Abstract
Amblyomma americanum tick serine protease inhibitor (serpin, AAS) 19, is a highly conserved protein that is characterized by its functional domain being 100% conserved across tick species. We also reported that AAS19 was an immunogenic tick saliva protein with anti-haemostatic functions and an inhibitor of trypsin-like proteases including five of the eight serine protease factors in the blood clotting cascade. In this study the goal was to validate the importance of AAS19 in A. americanum tick physiology, assess immunogenicity and investigate tick vaccine efficacy of yeast-expressed recombinant (r) AAS19. We confirm that AAS19 is important to A. americanum fitness and blood meal feeding. AAS19 mRNA disruption by RNAi silencing caused ticks to obtain blood meals that were 50% smaller than controls, and treated ticks being morphologically deformed with 100% of the deformed ticks dying in incubation. We show that rAAS19 is highly immunogenic in that two 500 µg inoculations mixed with TiterMax Gold adjuvant provoked antibody titers of more than 1:320000 that specifically reacted with native AAS19 in unfed and partially fed tick tissue. Since AAS19 is injected into animals during tick feeding, we challenge infested immunized rabbits twice to test if tick infestations of immunized rabbits could act as booster. While in the first infestation significantly smaller tick blood meals were observed on one of the two immunized rabbits, smaller blood meals were observed on both rabbits, but 60% of ticks that engorged on immunized rabbits in the second infestation failed to lay eggs. It is notable that ticks fed faster on immunized animals despite obtaining smaller blood meals. We conclude that rAAS19 is a potential component of cocktail tick vaccine.
Keywords: Amblyomma americanum, A. americanum serpin 19, tick vaccine antigens candidate
1. Introduction
Ticks and tick-borne diseases (TBD) pause enormous threats to global public and veterinary health. Ticks and important TBDs such as babesiosis, heartwater, and theileriosis are major source of economic loss in the livestock industry (Jongejan and Uilenberg, 2004). Presumably due to improved diagnostics, and climate change that is expanding geographic range of tick vectors, reported human TBDs are on the rise (Brownstein et al., 2005, Kalluri et al., 2007, Walker et al., 2008, Gray et al., 2009). By 2015, the USA Centers for Disease Control listed 14 reportable human TBD agents. Amblyomma americanum long considered a nuisance (Childs and Paddock, 2003), is now among medically important tick species. This tick is the principal vector for Ehrlichia chaffeensis and E. ewingii, the causative agents of human monocytic ehrlichiosis (Anderson et al., 1993, Wolf et al., 2000), causative agent of the southern tick-associated rash illness (STARI) (James et al., 2001, Masters et al., 2008), and the heartland virus (Savage et al., 2013). It is also involved in the epidemiology of Francisella tularensis (Taylor et al., 1991), and it also transmits Theileria cervi (Laird et al., 1988), E. chaffeensis, E. ewingii, and Borrelia lonestari (Varela-Stokes, 2007) to white tailed deer. Heavy A. americanum tick infestation has been reported to reduce productivity in cattle (Barnard et al., 1992, Barnard, 1985, Tolleson et al., 2012, Tolleson et al., 2010). In absence of tick vaccines against major TBD agents, killing of ticks using acaricides remains the most important tick control method. However serious limitations such as ticks quickly developing resistance to acaricides, environmental and food chain contamination threatens continuity of tick control programs (Graf et al., 2004, George et al., 2004, Ghosh et al., 2007). To solve the problem of acaricide resistance, immunization of animals against tick feeding has been advocated as a sustainable alternative (Opdebeeck et al., 1988, Willadsen, 2004, Sonenshine et al., 2006, de la Fuente and Kocan, 2006, de la Fuente et al., 2007, George, 2000). The rationale is that anti-tick vaccines will be effective against both acaricide susceptible and resistant tick populations (Willadsen, 2004, Merino et al., 2013, Mulenga et al., 2001, Mulenga et al., 1999). Commercialization of the vaccine against Rhipicephalus (Boophilus) microplus validated the feasibility of controlling ticks through immunization (Willadsen et al., 1995). Weaknesses of the R. microplus vaccine including effectiveness against one tick species (Rodriguez et al., 1995a, Rodriguez et al., 1995b, Garcia-Garcia et al., 2000), necessitates the search for effective target anti-tick vaccine antigens with potential to control multiple tick species (Mulenga et al., 2013a).
Our goal is to understand tick-feeding physiology as a means of discovering important tick saliva proteins that can be targeted for anti-tick vaccine development. In our laboratory, selection of potential tick vaccine targets is based on high amino acid conservation among ticks and that the candidate antigen is confirmed to be injected into its host. Through this pipeline we identified highly conserved A. americanum tick saliva serine protease inhibitor (serpin) (AAS) 19 characterized by 100% conservation of the functional domain reactive center loop in ixodid ticks (Porter et al., 2015). Kim et al. (2015a) recently showed that AAS19 mRNA is expressed in most tick tissues, that the native protein is injected into the host during tick feeding, and that this protein has anti-haemostic functions with broad inhibitor activity against trypsin, plasmin and five of the eight serine proteases (factors [f] Xa and XIa, strongly, fIIa [thrombin], factors IXa, and XIIa, moderately) in the mammalian blood clotting cascade. In this study the goal was to validate the significance of AAS19 in A. americanum tick feeding success and assess its anti-tick vaccine efficacy. We show that AAS19 is important to tick feeding success as revealed by RNAi silencing and that rAAS19 is a potential target in cocktail tick vaccine formulation.
2. MATERIALS AND METHODS
2.1. Ethics statement
All animal work was conducted and approved according to Texas A&M University Institutional Animal Care and Use Committee (AUP 2011-0207).
2.2. Tick feeding, dissection, and protein extractions
A. americanum ticks used in this study were purchased from Oklahoma State University tick laboratory (Stillwater, OK, USA). Ticks were maintained in 85% humidity chamber at 25°C before placing them on rabbits to feed. To feed, A. americanum ticks were placed onto the outer portion of the ear of specific pathogen-free (SPF) New Zealand rabbits and restricted in this location using orthopedic stockinettes adhered on the rabbit ear with Kamar Adhesive (Kamar Products Inc., Zionsville, IN, USA). To initiate feeding, 10 male ticks were placed into each ear stocking three days prior to adding 15 female ticks in each ear stocking (total of 30 female ticks per rabbit). To prepare tick protein extracts, five ticks from unfed and manually detached at 24, 72 and 120 h post tick attachment were rinsed in sterile 1X phosphate buffered saline (PBS) pH 7.4 and processed for dissections. Ticks were placed on a sterile glass slide and cut on the extreme anterior, posterior and lateral ends using a sterile razor blade. Tick organs including salivary glands (SG), midguts (MG), synganglion (SYN), Malpighian tubule (MT), ovary (OVR) and the remnants labeled as carcass (CA) were isolated and placed into IP lysis buffer with protease inhibitor cocktail (Thermo Scientific, Waltham, MA, USA). Protein extracts were homogenized and stored in −80°C.
2.3. RNAi silencing of AAS19 mRNA
RNAi-mediated silencing was performed as described (Mulenga et al., 2013b, Kim et al., 2014). Double stranded RNA (dsRNA) was synthesized using the Megascript RNAi kit (Thermo Scientific) targeting position 327–971 of AAS19 nucleotide sequence (NCBI Accession# GAYW01000076). The 644 base pair dsRNA target sequence was searched against tick sequences in GenBank to verify specificity. Using 2 µg of purified PCR product as template, dsRNA was synthesized using primers with added T7 promoter sequence in bold (For: 5′-TAATACGACTCACTATAGGGGTACGCCCTGGACGTCGCCAACG-3′ and Rev 5′-TAATACGACTCACTATAGGGGAGAGGTCGGCGTCAGCGGAG-3′). PCR primers for enhanced green fluorescent protein coding cDNA (EGFP; accession number JQ064510.1) with added T7 promoter sequence (Kim et al., 2014) were used to synthesize control EGFP-dsRNA. Two test groups of 15 female A. americanum ticks were injected with 0.5 – 1 µL (~3 µg/µL) EGFP-dsRNA or AAS19-dsRNA in nuclease free water as described (Kim et al., 2014). Injected ticks were kept for 24 h at 25°C in 85% humidity to recover before being placed on SPF New Zealand rabbits to feed.
The effect of AAS19 mRNA disruption on tick feeding success was investigated by assessing tick attachment and mortality rates, time to feed to repletion, engorgement weight (EW) as an index for amount of blood taken in by tick, and egg mass conversion ratio (EMCR) as measure of utilizing blood meal to produce eggs as described. Tick phenotypes during feeding were documented daily using the Canon EOS Rebel XS camera attached to a Canon Ultrasonic EF 100mm 1:2:8 USM Macro Lens (Canon USA Inc., Melville, NY, USA).
2.4. Validation of RNAi silencing
Disruption of AAS19 mRNA was verified by quantitative RT-PCR as described (Kim et al., 2014). Three ticks each that were injected with EGFP-dsRNA and AAS19-dsRNA were sampled at 48 h post-attachment by manual detachment. Ticks were processed individually. Tick organs (SG, MG, SYN, MT, OVR, CA) were dissected as described above. Extraction of mRNA using the Dynabead mRNA Direct Kit (Thermo Scientific) were performed following the manufacturer’s instructions. The extracted mRNA was quantified using the Infinite M200 Pro plate reader (Tecan, Männedorf, Switzerland). Template cDNA was synthesized from ~200 ng of mRNA using the Verso cDNA Synthesis Kit following the manufacturer’s instructions (Thermo Scientific). Approximately 50 ng cDNA and AAS19 forward (5′-GACAAGACGCACGGCAAAA-3′) and reverse (5′-GAAGTCCGGCGGCTCAT- 3′) primers in a final concentration of 900 nM each, were mixed with 2X SYBR Green Master Mix (Thermo Scientific) in triplicates and was subjected to qRT-PCR. For an internal control, A. americanum 40S ribosomal protein S4 (accession number GAGD01011247.1) (Koci et al., 2013) was used. Relative quantification (RQ) of AAS19 transcript was determined as described (Kim et al., 2014). AAS19 mRNA suppression was determined using the formula, S = 100-(RQT/RQC X 100) where S = mRNA suppression, RQT and RQC = RQ of tissues in AAS19-dsRNA injected and EGFP-dsRNA injected ticks, respectively. Data are presented as the mean (M) AAS19 mRNA suppression ± SEM.
