203:1534–1541. doi: 10.1093/infdis/jir138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akinsiku OT, Bansal A, Sabbaj S, Heath SL, Goepfert PA. Interleukin-2 production by polyfunctional HIV-1-specific CD8 T cells is associated with enhanced viral suppression. J Acquir Immune Defic Syndr. 2011;58:132–140. doi: 10.1097/QAI.0b013e318224d2e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bansal A, Carlson J, Yan J, Akinsiku OT, Schaefer M, Sabbaj S, et al. CD8 T cell response and evolutionary pressure to HIV-1 cryptic epitopes derived from antisense transcription. J Exp Med. 2010;207:51–59. doi: 10.1084/jem.20092060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim GG, Donnenberg VS, Donnenberg AD, Gooding W, Whiteside TL. A novel multiparametric flow cytometry-based cytotoxicity assay simultaneously immunophenotypes effector cells: comparisons to a 4 h 51Cr-release assay. J Immunol Methods. 2007;325:51–66. doi: 10.1016/j.jim.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiepiela P, Leslie AJ, Honeyborne I, Ramduth D, Thobakgale C, Chetty S, et al. Dominant influence of HLA-B in mediating the potential co-evolution of HIV and HLA. Nature. 2004;432:769–775. doi: 10.1038/nature03113. [DOI] [PubMed] [Google Scholar]
- 21.Shearer GM, Boasso A. Alloantigen-based AIDS vaccine: revisiting a “rightfully” discarded promising strategy. F1000 Med Rep. 2011;3:12. doi: 10.3410/M3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stott EJ. Anti-cell antibody in macaques. Nature. 1991;353:393. doi: 10.1038/353393a0. [DOI] [PubMed] [Google Scholar]
- 23.Luscher MA, Choy G, Embree JE, Nagelkerke NJ, Bwayo JJ, Njenga S, et al. Anti-HLA alloantibody is found in children but does not correlate with a lack of HIV type 1 transmission from infected mothers. AIDS Res Hum Retroviruses. 1998;14:99–107. doi: 10.1089/aid.1998.14.99. [DOI] [PubMed] [Google Scholar]
- 24.Luscher MA, Choy G, Njagi E, Bwayo JJ, Anzala AO, Ndinya-Achola JO, et al. Naturally occurring IgG anti-HLA alloantibody does not correlate with HIV type 1 resistance in Nairobi prostitutes. AIDS Res Hum Retroviruses. 1998;14:109–115. doi: 10.1089/aid.1998.14.109. [DOI] [PubMed] [Google Scholar]
- 25.Martin MP, Gao X, Lee JH, Nelson GW, Detels R, Goedert JJ, et al. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat Genet. 2002;31:429–434. doi: 10.1038/ng934. [DOI] [PubMed] [Google Scholar]
- 26.Winchester R, Pitt J, Charurat M, Magder LS, Goring HH, Landay A, et al. Mother-to-child transmission of HIV-1: strong association with certain maternal HLA-B alleles independent of viral load implicates innate immune mechanisms. J Acquir Immune Defic Syndr. 2004;36:659–670. doi: 10.1097/00126334-200406010-00002. [DOI] [PubMed] [Google Scholar]
- 27.Roelen DL, van Bree SP, van Beelen E, Schanz U, van Rood JJ, Claas FH. Cytotoxic T lymphocytes against HLA-B antigens are less naive than cytotoxic T lymphocytes against HLA-A antigens. Transplantation. 1994;57:446–450. doi: 10.1097/00007890-199402150-00023. [DOI] [PubMed] [Google Scholar]
- 28.Zhang L, van Bree S, van Rood JJ, Claas FH. The effect of individual HLA-A and -B mismatches on the generation of cytotoxic T lymphocyte precursors. Transplantation. 1990;50:1008–1010. doi: 10.1097/00007890-199012000-00022. [DOI] [PubMed] [Google Scholar]