Abstract
The androgen receptor (AR) remains the major oncogenic driver of prostate cancer, as evidenced by the efficacy of androgen deprivation therapy (ADT) in naïve patients, and the continued effectiveness of second generation ADTs in castration resistant disease. However, current ADTs are limited to interfering with AR ligand binding, either through suppression of androgen production or the use of competitive antagonists. Recent studies demonstrate 1) the expression of constitutively active AR splice variants that no longer depend on androgen, and 2) the ability of AR to signal in the cytoplasm independently of its transcriptional activity (non-genomic); thus highlighting the need to consider other ways to target AR. Herein, we review canonical AR signaling, but focus on AR non-genomic signaling, some of its downstream targets and how these effectors contribute to prostate cancer cell behavior. The goals of this review are to 1) re-highlight the continued importance of AR in prostate cancer as the primary driver, 2) discuss the limitations in continuing to use ligand binding as the sole targeting mechanism, 3) discuss the implications of AR non-genomic signaling in cancer progression and therapeutic resistance, and 4) address the need to consider non-genomic AR signaling mechanisms and pathways as a viable targeting strategy in combination with current therapies.
Keywords: Androgen Receptor, non-genomic signaling, prostate cancer, therapy
Introduction
Prostate cancer is a leading cause of cancer mortality in men in the U.S. [1]. The androgen receptor (AR) still remains the major oncogenic driver of prostate cancer, whether it be localized, castration resistant, or metastatic disease. Several lines of evidence support this AR dependence. First, androgen deprivation therapy (ADT) is wholly and dramatically effective at putting patients with naïve tumors into remission [2]. Second, even though these same patients will regress as their tumors become resistant to ADT, AR is retained and still highly expressed in those tumors, often amplified or mutated [3–5]. Third, treatment of these patients with more potent second generation ADTs, such as Enzalutamide or Abiraterone, leads to further suppression of the resistant tumors, even if it is ultimately not curative [6]. Fourth, laboratory studies demonstrate that genetically inducing the loss of AR in castration-resistant tumor cell lines, results in the death of these cells [7–9]. Recent evidence indicates that one resistance mechanism to second generation ADTs, is the generation of constitutively active AR splice variants that no longer depend on androgen [10]. A second resistance mechanism may involve AR function in the cytoplasm. Enzalutamide inhibits AR nuclear translocation [11] leading to cytoplasmic localized AR and elevated Src signaling [12]. Elevated Src activity, a known AR cytoplasmic target [13–15], is associated with a subset of Enzalutamide-resistant tumors [16]. These findings necessitate the re-evaluation of cytoplasmic AR functions, sometimes referred to as non-genomic signaling, and the implications for relying too heavily on targeting only the nuclear functions of AR.
I. The Androgen Receptor and Canonical Transcription
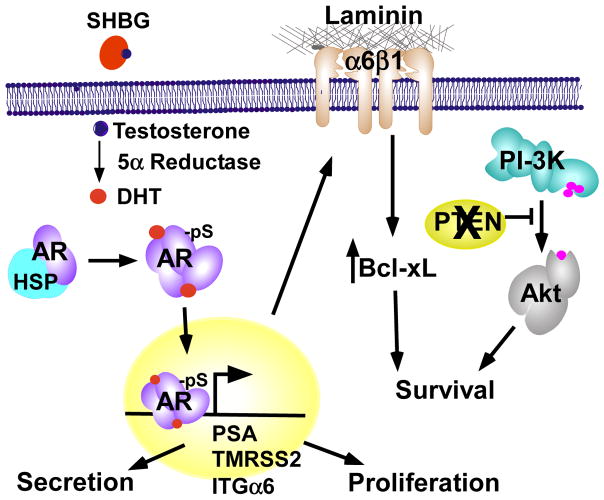
AR is a member of the nuclear steroid receptor family [17]. It is encoded by a single copy gene, nearly 90 Kb in size, and located on the q-arm of the X chromosome (Xq11.2). Once transcribed and translated, AR has a molecular weight around 80–110 kDa. AR, like other members of the steroid nuclear receptor family, is characterized by four functional domains. AR has an N-terminal transactivation domain (AF-1), a DNA binding domain (DBD) with two zinc fingers motifs and AR dimerization domain, a hinge region, and a ligand binding domain (LBD) containing a second activation domain (AF-2) [18–20]. AF-2 is involved in protein-protein interactions that aid in recruitment of co-activators that contain LXXLL motifs [21]. AR also has a poly-glutamine tract at residues 448–472 that varies in length between individuals [22]. This poly-glutamine stretch ranges from 8–31 repeats in normal individuals; however, expansion of this poly-glutamine tract is reported to cause spinal and bulbar muscular atrophy (also known as Kennedy’s Disease) [23]. Conversely, if the poly-glutamine stretch is shortened, AR is reported to be more transcriptionally active [24]. The hinge region possesses a nuclear localization sequence (NLS) which is exposed upon androgen binding [25, 26]. The LBD is composed of twelve alpha-helices and upon androgen binding, helix 12 becomes stabilized. This leads to the formation of a hydrophobic binding pocket for ligands such as androgen or other proteins that have the preferred FXXLF motif [22]. The LBD, in the absence of ligand, aides in keeping AR localized within the cytosol where it is kept in an inactivate conformation by heat shock proteins (Hsp) including Hsp 90, Hsp 70, Hsp 56, and p23. Circulating testosterone is delivered to AR-positive cells attached to sex hormone binding globulin (SBHG), and is then reduced intracellularly to dihydrotestosterone (DHT) by 5α-reductase (Figure 1). When DHT binds the LBD of AR, it displaces Hsps. AR then homo-dimerizes, is phosphorylated, undergoes a conformational change which exposes the nuclear localization sequence (NLS), and translocates to the nucleus.
Figure 1. Canonical androgen receptor-mediated transcription in tumor cells.
Circulating testosterone is delivered to AR positive cells attached to sex hormone binding globulin (SHBG), which is then reduced intracellularly to dihydroxytestosterone (DHT) by 5α-reductase. When DHT binds AR, it displaces heat shock protein (Hsps). AR then homo-dimerizes, is phosphorylated (pS), and translocates to the nucleus. Dimeric AR binds to androgen response elements in the promoters or enhancers of target genes to enhance or suppress their transcription. Targets include PSA and TMPRSS2 which are secreted into the lumen, integrin α6 (ITGα6) which promotes survival through adhesion to laminin, and cell cycle regulators that promote proliferation.
Once AR is in the nucleus, dimeric AR binds to androgen response elements (AREs) in the promoters or enhancers of target genes. ARE sequences are usually 6 base pair long “half site” direct or inverted repeats separated by 3 base pairs [18, 27, 28]. There are two classes of AREs; class I possess typical guanine residues and class II has atypical sequences and features that confer synergistic transcriptional activity to AR [28]. Interestingly, AR is also reported to bind upstream of promoters and enhancers of genes that do not possess a putative AREs [29, 30]. Once AR binds AREs, it recruits co-activators, co-repressors, and components of the pre-initiation complex [22, 31], which can either activate or repress transcription.
II. AR Function in Normal versus Cancerous Prostate
The human prostate gland is composed of epithelial-lined secretory ducts embedded in a smooth muscle-enriched stroma [32, 33]. The epithelium contains two cell types: a basal epithelium overlaid with luminal cells (Figure 2). AR is expressed within the luminal layer and in the stroma, where it has distinct roles. AR expression in the luminal cells is dispensable for gland development; however, it is required for the secretory function of luminal cells [20, 32–35]. In normal prostate epithelium, the primary function of AR is to induce expression of genes required to promote terminal differentiation, suppress proliferation, and promote secretion [36–40]. In contrast, proper gland development also requires AR expression in the stroma compartment. In the stroma, AR is thought to promote the production of growth factors, such as andromedins [41] or keratinocyte growth factor (KGF) [42], required for differentiation of the luminal cells [20, 34].
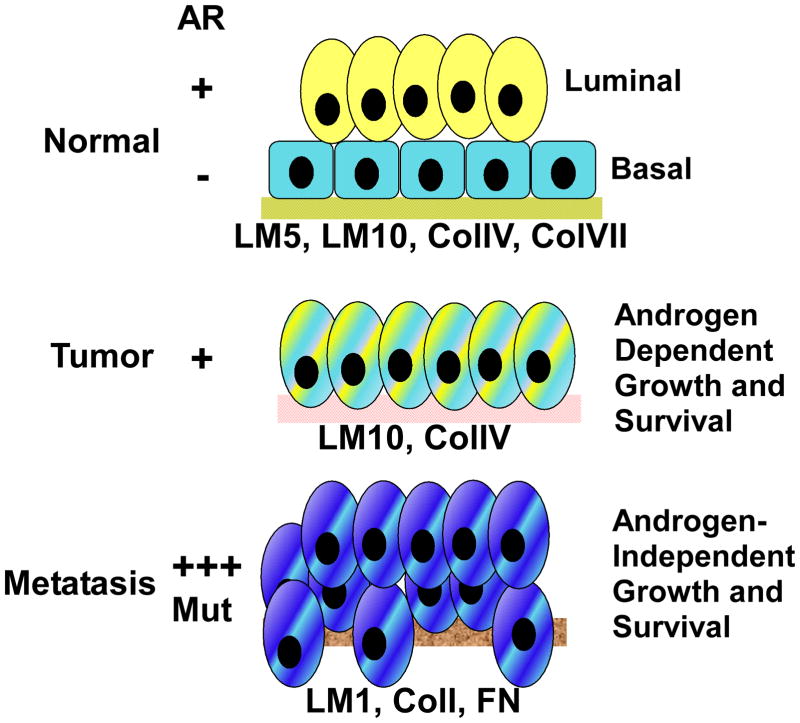
Figure 2. The journey of a normal prostate to prostate cancer.
The normal human gland consists of a basal cell layer bound to extracellular matrix (ECM) via integrins with a luminal layer on top expressing AR. AR functions in these cells to suppress growth and promote secretion. Basal cells are lost and ECM is altered in prostate tumors and the luminal-like cells co-express AR and integrins. AR drives androgen-dependent growth and survival of the tumor. Metastatic tumors become castration-resistant following ADT, which is accompanied by increased AR expression or mutational activation. Cells are invasive and the ECM composition is dictated by the metastatic site. Tumors are still dependent on AR for growth and survival, but no longer need physiological levels of androgen.
During prostate cancer development, the distinction between basal and luminal cells is lost, accompanied by a complete loss of discernable basal cells (Figure 2). The molecular basis for this apparent loss is unknown, but may involve some differentiation aspects related to AR. What results are tumor cells with markers and functions derived from both basal and luminal cells. For instance, prostate tumors often co-express both basal and luminal keratins, K5 and K8 respectively. They co-express basal cell integrins and luminal AR [34, 43–45]. One idea is that the tumor cells arise from a transient amplifying cell population or differentiation intermediate where these molecules may normally be transiently co-expressed [45].