2.5. Immunization of rabbits with recombinant (r) AAS19 and tick challenge infestation
SPF New Zealand white rabbits of approximately 10 – 12 weeks of age were immunized with TiterMax Gold adjuvant (Sigma, St. Louis, MO) or 514 µg of rAAS19 antigen in 1X PBS pH 7.4 mixed with an equivalent volume of adjuvant to 1 mL. Expression and purification of the immunizing antigen, rAAS19, in Pichia pastoris (X-33) was previously described in Kim et al., (2015a). Two immunizations were administered at days 0 and 30. Rabbits were inoculated subcutaneously and intradermally with ~200 µL of rAAS19: adjuvant or PBS: adjuvant mix into five injection sites. Two weeks after immunization, serum was collected to verify antibody response to rAAS19 by ELISA. Following immunization, rabbits were challenge infested with 40 adult female ticks (20 ticks per ear). In order to determine if repeated tick infestation of animals immunized with recombinant tick saliva proteins such as rAAS19 enhanced anti-tick immunity, rabbits were re-infested with 40 ticks at two weeks after the first infestation. Each rabbit received 20 ticks per ear. The effect(s) of immunization against tick feeding parameters were determined as described in RNAi silencing (section 2.3).
2.6. ELISA and western blotting analysis
Routine ELISA and western blotting analysis verified rabbit antibody response to rAAS19. For ELISA, 0.5 µg affinity purified rAAS19 was coated on 96 well plates overnight in coating buffer (0.1M sodium carbonate pH 9.5). Coated wells were subsequently blocked with 300 µL of 5% skim milk in PBS-Tween20 for 1h at room temperature (RT). Following appropriate incubations with pre-immune or immune sera (1:1000 to 1:320000), goat anti-rabbit IgG Horseradish Peroxidase (HRP) conjugated secondary antibody (Millipore, Billerica, MA, USA) and appropriate washes, wells were incubated with 1-Step Ultra TMB ELISA Substrate (Thermo Scientific) for 8 min at RT to detect antibody binding. Adding 2 M sulfuric acid stopped the reaction and OD was determined at A450nm using the Infinite M200 Pro plate reader (Tecan).
To determine if rAAS19 rabbit antisera specifically bound native AAS19, total protein extracts from dissected tick organs were subjected to routine western blotting analyses using antibodies to rAAS19. Given that the immunizing antigen was glycosylated (Kim et al., 2015a), we investigated if the observed antibody response was directed toward glycans on rAAS19 by including three controls: yeast expressed deglycosylated and non-deglycosylated (Kim et al., 2015a) and bacteria expressed rAAS19 (described below). Samples were loaded and resolved on a 10% SDS-PAGE gel and transferred onto a PVDF membrane. Membranes were blocked overnight at 4°C in 5% skim milk in PBS-Tween20. Following appropriate incubations with pre-immune or immune sera (1:10,000), the secondary Clean-Blot™ IP Detection Reagent HRP conjugated (1:40) (Thermo Scientific) antibody, and appropriate washes, membranes were incubated in Amersham ECL Prime Western Blotting Detection Reagent for 5 min and exposed on X-ray films for 10 and 30 min before developing films.
Recombinant AAS19 was expressed in bacteria using BL21 (DE3) pLysS Escherichia coli strain and the pRSETA plasmid expression system (Thermo Scientific) as previously described (Chalaire et al., 2011). Mature AAS19 protein open reading frame (Porter et al., 2015) was uni-directionally sub-cloned into pRSETA in BamHI and EcoRI sites using forward (5′-GGATTCGCAGAGCCCGACGAAGATGGCCG-3′) and reverse (5′-GAATTCTTAGAGGGCGTTAATTTCGCCCAG-3′) primers with added restriction enzyme sites in bold. Bacteria expressed rAAS19 were purified by eluting from PVDF membranes as described in Szewczyk et al. (1998) with slight modifications. To elute the protein off of the PVDF membrane, excised membrane was incubated with elution buffer (1% Triton X-100/2% SDS in 50mM Tris-HCl, pH 9.5) in 0.5 mL tubes for 30 minutes at RT with rapid shaking. Eluted proteins were collected, then transferred into a fresh tube and concentrated by acetone precipitation. Precipitates were re-suspended in 1X PBS pH 7.4.
2.7. Statistical analysis
Unpaired student t-test and Mann-Whitney analysis were used to determine if differences between AAS19-dsRNA and EGFP-dsRNA injected ticks were significant. One Way ANOVA and Tukey HSD, Fisher exact and Chi-Square test analyses were used to determine if tick-feeding performance on control and immunized rabbits were significantly different using the Prism 6 software (GraphPad Software, La Jolla, CA, USA). Data are reported as Mean ± SEM.
3. RESULTS
3.1. AAS19 silencing causes tick deformities and reduces tick blood meal size
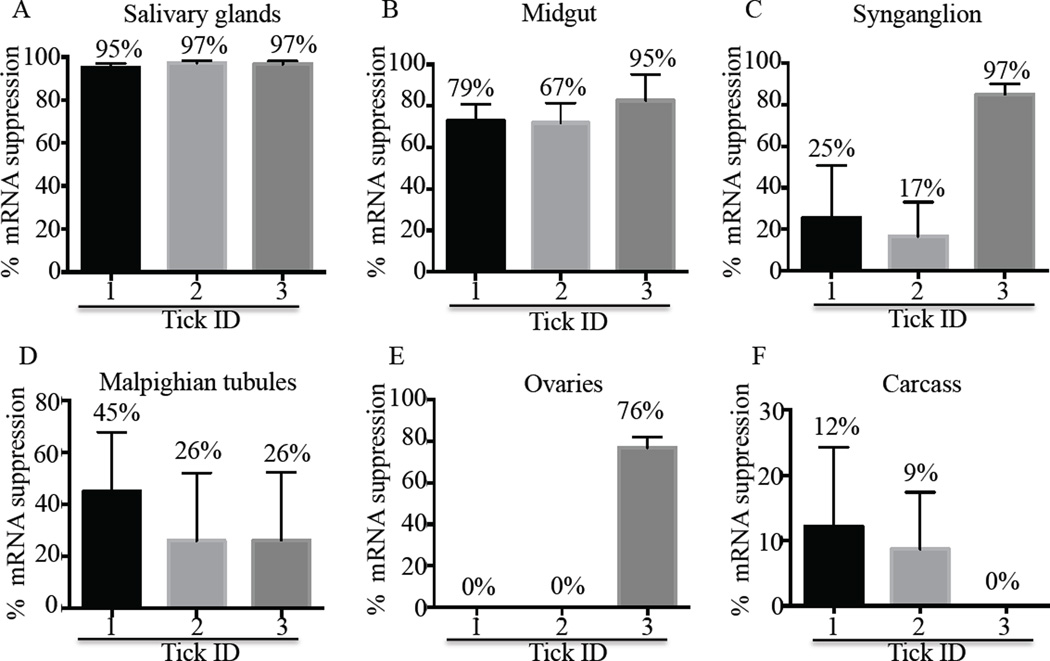
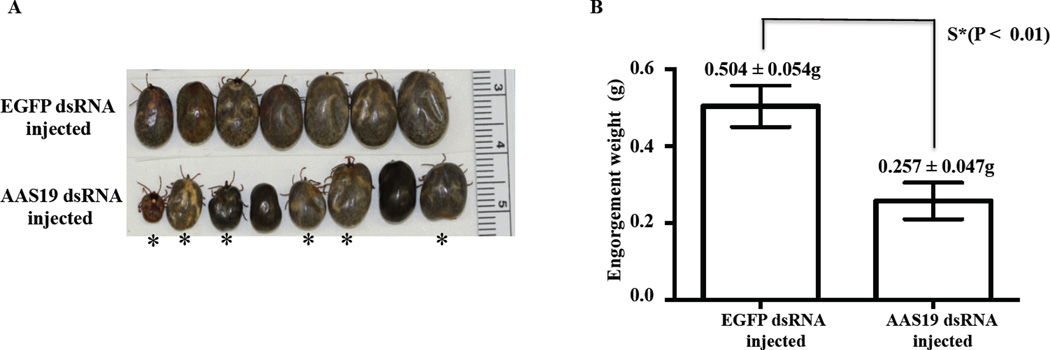
Figures 1 and 2 demonstrate that AAS19 is important to A. americanum tick feeding success as revealed by RNAi silencing analysis. Quantitative RT-PCR indicate that in three randomly sampled ticks, AAS19 mRNA suppression of 95–97%, 67–95%, 17–97%, 26–45%, 0–76%, and 0–12% in SG, MG, SY, MT, OV, and CA, respectively (Fig. 1), was achieved. Spontaneously detached ticks (Fig. 2A) were weighed to determine engorgement weight (EW) as an index for blood meal size. Mean EW of AAS19-dsRNA injected ticks (0.257 ± 0.04g) was ~50% smaller than EGFP-dsRNA injected ticks (0.504 ± 0.054g) with differences being statistically significant (P < 0.01) as revealed by unpaired student t-test (Fig. 2B). It is interesting to note that 75% (6/8) of the AAS19-dsRNA injected ticks were deformed (asterisks marked, Fig. 2A).
