Abstract
Background
Prevention of mother-to-child transmission (PMTCT) depends critically on HIV-positive women’s adherence to antiretroviral drugs during and after pregnancy. Adherence among pregnant and breastfeeding women remains a challenge across sub-Saharan Africa. Power dynamics within couples, such as intimate partner violence, has largely been neglected in research regarding PMTCT adherence.
Objective
This study aims to determine if there is a relationship between intimate partner violence and non-adherence to PMTCT.
Methods
In 2014, using a verbally administered cross-sectional survey at a large public health clinic in Lusaka, Zambia, 320 HIV-positive postpartum women, who were currently married or living with a man, provided information on their drug adherence during and after pregnancy, as well as relationship dynamics. Adherence was defined as the woman reporting she took or gave to the infant at least 80% of prescribed medication doses.
Results
Experiencing intimate partner violence was associated with decreased odds of adherence to PMTCT during and after pregnancy. Different forms of violence affected PMTCT adherence differentially. Physical violence had a less pronounced effect on non-adherence than emotional and sexual violence. A dose-response relationship between intimate partner violence and non-adherence was also observed.
Conclusions
Intimate partner violence is associated with non-adherence to PMTCT during and after pregnancy, which deserves increased attention in the effort to eliminate mother-to-child transmission.
Keywords: prevention of mother-to-child transmission, intimate partner violence, HIV, antiretroviral adherence, Zambia
Introduction
Antiretroviral (ARV) drugs during and after pregnancy can reduce the risk of mother-to-child transmission (MTCT) of HIV in utero, during childbirth, or while breastfeeding from 35% to less than 5% in low and middle-income countries (WHO, 2010a). Major strides have been made at the political and institutional level to increase coverage of ARVs for the purpose of prevention of mother-to-child transmission (PMTCT) in sub-Saharan Africa where 90% of HIV-positive mothers live (WHO, 2010b). Zambia is one of six countries in the region that has achieved PMTCT coverage of more than 75% (UNAIDS, 2012). Despite increasing access to highly efficacious combination antiretroviral therapy (cART) for PMTCT, hundreds of thousands of infants in sub-Saharan Africa continue to contract HIV from their mothers each year (WHO, 2013). In Zambia alone, despite a commendable downward trend, almost 10,000 infants are newly infected with HIV annually (UNICEF, 2012). A critical barrier to PMTCT in Zambia is HIV-positive women’s poor adherence to cART during pregnancy and the breastfeeding period (Ngoma et al., 2015).
While given less attention than scaling-up ARV services, PMTCT depends critically on HIV-positive pregnant and breastfeeding women’s adherence to cART. Recent studies suggest that HIV-positive individuals need to take at least 80% of prescribed cART doses to adequately suppress HIV (Gordon, Gharibian, Chong, & Chun, 2015; Kobin & Sheth, 2011). Suboptimal adherence to cART among HIV-positive pregnant and breastfeeding women not only increases the risk of MTCT, but also increases the likelihood of maternal HIV-related disease progression and drug resistance for both the mother and the infant (Nachega et al., 2007; Ngoma et al., 2015; Onono et al., 2015). Achieving high PMTCT adherence remains a challenge in many settings globally, including sub-Saharan Africa (Nachega et al., 2012). Indeed, research from Zambia indicates that PMTCT adherence ranges from 63% to 79% (Conkling et al., 2010; Megazzini et al., 2010; Stringer et al., 2010); however, these estimates are based on single dose Nevirapine (sdNVP) at the time of labor/delivery and not cART across the PMTCT cascade. Little is known about HIV-positive women’s ability to adhere to the newer extended cART PMTCT regimens in Zambia.
Often, HIV-positive women’s attitude, perceived norms, and personal agency are cited as ways to understand and address PMTCT adherence (Awiti Ujiji et al., 2011; Nyasulu & Naysulu, 2011; Varga & Brookes, 2008). The problem with examining PMTCT adherence using only intrapersonal constructs is that the sole responsibility to prevent MTCT is thereby placed on the HIV-positive mother, without taking into account the context in which these health behaviors occur and the large gender inequities that persist both within society and sexual relationships (Campbell et al., 2008).
Intimate partner violence (IPV) against women is one of the most compelling manifestations of unequal power in sexual relationships and the larger phenomenon of gender inequality (Blanc, 2001). IPV includes actual or threatened physical or sexual violence, or psychological and emotional abuse directed toward a partner/spouse that is part of a general strategy of power and control (Campbell, 2002; Johnson, 2008). Based on the most recent Zambian Demographic and Health Survey (ZDHS), IPV against women appears to be a normative and accepted behavior. Over 46% of women reported they believe a husband is justified in beating his wife for at least one specified reason and 47% of women reported experiencing IPV from their current partner/spouse (CSO, 2014). Qualitative research from Zambia indicates that refusing to have sex, discussing HIV testing or treatment, and disclosure of an HIV positive status to a male partner can initiate IPV against women (Human Rights Watch, 2007; Murray et al., 2006).