But by far the most striking aspect of prostate cancer, is the change in how AR behaves. While dispensable for survival and proliferation within normal prostate epithelium, it is absolutely required for prostate cancer survival and proliferation. The basis for this mechanistic switch is essentially unknown. However, the answer may lie in part with the relatively common occurrence of AR-regulated cancer-specific gene fusions. The first described fusions involved the juxtaposition of the 5′ ARE-containing promoter from the AR target gene, transmembrane protease serine 2 (TMPRSS2), to the coding sequence of several Ets family members including Erg, ETV1, ETV4, ETV5 and FLI1 [46, 47]. Therefore, the fusion leads to the production of Ets transcription factors, which potentially drive proliferation and suppress differentiation, under the control of AR. This could then confer on AR the ability to promote cell survival and proliferation in part by differentially targeting transcription to a new set of genes through Ets-like proteins [48–50].
However, not all tumors harbor these fusions [46]. Whole genome chromatin immunoprecipitation studies (ChIP-Seq) that interrogated sites of AR binding in tumor cells identified many targets not found in normal cells suggesting AR itself may bind aberrant targets in cancer cells [29, 51]. When combined with expression data, it was these off-target AR genes that more highly correlated with cancer pathogenesis [52].
One of the most well characterized AR target genes is PSA. PSA is a serine protease [53] secreted into the ducts of normal prostates, which can also be detected in the serum of men. Because AR signaling is dramatically increased in prostate cancer, detection of elevated levels of PSA in serum is a reflection of elevated activity in the gland and forms the basis for the PSA test. However, it can also reflect elevated activity due to other pathologies including prostatitis and BPH, limiting its specificity for cancer. Because elevated PSA is not required for tumorigenesis, but rather a bystander event, its levels may not truly reflect tumor burden or disease severity. This limited predictive value has caused the United States Preventative Task Force in 2012 to recommend against using the PSA test stating that PSA testing may lead to over-diagnosis and over-treatment [54]. Thus, understanding and discovery of improved biomarkers remains a critical need in the field of prostate cancer research.
III. Androgen Receptor Antagonists
Because most prostate tumors are exquisitely dependent on AR, inhibiting AR is the primary and most effective therapeutic strategy. Current ADTs are designed to prevent AR function by blocking androgen binding to AR. This is accomplished through three primary mechanisms. The first is to reduce androgen levels in the body or tissues using chemical castration agents such as luteinizing-hormone releasing hormones (LHRH) agonists or antagonists (i.e. leuprolide or abarelix). These function primarily by down-regulating the LHRH receptor and preventing androgen synthesis in the hypothalamus. Newer antagonists, abiraterone acetate, VT-464, and TAK-700, inhibit 17 α-hydroxylase/C17,20 lyase (CYP17A), an enzyme expressed in testicular, adrenal, and prostatic tumor tissue and is responsible for converting pregnenolone into androgen [55–59]. Another part of the androgen synthesis pathway is the conversion of androstenedione, a relatively weak androgen, into testosterone [60]. This reaction is catalyzed by aldo-keto reductase family 1 member 3 (AKR1C3) (also known as HSD17β) [60]. AKR1C3 inhibitors are currently under development [61]. A second mechanism to block androgen is to target 5-α reductase with finasteride and dutasteride, which blocks the intracellular conversion of testosterone to its higher binding affinity derivative DHT [62, 63].
The third approach is to block androgen binding to AR using competitive antagonists (i.e. bicalutamide, flutamide). However, these inhibitors have limitations. These include a 30-fold weaker binding affinity for AR LBD than DHT [64]. Also, a known AR T887A mutation allows these antagonists to act as agonists. Furthermore, they do not prevent AR nuclear translocation [65–68]. Combined targeting of circulating androgens and use of competitive antagonists, are commonly prescribed during ADT. ADT generally causes prostate cancer remission in 80–90% of patients, resulting in a median progression-free survival of 2.5–3 years [2, 69–71]. However, after this remission period, the cancer becomes castration resistant [2] and AR is still found to be persistently active during this phase of the disease. Given this notion of AR still being active and its ability to elude current competitive antagonists, next-generation AR antagonists that are non-competitive or have a greater AR affinity, were developed [72, 73]. One of these is enzalutamide (aka MDV3100) which, unlike bicalutamide, prevents AR nuclear translocation [73]. While initially effective, many responders later develop resistance after 47 weeks [6].
IV. Mechanisms that Confer Resistance to Anti-androgen Therapy
Despite attempts to improve therapeutic targeting of AR, resistance still remains a major obstacle. The over-expression or amplification of AR in 20–30% of castration-resistant cases [3–5] as well as gain-of-function point mutations have long been recognized as major resistance mechanisms [74]. AR mutations are observed in approximately 10–25% of CRPC [75, 76]. Amplified AR expression results in increased sensitivity to lower levels of androgen [77] and mutations often result in increased ability to bind other steroids, such as corticosteroids or estrogen. Some mutations convert antagonists to agonists [68, 78].
While enzalutamide displayed effectiveness initially in patients who failed standard ADT, many responders later developed resistance [6]. Several mechanisms for this resistance have been reported. One mechanism is a mutation in the LBD of AR which converts phenylalanine 876 to leucine (F876L) [16]. Another is emergence of AR splice variants lacking the LBD, creating a constitutively active AR [10, 79]. Finally, enzalutamide resistance also correlates with increased Src signaling.
The idea that tumor cells can make their own androgen, and thus elude the standard LHRH ADT treatment was recently demonstrated [32]. Androgens can be synthesized de novo from cholesterol via enzymatic steps that are catalyzed by cytochrome P450 (CYP) members [60, 80]. Cholesterol undergoes a cleavage reaction by the enzyme desmolase (CYP11A1) to convert it into pregnenolone that can be further converted into progesterone by 3β-hydroxysteroid dehydrogenase type 1 (3βHSD1) [60]. After these steps, pregnenolone or progesterone can be further converted into 17-OH pregnenolone or 17-OH progesterone by CYP17A1 [80]. These are further converted into the metabolic intermediates dehydroepiandrosterone (DHEA) or androstenedione respectively, which are converted to testosterone and then reduced to DHT by 5α reductase [80]. These enzymes are upregulated in CRPC to overcome the lack of circulating androgen. Further selection and dependency on this pathway was demonstrated by the recent discovery of a mutation in the androgen-synthesizing enzyme, 3β-hydroxysteroid dehydrogenase type 1 (3βHSD1) at residue N367T. This mutation confers resistance to poly-ubiquitylation leading to an accumulation of DHT [81]. It is unclear whether this mutation elicits resistance to abiraterone and other therapies. Enhanced androgen production can also be achieved by elevated expression of both type 1 and 2 5-α reductase [82]. Under normal conditions, type 1 5-α reductase is expressed in various cell types such as fibroblasts and skin cells while type 2 5-α reductase is expressed in prostate. However, both isozymes of 5-α reductase are expressed in prostate cancer [63].
AR regulates transcription by recruiting various co-activators such as p300-CBP and p160 [83, 84]. These are often over-expressed in prostate cancer resulting in increased AR transcriptional activity. The p160 co-activator, SRC-3, is degraded by the E3 ubiquitin ligase adaptor speckle-type poxvirus and zinc finger (POZ) domain protein (SPOP) [85]. However, in patients SPOP has a missense mutation in its substrate binding domain causing it not bind to SRC-3 and stabilizing SRC-3 [86, 87]. When SPOP is found mutated, DEK a SPOP substrate and onco-protein [88], was found to be up-regulated and contributed to tumor cell invasion [89].
Most recently, AR splice variants were discovered where exons 5 to 7 were deleted resulting in loss of the LBD [90]. This AR-variant (AR-V) is capable of nuclear translocation, ARE binding, and can activate AR target genes in the absence of androgen. Typically AR-V is detected in tumors that also express full length AR [91]. It is controversial as to whether AR-V function is dependent on full-length AR [90–92]. AR-V is detectable in castration resistant tumors and its expression is associated with shorter patient survival times and with enzalutamide resistance [79, 93–95]. Splice variant 7 of AR (ARV-7) has also drawn a lot of attention given its clinical significance in CRPC [10, 96].
AR can be activated by other signal transduction pathways, including growth factors, MAPK, Src, PKC, and PI3-K/Akt [97–101]. AR interaction with signaling scaffold proteins can lead to AR activation in the absence of ligand [102]. Receptor for activated C kinase 1 (RACK1), a protein kinase C (PKC) anchoring protein, was shown in a yeast two hybrid screen to be an AR interacting protein [103]. In vitro, RACK1 promotes AR nuclear translocation upon PKC activation in the absence of androgen [103]. Non-receptor tyrosine kinase Src can phosphorylate AR at Y534 within the hinge region of AR leading to increased AR translocation to the nucleus and enhanced transcriptional activation.
For all of these resistance mechanisms, there are currently no effective alternative therapies. Nor will their inhibition be accomplished by continuing to focus on agents that target exclusively the androgen component. A better understanding of these resistance mechanisms, how to measure or predict their occurrence, combined with a multi-targeted approach will be required before we can hope to fully eradicate castration-resistant prostate cancer.
V. Non-Nuclear AR Signaling
All of the therapy resistance mechanisms outlined above, largely focus on the nuclear transcriptional activity of AR. However, the use of newer therapies that displace AR from the nucleus into the cytoplasm may have unintended consequences as AR has known functions in the cytoplasm, which is often referred to as non-genomic signaling [104–107]. A primary characteristic of non-nuclear signaling is the rapidity with which it occurs. While still in the cytosol steroid receptors can undergo several protein-protein interactions within seconds to minutes after stimulation with steroids [108]. This ability is not limited to AR as it has been reported for several other steroid receptors including estrogen, glucocorticoids [109], thyroid hormone [110], and progesterone [111]. Signaling molecules activated by AR and other steroid receptors in a non-genomic fashion include Src family kinases (SFK), Ras, MAPK, Akt, L-type calcium channels, PKC, PLC, EGFR, and other second messenger proteins [112–117]. Furthermore, the cytoplasmic function of AR is observed in non-prostate cells, such as fibroblasts, where the levels of AR are relatively low, and AR translocation in response to androgen is not observed [118].