Figure 1. Validating the disruption of AAS19 mRNA in AAS19 double stranded (ds) RNA injected ticks.
Fifteen ticks were microinjected with 0.5–1 µL (~3 µg/µL) of AAS19- or EGFP- (control) dsRNA in nuclease free water. At 48 h post-attachment, three ticks per treatment of EGFP-dsRNA injected control and AAS19-dsRNA injected ticks, were manually detached. Tick organs including salivary glands (SG), midguts (MG), synganglion (SYN), Malpighian tubules (MT), ovaries (OV) and carcass (CA, tick remnants after removal of SG, MG, OV, and MT) were dissected and individually processed for mRNA extraction and then subjected to two-step quantitative (q) reverse transcriptase (RT)-PCR using AAS19 primers described in materials and methods section 2.4. Relative expression (RQ) of AAS19 mRNA was determined using the Comparative CT Method (ΔΔCT). Relative suppression of AAS19 mRNA was determined using the following formula, S = 100-(RQT/RQC X 100) where S = mRNA suppression, RQT and RQC = RQ of tissues of AAS19-dsRNA and EGFP-dsRNA injected ticks respectively.
Fig. 2. Phenotype and engorgement weights of AAS19 dsRNA injected ticks.
(A) Spontaneously detached ticks were photographed to document phenotypic changes of AAS19-dsRNA injected ticks compared to control EGFP-dsRNA injected ticks. Deformed ticks are asterisks marked (*). (B) After spontaneously detaching from the host, ticks were individually weighed to determine engorgement weights (EWs) as indices for amounts of blood imbibed by ticks. EWs were subjected to unpaired student t-test and Mann-Whitney analysis to determine statistical significance. Data is reported as mean (M) EWs (M ± SEM).
3.2. Native AAS19 protein is present in multiple tick organs
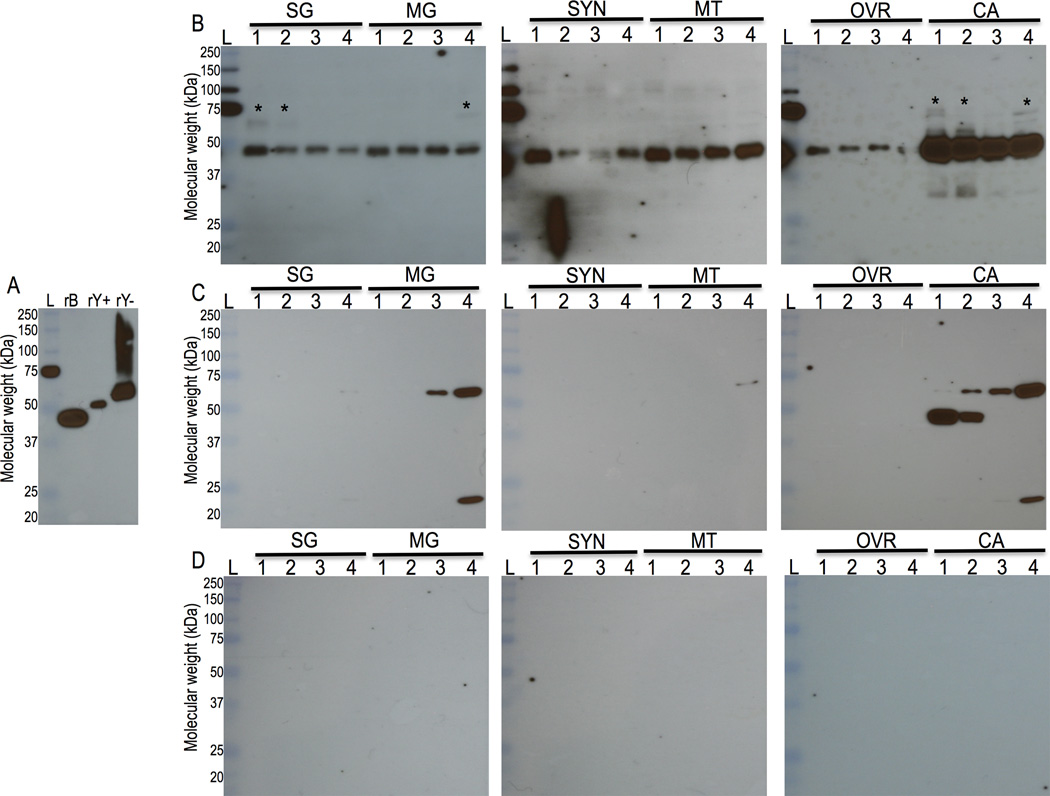
Immunization of rabbits with rAAS19 provoked antibody titers of more than 1:320,000 (not shown). Figure 3A shows that the antibody to rAAS19 specifically bound the immunizing antigen (glycosylated, lane rY−) and the two controls: yeast expressed deglycosylated (rY+) and bacteria expressed non-glycosylated (rB) forms, indicating that the antibody response may be directed at glycan and/or the protein backbone epitopes. Likewise, in figure 3B we observed a single native AAS19 protein band within the expected size range at below 50 kDa. It is interesting to note that, we detected higher molecular weight diffuse protein bands (asterisks marked), which we suspect could be glycosylated forms of native AAS19. The expected mature AAS19 is 388 amino acids long with 43 kDa calculated molecular weight (Porter et al., 2015). Based on data in figure 3, native AAS19 protein is constitutively present in multiple tick organs (SG, MG, OVR, SYN, CA, MT, and CA). We would like to note here that in figure 3C, serum of the pre-immune control bound some non-specific bands ~60 kDa in MG and CA. Additionally, a non-specific band of ~50 kDa was found in the CA in unfed and 24 h samples, but not in other stages. The serum from rabbit injected with PBS-adjuvant control did not bind anything (Fig. 3D).
Figure 3. Western blotting analyses.
Yeast and bacteria expressed rAAS19 (Fig. 3A) and crude tick saliva protein (Figs. 3B, C, and D) extracts were subjected to western blotting analysis using the rabbit antibody to yeast expressed rAAS19 as described in materials and methods section 2.6. Figure 3A, rB = E. coli expressed rAA19, rY+ and rY− = deglycosylated and glycosylated, respectively, Pichia pastoris expressed rAAS19. Crude protein extracts of dissected salivary glands (SG), midgut (MG), synganglion (SYN), Malpighian tubules (MT), ovaries (OVR) and the remnants as carcass (CA) were subjected to western blotting analyses using antibodies to yeast expressed rAAS19 (Fig. 3B), control PBS and adjuvant injected antibody (Fig. 3C), and rabbit pre-immune serum (Fig. 3D). Lanes 1, 2, 3, and 4 = Protein extracts of unfed, 24, 72, and 120 h fed ticks respectively. L = molecular weight ladder, Asterisks marked (*) indicate potential glycosylated native AAS19 form.
3.3. Immunization with rAAS19 enhances tick immunity induced by repeated tick infestation
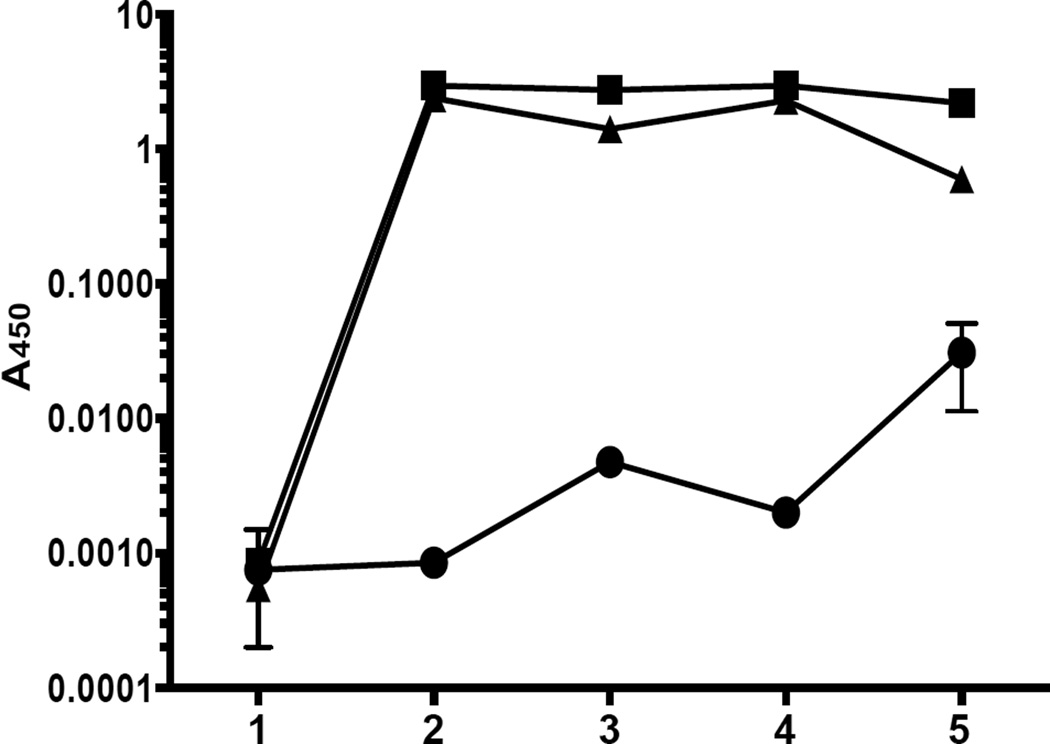
Figure 4 summarizes A450nm log10 of 1:20000 dilutions of immune sera to rAAS19 in tick-infested rabbits: control (filled circle) and immunized rabbits 1 and 2 (filled square and triangle respectively) collected at: (1) pre-immune (2) two weeks after the last immunization, (3) two and (4) four weeks post-first infestation, and (5) two weeks after second infestation. In the control rabbit, antibodies to rAAS19 increased with feeding from background at ~log10 of 0.001 to log10 of more than 0.01 after the second infestation. On the other hand repeated tick infestation of immunized rabbits did not result in increased antibody levels in immunized rabbits. While there was a nearly ten-fold increase between first and second infestations of control rabbits, there was a slight decrease in immunized rabbits after the second infestation (Fig. 4). Comparing data points five (two weeks after second tick infestation) of control and immunized rabbits, it is apparent that immunization of rabbits with rAAS19 boosted antibody levels to AAS19 by nearly a 100 fold.