IPV is associated with numerous adverse health outcomes among women globally (Campbell, 2002), including a higher risk of sexually-transmitted infections (STIs), including HIV (Harvey, Garcia-Moreno, & Butchart, 2007; Li et al., 2014). Women are also at increased risk of IPV after HIV-infection (Mulrenan et al., 2015). In addition, IPV is linked to poor reproductive health outcomes, such as low birth weight, preterm delivery, and maternal and infant mortality (Boy & Salihu, 2004; Emenike, Lawoko, & Dalal, 2008). Lastly, IPV reduces the likelihood of certain HIV-related health behaviors among women, such as HIV testing and adherence to cART for the woman’s own health (Hatcher, Smout, Turan, Christofides, & Stockl, 2015; Siemieniuk et al., 2013). Qualitative research from South Africa also indicates that HIV-positive women often cite IPV as a barrier to PMTCT (Hatcher et al., 2014; Mepham, Zondi, Mbuyazi, Mkhwanazi, & Newell, 2011). However, no studies to-date have quantitatively established a relationship between IPV and non-adherence to PMTCT in sub-Saharan Africa (Hatcher et al., 2015).
This article examines whether IPV against HIV-positive women is associated with non-adherence to cART during and after pregnancy in urban Zambia. This paper also explores whether the type, frequency, and severity of IPV differentially impact adherence to various PMTCT medication protocols. In order to create effective interventions, we need to understand both the nature of IPV, as well as its consequences (White, Smith, Koss, & Figueredo, 2000). The a priori hypothesis of this study is that women with more severe and frequent IPV will have the poorest adherence to PMTCT due to their lack of autonomy and decision-making ability within the family.
Methods
Study Design
A cross-sectional survey was conducted with postpartum HIV-positive women at a large public health center in Lusaka, Zambia, from April to August of 2014. Quantitative data were collected through a face-to-face survey with closed-ended questions on women’s self-reported PMTCT adherence and gender power dynamics within their current sexual relationship. The questionnaire was pretested during a pilot study in March of 2014. Trained Zambian research assistants verbally administered the survey in the local languages on paper forms in a private location at the health center. The questionnaire was written in English, but translated into the two most commonly spoken dialects in Lusaka. Participants received a small travel reimbursement for their time. Written informed consent or a thumbprint was obtained from all participants.
The four research assistants were all individuals who had previously participated in data collection for health research and were trained health care workers. They also attended a three-day training, which included in-depth discussion of research ethics. The study was designed and implemented in accordance with the World Health Organization Ethical and Safety Recommendations for Research on Domestic Violence Against Women (WHO, 2001). Women who reported IPV were offered referrals to the Young Women’s Christian Association (YWCA) in Lusaka for counseling and victim support services. The study was approved by the Colorado Multiple Institutional Review Board (COMIRB) and the Excellence in Research Ethics and Science (ERES) Converge in Lusaka, Zambia.
Study Participants
Participants were 320 HIV-positive postpartum mothers who had brought their infants for routine pediatric well-child care (e.g., immunizations) in the Department of Maternal and Child Health. The health center has a catchment population of over 160,000 individuals living in the surrounding low socioeconomic neighborhoods. Women who were HIV seropositive, over the age of 18 years, currently married or living with a man, and whose youngest infant was between 3 to 9 months of age, were invited to participate in the survey after completing their health care visit. Infant age criterion was selected to capture all of the essential postpartum PMTCT protocols and limit recall bias. Routine pediatric health care in Zambia recommends infants receive immunizations and other preventative care at 14 weeks; 6 months; and 9 months. Immunization compliance is high with only 3.2% of Zambian infants having no immunizations by 12 months of life (CSO, 2014).
Nurses determined eligibility for the study using the infant’s “Under-Five Card,” a mother’s copy of her child’s health record that she is required to bring to all pediatric health care visits and includes the child’s birth date, height and weight, immunizations, medications, and PMTCT. Nurses providing immunizations examined the Under-Five Card and verbally invited eligible women to participate in the survey by proceeding to a designated room in the clinic where research assistants awaited them. The response rate for eligible women was 85%.
