Abstract
Background and Aims
Pretreatment with clofibrate, a peroxisome proliferator-activated receptor alpha (PPARa) agonist, protects mice from acetaminophen (APAP) injury. Protection is not due to alterations in APAP metabolism and is dependent on PPARa expression. Gene array analysis revealed that mice receiving clofibrate have enhanced hepatic Vanin-1 (Vnn1) gene expression, a response that is also PPARa dependent.
Methods
We examined the role of Vnn1 by comparing the responses of Vnn1 knockout and wild type mice following APAP hepatotoxicity. APAP metabolism, hepatotoxicity and compensatory hepatocyte proliferation and immune responses were assessed.
Results
Vnn1 knockout mice are more susceptible to APAP hepatotoxicity despite no differences in hepatic glutathione content, gene expression of APAP metabolizing enzymes, or hepatic capacity to bioactivate or detoxify APAP ex vivo. Together, these data strongly suggest that the susceptibility of Vnn1 knockout mice is not due to differences in APAP metabolism. Immunochemistry revealed a lack of proliferating cell nuclear antigen-positive hepatocytes and F4/80-positive macrophages in and around areas of centrilobular necrosis in APAP-treated Vnn1 knockouts. Hepatic gene induction of pro-inflammatory cytokines was either significantly reduced or completely blunted in these mice. This was correlated with a reduction in early recruitment of cells positive for granulocyte differentiation antigen 1 or integrin alpha M. Heightened toxicity was also observed in CCl4 and ConA hepatitis models in the absence of Vnn1.
Conclusions
These results indicate that mice lacking Vnn1 have deficiencies in compensatory repair and immune responses following toxic APAP exposure and that these mechanisms may contribute to the enhanced hepatotoxicity seen.
Keywords: pantetheine hydrolase, cysteamine, clofibrate, peroxisome proliferators, liver
Introduction
Acetaminophen (APAP) is a widely used analgesic and antipyretic drug that is attributed to half of all acute liver failure cases in the United States [1]. Upon therapeutic administration, APAP is metabolized by hepatic glucuronidation or sulfation reactions and eliminated via multidrug resistance-associated proteins (Mrps) into blood and bile [2–4]. A small fraction of the parent compound undergoes bioactivation by cytochrome P450 to generate N-acetyl-p-benzoquinone imine (NAPQI), a reactive metabolite that is detoxified by glutathione (GSH). APAP overdose saturates detoxification pathways and enhances bioactivation. Excessive NAPQI generation causes GSH depletion, binding to macromolecules and oxidative stress with hepatic centrilobular necrosis ensuing [5].
Peroxisome proliferators (PPs), a class of peroxisome proliferator-activated receptor alpha (PPARa) agonists, protect rodents from APAP injury [6]. In mice, protection by clofibrate (CFB), a PP, does not appear to be due to alterations in APAP metabolism [7] or by increased detoxification by catalase [8] or NAD(P)H quinone oxidoreductase 1 (Nqo1) [9]. Protection is also independent of changes in APAP-protein adduct formation and GSH depletion [10], though it requires activation of PPARa [11].
Mice receiving CFB have a 7-fold induction in Vanin-1 (Vnn1) gene expression in liver [12]. Similar to the mechanism of PP-mediated protection, Vnn1 induction and expression are also PPARa dependent [12, 13]. The Vnn1 gene encodes for pantetheinase that hydrolyzes pantetheine into pantothenic acid (Vitamin B5) and cysteamine [14]. The aminothiol cysteamine is protective against lipid peroxidation [15, 16] and aldehyde overload [17]. Systemically, Vnn1 modulates tissue tolerance to stress [18] and this impacts immune function by regulating cell homing towards inflamed [19] or developing [20] tissues by an unknown mechanism.
Since protection from APAP by CFB is associated with Vnn1 induction [12] we investigated the susceptibility of Vnn1 knockout mice to different modes of liver injury. We more specifically show that mice lacking Vnn1 are sensitive to APAP hepatotoxicity. This occurs independently of changes in APAP bioactivation or detoxification, but is associated with a decrease in immune cell infiltration and hepatic repair in response to APAP injury. The identification of specific protective mechanisms mediated by Vnn1 may help developing more effective treatment of APAP overdose.
Materials and Methods
Animal Care and Treatment
Vnn1 knockout C57BL/6 mice were kept in a pathogen-free mouse facility [13]. Experiments were performed as approved by the Institutional Animal Care and Use Committee of the Université d’Aix Marseille, France. Male Vnn1 knockout and wild type mice receiving APAP treatment were fasted the night before receiving intraperitoneal (i.p.) injection with either APAP (400mg/kg in 50% propylene glycol) or vehicle. Food was returned at 8 hours and mice were sacrificed at 24 and 48 hours after treatment. For carbon tetrachloride (CCl4) treatment, 25µl/kg was delivered by i.p. injection in corn oil vehicle (25µl CCl4 / 10ml oil). Concanavalin A (ConA; 15mg/kg) was delivered by intravenous (i.v.) injection in saline. Studies using either CCl4 or ConA were terminated after 24hrs. Blood was collected by orbital bleeds while livers were either snap frozen in liquid nitrogen or fixed in formalin.
ALT Activity Assay
Hepatotoxicity was determined by measuring ALT activity in plasma samples using the Infinity GPT Reagent (Thermo Scientific, Waltham, MA) as recommended by the manufacturer and read on a 96-well plate reader (BioTek PowerwaveX, BioTek, Winooski, VT).
Histopathology and Immunohistochemistry
Paraffin sections were prepared from formalin-fixed livers. Hepatotoxicity was assessed following hematoxylin and eosin staining. Liver sections were scored on a 0–5 scale (1.0 intervals) according to the severity of centrilobular necrosis as previously performed [6]. Immunohistochemistry for proliferating cell nuclear antigen (PCNA) was probed using a kit (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. F4/80 was detected by incubating rat monoclonal primary antibody (Abcam, Cambridge, MA). Secondary antibodies were used before developing with 3, 3’ diaminobenzidine tetrahydrochloride (Vector Laboratories, Burlingame, CA).
Acetone-fixed frozen sections were stained with FITC-coupled anti-CD11b and PE-coupled Gr1 mAbs (BD Biosciences). Pictures were taken on a confocal microscope (Zeiss). Quantification was performed on 5–6 APAP-treated control or Vnn1-deficient mice 30 hours after APAP administration. Seven independent sections taken 30µm apart were prepared for each mouse and perivascular infiltrating cells were counted within each. Error bars represent the variability from seven pictures taken for each mouse.
qRT-PCR
Total RNA was isolated from frozen liver following homogenization in TRIzol (Invitrogen). cDNA was synthesized using M-MLV reverse transcriptase (Invitrogen) and random primers. qRT-PCR was performed on a 7500 Fast Real-Time PCR System (Applied Biosystems, Carlsbad, CA) using SYBR green and species-specific primer pairs for each gene (Supplementary Table 1). Gene expression was quantified by the ΔΔCT method and normalized to β-actin.
GSH Assay
Total hepatic GSH concentrations were quantified from frozen liver tissue by the recycling method as described [21]. Absorbance was read at 412nm, taking measurements at 30 second intervals for 2 minutes (Sigma Aldrich, St. Louis, MO).
In Vitro Metabolism of APAP
Hepatic microsomal and cytosolic fractions were prepared from frozen livers taken from untreated mice. In vitro biochemical analyses of APAP glucuronidation, sulfation and bioactivation were performed as described [22, 23].