The most well studied signaling molecule activated by steroid receptors is Src. Src is a cytosolic 60 kDa protein composed of seven functional domains: a myristoylated amino-terminus, a unique domain specific to each SFK member, a Src homology domain 3 (SH3), Src homology domain 2 (SH2), a linker region, a catalytic domain, and a COOH-terminal tail [119, 120]. The myristic acid moiety allows Src to localize to the inner membrane. When in its closed inactive conformation, the SH2 domain binds to its tyrosine phosphorylated Y530 tail and the SH3 domain binds to PXXP sequences within the linker region [119]. Upon activation, de-phosphorylation of the Y530 tail facilitates Src unfolding and activation loop phosphorylation site Y419 opens the catalytic domain. Further stabilization of activated Src or an alternative mechanism for activation can be enhanced by SH3-mediated binding to PXXP motifs in associated molecules. Although mutations in the C-terminal Y530 domain of Src generates a potent oncogene, such mutations are rare in human cancers. Nonetheless aggressive tumors have elevated Src tyrosine phosphorylation gene signatures that correlate with poor outcomes, including prostate cancer [12]. The mechanisms that lead to aberrant Src signaling in tumors is not completely clear, but growth factor signaling or elevated Src expression are known stimulators. Several SFKs are reportedly elevated in prostate cancer. Src is over-expressed in some prostate cancer cell lines as is a related member Abl [121, 122]. Other elevated SFKs in prostate cancer include Fyn and Yes [123, 124]. The SFK member FGR is significantly up-regulated in tissues from patients with castration-resistant disease [125]. Furthermore, elevation in SFK expression or activity is often correlated with poorer outcomes [125]. Thus, SFKs may play an important role in driving lethal prostate cancer, particularly in castration-resistant disease.
Within steroid-dependent tumors, some of the elevation in Src activity likely occurs through interactions with the receptors. While several steroid receptors can activate Src, the mechanisms involved appear to be different. For ER, Src can bind directly via its SH2 domain to tyrosine-phosphorylated ER. However, further studies demonstrate that ER-α and ER-β bind to LXXLL motifs in the scaffold protein modulator of non-genomic actions of the estrogen receptor (MNAR/PELP1) to interact with Src while localized to the cytosol [126, 127]. This ER-α-MNAR-Src complex leads to Src activation. For AR, the PXXP sequences within the AR linker domain mediate binding through the Src SH3 domain [5, 14, 128]. AR can also form a tertiary complex with MNAR/PELP1 and Src [129] (Figure 3). Initially, Src is inactive within this complex. However, when AR binds Src, this leads to the activation of Src in this complex (AR/MNAR/Src) and the subsequent activation of a downstream effector, MEK [129]. This complex is androgen dependent in LNCaP cells, but is constitutively active in a castration-resistant LNCaP derivative cell line, C4-2.
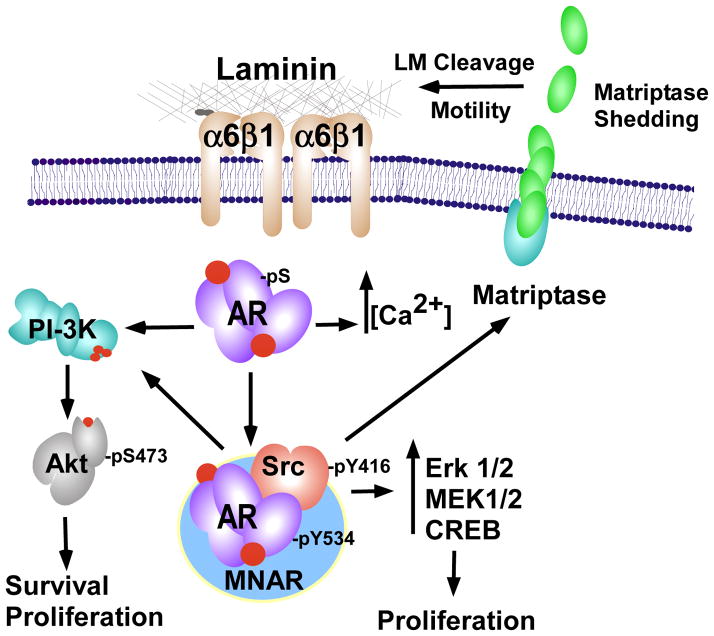
Figure 3. Non-genomic AR signaling.
Within the cytoplasm, active AR (mediated by androgen or constitutive kinase phosphorylation) binds the NMAR scaffold complex with Src. Src is activated by AR, and in turn AR is phosphorylated by Src (pY534). This complex can activate the MAPK/CREB pathways to promote proliferation. Src can also stimulate cell migration and Matrigel invasion through activation and shedding of Matriptase. PI3-K can be activated by AR either through Src or independently, leading to enhance proliferation and survival. AR can also independently stimulate intracellular calcium stores ([Ca2+]).
Another signaling molecule commonly activated by steroid receptors in a non-genomic fashion is MAPK [130]. Androgen stimulation of LNCaP or PC3 cells stably re-expressing wildtype AR for 2–60 minutes increased Erk-1/2 phosphorylation [130]. In another study, androgen activated Raf and Erk-2 within 2–5 minutes [14]. This was abrogated using either Casodex or Src inhibitor PP1 [14]. Introduction of AR into COS cells and subsequent androgen stimulation led to a complex of AR and Src [14]. Similar studies assessing non-genomic actions of AR and ER in an osteocytic cell line MLO-Y4 or COS cells highlighted the rapidity of steroid signaling and identified specific interactions between AR or ER and Src [14, 128].
Calcium signaling is a major player in prostate cancer progression [131]. Several studies demonstrated that non-genomic actions of AR can lead to increased intracellular calcium [132, 133]. Different mechanisms have been proposed, one involving a GPCR and another involving EGFR [134, 135]. Non-genomic action of AR on calcium signaling is also observed in other calcium-regulated cells, including neurites, smooth muscle, and heart [136–138].
VI. Functional Consequences of Non-Nuclear AR Signaling
Proliferation
Many different events are activated by AR signaling, including proliferation. AR transcriptional activity plays an important role in G1/S-phase transition [139] and castration-resistant prostate cancer [140]. However, non-genomic signaling also influences cell proliferation (Figure 3). In quiescent NIH3T3 fibroblasts, androgen induces S-phase entry through AR association with Src, which stimulates phosphatidylinositol 3-kinase (PI3-K). NIH3T3 cells express relatively low levels of AR and these events occur in the absence of AR nuclear localization [118]. Inhibition of Src in LNCaP cells decreased AR-dependent, but androgen-independent cell proliferation induced by IL-8 [141].
Cell Survival
Non-genomic AR signaling also promotes tumor cell survival. One example is the ability of AR to activate PI3-K [142]. Vas deferens epithelial cells and PC3 cells made to stably express AR and stimulated with androgen for 10–30 minutes induced p-AKTSer473 activation. This activation was blocked by PI3-K inhibitor LY294002 or AR antagonist bicalutamide [142]. Another study demonstrated that stimulating PC3-AR cells for 10–30 minutes induced phosphorylation of Forkhead protein FKHR-L1 at Ser256 and Bad at Ser112; both involved in promoting cell survival [143]. In this study, an interaction between AR and the PI3-K p85α subunit was detected. Further reports demonstrated that non-genomic AR triggered Src-MEK-1/2-cAMP-response element binding protein (CREB) activation. This pathway stimulated prostate tumor cell proliferation and enhanced survival capabilities [129] (Figure 3).
Invasion and Metastasis
Many of the signaling pathways activated by steroid receptors are known regulators of cell migration, invasion, and metastasis. So while AR-dependent non-genomic regulation of cell proliferation and survival are well established, its ability to control invasion and metastasis is not fully characterized. Since metastatic spread of cancer decreases the quality of life and ultimately leads to death, it is critical to understand the mechanisms that AR uses to drive metastasis.
Src plays a well-known role in regulating cell migration and invasion through phosphorylation of many substrates including p130Cas, Shc, FAK, p190RhoGAP, paxillin, and CDCP1 [144, 145]. Clinically, Src is implicated in driving bone metastasis. The majority of breast cancers that metastasizes to the bone possess active Src [146], and a Src signature is detected in breast cancer bone metastasis [147]. In in vivo studies using murine models of breast cancer, inhibition of Src decreased the size of metastatic tumors and delayed the appearance of bone metastasis [148, 149]. In prostate cancer models, inhibition of Src decreased prostate cancer cell adhesion, migration, and invasion, and targeting Src and Abl with dasatinib attenuated lymph node metastasis of orthotopic PC3 xenografts [13, 150–153].
The ability of non-genomic steroid signaling to facilitate migration, invasion, and metastasis was initially identified in breast cancer and later shown to facilitate movement of cholonic smooth muscle [154]. While correlations between elevated AR and Src activity and invasive migration were reported for prostate cancer, the mechanisms by which this occurred were not examined. A recent study demonstrated that the ability of AR to enhance invasion of prostate cancer cell lines occurred independently of its nuclear localization and was dependent on Src [13]. The study further identified matriptase activation by Src to be involved in promoting non-genomic AR prostate cancer cell invasion [13]. Specifically, the initial cleavage and shedding of minto the medium occurred within 20 minutes of androgen stimulation, required Src and AR, and occurred in cells expressing a non-nuclear form of AR (Figure 3). Interestingly, 24 hours later new matriptase mRNA was induced, which was shown to be dependent on nuclear AR [155]
VII. Clinical Importance of Targeting Non-genomic AR Signaling
Treatment of tumor cells with enzalutamide leads to high levels of cytosolic AR [12]. This has been tied to increased Src activation and cell motility and may be a plausible reason for recurrent disease. In the cytosol AR can activate Src and Src can phosphorylate AR at Y534 [156]. Interestingly, Y534 phosphorylation sensitizes AR to low levels of androgen [156], creating the possibility that cytoplasmic AR may stimulate signaling independent of ligand. As highlighted earlier, non-genomic signaling can also activate the MAPK signaling pathway [130]. Thus, non-genomic AR could increase MAPK signaling, which is coordinately dysregulated along with Akt signaling in advanced prostate cancers [157–159]. Additionally, enhanced Ras signaling reduces androgen dependency and Ras/MAPK activation is associated with metastatic lesions [160, 161].
These findings demonstrate that after ADT, AR non-genomic substrates may still need pharmacological inhibition and targeting these non-genomic signaling pathways could offer additional therapies. Indeed Src, PI3-K, and MAPK signaling inhibitors are clinically available. However, given that L-type calcium channels can be stimulated by AR, and the possibility of blocking their activity offers another potential therapeutic approach [132, 133]. Targeting other AR non-genomic downstream targets such as matriptase or MNAR may also be sagacious avenues worth scientific exploration.
However, clinical trials clearly indicate that attempting to target Src, PI-3-K, or Ras/MAPK in isolation is not effective in prostate cancer patients. The continued presence of AR-genomic signaling in this context still needs to be addressed and allows ‘escape’ from these targeted therapies. Thus, combination therapies in which enzalutamide-mediated shuttling of AR into the cytoplasm to prevent nuclear AR action, reduction in tumor cell synthesis of androgens with abiraterone to lower androgen levels, while also targeting Src, MAPK, calcium channels, or other targets to block non-genomic signaling may be required to effectively reduce the oncogenic dependency driven by AR.
VIII. Emerging Alternatives for Targeting AR
Current ADTs are designed to prevent AR signaling by blocking androgen binding to AR or by blocking circulating androgens. However, there are no FDA approved antagonists available that currently target cytosolic AR or the AR-V splice variant. One alternative strategy to target AR is to promote its destruction, either through inhibition of Hsp90 [162] or by introduction of an AR mRNA hammerhead ribozyme [163]. Targeting the DNA-binding domain (DBD) would also be plausible, as it would occlude AR from binding to ARE sequences and suppress genomic transcription.