Measures
Questions regarding medication adherence during and after pregnancy, the main outcome of interest, were developed by the primary investigator based on Simoni et al. (2006) and the 2010 Malawi DHS (National Statistical Office & ICF Macro, 2011). Using a visual analog scale (VAS) that was verbally explained to women by the research assistants, participants retrospectively reported on their estimate of cART adherence during and after pregnancy and whether they ingested sdNVP during labor/delivery. Participants also reported on their estimate of adherence to giving Niverapine (NVP) prophylaxis to their infant postpartum. Self-reporting by HIV-positive mothers is one of the most common methodologies used to collect data on PMTCT (Futterman et al., 2010; Mepham et al., 2011; Nassali et al., 2009). In addition, studies have shown that self-reported adherence to ART, including using the VAS, and plasma drug levels are significantly associated (Fabbiani et al., 2015; Fletcher et al., 2005; Murri et al., 2000). Although biomarkers were not available, research assistants did use PMTCT appointment records to validate women’s self-reported adherence.
At the time of the study, Zambia was following the WHO PMTCT Option A, where HIV-positive pregnant or breastfeeding women initiated lifelong ART only if they were eligible based on their CD4 count/clinical stage. HIV-positive pregnant or breastfeeding women who were not eligible for lifelong ART were given short-course PMTCT prophylaxis. Under both regimens, women are required to take cART during and after pregnancy as well as give their infant prophylaxis; although different drugs are prescribed for different lengths of time (WHO, 2010a). Data were collected for women on both regimens and regimen type was controlled for in the analysis.
Women reported on the proportion of prescribed cART doses they took while they were pregnant from 0 to 100%; if they ingested sdNVP during labor and delivery (only women on short course prophylaxis); how many of the prescribed cART doses they took since giving birth from 0 to 100%; and how many doses of NVP prophylaxis they gave to the infant from 0 to 100%. Only women who reported being offered medication were included in the analysis. Medical records were used to validate self-reports at the time of the survey. Adherence was evaluated as a binary variable, defined as a woman reporting she took (or gave to the infant) at least 80% of the prescribed medication doses during each respective time period (Gordon et al., 2015; Kobin & Sheth, 2011).
IPV, the independent variable of interest, was measured using a version of the Revised Conflict Tactics Scale (CTS2). The CTS2 is one of the most widely used IPV measurement tools worldwide and has strong reported psychometric properties (Straus, Hamby, BoneyMcCoy, & Sugarman, 1996). The version of the CTS2 used in this study came directly from the Zambian DHS Domestic Violence Module (CSO, 2009). The DHS Domestic Violence Module was first developed and standardized in 2000 and has been used in more than 80 surveys, including throughout sub-Saharan Africa (MacQuarrie, Winter, & Kishor, 2014). The module includes five items related to IPV: the woman’s acceptance of wife beating; if she has experienced any specific sexual, physical, or emotional IPV events from her current sexual partner; the frequency of those specific events in the past year; if any injuries occurred as the result of violence; and if she has ever hit her partner. There were three possible specific events for emotional violence, seven possible events for physical violence, and two possible events for sexual violence.
Covariates included the mother’s age, the infant’s age, education, parity, knowledge of PMTCT, number of household assets (a proxy for socioeconomic status), PMTCT regimen, and disclosure of HIV status to the husband/partner. PMTCT regimen was not controlled for in models for medication during childbirth, sdNVP, because this protocol is only indicated for women on the short-course prophylaxis regimen. Knowledge of PMTCT was measured by four questions on the survey (CSO, 2009) that were analyzed as a count variable based on correct answers, for example, “Can HIV be transmitted from a mother to her baby during pregnancy?” and “Are there any special drugs that a doctor or nurse can give to a woman infected with HIV to reduce the risk of transmission to the baby?”. Except PMTCT regimen, which was determined based on the medication a woman was taking during data analysis, all variables were directly from the Zambian DHS questionnaire (CSO, 2009).
Data Cleaning and Statistical Analysis
Survey data were double entered into CSPro and exported into Stata 12 for analysis. Surveys with more than 50% missing data were not included in the analyses (n=4). Missing data (2.3%) were imputed using multivariate chained equations in Stata 12 (Raghunathan, Lepkowski, Hoewyk, & Solenberger, 2001). Data converged, indicating that the multivariate chained model was a good fit for the dataset (StataCorp, 2009). Using the imputed dataset, simple linear regression and simple logistic regression models determined significant differences at the bivariate level in various participant characteristics between women who did and did not experience IPV. Multiple logistic regression models subsequently determined significant differences in the odds of adhering to each PMTCT medication protocol by experience with IPV after adjusting for covariates.