Figure 4. Effect of repeated tick infestation on antibody levels in immunized rabbits.
Affinity purified recombinant (r) AAS19 was subjected to routine ELISA as described in section 2.6. Data points are represented by (1) pre-immune (2) immune sera collected at two weeks after the booster, (3) two and (4) four weeks post-first tick infestation, and (5) two weeks after second tick infestation. Filled circle (●) = control rabbit inoculated with TiterMax Gold in PBS, filled triangle (▲) and square (■) = immunized rabbits one and two.
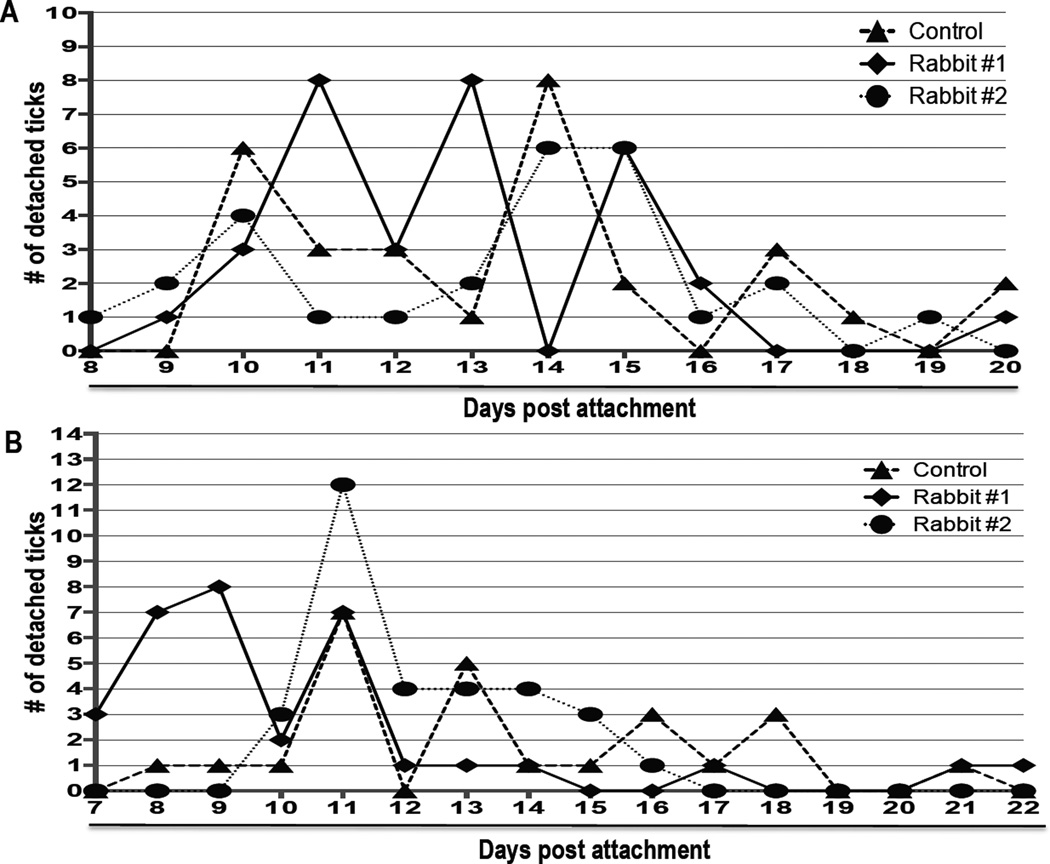
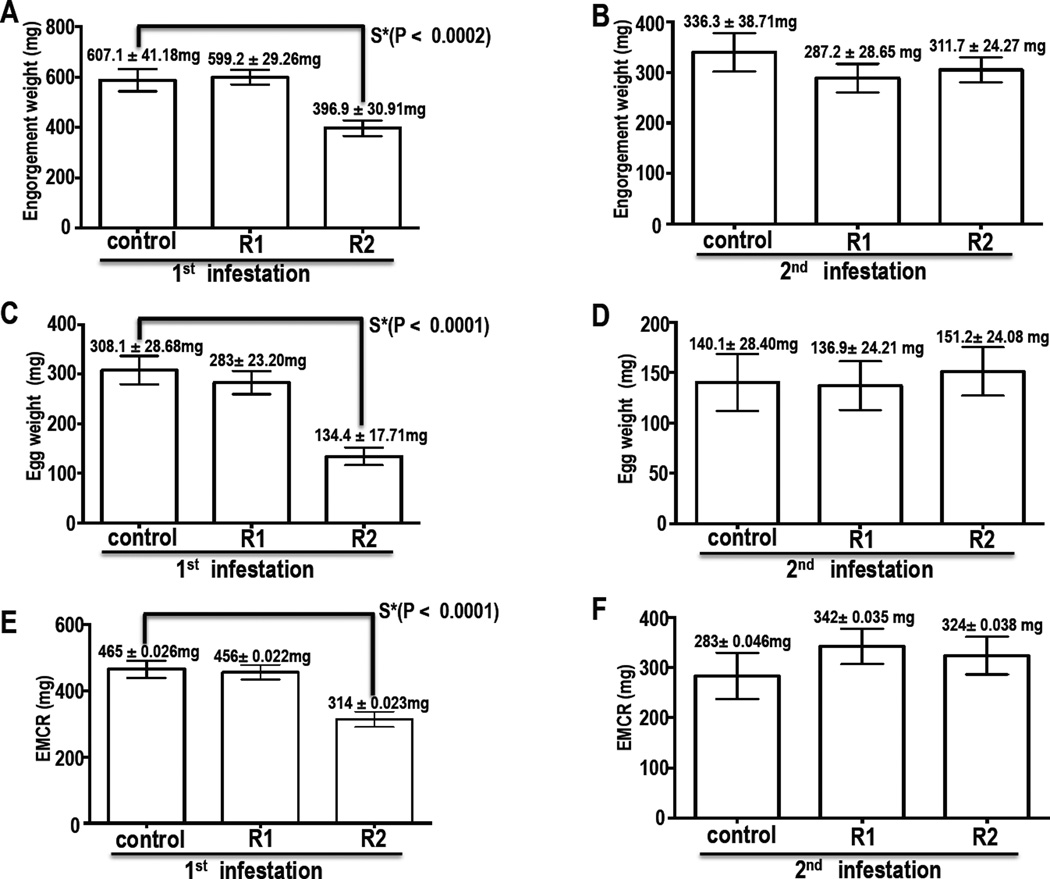
Figures 5 and 6 summarize the effects of rAAS19 immunization on A. americanum success during the first and second infestations. Immunization of rabbits with rAAS19 caused ticks to feed faster, but obtain smaller blood meal sizes overall (Figs. 5 and 6). In the first infestation, chi-square (and Fisher exact) test indicated that 91% of ticks that completed feeding within 15 days on immunized rabbit (R)1 (P = 0.0038) was statistically significant different from 79% of ticks that completed feeding on the control rabbit, while 85% of ticks completed feeding on R2 were apparently faster but not significantly different from the control (solid line) (Fig. 5A). Similarly in the second infestation, the observed 91% and 97% of ticks that completed feeding within 15 days on R1 and R2 respectively were different (P = 0.0285 and 0.0244, respectively) from 68% of ticks that completed feeding on the control rabbit (Fig. 5B). Despite feeding faster, ticks that fed on immunized rabbits obtained smaller blood meals as revealed by tick engorgement weight (EW) (Fig. 6). In the first infestation, One Way ANOVA [F (2, 84) = 11.87, P < 0.0001] and Tukey HSD analysis indicated that R1 Mean EW (599.2 ± 29.26) was not different from the control (607.1 ± 41.18), however R2 ticks were smaller with Mean EW (396.9 ± 30.91) significantly smaller [P < 0.0002] (Fig. 6A) than control. In the second infestation both R1 (287.2 ± 28.65 mg) and R2 (311.7 ± 24.27 mg) mean EW are apparently smaller, but not significantly different from control mean EW (336.3 ± 38.71mg) (Fig. 6B). It is notable that in figure 6B EW of ticks that fed on the control rabbit in the second infestation was smaller than the first infestation, which could point to the rabbit becoming resistant due to repeated infestations.
Figure 5. Effect of rAAS19 immunization on completion of feeding.
Ticks challenged on rAAS19 rabbits were observed through 3 weeks of feeding until detachment. (A) Detached ticks were recorded every 24 hours for 20 days for the first tick challenge infestation on control rabbit (broken lines) and rabbit (R)1 (solid lines) and R2 (dotted lines) that were immunized with rAAS19. (B) Detached ticks from second tick challenge infestation on control, R1, and R2 for 22 days. Each node represents the number of detached ticks. Data is reported as mean of number of detached ticks (M ± SEM).
Figure 6. Effect of rAAS19 immunization on blood meal size and egg laying.