Emerging methodologies to target the N-terminus of AR, independently of ligand, are currently being investigated. These agents include niphatenone B, Sintokamide A, EPI-506, and EPI-001. Each of these targets the AF-1 domain of AR in the N-terminus and prevents AR from binding to AREs [164–166]. Each of these pharmacological agents demonstrate promising results in vitro and in vivo by repressing androgen/AR induced transcription. The greatest benefit, is that they would be effective in cells harboring the AR-V or other splice variants lacking the LBD.
Finally, although rare, there is a subset of prostate tumors that express very low levels or do not express AR at all due to a number of possible mechanisms including methylation [167, 168]. In these cases, targeting AR will not be effective no matter how it is done. However, in these AR negative tumors, several downstream signaling cascades remain expressed or constitutively active, that may represent effective therapeutic targets. As we improve AR targeting at multiple levels, we may find that the frequency at which non-AR tumors arise increases. Thus, this last final challenge of defining the non-AR pathways that keep these tumors alive will remain when all the others have been solved.
Acknowledgments
The authors were supported by the National Cancer Institute of the National Institutes of Health under award number R01CA154835 (C.K.M.) and minority supplement (J.C.Z.), a UNCF/Merck Postdoctoral Science Research Fellowship award (J.C.Z), and the Van Andel Research Institute (VARI). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Pienta KJ, Smith DC. Advances in prostate cancer chemotherapy: a new era begins. CA Cancer J Clin. 2005;55:300–318. doi: 10.3322/canjclin.55.5.300. [DOI] [PubMed] [Google Scholar]
- 3.Visakorpi T, Hyytinen E, Koivisto P, Tanner M, Keinanen R, Palmberg C, Palotie A, Tammela T, Isola J, Kallioniemi OP. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat Genet. 1995;9:401–406. doi: 10.1038/ng0495-401. [DOI] [PubMed] [Google Scholar]
- 4.Gelmann EP. Molecular biology of the androgen receptor. J Clin Oncol. 2002;20:3001–3015. doi: 10.1200/JCO.2002.10.018. [DOI] [PubMed] [Google Scholar]
- 5.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, Rosenfeld MG, Sawyers CL. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 6.Scher HI, Beer TM, Higano CS, Anand A, Taplin ME, Efstathiou E, Rathkopf D, Shelkey J, Yu EY, Alumkal J, Hung D, Hirmand M, Seely L, Morris MJ, Danila DC, Humm J, Larson S, Fleisher M, Sawyers CL C. Prostate Cancer Foundation/Department of Defense Prostate Cancer Clinical Trials. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1–2 study. Lancet. 2010;375:1437–1446. doi: 10.1016/S0140-6736(10)60172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li TH, Zhao H, Peng Y, Beliakoff J, Brooks JD, Sun Z. A promoting role of androgen receptor in androgen-sensitive and -insensitive prostate cancer cells. Nucleic Acids Res. 2007;35:2767–2776. doi: 10.1093/nar/gkm198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liao X, Tang S, Thrasher JB, Griebling TL, Li B. Small-interfering RNA-induced androgen receptor silencing leads to apoptotic cell death in prostate cancer. Mol Cancer Ther. 2005;4:505–515. doi: 10.1158/1535-7163.MCT-04-0313. [DOI] [PubMed] [Google Scholar]
- 9.Cohen MB, Rokhlin OW. Mechanisms of prostate cancer cell survival after inhibition of AR expression. J Cell Biochem. 2009;106:363–371. doi: 10.1002/jcb.22022. [DOI] [PubMed] [Google Scholar]
- 10.Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, Chen Y, Mohammad TA, Chen Y, Fedor HL, Lotan TL, Zheng Q, De Marzo AM, Isaacs JT, Isaacs WB, Nadal R, Paller CJ, Denmeade SR, Carducci MA, Eisenberger MA, Luo J. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371:1028–1038. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffman-Censits J, Kelly WK. Enzalutamide: a novel antiandrogen for patients with castrate-resistant prostate cancer. Clin Cancer Res. 2013;19:1335–1339. doi: 10.1158/1078-0432.CCR-12-2910. [DOI] [PubMed] [Google Scholar]
- 12.Efstathiou E, Titus M, Wen S, Hoang A, Karlou M, Ashe R, Tu SM, Aparicio A, Troncoso P, Mohler J, Logothetis CJ. Molecular characterization of enzalutamide-treated bone metastatic castration-resistant prostate cancer. Eur Urol. 2015;67:53–60. doi: 10.1016/j.eururo.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zarif JC, Lamb LE, Schulz VV, Nollet EA, Miranti CK. Androgen receptor non-nuclear regulation of prostate cancer cell invasion mediated by Src and matriptase. Oncotarget. 2015;6:6862–6876. doi: 10.18632/oncotarget.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Migliaccio A, Castoria G, Di Domenico M, de Falco A, Bilancio A, Lombardi M, Barone MV, Ametrano D, Zannini MS, Abbondanza C, Auricchio F. Steroid-induced androgen receptor-oestradiol receptor beta-Src complex triggers prostate cancer cell proliferation. EMBO J. 2000;19:5406–5417. doi: 10.1093/emboj/19.20.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Izumi K, Zheng Y, Li Y, Zaengle J, Miyamoto H. Epidermal growth factor induces bladder cancer cell proliferation through activation of the androgen receptor. Int J Oncol. 2012;41:1587–1592. doi: 10.3892/ijo.2012.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korpal M, Korn JM, Gao X, Rakiec DP, Ruddy DA, Doshi S, Yuan J, Kovats SG, Kim S, Cooke VG, Monahan JE, Stegmeier F, Roberts TM, Sellers WR, Zhou W, Zhu P. An F876L mutation in androgen receptor confers genetic and phenotypic resistance to MDV3100 (enzalutamide) Cancer Discov. 2013;3:1030–1043. doi: 10.1158/2159-8290.CD-13-0142. [DOI] [PubMed] [Google Scholar]
- 17.Lamb DJ, Weigel NL, Marcelli M. Androgen receptors and their biology. Vitam Horm. 2001;62:199–230. doi: 10.1016/s0083-6729(01)62005-3. [DOI] [PubMed] [Google Scholar]
- 18.Dehm SM, Tindall DJ. Androgen receptor structural and functional elements: role and regulation in prostate cancer. Mol Endocrinol. 2007;21:2855–2863. doi: 10.1210/me.2007-0223. [DOI] [PubMed] [Google Scholar]
- 19.Litvinov IV, De Marzo AM, Isaacs JT. Is the Achilles’ heel for prostate cancer therapy a gain of function in androgen receptor signaling? J Clin Endocrinol Metab. 2003;88:2972–2982. doi: 10.1210/jc.2002-022038. [DOI] [PubMed] [Google Scholar]
- 20.Agoulnik IU, Weigel NL. Androgen receptor action in hormone-dependent and recurrent prostate cancer. J Cell Biochem. 2006;99:362–372. doi: 10.1002/jcb.20811. [DOI] [PubMed] [Google Scholar]
- 21.Heery DM, Kalkhoven E, Hoare S, Parker MG. A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature. 1997;387:733–736. doi: 10.1038/42750. [DOI] [PubMed] [Google Scholar]
- 22.Heemers HV, Tindall DJ. Androgen receptor (AR) coregulators: a diversity of functions converging on and regulating the AR transcriptional complex. Endocr Rev. 2007;28:778–808. doi: 10.1210/er.2007-0019. [DOI] [PubMed] [Google Scholar]
- 23.La Spada AR, Wilson EM, Lubahn DB, Harding AE, Fischbeck KH. Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature. 1991;352:77–79. doi: 10.1038/352077a0. [DOI] [PubMed] [Google Scholar]
- 24.Giovannucci E, Stampfer MJ, Krithivas K, Brown M, Dahl D, Brufsky A, Talcott J, Hennekens CH, Kantoff PW. The CAG repeat within the androgen receptor gene and its relationship to prostate cancer. Proc Natl Acad Sci USA. 1997;94:3320–3323. doi: 10.1073/pnas.94.7.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jenster G, Trapman J, Brinkmann AO. Nuclear import of the human androgen receptor. Biochem J. 1993;293(Pt 3):761–768. doi: 10.1042/bj2930761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou ZX, Sar M, Simental JA, Lane MV, Wilson EM. A ligand-dependent bipartite nuclear targeting signal in the human androgen receptor. Requirement for the DNA-binding domain and modulation by NH2-terminal and carboxyl-terminal sequences. J Biol Chem. 1994;269:13115–13123. [PubMed] [Google Scholar]
- 27.Claessens F, Verrijdt G, Schoenmakers E, Haelens A, Peeters B, Verhoeven G, Rombauts W. Selective DNA binding by the androgen receptor as a mechanism for hormone-specific gene regulation. J Steroid Biochem Mol Biol. 2001;76:23–30. doi: 10.1016/s0960-0760(00)00154-0. [DOI] [PubMed] [Google Scholar]
- 28.Reid KJ, Hendy SC, Saito J, Sorensen P, Nelson CC. Two classes of androgen receptor elements mediate cooperativity through allosteric interactions. J Biol Chem. 2001;276:2943–2952. doi: 10.1074/jbc.M009170200. [DOI] [PubMed] [Google Scholar]
- 29.Massie CE, Adryan B, Barbosa-Morais NL, Lynch AG, Tran MG, Neal DE, Mills IG. New androgen receptor genomic targets show an interaction with the ETS1 transcription factor. EMBO Rep. 2007;8:871–878. doi: 10.1038/sj.embor.7401046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waghray A, Feroze F, Schober MS, Yao F, Wood C, Puravs E, Krause M, Hanash S, Chen YQ. Identification of androgen-regulated genes in the prostate cancer cell line LNCaP by serial analysis of gene expression and proteomic analysis. Proteomics. 2001;1:1327–1338. doi: 10.1002/1615-9861(200110)1:10<1327::AID-PROT1327>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 31.Roeder RG. The role of general initiation factors in transcription by RNA polymerase II. Trends in Biochem Sci. 1996;21:327–335. [PubMed] [Google Scholar]
- 32.Abate-Shen C, Shen MM. Molecular genetics of prostate cancer. Genes Dev. 2000;14:2410–2434. doi: 10.1101/gad.819500. [DOI] [PubMed] [Google Scholar]