Statistical Analysis
Results are expressed as means ± standard error. Statistical analysis of the data was compared using the student’s t test or two way ANOVA when gender and genotypes were combined in the CCl4 experiment, followed by post-hoc analysis. Differences were considered significant at p<0.05.
Results
Vanin-1 null mice are sensitive to acetaminophen-induced hepatotoxicity
Previous gene array studies in our laboratory established an association between hepatic Vnn1 gene upregulation and protection against APAP in mice receiving CFB pretreatment [12]. To determine whether Vnn1 participates to hepatoprotection, Vnn1-null mice were treated with APAP. Liver toxicity was assessed by plasma alanine aminotransferase (ALT) activity and histopathological analysis of liver sections. At 24 hours the mean plasma ALT activity was three-fold higher in knockouts compared to wild type mice, 768 ± 161 U/L and 255 ± 75 U/L, respectively (Table 1). By 48 hours, ALT activity was four times higher in Vnn1 knockouts (936 ± 151 versus 223 ± 55 U/L in wild type). Histopathological analysis of liver sections revealed that Vnn1 knockout mice tended to have a higher hepatic necrosis score compared to wild types at 24 hours, and that this trend became significant at 48 hours. Therefore, mice lacking Vnn1 are susceptible to APAP hepatotoxicity, consistent with a hepatoprotective role for Vnn1.
Table 1.
Plasma ALT Activity and Hepatic Necrosis after APAP Treatment.
| Plasma ALT Activity1 |
Hepatic Necrosis2 |
|||||||
|---|---|---|---|---|---|---|---|---|
| (U/L) | (% Mice with Score ≥ 2) | _____ | ||||||
| Genotype | Control | 24hr APAP | 48hr APAP | Control | 24hr APAP | 48hr APAP | ||
| Wild Type | 25 ± 2 | 255 ± 75 | 223 ± 55 | 0 | 0 | 40 | ||
| Vnn1 Knockout | 22 ± 2 | 768 ± 161* | 936 ± 151* | 0 | 20 | 80* |
ALT activity (n = 4–10) presented as mean ± SE.
Hepatic histopathology (n = 4–5) was scored on a 0–5 scale as previously described [6].
p < 0.05 compared to wild type.
Susceptibility of Vanin-1 null mice to APAP is not due to differential metabolism
Enhanced APAP toxicity may be caused by increased bioactivation to reactive NAPQI or by decreased ability of the liver to detoxify APAP or its reactive intermediate. Therefore, the higher susceptibility of Vnn1 knockout mice could result from differences in the expression of APAP metabolizing or detoxifying enzymes. qRT-PCR was performed on livers collected from naïve, fasted Vnn1 knockout and wild type mice. No differences in hepatic mRNA expression of APAP bioactivating enzymes Cyp1a2, 3a11, 2e1, detoxifying enzyme UDP-glucuronosyltransferase 1a6 (Ugt1a6) or genes involved in the disposition of APAP metabolites, such as Abcc2, 3 and 4 (Mrp2-4) were observed between genotypes (Supplementary Fig. 1A, B).
To ensure that the enhanced susceptibility of Vnn1 knockout mice to APAP toxicity is not due to differences in the livers’ ability to bioactivate or detoxify APAP, hepatic metabolism of APAP was performed in vitro. Microsomal incubations were prepared from frozen livers of both genotypes and their ability to bioactivate APAP was assessed by measuring the formation of APAP-NAC metabolite following the addition of NAC, NADP and APAP. Complete incubations from both Vnn1 knockout and wild type livers formed APAP-NAC at similar rates (Supplementary Fig. 2A), indicating that a comparable amount of NAPQI was being generated in liver preparations from both genotypes.
The capacity of the liver to detoxify APAP by glucuronidation and sulfation was then assessed in vitro. APAP-Gluc formation was determined by incubating microsomes with APAP and UDPGA, while APAP-Sulf formation was monitored by incubating cytosolic fractions with PAPS, dithiothreitol (DTT) and APAP. In both instances, incubations from Vnn1 knockout and wild type livers generated metabolites at similar rates (Supplementary Fig. 2B and C, respectively). Collectively, gene expression and in vitro APAP metabolism analyses demonstrate that the increased susceptibility of Vnn1 knockout mice to APAP hepatotoxicity is unlikely due to alteration in bioactivation, detoxification or disposition of APAP.
Vanin-1 null mice have no deficiencies in APAP detoxification capacity by GSH
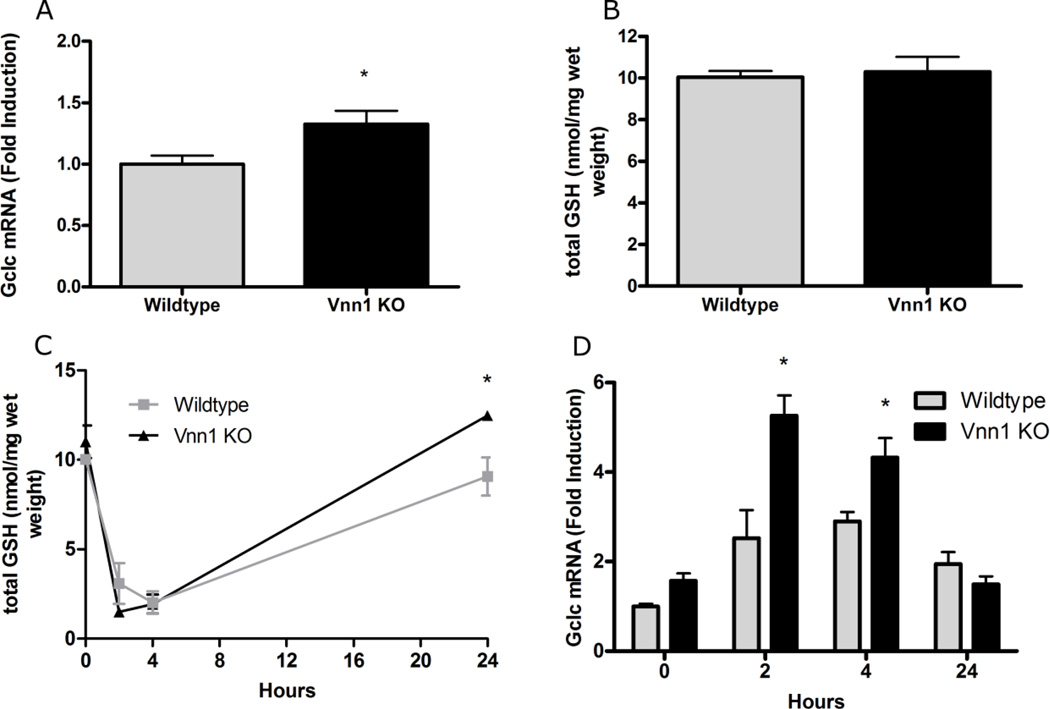
GSH status conditions the extent of damage following APAP treatment, as APAP binding to cellular macromolecules is not detected until GSH is sufficiently depleted [24]. To investigate whether Vnn1 knockout mice have altered GSH synthesis and/or content, we examined the hepatic mRNA expression of the catalytic subunit of gamma glutamylcysteine synthetase (Gclc). Vnn1 knockout mice exhibited 33% greater expression of Gclc when compared to wild types (Fig. 1A). However, basal GSH concentration in livers was equivalent (Fig. 1B). It is therefore unlikely that the susceptibility of Vnn1 knockout mice to APAP hepatotoxicity is due to altered GSH content in liver.