Ticks were allowed to feed on control and immunized rabbits (R)1 and 2 to repletion. Engorgement weights (EW) of detached ticks that fed on control and immunized rabbit groups were recorded from first (A) and second (B) infestations as indices for amounts of blood imbibed by ticks. Engorged ticks were incubated at 25°C at 85–90% relative humidity until oviposition. Eggs were collected and weighed from the first (C) and second (D) infestations. To determine the egg mass conversion ratio (EMCR), as a measure of the tick’s ability to utilize the blood meal to produce eggs, from first (E) and second (F) infestations, weights of egg masses were divided by engorgement mass. EW, egg weights and EMCRs were subjected to One-Way ANOVA and Tukey’s analysis to determine statistical significance. Data is reported as mean of EW, egg weights or EMCR (M ± SEM).
The egg mass of ticks that fed on R2 (134.4 ± 17.71 mg) was significantly lower (P<0.0001), whereas R1 (283.4 ± 23.20 mg) was apparently lower but not significantly different than the control (308.1 ± 280.68 mg) (Fig. 6C), as revealed by Tukey HSD analysis. In the second infestation there were no significant differences in egg weight between all groups (Fig. 6D). The effect of rAA19 immunization on the tick's ability to convert the blood meal to eggs was inconclusive (Figs. 5E and 5F). The egg mass conversion ratio (EMCR) for ticks that fed on R2 (0.314 ± 0.023 mg) was significantly lower (P<0.0001) than the control (0.465 ± 0.026 mg) (Fig. 5E), however there was no effect on ticks that fed on R1 in both first and second infestations, and for ticks that fed on R2 in the second infestation (Figs. 6E and 6F).
Data summarized in table 1 show that while immunization did not apparently affect the ability of ticks to lay eggs in the first infestation, significantly more ticks that fed on immunized rabbits failed to lay eggs in the second infestation. Chi-square (Fisher exact) test revealed that the observed 61% (P = 0.0167) R1 and 68% (P = 0.0010) R2 of ticks that did not lay eggs in the second infestation were significantly higher than the 48% from the control rabbit. Other parameters summarized in table 1 were not different from the control. Although we placed equal numbers of ticks on the control and rAAS19-immunized rabbits, there was a difference in the number of engorged ticks per rabbit. We observed that some engorged ticks were crushed after they detached from the host and before we were able to collect them, which caused the differences in the number of engorged ticks.
Table 1.
Effect of rAAS19 immunization on Amblyomma americanum egg laying
| 1st infestation | 2nd infestation | |||||
|---|---|---|---|---|---|---|
| Parameters | Control rabbit |
Rabbit 1 |
Rabbit 2 |
Control rabbit |
Rabbit 1 |
Rabbit 2 |
| Egg Weight (mg) | 308.18 | 283 | 134.40 | 140.14 | 136.9 | 151.16 |
|
Egg Mass Conversion Ratio (mg) |
0.465 | 0.456 | 0.313 | 0.283 | 0.342 | 0.32 |
| % Ticks failed to lay eggs | 6.9 (2/29) |
6.25 (2/32) |
3.71 (1/26) |
48 (12/25) |
60.6 (20/33) |
67.74 (21/31) |
4. DISCUSSION
We previously reported that AAS19 was a highly conserved serine protease inhibitor (serpin) that was characterized by its functional domain reactive center loop being 100% conserved in ixodid ticks where we could find data (Porter et al., 2015). In another study we showed that AAS19 mRNA was expressed in multiple tick organs, and that native AAS19 was an immunogenic tick saliva protein as indicated by antibodies to replete fed tick saliva proteins specifically binding yeast expressed rAAS19. Further, we showed that rAAS19 has anti-haemostatic functions and that it is an inhibitor of trypsin-like proteases including five of the eight serine proteases in the blood clotting cascade (Kim et al., 2015a). These earlier findings collectively suggested the potential for AAS19 to regulate important functions in tick feeding success. In this study we confirm that AAS19 plays important roles in tick feeding physiology and overall tick fitness as revealed by the observed deformities and significant reductions in blood meal sizes of AAS19 dsRNA injected ticks. The cause of the observed deformations in AAS19-dsRNA injected ticks is unknown at this point. However, we speculate that uncontrolled activities of a yet unknown host and/or tick derived protease that is under control of AAS19 caused this. Similar to observations in this study, silencing of a serpin in D. melanogaster impaired arthropod morphology (Hashimoto et al., 2003). The role of inhibitory serpins is to terminate protease actions beyond what is physiologically allowable. Several lines of research have demonstrated that uncontrolled protease activity do destroy self-tissue, particularly in disease situations (Owen and Campbell, 1999, Nakatani et al., 2001, Straka, 2011). We hypothesize that the protease that is controlled by AAS19 is important to tick physiology, but if left uncontrolled it impairs in biochemical homeostasis, and identifying the AAS19 controlled protease is highly desirable.
The long-term goal of our research is to find target antigens for tick vaccine development. Thus, a goal of this study was to investigate immunogenicity and anti-tick vaccine efficacy rAAS19. The observation that rAAS19 provoked titers of up to 1:320000 confirmed immunogenicity of this antigen. There is evidence that antibodies to glycosylated immunogens such as rAAS19 may predominantly be directed to glycans on the protein, and not the protein backbone (Tellam et al., 1992, Rodriguez-Valle et al., 2001, Eberl et al., 2001, Kariuki et al., 2008). The observation that antibodies to yeast expressed rAAS19, also reacted with bacteria expressed AAS19 rules this out. Of significant interest to us, the antibody response to rAAS19 also reacted with epitopes on glycosylated and non-glycosylated forms of native AAS19 as revealed by western blotting analysis data. The observation that native AAS19 protein is present in multiple tick organs and the fact that ticks inject this protein into the host during feeding (Kim et al., 2015a) suggests that this protein may function inside the tick and at the tick-feeding site. This observation is not unique to AAS19. Similar characteristics have been observed for multiple other tick saliva proteins including AV422 (Mulenga et al., 2013b, Radulovic et al., 2014, Tirloni et al., 2014, 2015), insulin-like growth factor binding protein-related protein 1 (Radulovic et al., 2015), calreticulin (Sanders et al., 1999, Kim et al., 2015b), and tick histamine release factor (Mulenga et al., 2003). Functions of AAS19 in the tick and at the tick-feeding site are not well established other than previously reported anti-haemostatic properties (Kim et al., 2015). Identifying AAS19 native targets in the tick and the tick-host interface would be the next step to better understand the role(s) of AAS19 in tick feeding physiology.
Trager (1939a, 1939b) observed that repeated infestations with Dermacentor variabilis larvae provoked tick-feeding resistance in rabbits (Trager et al., 1939a, 1939b). Over the years several lines of research have attempted to replicate these studies through immunization of animals with crude tick saliva gland protein extracts (Brown et al, 1984, Shapiro et al., 1989, Nyindo et al., 1989, Banerjee et al., 1990, Kovar, 2004, Willadsen, 2006, Narasimhan et al., 2007, Jittapalapong et al., 2008) and since the 1990s with recombinant tick saliva proteins (Tsuda et al., 2001, Mulenga et al., 1999, You, 2005, Dai et al., 2009, Guo et al., 2009). The common practice in tick vaccine efficacy studies is to challenge infests immunized animals once (Tsuda et al., 2001, Mulenga et al., 1999, You, 2005, Dai et al., 2009, Guo et al., 2009, Galay et al., 2014). In this study, a different approach was used. We challenge infested the control and immunized rabbits twice. Given the fact that AAS19 is injected into animals during tick feeding (Kim et al., 2015a), we reasoned that repeated inoculation of native AAS19 could serve as a booster and enhance protective tick immunity conferred by rAAS19 immunization. It is interesting to note that, although in the first infestation, most tick feeding parameters were not affected except for smaller tick blood meal sizes observed on one of the two rAAS19-immunized rabbits. The effects of immunization in the second infestation were dramatic as ticks that fed on both immunized rabbits were not only smaller but majority of these ticks failed to lay eggs. The implication of this observation is that over time, immunization of animals with rAAS19 could reduce the tick population. The other practical implication could be toward finding ways to immunize wildlife against tick feeding. Traditional immunization protocols that require primary and booster injections are impractical in wildlife because of the difficulty to identify animals that require booster injections. Thus discovery of antigens such as rAAS19 in which tick infestation of immunized animals will serve as a booster will make it practical to deliver anti-tick vaccines to wildlife. In this way we can deliver tick vaccine antigens such as rAAS19 to wildlife once, and subsequent tick infestations will serve as booster and potentially the level of protection increasing with each subsequent infestation. Wildlife is central to both animal and human TBDs epidemiology as wild animals serve both as blood meal sources of vector ticks and reservoirs for TBD agents. For instance, white tailed deer is the major blood meal source for A. americanum and Ixodes scapularis, the two medically important tick species that transmit 9 of the 14 reportable human TBD agents listed by USA Centers for Disease Control (www.cdc.gov). There is evidence that deer populations were associated with human cases of TBDs in several localities around in the USA (Piesman et al., 1979, Nieto et al., 2012, Raizman et al., 2013, Wiznia et al., 2013, Mays et al., 2014). In the US livestock industry, the threat of R. microplus and R. annulatus including its vectored deadly Babesia cattle parasites have been eradicated for more than 50 years (Walker, 2011, Giles et al., 2014). Re-establishment of R. microplus and R. annulatus ticks and their associated parasites are prevalent in Mexico (Lohmeyer et al., 2011; Busch et al., 2014). Re-increasing deer population threatens continued success of preventing R. microplus from Mexico establishing in the USA (Lohmeyer et al., 2011, Pound et al., 2010). Deer infested with acaricide resistant ticks from Mexico were found inside the USA border (Busch et al., 2014, Rodriguez-Vivas et al., 2014). Thus finding ways to effectively immunize deer against ticks could make significant contributions toward controlling human TBDs, which encourages the need to develop alternative control methods such as effective anti-tick vaccines.