Dummy variables were created for emotional, physical, and sexual violence, as well as experiencing any IPV. This is a standard way of using the DHS Domestic Violence Module (Alio, Nana, & Salihu, 2009; Kishor & Johnson, 2004; Lawoko, Dalal, Jiayou, & Jansson, 2007). In addition, to better understand the nature of IPV, calculated was the number of violent events a woman experienced from her current partner by summing the total number of specific emotional, physical, or sexual events a woman reported out of a total of 12 possible events. In addition, a dummy variable for any injuries as a result of violence was created to capture the severity of physical/sexual IPV.
Results
Participant characteristics
The total sample size was 320 HIV-positive women (see Table 1). The mean age of the women was 29 years. On average, women had given birth to just over three children, with the youngest being approximately 6 months of age. Over 70% of women had completed primary education, but only 14% had completed secondary education. On average, women reported having 9 household items out of possible of 21 total listed items. Over 74% of women had electricity, 37% had a refrigerator, and only 4% owned a vehicle. Knowledge of PMTCT was high with over 87% of participants correctly answering all four PMTCT-related questions. Women had been in their current sexual relationship on average for 7 years. Among the women who reported knowing their partner’s HIV status (80%, n=256), 35% reported being serodiscordant with the male partner having an HIV-negative status (according the woman). Disclosure of one’s HIV positive status to their husband/partner was high with 92% of women reporting they told their partner they were HIV-positive. Lastly, acceptance of IPV was also high, with 66% of women agreeing a husband is justified in beating his wife for at least one specified reason, such as refusing sex, burning the food, or neglecting the children.
Table 1.
Participant characteristics stratified by experiencing intimate partner violence.
| Demographic Characteristics | Total (N=320) | Never experienced IPV (n=125) | Experienced IPV (n=195) | Test of Difference 1
|
|
|---|---|---|---|---|---|
| OR/Coef. | p-value | ||||
| Age (years) | 29.1 (5.9) | 29.5 (6.5) | 28.8 (5.4) | −0.56 | 0.404 |
| Age of infant (months) | 5.8 (2.3) | 5.9 (2.3) | 5.8 (2.3) | −0.11 | 0.687 |
| Number of children | 3.4 (1.7) | 3.5 (1.8) | 3.4 (1.6) | −0.08 | 0.684 |
| Completed primary education | 72.0% | 69.6% | 74.0% | 1.21 | 0.442 |
| Completed secondary education | 13.7% | 11.0% | 15.0% | 1.55 | 0.214 |
| Number of household assets 2 | 8.6 (3.2) | 8.6 (3.3) | 8.7 (3.0) | 0.052 | 0.893 |
| Length of relationship (years) | 6.6 (5.4) | 7.8 (6.1) | 5.9 (4.8) | −1.46 | 0.019 |
| Husband/partner tested for HIV | 80.3% | 85.0% | 77.1% | 0.55 | 0.051 |
| Discordant couple (n=256) 3 | 35.0% | 33.6% | 35.6% | 0.90 | 0.691 |
| PMTCT knowledge (correctly answered all four questions) | 87.2% | 88.0% | 86.7% | 0.88 | 728 |
| Disclosed to husband/partner HIV status | 91.6% | 96.2% | 89.0% | 0.28 | 0.024 |
| Acceptance of Wife Beating | |||||
| Believe that wife beating is justified for at least one reason | 66.6% | 44.8% | 80.9% | 5.09 | 0.000 |
| Power Dynamics Within Couples | |||||
| Lower income than partner | 71.2% | 80.3% | 65.2% | 0.50 | 0.059 |
| Partner has final say over money | 61.5% | 43.7% | 73.2% | 0.29 | 0.000 |
| Number of controlling behaviors 4 | 3.4 (2.1) | 2.4 (1.8) | 4.1 (1.9) | 1.69 | 0.000 |
| Ever hit partner | 8.4% | 5.4% | 10.3% | 1.99 | 0.141 |
| PMTCT Adherence | |||||
| >80% cART during pregnancy (n=271)5 | 88.2% | 96.0% | 83.5% | 0.21 | 0.004 |
| Took sdNVP during childbirth (n=131) | 92.4% | 97.6% | 90.0% | 0.25 | 0.164 |
| >80% cART during postnatal (n=285) | 90.9% | 98.2% | 86.3% | 0.12 | 0.004 |
| >80% infant NVP prophylaxis (n=303) | 85.5% | 97.5% | 77.8% | 0.09 | 0.000 |
| Total | 100% | 39.1% | 60.9% | ||
Notes. Statistics are summarized as Mean (standard deviation) unless otherwise stated.