Fig. 1. Glutathione Status in Livers from Naïve Wild type and Vanin-1 Knockout Mice.
(A and C) qRT-PCR was performed to quantify hepatic Gclc mRNA expression among fasted (A) and APAP treated (C) livers from both genotypes. (B and D) Total GSH content was examined in frozen livers from naïve (B) and APAP treated (D) Vnn1 knockout and wild type mice. The data are presented as mean ± SEM (n = 4–7 animals). An asterisk (*) represents a significant difference (p < 0.05) between wild type and Vnn1 knockout mice at the same time point.
To test whether the livers of Vnn1-null mice have an altered capacity to regenerate GSH or to neutralize NAPQI via GSH conjugation, a time-dependent GSH depletion study was performed following APAP dosing. APAP-treated mice were sacrificed at 2, 4 or 24 hours post-injection. A >70% depletion of total hepatic GSH was observed in both genotypes between 2 and 4 hours (Fig. 1C). Interestingly, GSH replenishment was more pronounced in Vnn1 knockout mice at 24 hours. Induction of hepatic Gclc gene expression was also greater at 2 and 4 hours after APAP treatment in the Vnn1 knockout mice relative to wild types (Fig. 1D). Together, these data show that hepatic basal GSH content and utilization after APAP treatment in Vnn1 knockout and wild type mice are similar, suggesting that the enhanced APAP toxicity seen in knockout mice is not due to impaired detoxification by GSH or its replenishment.
Vanin-1 knockout mice exhibit deficiencies in compensatory hepatocellular proliferation and F4/80-positive macrophage infiltration following toxic acetaminophen insult
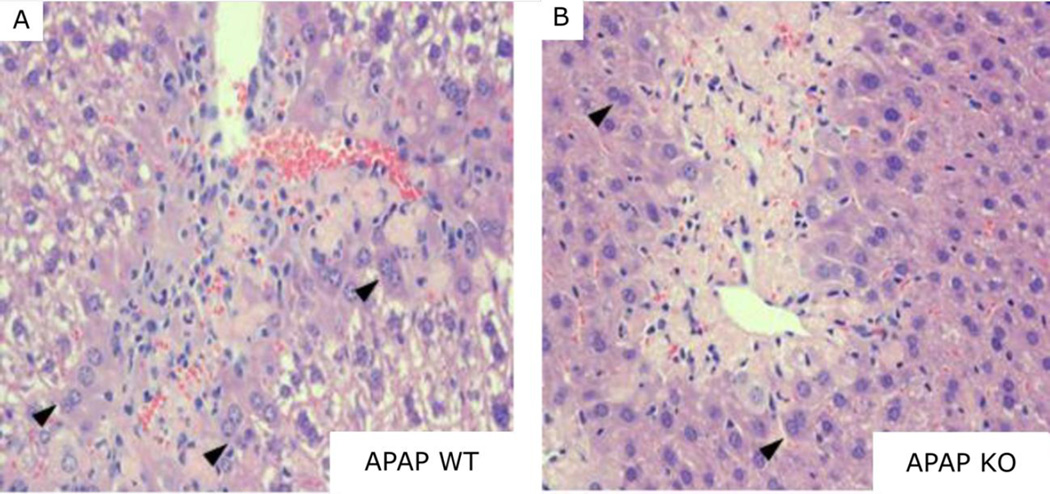
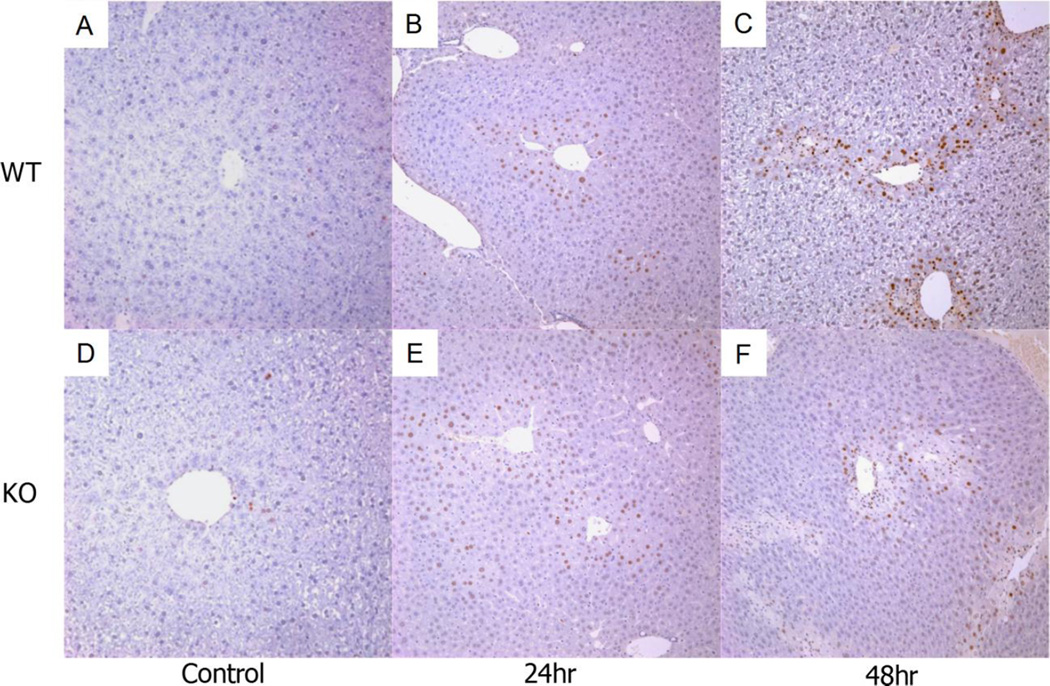
Activation of repair mechanisms also determines the extent of hepatotoxicity following APAP injury [25]. This involves the recruitment of immune cells to sites of damage and the proliferation of viable hepatocytes surrounding necrotic areas in centrilobular regions. Disruption of these mechanisms could result in enhanced APAP toxicity. To investigate this, we analyzed H&E stained liver sections from various mice 48 hours after APAP treatment. The number of infiltrating immune cells generally correlates with the severity of damage [26, 27]. Surprisingly, Vnn1 knockout mice had noticeably less infiltration within areas of centrilobular necrosis compared to wild type mice (Fig. 2) despite having more severe hepatocellular damage. The knockouts also displayed decreased numbers of bi-nucleated hepatocytes surrounding the areas of injury (Fig. 2B), reflecting reduced compensatory hepatocyte proliferation. Assessment of mitotic PCNA-positive hepatocytes was performed on liver sections 24 and 48 hours following APAP treatment. Mitotic cells were identified as early as 24 hours (Fig. 3A, D) in damaged centrilobular areas in both genotypes but in much larger proportion in wild type mice at 48 hours (Fig. 3C, F), indicating a delayed deficiency of cellular proliferation in Vnn1 knockouts in response to APAP injury.
Fig. 2. Histopathological Analysis of Livers from Vnn1 Wild type and Null Mice Receiving Acetaminophen.
48 hours after 400mg/kg APAP treatment, less infiltration by immune cells (small blue nuclei within necrotic area) is evident within areas of centrilobular necrosis in (B) Vnn1 null mice relative to (A) wild types. Also, less compensatory proliferation of hepatocytes is seen surrounding the necrotic areas as illustrated by the presence of fewer binucleated hepatocytes (arrowheads). Magnification = 200×.