- 33.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 34.Lamb LE, Knudsen BS, Miranti CK. E-cadherin-mediated survival of androgen-receptor-expressing secretory prostate epithelial cells derived from a stratified in vitro differentiation model. J Cell Sci. 2010;123:266–276. doi: 10.1242/jcs.054502. [DOI] [PubMed] [Google Scholar]
- 35.Wu CT, Altuwaijri S, Ricke WA, Huang SP, Yeh S, Zhang C, Niu Y, Tsai MY, Chang C. Increased prostate cell proliferation and loss of cell differentiation in mice lacking prostate epithelial androgen receptor. Proc Natl Acad Sci USA. 2007;104:12679–12684. doi: 10.1073/pnas.0704940104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sensibar JA. Analysis of cell death and cell proliferation in embryonic stages, normal adult, and aging prostates in human and animals. Microsc Res Tech. 1995;30:342–350. doi: 10.1002/jemt.1070300409. [DOI] [PubMed] [Google Scholar]
- 37.Yadav N, Heemers HV. Androgen action in the prostate gland. Minerva Urol Nefrol. 2012;64:35–49. [PubMed] [Google Scholar]
- 38.Niu Y, Altuwaijri S, Lai KP, Wu CT, Ricke WA, Messing EM, Yao J, Yeh S, Chang C. Androgen receptor is a tumor suppressor and proliferator in prostate cancer. Proc Natl Acad Sci USA. 2008;105:12182–12187. doi: 10.1073/pnas.0804700105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whitacre DC, Chauhan S, Davis T, Gordon D, Cress AE, Miesfeld RL. Androgen induction of in vitro prostate cell differentiation. Cell Growth Differ. 2002;13:1–11. [PubMed] [Google Scholar]
- 40.Berger R, Febbo PG, Majumder PK, Zhao JJ, Mukherjee S, Signoretti S, Campbell KT, Sellers WR, Roberts TM, Loda M, Golub TR, Hahn WC. Androgen-induced differentiation and tumorigenicity of human prostate epithelial cells. Cancer Res. 2004;64:8867–8875. doi: 10.1158/0008-5472.CAN-04-2938. [DOI] [PubMed] [Google Scholar]
- 41.Lu W, Luo Y, Kan M, McKeehan WL. Fibroblast growth factor-10. A second candidate stromal to epithelial cell andromedin in prostate. J Biol Chem. 1999;274:12827–12834. doi: 10.1074/jbc.274.18.12827. [DOI] [PubMed] [Google Scholar]
- 42.Planz B, Aretz HT, Wang Q, Tabatabaei S, Kirley SD, Lin CW, McDougal WS. Immunolocalization of the keratinocyte growth factor in benign and neoplastic human prostate and its relation to androgen receptor. Prostate. 1999;41:233–242. doi: 10.1002/(sici)1097-0045(19991201)41:4<233::aid-pros3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 43.Brawer MK, Peehl DM, Stamey TA, Bostwick DG. Keratin immunoreactivity in the benign and neoplastic human prostate. Cancer Res. 1985;45:3663–3667. [PubMed] [Google Scholar]
- 44.Nagle RB, Ahmann FR, McDaniel KM, Paquin ML, Clark VA, Celniker A. Cytokeratin characterization of human prostatic carcinoma and its derived cell lines. Cancer Res. 1987;47:281–286. [PubMed] [Google Scholar]
- 45.Berger PL, Frank SB, Schulz VV, Nollet EA, Edick MJ, Holly B, Chang TT, Hostetter G, Kim S, Miranti CK. Transient induction of ING4 by Myc drives prostate epithelial cell differentiation and its disruption drives prostate tumorigenesis. Cancer Res. 2014;74:3357–3368. doi: 10.1158/0008-5472.CAN-13-3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barbieri CE, Tomlins SA. The prostate cancer genome: perspectives and potential. Urol Oncol. 2014;32:53 e15–22. doi: 10.1016/j.urolonc.2013.08.025. [DOI] [PubMed] [Google Scholar]
- 47.Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, Varambally S, Cao X, Tchinda J, Kuefer R, Lee C, Montie JE, Shah RB, Pienta KJ, Rubin MA, Chinnaiyan AM. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–648. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 48.Hollenhorst PC, Jones DA, Graves BJ. Expression profiles frame the promoter specificity dilemma of the ETS family of transcription factors. Nucleic Acids Res. 2004;32:5693–5702. doi: 10.1093/nar/gkh906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hollenhorst PC, McIntosh LP, Graves BJ. Genomic and biochemical insights into the specificity of ETS transcription factors. Annu Rev Biochem. 2011;80:437–471. doi: 10.1146/annurev.biochem.79.081507.103945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hollenhorst PC, Paul L, Ferris MW, Graves BJ. The ETS gene ETV4 is required for anchorage-independent growth and a cell proliferation gene expression program in PC3 prostate cells. Genes Cancer. 2011;1:1044–1052. doi: 10.1177/1947601910395578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Massie CE, Mills IG. Global identification of androgen response elements. Meth Mol Biol. 2011;776:255–273. doi: 10.1007/978-1-61779-243-4_15. [DOI] [PubMed] [Google Scholar]
- 52.Massie CE, Lynch A, Ramos-Montoya A, Boren J, Stark R, Fazli L, Warren A, Scott H, Madhu B, Sharma N, Bon H, Zecchini V, Smith DM, Denicola GM, Mathews N, Osborne M, Hadfield J, Macarthur S, Adryan B, Lyons SK, Brindle KM, Griffiths J, Gleave ME, Rennie PS, Neal DE, Mills IG. The androgen receptor fuels prostate cancer by regulating central metabolism and biosynthesis. EMBO J. 2011;30:2719–2733. doi: 10.1038/emboj.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lilja H. A kallikrein-like serine protease in prostatic fluid cleaves the predominant seminal vesicle protein. J Clin Invest. 1985;76:1899–1903. doi: 10.1172/JCI112185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moyer VA U.S.P.S.T. Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:120–134. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 55.Attard G, Belldegrun AS, de Bono JS. Selective blockade of androgenic steroid synthesis by novel lyase inhibitors as a therapeutic strategy for treating metastatic prostate cancer. BJU Int. 2005;96:1241–1246. doi: 10.1111/j.1464-410X.2005.05821.x. [DOI] [PubMed] [Google Scholar]
- 56.Hara T, Kouno J, Kaku T, Takeuchi T, Kusaka M, Tasaka A, Yamaoka M. Effect of a novel 17,20-lyase inhibitor, orteronel (TAK-700), on androgen synthesis in male rats. J Steroid Biochem Mol Biol. 2013;134:80–91. doi: 10.1016/j.jsbmb.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 57.Soifer HS, Souleimanian N, Wu S, Voskresenskiy AM, Collak FK, Cinar B, Stein CA. Direct regulation of androgen receptor activity by potent CYP17 inhibitors in prostate cancer cells. J Biol Chem. 2012;287:3777–3787. doi: 10.1074/jbc.M111.261933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamaoka M, Hara T, Araki H, Kaku T, Hitaka T, Tasaka A, Kusaka M. Effect of an investigational CYP17A1 inhibitor, orteronel (TAK-700), on estrogen- and corticoid-synthesis pathways in hypophysectomized female rats and on the serum estradiol levels in female cynomolgus monkeys. J Steroid Biochem Mol Biol. 2013;138:298–306. doi: 10.1016/j.jsbmb.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 59.Toren PJ, Kim S, Pham S, Mangalji A, Adomat H, Guns ES, Zoubeidi A, Moore W, Gleave ME. Anticancer activity of a novel selective CYP17A1 inhibitor in preclinical models of castrate-resistant prostate cancer. Mol Cancer Ther. 2015;14:59–69. doi: 10.1158/1535-7163.MCT-14-0521. [DOI] [PubMed] [Google Scholar]
- 60.Yamaoka M, Hara T, Kusaka M. Overcoming persistent dependency on androgen signaling after progression to castration-resistant prostate cancer. Clin Cancer Res. 2010;16:4319–4324. doi: 10.1158/1078-0432.CCR-10-0255. [DOI] [PubMed] [Google Scholar]
- 61.Watanabe K, Kakefuda A, Yasuda M, Enjo K, Kikuchi A, Furutani T, Naritomi Y, Otsuka Y, Okada M, Ohta M. Discovery of 2-methyl-1-{1-[(5-methyl-1H-indol-2-yl)carbonyl]piperidin-4-yl}propan-2-ol: a novel, potent and selective type 5 17beta-hydroxysteroid dehydrogenase inhibitor. Bioorg Med Chem. 2013;21:5261–5270. doi: 10.1016/j.bmc.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 62.Deslypere JP, Young M, Wilson JD, McPhaul MJ. Testosterone and 5 alpha-dihydrotestosterone interact differently with the androgen receptor to enhance transcription of the MMTV-CAT reporter gene. Mol Cell Endocrinol. 1992;88:15–22. doi: 10.1016/0303-7207(92)90004-p. [DOI] [PubMed] [Google Scholar]
- 63.Xu Y, Dalrymple SL, Becker RE, Denmeade SR, Isaacs JT. Pharmacologic basis for the enhanced efficacy of dutasteride against prostatic cancers. Clin Cancer Res. 2006;12:4072–4079. doi: 10.1158/1078-0432.CCR-06-0184. [DOI] [PubMed] [Google Scholar]
- 64.Kolvenbag GJ, Furr BJ, Blackledge GR. Receptor affinity and potency of non-steroidal antiandrogens: translation of preclinical findings into clinical activity. Prostate Cancer Prostatic Dis. 1998;1:307–314. doi: 10.1038/sj.pcan.4500262. [DOI] [PubMed] [Google Scholar]
- 65.Mohler JL, Gregory CW, Ford OH, 3rd, Kim D, Weaver CM, Petrusz P, Wilson EM, French FS. The androgen axis in recurrent prostate cancer. Clin Cancer Res. 2004;10:440–448. doi: 10.1158/1078-0432.ccr-1146-03. [DOI] [PubMed] [Google Scholar]
- 66.Schuurmans AL, Bolt J, Veldscholte J, Mulder E. Regulation of growth of LNCaP human prostate tumor cells by growth factors and steroid hormones. J Steroid Biochem Mol Biol. 1991;40:193–197. doi: 10.1016/0960-0760(91)90182-5. [DOI] [PubMed] [Google Scholar]
- 67.Veldscholte J, Berrevoets CA, Brinkmann AO, Grootegoed JA, Mulder E. Anti-androgens and the mutated androgen receptor of LNCaP cells: differential effects on binding affinity, heat-shock protein interaction, and transcription activation. Biochemistry. 1992;31:2393–2399. doi: 10.1021/bi00123a026. [DOI] [PubMed] [Google Scholar]