The expected effect of anti-tick immunization on tick feeding success would be slowing down tick feeding efficiency. It was surprising to note in this study that majority of ticks that fed on the immunized rabbits detached from the host quicker than the control. The implication of ticks that fed on rAAS19 completing to feed at a much faster pace is unknown at the moment. It is notable that despite feeding faster, these ticks were able to oviposit. Unfortunately, we were not able to evaluate the egg hatching to validate if eggs that were laid by ticks that engorged faster were viable. Consistent with previous data (Merino et al., 2011, Almazan et al., 2010), we also observed that antibody titers to rAAS19 increased with tick feeding in control rabbits. In contrast, we observed an interesting phenomenon in rAAS19-immunized rabbits, that antibody titers to rAAS19 dropped following tick infestation before rising back up at four weeks post infestation.
Another interesting observation from our study was that, while RNAi silencing caused morphological deformities in AAS19 dsRNA injected ticks; ticks that engorged on rAAS19-immunized rabbits were normal albeit smaller. The discrepancy of this effect cannot be explained at this point. It is potentially possible that we achieved more suppression of protein function with RNAi silencing than with antibodies to rAAS19. Given that we used a long, 644 base pair dsRNA, there is a possibility that the observed deformities could also be due to off target effects. In RNAi silencing, off target effects can occur if the target sequence has a match of as little as 11 contiguous nucleotides with another sequence (Qiu et al., 2005, Jackson et al., 2003). However, we believe that the possibility for off target effects is remote in that when subjected to phylogeny analysis with 120 other A. americanum serpins, AAS19 amino acid sequence segregated alone (Porter et al., 2015) indicating that this protein did not have a close relative among A. americanum serpins. Furthermore, when scanned against tick sequence entries in GenBank, AAS19 dsRNA target sequence did not return matches that were more than 21 nucleic acids long.
In conclusion, this study builds on the biology of AAS19, a uniquely highly conserved tick serpin that likely play yet unknown important roles both in the tick and at the tick-feeding site. Based on tick vaccine efficacy data in this study, rAAS19 is partially protective as a standalone target antigen. However, it represents a potential component of a cocktail tick vaccine antigen. Efforts to test tick vaccine efficacy of rAAS19 in a cocktail antigen mixture are underway.
ACKNOWLEDGEMENTS
This research was supported by National Institutes of Health grants (AI081093, AI093858, AI074789, AI074789-01A1S1) to AM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Almazan C, Lagunes R, Villar M, Canales M, Rosario-Cruz R, Jongejan F, de la Fuente J. Identification and characterization of Rhipicephalus (Boophilus) microplus candidate protective antigens for the control of cattle tick infestations. Parasitol. Res. 2010;106:471–479. doi: 10.1007/s00436-009-1689-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BE, Sims KG, Olson JG, Childs JE, Piesman JF, Happ CM, Maupin GO, Johnson BJ. Amblyomma americanum: a potential vector of human ehrlichiosis. Am. J. Trop. Med. Hyg. 1993;49:239–244. doi: 10.4269/ajtmh.1993.49.239. [DOI] [PubMed] [Google Scholar]
- Banerjee DP, Momin RR, Samantaray S. Immunization of cattle (Bos indicus X Bos taurus) against Hyalomma anatolicum anatolicum using antigens derived from tick salivary gland extracts. Int. J. Parasitol. 1990;20:969–972. doi: 10.1016/0020-7519(90)90037-n. [DOI] [PubMed] [Google Scholar]
- Barnard DR, Ervin RT, Epplin FM. Bio-Economic Impact of Amblyomma americanum in Beef Cattle Production Systems. Tick Vector Biology. 1992:55–69. [Google Scholar]
- Barnard DR. Injury thresholds and production loss functions for the lone star tick, Amblyomma americanum (Acari: Ixodidae), on pastured, preweaner beef cattle, Bos taurus. J. Econ. Entomol. 1985;78:852–855. doi: 10.1093/jee/78.4.852. [DOI] [PubMed] [Google Scholar]
- Brown SJ, Shapiro SZ, Askenase PW. Characterization of tick antigens inducing host immune resistance. I. Immunization of guinea pigs with Amblyomma americanum-derived salivary gland extracts and identification of an important salivary gland protein antigen with guinea pig anti-tick antibodies. J. Immunol. 1984;133:3319–3325. [PubMed] [Google Scholar]
- Brownstein JS, Holford TR, Fish D. Effect of Climate Change on Lyme Disease Risk in North America. Ecohealth. 2005;2:38–46. doi: 10.1007/s10393-004-0139-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch JD, Stone NE, Nottingham R, Araya-Anchetta A, Lewis J, Hochhalter C, Giles JR, Gruendike J, Freeman J, Buckmeier G, Bodine D, Duhaime R, Miller RJ, Davey RB, Olafson PU, Scoles GA, Wagner DM. Widespread movement of invasive cattle fever ticks (Rhipicephalus microplus) in southern Texas leads to shared local infestations on cattle and deer. Parasit. Vectors. 2014;7 doi: 10.1186/1756-3305-7-188. 188-3305-7-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalaire KC, Kim TK, Garcia-Rodriguez H, Mulenga A. Amblyomma americanum (L.) (Acari: Ixodidae) tick salivary gland serine protease inhibitor (serpin) 6 is secreted into tick saliva during tick feeding. J. Exp. Biol. 2011;214:665–673. doi: 10.1242/jeb.052076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs JE, Paddock CD. The ascendancy of Amblyomma americanum as a vector of pathogens affecting humans in the United States. Annu. Rev. Entomol. 2003;48:307–337. doi: 10.1146/annurev.ento.48.091801.112728. [DOI] [PubMed] [Google Scholar]
- Dai J, Wang P, Adusumilli S, Booth CJ, Narasimhan S, Anguita J, Fikrig E. Antibodies against a tick protein, Salp15, protect mice from the Lyme disease agent. Cell. Host Microbe. 2009;6:482–492. doi: 10.1016/j.chom.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente J, Almazan C, Canales M, Perez de la Lastra JM, Kocan KM, Willadsen P. A ten-year review of commercial vaccine performance for control of tick infestations on cattle. Anim. Health. Res. Rev. 2007;8:23–28. doi: 10.1017/S1466252307001193. [DOI] [PubMed] [Google Scholar]
- de la Fuente J, Kocan KM. Strategies for development of vaccines for control of ixodid tick species. Parasite Immunol. 2006;28:275–283. doi: 10.1111/j.1365-3024.2006.00828.x. [DOI] [PubMed] [Google Scholar]
- Eberl M, Langermans JA, Vervenne RA, Nyame AK, Cummings RD, Thomas AW, Coulson PS, Wilson RA. Antibodies to glycans dominate the host response to schistosome larvae and eggs: is their role protective or subversive? J. Infect. Dis. 2001;183:1238–1247. doi: 10.1086/319691. [DOI] [PubMed] [Google Scholar]
- Galay RL, Miyata T, Umemiya-Shirafuji R, Maeda H, Kusakisako K, Tsuji N, Mochizuki M, Fujisaki K, Tanaka T. Evaluation and comparison of the potential of two ferritins as anti-tick vaccines against Haemaphysalis longicornis. Parasit. Vectors. 2014;7 doi: 10.1186/s13071-014-0482-x. 482-014-0482-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Garcia JC, Montero C, Redondo M, Vargas M, Canales M, Boue O, Rodriguez M, Joglar M, Machado H, Gonzalez IL, Valdes M, Mendez L, de la Fuente J. Control of ticks resistant to immunization with Bm86 in cattle vaccinated with the recombinant antigen Bm95 isolated from the cattle tick, Boophilus microplus. Vaccine. 2000;18:2275–2287. doi: 10.1016/s0264-410x(99)00548-4. [DOI] [PubMed] [Google Scholar]
- George JE. Present and future technologies for tick control. Ann. N. Y. Acad. Sci. 2000;916:583–588. doi: 10.1111/j.1749-6632.2000.tb05340.x. [DOI] [PubMed] [Google Scholar]
- George JE, Pound JM, Davey RB. Chemical control of ticks on cattle and the resistance of these parasites to acaricides. Parasitology. 2004;129(Suppl):S353–S366. doi: 10.1017/s0031182003004682. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Azhahianambi P, Yadav MP. Upcoming and future strategies of tick control: a review. J. Vector Borne Dis. 2007;44:79–89. [PubMed] [Google Scholar]
- Giles JR, Peterson AT, Busch JD, Olafson PU, Scoles GA, Davey RB, Pound JM, Kammlah DM, Lohmeyer KH, Wagner DM. Invasive potential of cattle fever ticks in the southern United States. Parasit. Vectors. 2014;7 doi: 10.1186/1756-3305-7-189. 189-3305-7-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf JF, Gogolewski R, Leach-Bing N, Sabatini GA, Molento MB, Bordin EL, Arantes GJ. Tick control: an industry point of view. Parasitology. 2004;129(Suppl):S427–S442. doi: 10.1017/s0031182004006079. [DOI] [PubMed] [Google Scholar]