Fig. 3. Hepatic PCNA Protein Expression in Vanin 1 Knockout and Wild type Mice following Toxic Acetaminophen Exposure.
Representative sections of control-treated liver from (A) wild type or (D) Vnn1 knockout mice. Hepatic PCNA protein is enhanced similarly in (B) wild type mice and (E) Vnn1 knockouts 24 hours following toxic APAP exposure. At 48 hours, staining is more intense in (C) wild type mice than (F) Vnn1 knockouts. Magnification = 100×.
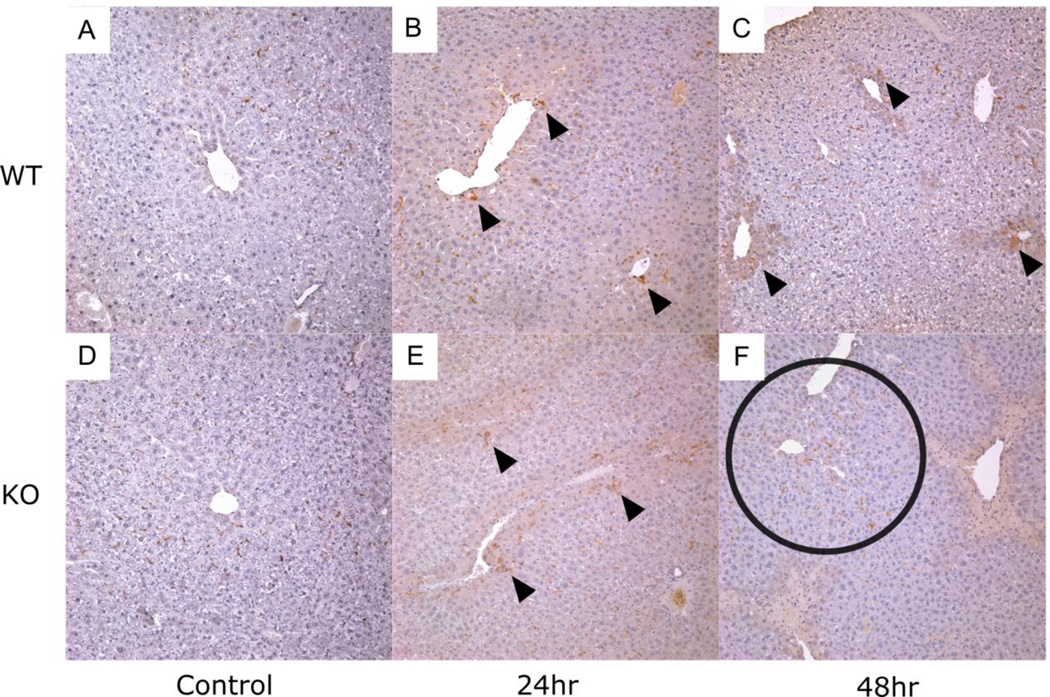
Liver sections were also stained with anti-F4/80 antibody, a marker of resident macrophages. Once again, no differences in staining were observed between genotypes at 24 hours (Fig. 4B, E). In contrast, by 48 hours, F4/80 positive cells were less likely to be found within areas of necrosis in Vnn1 knockouts (Fig. 4C, F). Instead, F4/80-positive cells in the knockout mice primarily resided in periportal regions despite the clear presence of centrilobular necrosis, suggesting an abnormal localization and/or activation of these cells 48 hours after injury.
Fig. 4. Hepatic F4/80 Protein Expression in Vnn1 Knockout and Wild type Mice following Toxic Acetaminophen Exposure.
Representative sections of control-treated liver from (A) wild type or (D) Vnn1 knockout mice. Arrows illustrate similar distribution of F4/80-positive cells in (B) wild type and (E) Vnn1 knockouts after 24 hours. At 48 hours, fewer F4/80-positive cells are evident within areas of centrilobular necrosis in (F) Vnn1 knockouts relative to (C) wild type mice. Instead, F4/80-positive cells remain in periportal regions (circled). Magnification = 100×.
Altered hepatic myeloid cell infiltration and activation in Vanin-1 knockout mice following acetaminophen intoxication
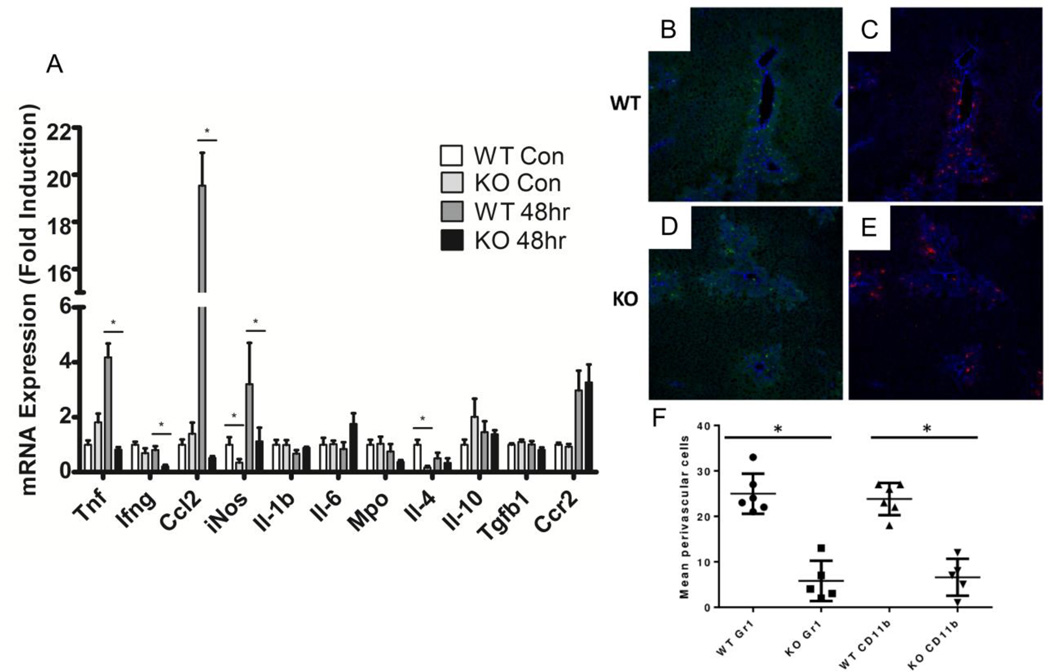
In addition to the reduced numbers of hepatic F4/80 positive cells after APAP treatment [28, 29], blood-derived monocytes are recruited towards the lesion, a process involving the chemokine receptor 2 (Ccr2)/ chemokine ligand 2 (Ccl2) axis. Tissue or blood-derived macrophages then polarize by integrating microenvironmental signals. To investigate whether these processes could be altered in Vnn1 knockout mice we quantified by qRT-PCR the relative gene expression of various cytokine and chemokines in whole liver obtained 48 hours following APAP treatment. In vehicle-treated mice, gene expression of inducible nitric oxide synthase (iNos), an inflammation marker, and interleukin 4 (Il-4), which polarizes macrophages towards a repair phenotype, were reduced in Vnn1 knockouts by 2.9 and 4.3 fold, respectively (Fig. 5A). Following 48hr APAP treatment, pro-inflammatory cytokines interferon gamma (Ifng), iNos and tumor necrosis factor (Tnf) were damped in knockout mice by 2.7, 2.8 and 5.1 fold. Ccl2 was induced by almost 20 fold in wild type mice following APAP, though induction was completely blunted in Vnn1 knockouts. This suggests a decrease in chemotactic and cytokine signaling in Vnn1 knockout mice in response to APAP, particularly in genes with a pro-inflammatory phenotype.