- 68.Veldscholte J, Ris-Stalpers C, Kuiper GG, Jenster G, Berrevoets C, Claassen E, van Rooij HC, Trapman J, Brinkmann AO, Mulder E. A mutation in the ligand binding domain of the androgen receptor of human LNCaP cells affects steroid binding characteristics and response to anti-androgens. Biochem Biophys Res Commun. 1990;173:534–540. doi: 10.1016/s0006-291x(05)80067-1. [DOI] [PubMed] [Google Scholar]
- 69.Hellerstedt BA, Pienta KJ. The current state of hormonal therapy for prostate cancer. CA Cancer J Clin. 2002;52:154–179. doi: 10.3322/canjclin.52.3.154. [DOI] [PubMed] [Google Scholar]
- 70.Shah RB, Mehra R, Chinnaiyan AM, Shen R, Ghosh D, Zhou M, Macvicar GR, Varambally S, Harwood J, Bismar TA, Kim R, Rubin MA, Pienta KJ. Androgen-independent prostate cancer is a heterogeneous group of diseases: lessons from a rapid autopsy program. Cancer Res. 2004;64:9209–9216. doi: 10.1158/0008-5472.CAN-04-2442. [DOI] [PubMed] [Google Scholar]
- 71.Bracarda S, de Cobelli O, Greco C, Prayer-Galetti T, Valdagni R, Gatta G, de Braud F, Bartsch G. Cancer of the prostate. Crit Rev Oncol Hematol. 2005;56:379–396. doi: 10.1016/j.critrevonc.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 72.Jones JO, Diamond MI. A cellular conformation-based screen for androgen receptor inhibitors. ACS Chem Biol. 2008;3:412–418. doi: 10.1021/cb800054w. [DOI] [PubMed] [Google Scholar]
- 73.Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, Wongvipat J, Smith-Jones PM, Yoo D, Kwon A, Wasielewska T, Welsbie D, Chen CD, Higano CS, Beer TM, Hung DT, Scher HI, Jung ME, Sawyers CL. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–790. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Linja MJ, Visakorpi T. Alterations of androgen receptor in prostate cancer. J Steroid Biochem Mol Biol. 2004;92:255–264. doi: 10.1016/j.jsbmb.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 75.Gaddipati JP, McLeod DG, Heidenberg HB, Sesterhenn IA, Finger MJ, Moul JW, Srivastava S. Frequent detection of codon 877 mutation in the androgen receptor gene in advanced prostate cancers. Cancer Res. 1994;54:2861–2864. [PubMed] [Google Scholar]
- 76.Taplin ME, Rajeshkumar B, Halabi S, Werner CP, Woda BA, Picus J, Stadler W, Hayes DF, Kantoff PW, Vogelzang NJ, Small EJ Cancer, B.S. Leukemia Group. Androgen receptor mutations in androgen-independent prostate cancer: Cancer and Leukemia Group B Study 9663. J Clin Oncol. 2003;21:2673–2678. doi: 10.1200/JCO.2003.11.102. [DOI] [PubMed] [Google Scholar]
- 77.Waltering KK, Helenius MA, Sahu B, Manni V, Linja MJ, Janne OA, Visakorpi T. Increased expression of androgen receptor sensitizes prostate cancer cells to low levels of androgens. Cancer Res. 2009;69:8141–8149. doi: 10.1158/0008-5472.CAN-09-0919. [DOI] [PubMed] [Google Scholar]
- 78.Taplin ME, Bubley GJ, Shuster TD, Frantz ME, Spooner AE, Ogata GK, Keer HN, Balk SP. Mutation of the androgen-receptor gene in metastatic androgen-independent prostate cancer. N Engl J Med. 1995;332:1393–1398. doi: 10.1056/NEJM199505253322101. [DOI] [PubMed] [Google Scholar]
- 79.Li Y, Chan SC, Brand LJ, Hwang TH, Silverstein KA, Dehm SM. Androgen receptor splice variants mediate enzalutamide resistance in castration-resistant prostate cancer cell lines. Cancer Res. 2013;73:483–489. doi: 10.1158/0008-5472.CAN-12-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rainey WE, Nakamura Y. Regulation of the adrenal androgen biosynthesis. J Steroid Biochem Mol Biol. 2008;108:281–286. doi: 10.1016/j.jsbmb.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chang KH, Li R, Kuri B, Lotan Y, Roehrborn CG, Liu J, Vessella R, Nelson PS, Kapur P, Guo X, Mirzaei H, Auchus RJ, Sharifi N. A gain-of-function mutation in DHT synthesis in castration-resistant prostate cancer. Cell. 2013;154:1074–1084. doi: 10.1016/j.cell.2013.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Titus MA, Gregory CW, Ford OH, 3rd, Schell MJ, Maygarden SJ, Mohler JL. Steroid 5alpha-reductase isozymes I and II in recurrent prostate cancer. Clin Cancer Res. 2005;11:4365–4371. doi: 10.1158/1078-0432.CCR-04-0738. [DOI] [PubMed] [Google Scholar]
- 83.Culig Z, Comuzzi B, Steiner H, Bartsch G, Hobisch A. Expression and function of androgen receptor coactivators in prostate cancer. J Steroid Biochem Mol Biol. 2004;92:265–271. doi: 10.1016/j.jsbmb.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 84.Heemers HV, Sebo TJ, Debes JD, Regan KM, Raclaw KA, Murphy LM, Hobisch A, Culig Z, Tindall DJ. Androgen deprivation increases p300 expression in prostate cancer cells. Cancer Res. 2007;67:3422–3430. doi: 10.1158/0008-5472.CAN-06-2836. [DOI] [PubMed] [Google Scholar]
- 85.Geng C, He B, Xu L, Barbieri CE, Eedunuri VK, Chew SA, Zimmermann M, Bond R, Shou J, Li C, Blattner M, Lonard DM, Demichelis F, Coarfa C, Rubin MA, Zhou P, O’Malley BW, Mitsiades N. Prostate cancer-associated mutations in speckle-type POZ protein (SPOP) regulate steroid receptor coactivator 3 protein turnover. Proc Natl Acad Sci USA. 2013;110:6997–7002. doi: 10.1073/pnas.1304502110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Barbieri CE, Baca SC, Lawrence MS, Demichelis F, Blattner M, Theurillat JP, White TA, Stojanov P, Van Allen E, Stransky N, Nickerson E, Chae SS, Boysen G, Auclair D, Onofrio RC, Park K, Kitabayashi N, MacDonald TY, Sheikh K, Vuong T, Guiducci C, Cibulskis K, Sivachenko A, Carter SL, Saksena G, Voet D, Hussain WM, Ramos AH, Winckler W, Redman MC, Ardlie K, Tewari AK, Mosquera JM, Rupp N, Wild PJ, Moch H, Morrissey C, Nelson PS, Kantoff PW, Gabriel SB, Golub TR, Meyerson M, Lander ES, Getz G, Rubin MA, Garraway LA. Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer. Nat Genet. 2012;44:685–689. doi: 10.1038/ng.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lindberg J, Klevebring D, Liu W, Neiman M, Xu J, Wiklund P, Wiklund F, Mills IG, Egevad L, Gronberg H. Exome sequencing of prostate cancer supports the hypothesis of independent tumour origins. Eur Urol. 2013;63:347–353. doi: 10.1016/j.eururo.2012.03.050. [DOI] [PubMed] [Google Scholar]
- 88.Sanden C, Gullberg U. The DEK oncoprotein and its emerging roles in gene regulation. Leukemia. 2015;29:1632–1636. doi: 10.1038/leu.2015.72. [DOI] [PubMed] [Google Scholar]
- 89.Theurillat JP, Udeshi ND, Errington WJ, Svinkina T, Baca SC, Pop M, Wild PJ, Blattner M, Groner AC, Rubin MA, Moch H, Prive GG, Carr SA, Garraway LA. Prostate cancer. Ubiquitylome analysis identifies dysregulation of effector substrates in SPOP-mutant prostate cancer. Science. 2014;346:85–89. doi: 10.1126/science.1250255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li Y, Alsagabi M, Fan D, Bova GS, Tewfik AH, Dehm SM. Intragenic rearrangement and altered RNA splicing of the androgen receptor in a cell-based model of prostate cancer progression. Cancer Res. 2011;71:2108–2117. doi: 10.1158/0008-5472.CAN-10-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Watson PA, Chen YF, Balbas MD, Wongvipat J, Socci ND, Viale A, Kim K, Sawyers CL. Constitutively active androgen receptor splice variants expressed in castration-resistant prostate cancer require full-length androgen receptor. Proc Natl Acad Sci USA. 2010;107:16759–16765. doi: 10.1073/pnas.1012443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li Y, Hwang TH, Oseth LA, Hauge A, Vessella RL, Schmechel SC, Hirsch B, Beckman KB, Silverstein KA, Dehm SM. AR intragenic deletions linked to androgen receptor splice variant expression and activity in models of prostate cancer progression. Oncogene. 2012;31:4759–4767. doi: 10.1038/onc.2011.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Guo Z, Yang X, Sun F, Jiang R, Linn DE, Chen H, Chen H, Kong X, Melamed J, Tepper CG, Kung HJ, Brodie AM, Edwards J, Qiu Y. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res. 2009;69:2305–2313. doi: 10.1158/0008-5472.CAN-08-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hornberg E, Ylitalo EB, Crnalic S, Antti H, Stattin P, Widmark A, Bergh A, Wikstrom P. Expression of androgen receptor splice variants in prostate cancer bone metastases is associated with castration-resistance and short survival. PloS One. 2011;6:e19059. doi: 10.1371/journal.pone.0019059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang X, Morrissey C, Sun S, Ketchandji M, Nelson PS, True LD, Vakar-Lopez F, Vessella RL, Plymate SR. Androgen receptor variants occur frequently in castration resistant prostate cancer metastases. PloS One. 2011;6:e27970. doi: 10.1371/journal.pone.0027970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Luo J, Pienta KJ. Words of wisdom: re: androgen receptor splice variants mediate enzalutamide resistance in castration-resistant prostate cancer cell lines. Eur Urol. 2013;64:339–340. doi: 10.1016/j.eururo.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 97.Wu JD, Haugk K, Woodke L, Nelson P, Coleman I, Plymate SR. Interaction of IGF signaling and the androgen receptor in prostate cancer progression. J Cell Biochem. 2006;99:392–401. doi: 10.1002/jcb.20929. [DOI] [PubMed] [Google Scholar]
- 98.Arnold JT, Isaacs JT. Mechanisms involved in the progression of androgen-independent prostate cancers: it is not only the cancer cell’s fault. Endocr Relat Cancer. 2002;9:61–73. doi: 10.1677/erc.0.0090061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Craft N, Shostak Y, Carey M, Sawyers CL. A mechanism for hormone-independent prostate cancer through modulation of androgen receptor signaling by the HER-2/neu tyrosine kinase. Nat Med. 1999;5:280–285. doi: 10.1038/6495. [DOI] [PubMed] [Google Scholar]