- Gray JS, Dautel H, Estrada-Pena A, Kahl O, Lindgren E. Effects of climate change on ticks and tick-borne diseases in Europe. Interdiscip. Perspect. Infect. Dis. 2009;2009:593232. doi: 10.1155/2009/593232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Booth CJ, Paley MA, Wang X, DePonte K, Fikrig E, Narasimhan S, Montgomery RR. Inhibition of neutrophil function by two tick salivary proteins. Infect. Immun. 2009;77:2320–2329. doi: 10.1128/IAI.01507-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto C, Kim DR, Weiss LA, Miller JW, Morisato D. Spatial regulation of developmental signaling by a serpin. Dev. Cell. 2003;5:945–950. doi: 10.1016/s1534-5807(03)00338-1. [DOI] [PubMed] [Google Scholar]
- Jackson AL, Bartz SR, Schelter J, Kobayashi SV, Burchard J, Mao M, Li B, Cavet G, Linsley PS. Expression profiling reveals off-target gene regulation by RNAi. Nat. Biotechnol. 2003;21:635–637. doi: 10.1038/nbt831. [DOI] [PubMed] [Google Scholar]
- James AM, Liveris D, Wormser GP, Schwartz I, Montecalvo MA, Johnson BJ. Borrelia lonestari infection after a bite by an Amblyomma americanum tick. J. Infect. Dis. 2001;183:1810–1814. doi: 10.1086/320721. [DOI] [PubMed] [Google Scholar]
- Jittapalapong S, Phichitrasilp T, Chanphao H, Rerkamnuychoke W, Stich RW. Immunization with tick salivary gland extracts. Ann. N. Y. Acad. Sci. 2008;1149:200–204. doi: 10.1196/annals.1428.083. [DOI] [PubMed] [Google Scholar]
- Jongejan F, Uilenberg G. The global importance of ticks. Parasitology. 2004;129(Suppl):S3–S14. doi: 10.1017/s0031182004005967. [DOI] [PubMed] [Google Scholar]
- Kalluri S, Gilruth P, Rogers D, Szczur M. Surveillance of arthropod vector-borne infectious diseases using remote sensing techniques: a review. PLoS Pathog. 2007;3:1361–1371. doi: 10.1371/journal.ppat.0030116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariuki TM, Farah IO, Wilson RA, Coulson PS. Antibodies elicited by the secretions from schistosome cercariae and eggs are predominantly against glycan epitopes. Parasite Immunol. 2008;30:554–562. doi: 10.1111/j.1365-3024.2008.01054.x. [DOI] [PubMed] [Google Scholar]
- Kim TK, Curran J, Mulenga A. Dual silencing of long and short Amblyomma americanum acidic chitinase forms weakens the tick cement cone stability. J. Exp. Biol. 2014;217:3493–3503. doi: 10.1242/jeb.107979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TK, Tirloni L, Radulovic Z, Lewis L, Bakshi M, Hill C, da Silva Vaz I, Jr, Logullo C, Termignoni C, Mulenga A. Conserved Amblyomma americanum tick Serpin19, an inhibitor of blood clotting factors Xa and XIa, trypsin and plasmin, has anti-haemostatic functions. Int. J. Parasitol. 2015a;45:613–627. doi: 10.1016/j.ijpara.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TK, Ibelli AM, Mulenga A. Amblyomma americanum tick calreticulin binds C1q but does not inhibit activation of the classical complement cascade. Ticks Tick Borne Dis. 2015b;6:91–101. doi: 10.1016/j.ttbdis.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koci J, Simo L, Park Y. Validation of internal reference genes for real-time quantitative polymerase chain reaction studies in the tick, Ixodes scapularis (Acari: Ixodidae) J. Med. Entomol. 2013;50:79–84. doi: 10.1603/me12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovar L. Tick saliva in anti-tick immunity and pathogen transmission. Folia Microbiol. (Praha) 2004;49:327–336. doi: 10.1007/BF02931051. [DOI] [PubMed] [Google Scholar]
- Laird JS, Kocan AA, Kocan KM, Presley SM, Hair JA. Susceptibility of Amblyomma americanum to natural and experimental infections with Theileria cervi. J. Wildl. Dis. 1988;24:679–683. doi: 10.7589/0090-3558-24.4.679. [DOI] [PubMed] [Google Scholar]
- Lohmeyer KH, Pound JM, May MA, Kammlah DM, Davey RB. Distribution of Rhipicephalus (Boophilus) microplus and Rhipicephalus (Boophilus) annulatus (Acari: Ixodidae) infestations detected in the United States along the Texas/Mexico border. J. Med. Entomol. 2011;48:770–774. doi: 10.1603/me10209. [DOI] [PubMed] [Google Scholar]
- Masters EJ, Grigery CN, Masters RW. STARI, or Masters disease: Lone Star tick-vectored Lyme-like illness. Infect. Dis. Clin. North Am. 2008;22:361–376. viii. doi: 10.1016/j.idc.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Mays SE, Hendricks BM, Paulsen DJ, Houston AE, Trout Fryxell RT. Prevalence of five tick-borne bacterial genera in adult Ixodes scapularis removed from white-tailed deer in western Tennessee. Parasit. Vectors. 2014;7 doi: 10.1186/s13071-014-0473-y. 473-014-0473-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino O, Alberdi P, Perez de la, Lastra JM, de la Fuente J. Tick vaccines and the control of tick-borne pathogens. Front. Cell. Infect. Microbiol. 2013;3:30. doi: 10.3389/fcimb.2013.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino O, Almazan C, Canales M, Villar M, Moreno-Cid JA, Estrada-Pena A, Kocan KM, de la Fuente J. Control of Rhipicephalus (Boophilus) microplus infestations by the combination of subolesin vaccination and tick autocidal control after subolesin gene knockdown in ticks fed on cattle. Vaccine. 2011;29:2248–2254. doi: 10.1016/j.vaccine.2011.01.050. [DOI] [PubMed] [Google Scholar]
- Mulenga A, Kim T, Ibelli AM. Amblyomma americanum tick saliva serine protease inhibitor 6 is a cross-class inhibitor of serine proteases and papain-like cysteine proteases that delays plasma clotting and inhibits platelet aggregation. Insect Mol. Biol. 2013a;22:306–319. doi: 10.1111/imb.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulenga A, Kim TK, Ibelli AM. Deorphanization and target validation of cross-tick species conserved novel Amblyomma americanum tick saliva protein. Int. J. Parasitol. 2013b;43:439–451. doi: 10.1016/j.ijpara.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulenga A, Sugimoto C, Sako Y, Ohashi K, Musoke A, Shubash M, Onuma M. Molecular characterization of a Haemaphysalis longicornis tick salivary gland-associated 29-kilodalton protein and its effect as a vaccine against tick infestation in rabbits. Infect. Immun. 1999;67:1652–1658. doi: 10.1128/iai.67.4.1652-1658.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulenga A, Sugino M, Nakajim M, Sugimoto C, Onuma M. Tick-Encoded serine proteinase inhibitors (serpins); potential target antigens for tick vaccine development. J. Vet. Med. Sci. 2001;63:1063–1069. doi: 10.1292/jvms.63.1063. [DOI] [PubMed] [Google Scholar]
- Mulenga A, Macaluso KR, Simser JA, Azad AF. The American dog tick, Dermacentor variabilis, encodes a functional histamine release factor homolog. Insect Biochem. Mol. Biol. 2003;33:911–919. doi: 10.1016/s0965-1748(03)00097-3. [DOI] [PubMed] [Google Scholar]
- Nakatani K, Takeshita S, Tsujimoto H, Kawamura Y, Sekine I. Inhibitory effect of serine protease inhibitors on neutrophil-mediated endothelial cell injury. J. Leukoc. Biol. 2001;69:241–247. [PubMed] [Google Scholar]
- Narasimhan S, Deponte K, Marcantonio N, Liang X, Royce TE, Nelson KF, Booth CJ, Koski B, Anderson JF, Kantor F, Fikrig E. Immunity against Ixodes scapularis salivary proteins expressed within 24 hours of attachment thwarts tick feeding and impairs Borrelia transmission. PLoS One. 2007;2:e451. doi: 10.1371/journal.pone.0000451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto NC, Teglas MB, Stewart KM, Wasley T, Wolff PL. Detection of relapsing fever spirochetes (Borrelia hermsii and Borrelia coriaceae) in free-ranging mule deer (Odocoileus hemionus) from Nevada, United States. Vector Borne Zoonotic Dis. 2012;12:99–105. doi: 10.1089/vbz.2011.0716. [DOI] [PubMed] [Google Scholar]
- Nyindo M, Essuman S, Dhadialla TS. Immunization against ticks: use of salivary gland antigens and infestations with Rhipicephalus appendiculatus (Acari: Ixodidae) in rabbits. J. Med. Entomol. 1989;26:430–434. doi: 10.1093/jmedent/26.5.430. [DOI] [PubMed] [Google Scholar]
- Opdebeeck JP, Wong JY, Jackson LA, Dobson C. Vaccines to protect Hereford cattle against the cattle tick, Boophilus microplus. Immunology. 1988;63:363–367. [PMC free article] [PubMed] [Google Scholar]
- Owen CA, Campbell EJ. The cell biology of leukocyte-mediated proteolysis. J. Leukoc. Biol. 1999;65:137–150. doi: 10.1002/jlb.65.2.137. [DOI] [PubMed] [Google Scholar]
- Piesman J, Spielman A, Etkind P, Ruebush TK, 2nd, Juranek DD. Role of deer in the epizootiology of Babesia microti in Massachusetts, USA. J. Med. Entomol. 1979;15:537–540. doi: 10.1093/jmedent/15.5-6.537. [DOI] [PubMed] [Google Scholar]