Fig. 5. Cytokine Expression and Immune Cell Analyses of Acetaminophen-treated Wild Type and Vnn1 Knockout Mice.
(A) APAP-mediated gene induction of pro-inflammatory cytokines was blunted in Vnn1 knockout mice 48 hours after treatment. (B–F) Early hepatic recruitment of CD11b (B and D; green staining) and Gr-1 (C and E; red staining) positive cells 30 hours after APAP treatment. Anti CD-31 staining (blue) used as an endothelial marker. (F) Quantification revealed fewer infiltrating Gr-1 and CD11b positive cells in livers from KO mice. Mean ± SEM (n = 4–6 animals). Asterisks (*) represent a statistical difference (p < 0.05) between genotypes.
To investigate whether recruitment of neutrophils and monocytes is modified in Vnn1 knockouts, we performed dual-staining immunofluorescence for granulocyte differentiation antigen 1 (Gr-1) and integrin alpha M (CD11b) on frozen liver sections 30 hours following APAP treatment. Neutrophils express high levels of Gr-1 and CD11b antigens whereas monocytes preferentially express CD11b and display lower Gr-1 levels. CD31 was probed as an endothelial marker. Cells positive for either Gr-1 or CD11b were less prevalent in APAP-treated Vnn1 knockout mice in comparison to wild type as evidenced by punctate staining in and surrounding areas of necrosis (Fig. 5B–E). In both genotypes, the majority of positive cells expressed both markers and localized to centrilobular areas. Quantification of either marker indicated that, on average, positive cells were reduced by five times in Vnn1 knockout livers compared to wild type (Fig. 5F), signifying that recruitment of Gr-1+ and CD11b+ cells is compromised in response to APAP hepatotoxicity in Vnn1 knockout mice.
Vanin-1 null mice are susceptible to injury following acute treatment of other hepatotoxicants
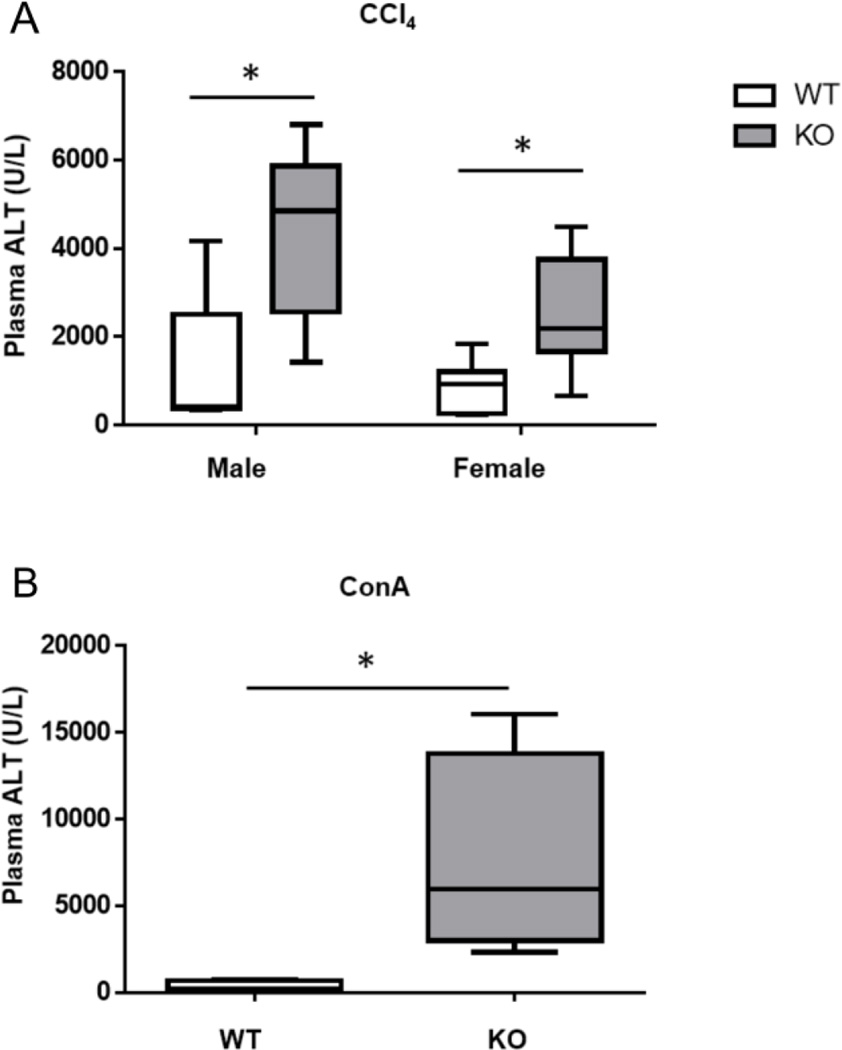
The present studies demonstrate that mice lacking Vnn1 are susceptible to injury following APAP treatment. To determine whether this susceptibility is extended to other hepatotoxicants, we administered CCl4 or ConA to wild type and Vnn1 knockout mice. Like APAP, CCl4 treatment results in the production of a reactive intermediate (CCl3−) and subsequent centrilobular necrosis. CCl4 treatment (25ul/kg, i.p.) resulted in enhanced toxicity in male (means; KO=4340 vs. WT=1230) and female (KO=2510 vs. WT=866) Vnn1 knockouts as measured by plasma ALT activity, a significant result when the genders are combined and compared by genotype (Fig. 6A; 2way ANOVA analysis: Gender effect not significant; Genotype effect: p<0.001).
Fig. 6. Plasma ALT Activity of Wild Type and Vnn1 Knockout Mice Receiving Carbon Tetrachloride or Concanavalin A Treatment.
Vnn1 knockout mice were more susceptible to elevations in plasma ALT 24 hours following (A) carbon tetrachloride (i.p., 25µl/kg in corn oil) or (B) concanavalin A (i.v., 15mg/kg in saline) treatment. The data are presented as the median, in boxes ranging from the 25th to 75th percentiles with whiskers corresponding to the minimum and maximum values (n = 4–6 animals). An asterisk (*) represents a significant difference (p < 0.01) between wild type and Vnn1 knockout mice.
To determine whether Vnn1 knockouts are susceptible to a hepatotoxicant that acts independently of reactive metabolite generation, mice were treated with ConA. ConA treatment is a model of autoimmune hepatitis, resulting in T cell and macrophage-dependent liver injury [30]. Following an i.v. injection of 15mg/kg ConA, Vnn1 knockout mice were strikingly more susceptible to increased ALT than wild types (7571±2985 vs. 315±141, respectively) (Fig. 6B). Collectively, these results indicate that a loss of Vnn1 results in greater susceptibility to injury from multiple classes of hepatotoxicants.
Discussion
In the present studies we investigated the susceptibility of Vnn1 knockout mice to APAP hepatotoxicity. APAP treatment resulted in enhanced hepatotoxicity in the knockouts at both 24 and 48 hours as measured by plasma ALT activity and histopathological scoring of liver sections. Increased toxicity occurs despite no observed differences in gene expression of APAP metabolizing enzymes or hepatic efflux transporters. In vitro APAP metabolism studies confirmed that there are no significant alterations in the capacity of livers from Vnn1 knockout mice to bioactivate or detoxify APAP by CYP450 or glucuronidation and sulfation reactions, respectively.