- 100.Manin M, Baron S, Goossens K, Beaudoin C, Jean C, Veyssiere G, Verhoeven G, Morel L. Androgen receptor expression is regulated by the phosphoinositide 3-kinase/Akt pathway in normal and tumoral epithelial cells. Biochem J. 2002;366:729–736. doi: 10.1042/BJ20020585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sharma M, Chuang WW, Sun Z. Phosphatidylinositol 3-kinase/Akt stimulates androgen pathway through GSK3beta inhibition and nuclear beta-catenin accumulation. J Biol Chem. 2002;277:30935–30941. doi: 10.1074/jbc.M201919200. [DOI] [PubMed] [Google Scholar]
- 102.Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol. 2005;23:8253–8261. doi: 10.1200/JCO.2005.03.4777. [DOI] [PubMed] [Google Scholar]
- 103.Rigas AC, Ozanne DM, Neal DE, Robson CN. The scaffolding protein RACK1 interacts with androgen receptor and promotes cross-talk through a protein kinase C signaling pathway. J Biol Chem. 2003;278:46087–46093. doi: 10.1074/jbc.M306219200. [DOI] [PubMed] [Google Scholar]
- 104.Revelli A, Massobrio M, Tesarik J. Nongenomic actions of steroid hormones in reproductive tissues. Endocr Rev. 1998;19:3–17. doi: 10.1210/edrv.19.1.0322. [DOI] [PubMed] [Google Scholar]
- 105.Foradori CD, Weiser MJ, Handa RJ. Non-genomic actions of androgens. Front Neuroendocrin. 2008;29:169–181. doi: 10.1016/j.yfrne.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wehling M. Specific, nongenomic actions of steroid hormones. Annu Rev Physiol. 1997;59:365–393. doi: 10.1146/annurev.physiol.59.1.365. [DOI] [PubMed] [Google Scholar]
- 107.Heinlein CA, Chang C. The roles of androgen receptors and androgen-binding proteins in nongenomic androgen actions. Mol Endocrinol. 2002;16:2181–2187. doi: 10.1210/me.2002-0070. [DOI] [PubMed] [Google Scholar]
- 108.Falkenstein E, Tillmann HC, Christ M, Feuring M, Wehling M. Multiple actions of steroid hormones--a focus on rapid, nongenomic effects. Pharmacol Rev. 2000;52:513–556. [PubMed] [Google Scholar]
- 109.McEwen BS, Krey LC, Luine VN. Steroid hormone action in the neuroendocrine system: when is the genome involved? Res Publ Assoc Res Nerv Ment Dis. 1978;56:255–268. [PubMed] [Google Scholar]
- 110.Kalyanaraman H, Schwappacher R, Joshua J, Zhuang S, Scott BT, Klos M, Casteel DE, Frangos JA, Dillmann W, Boss GR, Pilz RB. Nongenomic thyroid hormone signaling occurs through a plasma membrane-localized receptor. Sci Signal. 2014;7:ra48. doi: 10.1126/scisignal.2004911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Boonyaratanakornkit V, Scott MP, Ribon V, Sherman L, Anderson SM, Maller JL, Miller WT, Edwards DP. Progesterone receptor contains a proline-rich motif that directly interacts with SH3 domains and activates c-Src family tyrosine kinases. Mol Cell. 2001;8:269–280. doi: 10.1016/s1097-2765(01)00304-5. [DOI] [PubMed] [Google Scholar]
- 112.Migliaccio A, Piccolo D, Castoria G, Di Domenico M, Bilancio A, Lombardi M, Gong W, Beato M, Auricchio F. Activation of the Src/p21ras/Erk pathway by progesterone receptor via cross-talk with estrogen receptor. EMBO J. 1998;17:2008–2018. doi: 10.1093/emboj/17.7.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kreisberg JI, Malik SN, Prihoda TJ, Bedolla RG, Troyer DA, Kreisberg S, Ghosh PM. Phosphorylation of Akt (Ser473) is an excellent predictor of poor clinical outcome in prostate cancer. Cancer Res. 2004;64:5232–5236. doi: 10.1158/0008-5472.CAN-04-0272. [DOI] [PubMed] [Google Scholar]
- 114.Gorczynska E, Handelsman DJ. Androgens rapidly increase the cytosolic calcium concentration in Sertoli cells. Endocrinology. 1995;136:2052–2059. doi: 10.1210/endo.136.5.7720654. [DOI] [PubMed] [Google Scholar]
- 115.Sun M, Yang L, Feldman RI, Sun XM, Bhalla KN, Jove R, Nicosia SV, Cheng JQ. Activation of phosphatidylinositol 3-kinase/Akt pathway by androgen through interaction of p85alpha, androgen receptor, and Src. J Biol Chem. 2003;278:42992–43000. doi: 10.1074/jbc.M306295200. [DOI] [PubMed] [Google Scholar]
- 116.Goueli SA. Differential regulation of prostatic protein kinase C isozymes by androgens. FEBS Lett. 1990;264:53–55. doi: 10.1016/0014-5793(90)80762-8. [DOI] [PubMed] [Google Scholar]
- 117.Nguyen TV, Yao M, Pike CJ. Androgens activate mitogen-activated protein kinase signaling: role in neuroprotection. J Neurochem. 2005;94:1639–1651. doi: 10.1111/j.1471-4159.2005.03318.x. [DOI] [PubMed] [Google Scholar]
- 118.Castoria G, Lombardi M, Barone MV, Bilancio A, Di Domenico M, Bottero D, Vitale F, Migliaccio A, Auricchio F. Androgen-stimulated DNA synthesis and cytoskeletal changes in fibroblasts by a nontranscriptional receptor action. J Cell Biol. 2003;161:547–556. doi: 10.1083/jcb.200211099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Guarino M. Src signaling in cancer invasion. J Cell physiol. 2010;223:14–26. doi: 10.1002/jcp.22011. [DOI] [PubMed] [Google Scholar]
- 120.Yeatman TJ. A renaissance for SRC. Nat Rev Cancer. 2004;4:470–480. doi: 10.1038/nrc1366. [DOI] [PubMed] [Google Scholar]
- 121.Asim M, Siddiqui IA, Hafeez BB, Baniahmad A, Mukhtar H. Src kinase potentiates androgen receptor transactivation function and invasion of androgen-independent prostate cancer C4-2 cells. Oncogene. 2008;27:3596–3604. doi: 10.1038/sj.onc.1211016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Drake JM, Graham NA, Stoyanova T, Sedghi A, Goldstein AS, Cai H, Smith DA, Zhang H, Komisopoulou E, Huang J, Graeber TG, Witte ON. Oncogene-specific activation of tyrosine kinase networks during prostate cancer progression. Proc Natl Acad Sci USA. 2012;109:1643–1648. doi: 10.1073/pnas.1120985109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chatterji T, Varkaris AS, Parikh NU, Song JH, Cheng CJ, Schweppe RE, Alexander S, Davis JW, Troncoso P, Friedl P, Kuang J, Lin SH, Gallick GE. Yes-mediated phosphorylation of focal adhesion kinase at tyrosine 861 increases metastatic potential of prostate cancer cells. Oncotarget. 2015;6:10175–10194. doi: 10.18632/oncotarget.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Posadas EM, Al-Ahmadie H, Robinson VL, Jagadeeswaran R, Otto K, Kasza KE, Tretiakov M, Siddiqui J, Pienta KJ, Stadler WM, Rinker-Schaeffer C, Salgia R. FYN is overexpressed in human prostate cancer. BJU Int. 2009;103:171–177. doi: 10.1111/j.1464-410X.2008.08009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Edwards J, Krishna NS, Witton CJ, Bartlett JM. Gene amplifications associated with the development of hormone-resistant prostate cancer. Clin Cancer Res. 2003;9:5271–5281. [PubMed] [Google Scholar]
- 126.Wong CW, McNally C, Nickbarg E, Komm BS, Cheskis BJ. Estrogen receptor-interacting protein that modulates its nongenomic activity-crosstalk with Src/Erk phosphorylation cascade. Proc Natl Acad Sci USA. 2002;99:14783–14788. doi: 10.1073/pnas.192569699. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 127.Barletta F, Wong CW, McNally C, Komm BS, Katzenellenbogen B, Cheskis BJ. Characterization of the interactions of estrogen receptor and MNAR in the activation of cSrc. Mol Endocrinol. 2004;18:1096–1108. doi: 10.1210/me.2003-0335. [DOI] [PubMed] [Google Scholar]
- 128.Kousteni S, Bellido T, Plotkin LI, O’Brien CA, Bodenner DL, Han L, Han K, DiGregorio GB, Katzenellenbogen JA, Katzenellenbogen BS, Roberson PK, Weinstein RS, Jilka RL, Manolagas SC. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell. 2001;104:719–730. [PubMed] [Google Scholar]
- 129.Unni E, Sun S, Nan B, McPhaul MJ, Cheskis B, Mancini MA, Marcelli M. Changes in androgen receptor nongenotropic signaling correlate with transition of LNCaP cells to androgen independence. Cancer Res. 2004;64:7156–7168. doi: 10.1158/0008-5472.CAN-04-1121. [DOI] [PubMed] [Google Scholar]
- 130.Peterziel H, Mink S, Schonert A, Becker M, Klocker H, Cato AC. Rapid signalling by androgen receptor in prostate cancer cells. Oncogene. 1999;18:6322–6329. doi: 10.1038/sj.onc.1203032. [DOI] [PubMed] [Google Scholar]
- 131.Flourakis M, Prevarskaya N. Insights into Ca2+ homeostasis of advanced prostate cancer cells. Biochim Biophys Acta. 2009;1793:1105–1109. doi: 10.1016/j.bbamcr.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 132.Foradori CD, Werner SB, Sandau US, Clapp TR, Handa RJ. Activation of the androgen receptor alters the intracellular calcium response to glutamate in primary hippocampal neurons and modulates sarco/endoplasmic reticulum calcium ATPase 2 transcription. Neuroscience. 2007;149:155–164. doi: 10.1016/j.neuroscience.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 133.Steinsapir J, Socci R, Reinach P. Effects of androgen on intracellular calcium of LNCaP cells. Biochem Biophys Res Commun. 1991;179:90–96. doi: 10.1016/0006-291x(91)91338-d. [DOI] [PubMed] [Google Scholar]
- 134.Sun YH, Gao X, Tang YJ, Xu CL, Wang LH. Androgens induce increases in intracellular calcium via a G protein-coupled receptor in LNCaP prostate cancer cells. J Androl. 2006;27:671–678. doi: 10.2164/jandrol.106.000554. [DOI] [PubMed] [Google Scholar]
- 135.Oliver VL, Poulios K, Ventura S, Haynes JM. A novel androgen signalling pathway uses dihydrotestosterone, but not testosterone, to activate the EGF receptor signalling cascade in prostate stromal cells. Brit J Pharmacol. 2013;170:592–601. doi: 10.1111/bph.12307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gonzalez-Montelongo MC, Marin R, Gomez T, Diaz M. Androgens are powerful non-genomic inducers of calcium sensitization in visceral smooth muscle. Steroids. 2010;75:533–538. doi: 10.1016/j.steroids.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 137.Estrada M, Uhlen P, Ehrlich BE. Ca2+ oscillations induced by testosterone enhance neurite outgrowth. J Cell Aci. 2006;119:733–743. doi: 10.1242/jcs.02775. [DOI] [PubMed] [Google Scholar]