- Porter L, Radulovic Z, Kim T, Braz GR, Da Silva Vaz I, Jr, Mulenga A. Bioinformatic analyses of male and female Amblyomma americanum tick expressed serine protease inhibitors (serpins) Ticks Tick Borne Dis. 2015;6:16–30. doi: 10.1016/j.ttbdis.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pound JM, George JE, Kammlah DM, Lohmeyer KH, Davey RB. Evidence for role of white-tailed deer (Artiodactyla: Cervidae) in epizootiology of cattle ticks and southern cattle ticks (Acari: Ixodidae) in reinfestations along the Texas/Mexico border in south Texas: a review and update. J. Econ. Entomol. 2010;103:211–218. doi: 10.1603/EC09359. [DOI] [PubMed] [Google Scholar]
- Qiu S, Adema CM, Lane T. A computational study of off-target effects of RNA interference. Nucleic Acids Res. 2005;33:1834–1847. doi: 10.1093/nar/gki324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulovic ZM, Kim TK, Porter LM, Sze SH, Lewis L, Mulenga A. A 24–48 h fed Amblyomma americanum tick saliva immuno-proteome. BMC Genomics. 2014;15 doi: 10.1186/1471-2164-15-518. 518-2164-15-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulovic ZM, Porter LM, Kim TK, Bakshi M, Mulenga A. Amblyomma americanum tick saliva insulin-like growth factor binding protein-related protein 1 binds insulin but not insulin-like growth factors. Insect Mol. Biol. 2015;24:539–550. doi: 10.1111/imb.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raizman EA, Holland JD, Shukle JT. White-tailed deer (Odocoileus virginianus) as a potential sentinel for human Lyme disease in Indiana. Zoonoses Public. Health. 2013;60:227–233. doi: 10.1111/j.1863-2378.2012.01518.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez M, Massard CL, da Fonseca AH, Ramos NF, Machado H, Labarta V, de la Fuente J. Effect of vaccination with a recombinant Bm86 antigen preparation on natural infestations of Boophilus microplus in grazing dairy and beef pure and cross-bred cattle in Brazil. Vaccine. 1995a;13:1804–1808. doi: 10.1016/0264-410x(95)00119-l. [DOI] [PubMed] [Google Scholar]
- Rodriguez M, Penichet ML, Mouris AE, Labarta V, Luaces LL, Rubiera R, Cordoves C, Sanchez PA, Ramos E, Soto A. Control of Boophilus microplus populations in grazing cattle vaccinated with a recombinant Bm86 antigen preparation. Vet. Parasitol. 1995b;57:339–349. doi: 10.1016/0304-4017(94)00678-6. [DOI] [PubMed] [Google Scholar]
- Rodriguez Valle M, Montero C, Machado H, Joglar M, de la Fuente J, Garcia-Garcia JC. The evaluation of yeast derivatives as adjuvants for the immune response to the Bm86 antigen in cattle. BMC Biotechnol. 2001;1:2. doi: 10.1186/1472-6750-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Vivas RI, Perez-Cogollo LC, Rosado-Aguilar JA, Ojeda-Chi MM, Trinidad-Martinez I, Miller RJ, Li AY, de Leon AP, Guerrero F, Klafke G. Rhipicephalus (Boophilus) microplus resistant to acaricides and ivermectin in cattle farms of Mexico. Rev. Bras. Parasitol. Vet. 2014;23:113–122. doi: 10.1590/s1984-29612014044. [DOI] [PubMed] [Google Scholar]
- Sanders ML, Glass GE, Nadelman RB, Wormser GP, Scott AL, Raha S, Ritchie BC, Jaworski DC, Schwartz BS. Antibody levels to recombinant tick calreticulin increase in humans after exposure to Ixodes scapularis (Say) and are correlated with tick engorgement indices. Am. J. Epidemiol. 1999;149:777–784. doi: 10.1093/oxfordjournals.aje.a009887. [DOI] [PubMed] [Google Scholar]
- Savage HM, Godsey MS, Jr, Lambert A, Panella NA, Burkhalter KL, Harmon JR, Lash RR, Ashley DC, Nicholson WL. First detection of heartland virus (Bunyaviridae: Phlebovirus) from field collected arthropods. Am. J. Trop. Med. Hyg. 2013;89:445–452. doi: 10.4269/ajtmh.13-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro SZ, Voigt WP, Ellis JA. Acquired resistance to ixodid ticks induced by tick cement antigen. Exp. Appl. Acarol. 1989;7:33–41. doi: 10.1007/BF01200451. [DOI] [PubMed] [Google Scholar]
- Sonenshine DE, Kocan KM, de la Fuente J. Tick control: further thoughts on a research agenda. Trends Parasitol. 2006;22:550–551. doi: 10.1016/j.pt.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Straka M. Oral manifestations of diabetes mellitus and influences of periodontological treatment on diabetes mellitus. Bratisl. Lek. Listy. 2011;112:416–420. [PubMed] [Google Scholar]
- Szewczyk B, Pilat Z, Bienkowska-Szewczyk K, Summers DF. Elution of glycoproteins from replicas of sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels. Electrophoresis. 1998;19:220–223. doi: 10.1002/elps.1150190214. [DOI] [PubMed] [Google Scholar]
- Taylor JP, Istre GR, McChesney TC, Satalowich FT, Parker RL, McFarland LM. Epidemiologic characteristics of human tularemia in the southwest-central states, 1981–1987. Am. J. Epidemiol. 1991;133:1032–1038. doi: 10.1093/oxfordjournals.aje.a115812. [DOI] [PubMed] [Google Scholar]
- Tellam RL, Smith D, Kemp DH, Willadsen P. Vaccination against ticks. In: Yong WK, editor. Animal Parasite Control Utilizing Biotechnology. Boca Raton: CRC Press; 1992. p. 303. [Google Scholar]
- Tirloni L, Islam MS, Kim TK, Diedrich JK, Yates JR, 3rd, Pinto AF, Mulenga A, You MJ, Da Silva Vaz I., Jr Saliva from nymph and adult females of Haemaphysalis longicornis: a proteomic study. Parasit. Vectors. 2015;8 doi: 10.1186/s13071-015-0918-y. 338-015-0918-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirloni L, Reck J, Terra RM, Martins JR, Mulenga A, Sherman NE, Fox JW, Yates JR, 3rd, Termignoni C, Pinto AF, Vaz Ida S., Jr Proteomic analysis of cattle tick Rhipicephalus (Boophilus) microplus saliva: a comparison between partially and fully engorged females. PLoS One. 2014;9:e94831. doi: 10.1371/journal.pone.0094831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager W. Further observations on acquired immunity to the tick Dermacentor variabilis Say. J. Parasitol. 1939a;25:137–139. [Google Scholar]
- Trager W. Acquired Immunity to Ticks. J. Parasitol. 1939b;25:57–81. [Google Scholar]
- Tolleson DR, Carstens GE, Welsh TH, Jr, Teel PD, Strey OF, Longnecker MT, Prince SD, Banik KK. Plane of nutrition by tick-burden interaction in cattle: effect on growth and metabolism. J. Anim. Sci. 2012;90:3442–3450. doi: 10.2527/jas.2011-5066. [DOI] [PubMed] [Google Scholar]
- Tolleson DR, Teel PD, Stuth JW, Strey OF, Welsh TH, Jr, Carstensr GE, Longnecker MT, Banik KK, Prince SD. Effects of a lone star tick (Amblyomma americanum) burden on performance and metabolic indicators in growing beef steers. Vet. Parasitol. 2010;173:99–106. doi: 10.1016/j.vetpar.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Tsuda A, Mulenga A, Sugimoto C, Nakajima M, Ohashi K, Onuma M. cDNA cloning, characterization and vaccine effect analysis of Haemaphysalis longicornis tick saliva proteins. Vaccine. 2001;19:4287–4296. doi: 10.1016/s0264-410x(01)00148-7. [DOI] [PubMed] [Google Scholar]
- Varela-Stokes AS. Transmission of bacterial agents from lone star ticks to white-tailed deer. J. Med. Entomol. 2007;44:478–483. doi: 10.1603/0022-2585(2007)44[478:tobafl]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Walker DH, Paddock CD, Dumler JS. Emerging and re-emerging tick-transmitted rickettsial and ehrlichial infections. Med. Clin. North Am. 2008;92:1345–1361. x. doi: 10.1016/j.mcna.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Walker AR. Eradication and control of livestock ticks: biological, economic and social perspectives. Parasitology. 2011;138:945–959. doi: 10.1017/S0031182011000709. [DOI] [PubMed] [Google Scholar]
- Willadsen P. Vaccination against ectoparasites. Parasitology. 2006;133(Suppl):S9–S25. doi: 10.1017/S0031182006001788. [DOI] [PubMed] [Google Scholar]
- Willadsen P. Anti-tick vaccines. Parasitology. 2004;129(Suppl):S367–S387. doi: 10.1017/s0031182003004657. [DOI] [PubMed] [Google Scholar]
- Willadsen P, Bird P, Cobon GS, Hungerford J. Commercialisation of a recombinant vaccine against Boophilus microplus. Parasitology. 1995;110(Suppl):S43–S50. doi: 10.1017/s0031182000001487. [DOI] [PubMed] [Google Scholar]
- Wiznia DH, Christos PJ, LaBonte AM. The use of deer vehicle accidents as a proxy for measuring the degree of interaction between human and deer populations and its correlation with the incidence rate of Lyme disease. J. Environ. Health. 2013;75:32–39. [PMC free article] [PubMed] [Google Scholar]
- Wolf L, McPherson T, Harrison B, Engber B, Anderson A, Whitt P. Prevalence of Ehrlichia ewingii in Amblyomma americanum in North Carolina. J. Clin. Microbiol. 2000;38:2795. doi: 10.1128/jcm.38.7.2795-2795.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You MJ. Immunization of mice with recombinant P27/30 protein confers protection against hard tick Haemaphysalis longicornis (Acari: Ixodidae) infestation. J. Vet. Sci. 2005;6:47–51. [PubMed] [Google Scholar]