GSH metabolism plays an essential role in APAP detoxication. Although Vnn1 impacts GSH content in BALB/c mice [31, 32], control and Vnn1 deficient C57BL/6 mice have comparable basal hepatic GSH levels and, furthermore, no differences in GSH utilization after APAP treatment until the recovery phase (24 hour time point) at which time GSH repletion is augmented in Vnn1 knockout mice. This could result from enhanced gGCS expression and/or activity in the absence of cystamine in Vnn1 deficient mice during detoxication. Overall, our data suggest that the susceptibility of Vnn1 knockouts is not due to a decrease in the capacity of the liver to detoxify APAP via GSH.
Compensatory hepatocellular proliferation also contributes to the outcome of acute liver injury. This process might be altered in Vnn1 knockout mice, which show a decrease in bi-nucleated and PCNA-positive hepatocytes after toxic APAP exposure surrounding foci of centrilobular necrosis. This is in agreement with the beneficial effect of Vnn1 expression during chondroblast expansion [33], suggesting a possible role for Vnn1 in cell proliferation.
Vnn1 knockout mice displayed less infiltration of immune cells, including F4/80-positive macrophages 48 hours after APAP treatment despite having more necrosis, an unexpected result given that the degree of infiltration generally correlates with the severity of APAP injury [26, 27]. This finding is in agreement with similar observations in an infectious model [19], and could be explained by the significant reduction in the production of the Ccl2 chemokine, previously shown to recruit myeloid cells with hepatoprotective potential in part via the clearance of necrotic cells [28, 34]. As a consequence, the level of expression of four out of seven pro-inflammatory immune cell polarization genes was reduced in Vnn1 knockout livers 48 hours after APAP treatment. A lack of Vnn1 has been linked to deficiencies in immune cell infiltration in other mouse models of tissue repair [20] or intestinal inflammation [32]. Similarly, Vnn2, another pantheinase isoform present on human neutrophils and CD-14 positive monocytes regulates leukocyte migration and adhesion [35, 36], possibly through the engagement of lipid rafts on the cell surface [37]. Another report describes impairment of arterial repair following ischemic stress due to lack of myofibroblast activation in the absence of Vnn1 [38]. Thus, Vnn1 may have important cell signaling functions involved in the recruitment, activation and crosstalk of immune and/or stromal cells involved in repair processes.
To further explore the role of Vnn1 as a genetic determinant of chemical-induced liver injuries, knockout mice were also treated with CCl4, a toxicant similar to APAP in that it causes centrilobular necrosis following bioactivation of the parent compound to a reactive metabolite. The results with CCl4 reported here indicate that the enhanced susceptibility of Vnn1 knockout mice to liver injury is not unique to APAP.
To determine whether the mechanism of susceptibility is independent of the scavenging of reactive oxygen species, we treated mice with ConA, a model toxicant used to assess T cell and macrophage-dependent liver injury. Instead of the production of a reactive metabolite, the mechanism of toxicity is the primary result of immune cell activation by ConA leading to cytokine-induced hepatocyte cell death [39, 40]. Plasma ALT 24 hours following ConA treatment was drastically enhanced in the knockout mice, giving further evidence that Vnn1 affects the immune response following toxicant exposure. Furthermore, this provides additional evidence (along with the metabolism studies presented here) that the mechanisms for enhanced susceptibility of Vnn1 knockout mice to hepatotoxicants are independent of the scavenging of a reactive metabolite. Overall, the analysis and results with three distinct hepatotoxicants in Vnn1 knockout mice validate our previous observation that treatment with CFB, which induces Vnn1, protects against hepatotoxicants other than APAP.
Cysteamine, the catalytic product of pantetheine hydrolysis, has been used clinically as an antidote for APAP overdose [41]; however, the mechanism by which cysteamine acts has not been elucidated. Cysteine and other APAP antidotes that enhance GSH synthesis lose their protective effect when GSH synthesis is inhibited by buthionine sulfoximine treatment. Cysteamine remains protective during these conditions [42], suggesting that the mechanism of protection is independent of GSH synthesis. Furthermore, the estimated 20–50µM cysteamine concentration in mouse liver [12, 43] is appreciably lower than other non-protein thiols such as GSH, suggesting that direct binding of NAPQI by cysteamine is minimal.
Mice that are protected from APAP by CFB pretreatment have increased hepatic concentrations of oxidized cystamine (and enhanced Vnn1 gene expression), while concentrations of reduced cysteamine are unchanged [12]. One can anticipate that under acute oxidative stress, cystamine dominates over cysteamine. Oxidized glutathione, cystine and cystamine are all thought to regulate enzymatic pathways by protein disulfide exchange, resulting in modifications of enzyme activity mediated by critical cysteine sites. Specifically, in vitro assays have shown that cystamine can inhibit key enzymes involved in tissue response to stress including PKCe, transglutaminase and caspase 3 [44–47]. In this way, it is likely that oxidized cystamine acts as a sensor when the cellular redox status is altered, regulating thiol-sensitive cellular processes involved in response to stress.
Gene array studies revealed that Vnn1 was the most significantly up-regulated gene in the differential comparison of wild type and PPARa-null mice pretreated with CFB and challenged with APAP. The observed 53-fold difference in gene expression is in great part due to a much lower basal expression of Vnn1 in the mutant mice [12]. This is conceivably one of the few examples in the literature where gene array analyses have provided a highly credible mechanistic lead (Vnn1 gene expression) subsequently proven through functional toxicity studies to be a novel genetic determinant of APAP hepatotoxicity. The studies presented here show that Vnn1 regulates the recruitment of specific populations of immune cells following APAP toxicity, possibly through modulation of redox-sensitive enzymatic activities.
Supplementary Material
Highlights.
Enhanced susceptibility of Vanin-1 null mice to acetaminophen hepatotoxicity
Absence of Vanin-1 results in deficiency in compensatory liver repair
Altered immunity in livers from acetaminophen treated Vanin-1 null mice
PPAR-alpha activation induces Vanin-1 and protects against acetaminophen toxicity
Acknowledgments
Financial Support: This work was supported by the National Institutes of Health Grant (DK069557).
Abbreviations
- PAPS
3’-Phosphoadenosine 5’-Phosphosulfate
- DTNB
5,5’-Dithio-Bis(2-Nitrobenzoic Acid)
- APAP
Acetaminophen
- ALT
Alanine Aminotrasferase
- BSA
Bovine Serum Albumin
- CCl4
Carbon tetrachloride
- Ccr2
C-C Chemokine Receptor 2
- CFB
Clofibrate
- CD
Cluster of Differentiation
- ConA
Concanavalin A
- CYP450
Cytochrome P450
- gGCS
Gamma-Glutamylcysteine Synthetase
- Gclc
Glutamate-cysteine ligase catalytic subunit
- GSH
Glutathione
- GSSG
Glutathione Disulfide
- iNos
Inducible Nitric Oxide Synthase
- Ifng
Interferon Gamma
- Il
Interleukin
- Mrp
Multidrug Resistance Associated Protein
- Mpo
Myeloperoxidase
- NAC
N-acetyl cysteine
- NAPQI
N-acetyl-p-benzoquinone imine
- Nqo1
NAD(P)H:Quinone Oxidoreductase 1
- PPARa
Peroxisome Proliferator-Activated Receptor Alpha
- PCNA
Proliferating Cell Nuclear Antigen
- Tgfβ1
Transforming Growth Factor Beta 1
- Tnf
Tumor Necrosis Factor
- Ugt
UDP-glucuronosyltransferase
- Vanin-1; Vnn1
Vascular Non-Inflammatory Molecule 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author’s Contributions:
Study concept and design: Ferreira, Galland, Naquet, Manautou
Acquisition of data: Ferreira, Goedken, Rommelaere, Chasson, Galland.