- 138.Beesley RD, Palmer BM, Casson PR, Toth MJ. Effects of testosterone on cardiomyocyte calcium homeostasis and contractile function in female rats. Exp Physiol. 2013;98:161–171. doi: 10.1113/expphysiol.2012.067009. [DOI] [PubMed] [Google Scholar]
- 139.Comstock CE, Knudsen KE. The complex role of AR signaling after cytotoxic insult: implications for cell-cycle-based chemotherapeutics. Cell Cycle. 2007;6:1307–1313. doi: 10.4161/cc.6.11.4353. [DOI] [PubMed] [Google Scholar]
- 140.Wang Q, Li W, Zhang Y, Yuan X, Xu K, Yu J, Chen Z, Beroukhim R, Wang H, Lupien M, Wu T, Regan MM, Meyer CA, Carroll JS, Manrai AK, Janne OA, Balk SP, Mehra R, Han B, Chinnaiyan AM, Rubin MA, True L, Fiorentino M, Fiore C, Loda M, Kantoff PW, Liu XS, Brown M. Androgen receptor regulates a distinct transcription program in androgen-independent prostate cancer. Cell. 2009;138:245–256. doi: 10.1016/j.cell.2009.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lee LF, Louie MC, Desai SJ, Yang J, Chen HW, Evans CP, Kung HJ. Interleukin-8 confers androgen-independent growth and migration of LNCaP: differential effects of tyrosine kinases Src and FAK. Oncogene. 2004;23:2197–2205. doi: 10.1038/sj.onc.1207344. [DOI] [PubMed] [Google Scholar]
- 142.Baron S, Manin M, Beaudoin C, Leotoing L, Communal Y, Veyssiere G, Morel L. Androgen receptor mediates non-genomic activation of phosphatidylinositol 3-OH kinase in androgen-sensitive epithelial cells. J Biol Chem. 2004;279:14579–14586. doi: 10.1074/jbc.M306143200. [DOI] [PubMed] [Google Scholar]
- 143.Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L) Cell. 1996;87:619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- 144.Spassov DS, Wong CH, Sergina N, Ahuja D, Fried M, Sheppard D, Moasser MM. Phosphorylation of Trask by Src kinases inhibits integrin clustering and functions in exclusion with focal adhesion signaling. Mol Cell Biol. 2011;31:766–782. doi: 10.1128/MCB.00841-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Summy JM, Gallick GE. Src family kinases in tumor progression and metastasis. Cancer Metastasis Rev. 2003;22:337–358. doi: 10.1023/a:1023772912750. [DOI] [PubMed] [Google Scholar]
- 146.Planas-Silva MD, Bruggeman RD, Grenko RT, Stanley Smith J. Role of c-Src and focal adhesion kinase in progression and metastasis of estrogen receptor-positive breast cancer. Biochem Biophys Res Commun. 2006;341:73–81. doi: 10.1016/j.bbrc.2005.12.164. [DOI] [PubMed] [Google Scholar]
- 147.Zhang XH, Wang Q, Gerald W, Hudis CA, Norton L, Smid M, Foekens JA, Massague J. Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer Cell. 2009;16:67–78. doi: 10.1016/j.ccr.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Myoui A, Nishimura R, Williams PJ, Hiraga T, Tamura D, Michigami T, Mundy GR, Yoneda T. C-SRC tyrosine kinase activity is associated with tumor colonization in bone and lung in an animal model of human breast cancer metastasis. Cancer Res. 2003;63:5028–5033. [PubMed] [Google Scholar]
- 149.Rucci N, Recchia I, Angelucci A, Alamanou M, Del Fattore A, Fortunati D, Susa M, Fabbro D, Bologna M, Teti A. Inhibition of protein kinase c-Src reduces the incidence of breast cancer metastases and increases survival in mice: implications for therapy. J Pharmacol Exp Ther. 2006;318:161–172. doi: 10.1124/jpet.106.102004. [DOI] [PubMed] [Google Scholar]
- 150.Nam S, Kim D, Cheng JQ, Zhang S, Lee JH, Buettner R, Mirosevich J, Lee FY, Jove R. Action of the Src family kinase inhibitor, dasatinib (BMS-354825), on human prostate cancer cells. Cancer Res. 2005;65:9185–9189. doi: 10.1158/0008-5472.CAN-05-1731. [DOI] [PubMed] [Google Scholar]
- 151.Recchia I, Rucci N, Festuccia C, Bologna M, MacKay AR, Migliaccio S, Longo M, Susa M, Fabbro D, Teti A. Pyrrolopyrimidine c-Src inhibitors reduce growth, adhesion, motility and invasion of prostate cancer cells in vitro. Eur J Cancer. 2003;39:1927–1935. doi: 10.1016/s0959-8049(03)00394-0. [DOI] [PubMed] [Google Scholar]
- 152.Slack JK, Adams RB, Rovin JD, Bissonette EA, Stoker CE, Parsons JT. Alterations in the focal adhesion kinase/Src signal transduction pathway correlate with increased migratory capacity of prostate carcinoma cells. Oncogene. 2001;20:1152–1163. doi: 10.1038/sj.onc.1204208. [DOI] [PubMed] [Google Scholar]
- 153.Park SI, Zhang J, Phillips KA, Araujo JC, Najjar AM, Volgin AY, Gelovani JG, Kim SJ, Wang Z, Gallick GE. Targeting SRC family kinases inhibits growth and lymph node metastases of prostate cancer in an orthotopic nude mouse model. Cancer Res. 2008;68:3323–3333. doi: 10.1158/0008-5472.CAN-07-2997. [DOI] [PubMed] [Google Scholar]
- 154.Gonzalez-Montelongo MC, Marin R, Gomez T, Marrero-Alonso J, Diaz M. Androgens induce nongenomic stimulation of colonic contractile activity through induction of calcium sensitization and phosphorylation of LC20 and CPI-17. Mol Endocrinol. 2010;24:1007–1023. doi: 10.1210/me.2009-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kiyomiya K, Lee MS, Tseng IC, Zuo H, Barndt RJ, Johnson MD, Dickson RB, Lin CY. Matriptase activation and shedding with HAI-1 is induced by steroid sex hormones in human prostate cancer cells, but not in breast cancer cells. A J Physiol Cell Physiol. 2006;291:C40–49. doi: 10.1152/ajpcell.00351.2005. [DOI] [PubMed] [Google Scholar]
- 156.Guo Z, Dai B, Jiang T, Xu K, Xie Y, Kim O, Nesheiwat I, Kong X, Melamed J, Handratta VD, Njar VC, Brodie AM, Yu LR, Veenstra TD, Chen H, Qiu Y. Regulation of androgen receptor activity by tyrosine phosphorylation. Cancer Cell. 2006;10:309–319. doi: 10.1016/j.ccr.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 157.Kinkade CW, Castillo-Martin M, Puzio-Kuter A, Yan J, Foster TH, Gao H, Sun Y, Ouyang X, Gerald WL, Cordon-Cardo C, Abate-Shen C. Targeting AKT/mTOR and ERK MAPK signaling inhibits hormone-refractory prostate cancer in a preclinical mouse model. J Clin Invest. 2008;118:3051–3064. doi: 10.1172/JCI34764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Malik SN, Brattain M, Ghosh PM, Troyer DA, Prihoda T, Bedolla R, Kreisberg JI. Immunohistochemical demonstration of phospho-Akt in high Gleason grade prostate cancer. Clin Cancer Res. 2002;8:1168–1171. [PubMed] [Google Scholar]
- 159.Thomas GV, Horvath S, Smith BL, Crosby K, Lebel LA, Schrage M, Said J, De Kernion J, Reiter RE, Sawyers CL. Antibody-based profiling of the phosphoinositide 3-kinase pathway in clinical prostate cancer. Clin Cancer Res. 2004;10:8351–8356. doi: 10.1158/1078-0432.CCR-04-0130. [DOI] [PubMed] [Google Scholar]
- 160.Bakin RE, Gioeli D, Bissonette EA, Weber MJ. Attenuation of Ras signaling restores androgen sensitivity to hormone-refractory C4-2 prostate cancer cells. Cancer Res. 2003;63:1975–1980. [PubMed] [Google Scholar]
- 161.Mulholland DJ, Kobayashi N, Ruscetti M, Zhi A, Tran LM, Huang J, Gleave M, Wu H. Pten loss and RAS/MAPK activation cooperate to promote EMT and metastasis initiated from prostate cancer stem/progenitor cells. Cancer Res. 2012;72:1878–1889. doi: 10.1158/0008-5472.CAN-11-3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Solit DB, Zheng FF, Drobnjak M, Munster PN, Higgins B, Verbel D, Heller G, Tong W, Cordon-Cardo C, Agus DB, Scher HI, Rosen N. 17-Allylamino-17-demethoxygeldanamycin induces the degradation of androgen receptor and HER-2/neu and inhibits the growth of prostate cancer xenografts. Clin Cancer Res. 2002;8:986–993. [PubMed] [Google Scholar]
- 163.Zegarra-Moro OL, Schmidt LJ, Huang H, Tindall DJ. Disruption of androgen receptor function inhibits proliferation of androgen-refractory prostate cancer cells. Cancer Res. 2002;62:1008–1013. [PubMed] [Google Scholar]
- 164.Andersen RJ, Mawji NR, Wang J, Wang G, Haile S, Myung JK, Watt K, Tam T, Yang YC, Banuelos CA, Williams DE, McEwan IJ, Wang Y, Sadar MD. Regression of castrate-recurrent prostate cancer by a small-molecule inhibitor of the amino-terminus domain of the androgen receptor. Cancer Cell. 2010;17:535–546. doi: 10.1016/j.ccr.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 165.Sadar MD, Williams DE, Mawji NR, Patrick BO, Wikanta T, Chasanah E, Irianto HE, Soest RV, Andersen RJ. Sintokamides A to E, chlorinated peptides from the sponge Dysidea sp. that inhibit transactivation of the N-terminus of the androgen receptor in prostate cancer cells. Organic Lett. 2008;10:4947–4950. doi: 10.1021/ol802021w. [DOI] [PubMed] [Google Scholar]
- 166.Meimetis LG, Williams DE, Mawji NR, Banuelos CA, Lal AA, Park JJ, Tien AH, Fernandez JG, de Voogd NJ, Sadar MD, Andersen RJ. Niphatenones, glycerol ethers from the sponge Niphates digitalis block androgen receptor transcriptional activity in prostate cancer cells: structure elucidation, synthesis, and biological activity. J Med Chem. 2012;55:503–514. doi: 10.1021/jm2014056. [DOI] [PubMed] [Google Scholar]
- 167.Sasaki M, Tanaka Y, Perinchery G, Dharia A, Kotcherguina I, Fujimoto S, Dahiya R. Methylation and inactivation of estrogen, progesterone, and androgen receptors in prostate cancer. J Natl Cancer Inst. 2002;94:384–390. doi: 10.1093/jnci/94.5.384. [DOI] [PubMed] [Google Scholar]
- 168.Lin HK, Wang L, Hu YC, Altuwaijri S, Chang C. Phosphorylation-dependent ubiquitylation and degradation of androgen receptor by Akt require Mdm2 E3 ligase. EMBO J. 2002;21:4037–4048. doi: 10.1093/emboj/cdf406. [DOI] [PMC free article] [PubMed] [Google Scholar]