Analysis and interpretation of data: Ferreira, Goedken, Galland, Naquet, Manautou.
Drafting of manuscript: Ferreira, Naquet, Manautou.
Critical revision: Naquet, Manautou.
Statistical analysis: Ferreira, Naquet.
Conflict of Interest: The authors report no conflict of interest.
References
- 1.Lee WM. The case for limiting acetaminophen-related deaths: smaller doses and unbundling the opioid-acetaminophen compounds. Clin Pharmacol Ther. 2010;88:289–292. doi: 10.1038/clpt.2010.164. [DOI] [PubMed] [Google Scholar]
- 2.Zamek-Gliszczynski MJ, Hoffmaster KA, Tian X, Zhao R, Polli JW, Humphreys JE, et al. Multiple mechanisms are involved in the biliary excretion of acetaminophen sulfate in the rat: role of Mrp2 and Bcrp1. Drug Metab Dispos. 2005;33:1158–1165. doi: 10.1124/dmd.104.002188. [DOI] [PubMed] [Google Scholar]
- 3.Zamek-Gliszczynski MJ, Nezasa K, Tian X, Bridges AS, Lee K, Belinsky MG, et al. Evaluation of the role of multidrug resistance-associated protein (Mrp) 3 and Mrp4 in hepatic basolateral excretion of sulfate and glucuronide metabolites of acetaminophen, 4-methylumbelliferone, and harmol in Abcc3−/− and Abcc4−/− mice. J Pharmacol Exp Ther. 2006;319:1485–1491. doi: 10.1124/jpet.106.110106. [DOI] [PubMed] [Google Scholar]
- 4.Manautou JE, de Waart DR, Kunne C, Zelcer N, Goedken M, Borst P, et al. Altered disposition of acetaminophen in mice with a disruption of the Mrp3 gene. Hepatology. 2005;42:1091–1098. doi: 10.1002/hep.20898. [DOI] [PubMed] [Google Scholar]
- 5.Hinson JA, Roberts DW, James LP. Mechanisms of acetaminophen-induced liver necrosis. Handb Exp Pharmacol. 2010:369–405. doi: 10.1007/978-3-642-00663-0_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manautou JE, Hoivik DJ, Tveit A, Hart SG, Khairallah EA, Cohen SD. Clofibrate pretreatment diminishes acetaminophen's selective covalent binding and hepatotoxicity. Toxicol Appl Pharmacol. 1994;129:252–263. doi: 10.1006/taap.1994.1250. [DOI] [PubMed] [Google Scholar]
- 7.Chen C, Hennig GE, McCann DJ, Manautou JE. Effects of clofibrate and indocyanine green on the hepatobiliary disposition of acetaminophen and its metabolites in male CD-1 mice. Xenobiotica. 2000;30:1019–1032. doi: 10.1080/00498250010002252. [DOI] [PubMed] [Google Scholar]
- 8.Chen C, Hennig GE, Whiteley HE, Manautou JE. Protection against acetaminophen hepatotoxicity by clofibrate pretreatment: role of catalase induction. J Biochem Mol Toxicol. 2002;16:227–234. doi: 10.1002/jbt.10043. [DOI] [PubMed] [Google Scholar]
- 9.Moffit JS, Aleksunes LM, Kardas MJ, Slitt AL, Klaassen CD, Manautou JE. Role of NAD(P)H:quinone oxidoreductase 1 in clofibrate-mediated hepatoprotection from acetaminophen. Toxicology. 2007;230:197–206. doi: 10.1016/j.tox.2006.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manautou JE, Emeigh Hart SG, Khairallah EA, Cohen SD. Protection against acetaminophen hepatotoxicity by a single dose of clofibrate: effects on selective protein arylation and glutathione depletion. Fundam Appl Toxicol. 1996;29:229–237. doi: 10.1006/faat.1996.0026. [DOI] [PubMed] [Google Scholar]
- 11.Chen C, Hennig GE, Whiteley HE, Corton JC, Manautou JE. Peroxisome proliferator-activated receptor alpha-null mice lack resistance to acetaminophen hepatotoxicity following clofibrate exposure. Toxicol Sci. 2000;57:338–344. doi: 10.1093/toxsci/57.2.338. [DOI] [PubMed] [Google Scholar]
- 12.Moffit JS, Koza-Taylor PH, Holland RD, Thibodeau MS, Beger RD, Lawton MP, et al. Differential gene expression in mouse liver associated with the hepatoprotective effect of clofibrate. Toxicol Appl Pharmacol. 2007;222:169–179. doi: 10.1016/j.taap.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rommelaere S, Millet V, Gensollen T, Bourges C, Eeckhoute J, Hennuyer N, et al. PPARalpha regulates the production of serum Vanin-1 by liver. FEBS Lett. 2013;587:3742–3748. doi: 10.1016/j.febslet.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 14.Pitari G, Malergue F, Martin F, Philippe JM, Massucci MT, Chabret C, et al. Pantetheinase activity of membrane-bound Vanin-1: lack of free cysteamine in tissues of Vanin-1 deficient mice. FEBS Lett. 2000;483:149–154. doi: 10.1016/s0014-5793(00)02110-4. [DOI] [PubMed] [Google Scholar]
- 15.Winterbourn CC, Metodiewa D. Reactivity of biologically important thiol compounds with superoxide and hydrogen peroxide. Free Radic Biol Med. 1999;27:322–328. doi: 10.1016/s0891-5849(99)00051-9. [DOI] [PubMed] [Google Scholar]
- 16.Skrede S, Christophersen BO. Effects of cystamine and cysteamine on the peroxidation of lipids and the release of proteins from mitochondria. Biochem J. 1966;101:37–41. doi: 10.1042/bj1010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wood PL, Khan MA, Moskal JR. Cellular thiol pools are responsible for sequestration of cytotoxic reactive aldehydes: central role of free cysteine and cysteamine. Brain Res. 2007;1158:158–163. doi: 10.1016/j.brainres.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Naquet P, Pitari G, Duprè S, Galland F. Role of the Vnn1 pantetheinase in tissue tolerance to stress. Biochem Soc Trans. 2014;42:1094–1100. doi: 10.1042/BST20140092. [DOI] [PubMed] [Google Scholar]
- 19.Meghari S, Berruyer C, Lepidi H, Galland F, Naquet P, Mege JL. Vanin-1 controls granuloma formation and macrophage polarization in Coxiella burnetii infection. Eur J Immunol. 2007;37:24–32. doi: 10.1002/eji.200636054. [DOI] [PubMed] [Google Scholar]
- 20.Aurrand-Lions M, Galland F, Bazin H, Zakharyev VM, Imhof BA, Naquet P. Vanin-1, a novel GPI-linked perivascular molecule involved in thymus homing. Immunity. 1996;5:391–405. doi: 10.1016/s1074-7613(00)80496-3. [DOI] [PubMed] [Google Scholar]
- 21.Rahman I, Kode A, Biswas SK. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc. 2006;1:3159–3165. doi: 10.1038/nprot.2006.378. [DOI] [PubMed] [Google Scholar]
- 22.Manautou JE, Tveit A, Hoivik DJ, Khairallah EA, Cohen SD. Protection by clofibrate against acetaminophen hepatotoxicity in male CD-1 mice is associated with an early increase in biliary concentration of acetaminophen-glutathione adducts. Toxicol Appl Pharmacol. 1996;140:30–38. doi: 10.1006/taap.1996.0194. [DOI] [PubMed] [Google Scholar]
- 23.Reisman SA, Csanaky IL, Aleksunes LM, Klaassen CD. Altered disposition of acetaminophen in Nrf2-null and Keap1-knockdown mice. Toxicol Sci. 2009;109:31–40. doi: 10.1093/toxsci/kfp047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitchell JR, Jollow DJ, Potter WZ, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. The Journal of pharmacology and experimental therapeutics. 1973;187:211–217. [PubMed] [Google Scholar]
- 25.Jaeschke H, Williams CD, Ramachandran A, Bajt ML. Acetaminophen hepatotoxicity and repair: the role of sterile inflammation and innate immunity. Liver Int. 2011 doi: 10.1111/j.1478-3231.2011.02501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laskin DL, Laskin JD. Role of macrophages and inflammatory mediators in chemically induced toxicity. Toxicology. 2001;160:111–118. doi: 10.1016/s0300-483x(00)00437-6. [DOI] [PubMed] [Google Scholar]
- 27.Laskin DL, Pilaro AM, Ji S. Potential role of activated macrophages in acetaminophen hepatotoxicity. II. Mechanism of macrophage accumulation and activation. Toxicol Appl Pharmacol. 1986;86:216–226. doi: 10.1016/0041-008x(86)90052-9. [DOI] [PubMed] [Google Scholar]
- 28.Dambach DM, Watson LM, Gray KR, Durham SK, Laskin DL. Role of CCR2 in macrophage migration into the liver during acetaminophen-induced hepatotoxicity in the mouse. Hepatology. 2002;35:1093–1103. doi: 10.1053/jhep.2002.33162. [DOI] [PubMed] [Google Scholar]
- 29.Holt MP, Cheng L, Ju C. Identification and characterization of infiltrating macrophages in acetaminophen-induced liver injury. Journal of leukocyte biology. 2008;84:1410–1421. doi: 10.1189/jlb.0308173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang HX, Liu M, Weng SY, Li JJ, Xie C, He HL, et al. Immune mechanisms of Concanavalin A model of autoimmune hepatitis. World J Gastroenterol. 2012;18:119–125. doi: 10.3748/wjg.v18.i2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berruyer C, Martin FM, Castellano R, Macone A, Malergue F, Garrido-Urbani S, et al. Vanin-1−/− mice exhibit a glutathione-mediated tissue resistance to oxidative stress. Mol Cell Biol. 2004;24:7214–7224. doi: 10.1128/MCB.24.16.7214-7224.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin F, Penet MF, Malergue F, Lepidi H, Dessein A, Galland F, et al. Vanin-1(−/−) mice show decreased NSAID- and Schistosoma-induced intestinal inflammation associated with higher glutathione stores. J Clin Invest. 2004;113:591–597. doi: 10.1172/JCI19557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson KA, Yao W, Lane NE, Naquet P, Terkeltaub RA. Vanin-1 pantetheinase drives increased chondrogenic potential of mesenchymal precursors in ank/ank mice. Am J Pathol. 2008;172:440–453. doi: 10.2353/ajpath.2008.070753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Campion SN, Johnson R, Aleksunes LM, Goedken MJ, van Rooijen N, Scheffer GL, et al. Hepatic Mrp4 induction following acetaminophen exposure is dependent on Kupffer cell function. Am J Physiol Gastrointest Liver Physiol. 2008;295:G294–G304. doi: 10.1152/ajpgi.00541.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suzuki K, Watanabe T, Sakurai S, Ohtake K, Kinoshita T, Araki A, et al. A novel glycosylphosphatidyl inositol-anchored protein on human leukocytes: a possible role for regulation of neutrophil adherence and migration. J Immunol. 1999;162:4277–4284. [PubMed] [Google Scholar]
- 36.Sendo D, Takeda Y, Ishikawa H, Sendo F, Araki Y. Localization of GPI-80, a beta2-integrin-associated glycosylphosphatidyl-inositol anchored protein, on strongly CD14-positive human monocytes. Immunobiology. 2003;207:217–221. doi: 10.1078/0171-2985-00235. [DOI] [PubMed] [Google Scholar]
- 37.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 38.Dammanahalli KJ, Stevens S, Terkeltaub R. Vanin-1 pantetheinase drives smooth muscle cell activation in post-arterial injury neointimal hyperplasia. PLoS One. 2012;7:e39106. doi: 10.1371/journal.pone.0039106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang X, Wei HX, Rui S, Wei H, Tian Z. Opposite effects of high and low doses of interleukin-2 on T cell-mediated hepatitis in mice (interleukin-2 on hepatitis) Hepatol Int. 2010;4:641–648. doi: 10.1007/s12072-010-9196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knolle PA, Gerken G, Loser E, Dienes HP, Gantner F, Tiegs G, et al. Role of sinusoidal endothelial cells of the liver in concanavalin A-induced hepatic injury in mice. Hepatology. 1996;24:824–829. doi: 10.1002/hep.510240413. [DOI] [PubMed] [Google Scholar]
- 41.Prescott LF, Newton RW, Swainson CP, Wright N, Forrest AR, Matthew H. Successful treatment of severe paracetamol overdosage with cysteamine. Lancet. 1974;1:588–592. doi: 10.1016/s0140-6736(74)92649-x. [DOI] [PubMed] [Google Scholar]
- 42.Miners JO, Drew R, Birkett DJ. Mechanism of action of paracetamol protective agents in mice in vivo. Biochem Pharmacol. 1984;33:2995–3000. doi: 10.1016/0006-2952(84)90599-9. [DOI] [PubMed] [Google Scholar]
- 43.Duffel MW, Logan DJ, Ziegler DM. Cysteamine and cystamine. Methods Enzymol. 1987;143:149–154. doi: 10.1016/0076-6879(87)43027-9. [DOI] [PubMed] [Google Scholar]
- 44.Cappiello M, Voltarelli M, Cecconi I, Vilardo PG, Dal Monte M, Marini I, et al. Specifically targeted modification of human aldose reductase by physiological disulfides. J Biol Chem. 1996;271:33539–33544. doi: 10.1074/jbc.271.52.33539. [DOI] [PubMed] [Google Scholar]
- 45.Lesort M, Lee M, Tucholski J, Johnson GV. Cystamine inhibits caspase activity. Implications for the treatment of polyglutamine disorders. J Biol Chem. 2003;278:3825–3830. doi: 10.1074/jbc.M205812200. [DOI] [PubMed] [Google Scholar]
- 46.Mallis RJ, Buss JE, Thomas JA. Oxidative modification of H-ras: S-thiolation and S-nitrosylation of reactive cysteines. Biochem J. 2001;355:145–153. doi: 10.1042/0264-6021:3550145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nurminskaya MV, Belkin AM. Cellular functions of tissue transglutaminase. International review of cell and molecular biology. 2012;294:1–97. doi: 10.1016/B978-0-12-394305-7.00001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.