Abbr. cART=Combination antiretroviral therapy. IVP=Intimate partner violence. NVP=Nevirapine. PMTCT=Prevention of mother-to-child transmission. OR=Odds ratio. SD=Standard deviation. sdNVP=Single dose NVP.
Unadjusted models
Out of a possible list of 21 items
Of the women who knew their partner’s status
Out of a possible list of 6 behaviors
Different sample size because women were offered medication differentially for each protocol.
p<0.05
p<0.01
P<0.001 level of significant difference between IPV and no IPV groups.
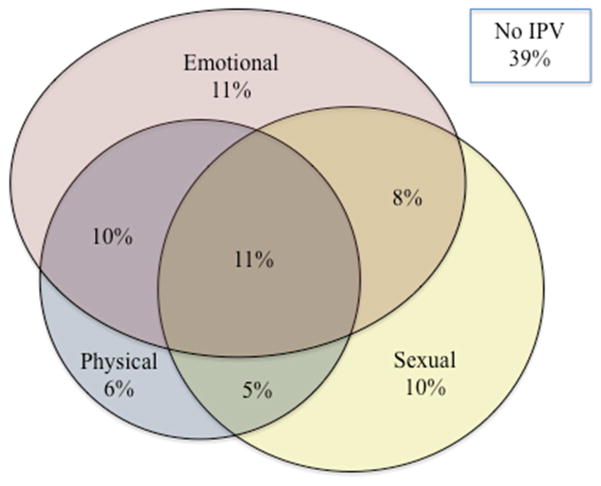
Figure 1 displays the descriptive results for women’s experiences with IPV. The majority of women (61%) reported experiencing at least one violent event in their current relationship. The most prevalent form of IPV was emotional violence, with 40% of women reporting at least one emotionally violent event, such as the husband/partner humiliating her in public or threatening to hurt her. Over one-third of women also reported sexual violence in the form of being physically forced to have sex or forced to perform a sexual act when she did not want to. In addition, over 30% of women reported at least one physically violent event, such as being slapped, punched, kicked, dragged, or beat up. Lastly, over 10% of women reported experiencing all forms of IPV (i.e., emotional, sexual, and physical). Of the women who experienced physical/sexual IPV (n=157), 57% experienced an injury as a result of the violence, such as aches, pains, or bruises.
Figure 1.
Women’s Experiences with Intimate Partner Violence (n=320)
The majority of women (71%) reported having less income than their male partner (see Table 1). In addition, over 60% of women said their husband/partner has the final decision over how money is spent. Over 90% of women experienced at least one controlling behavior from their husband/partner and the mean number of controlling behaviors was 3.4 (out of a possible of 6 behaviors). Only 8% of women reported ever hitting their current partner. Characteristics associated with experiencing IPV at the bivariate level were: shorter length of relationship; non-disclosure of HIV status to the husband/partner; acceptance of wife beating; partner having the final say over money; and the mean number of partner controlling behaviors.
PMTCT Adherence
Among women who were offered PMTCT, during pregnancy, 12% reported inadequate cART adherence (i.e., self-reported adherence of less than 80% of prescribed medication doses). During childbirth, 8% of women on the short-course prophylaxis regimen reported non-adherence to ingesting sdNVP during labor/delivery. During the postpartum time period, 9% of women reported inadequate cART adherence. Lastly, 14% of women reported inadequate adherence to giving the infant prophylaxis. At the bivariate level, experiencing IPV was negatively associated with adherence to all PMTCT medication except sdNVP during childbirth (see Table 1).
Intimate Partner Violence and PMTCT Adherence
In the adjusted models, experiencing IPV remained significantly associated with non-adherence to all of the PMTCT protocols except sdNVP during childbirth (see Table 2). Women who experienced IPV had a 74% reduced odds of adherence to drugs during pregnancy (p<0.05); an 89% reduced odds of adherence to drugs postpartum (p<0.01); and a 91% reduced odds of adherence to giving the infant prophylaxis (p<0.001) compared to women who did not experience any IPV.
Table 2.
Logistic regression results for the odds of adhering to prevention of mother-to-child transmission (PMTCT) protocols when women have experienced intimate partner violence and by covariates.
| Factor | cART adherence >80% during pregnancy (n=271) 1 | Took sdNVP during childbirth (n=131) 1 | cART adherence >80% during postpartum (n=285) 1 | Infant NVP prophylaxis adherence >80% during postpartum (n=303) 1 |
|---|---|---|---|---|
|
|
|
|||
| Experienced IPV | 0.26* (0.08 – 0.88) | 0.34 (0.04 – 3.07) | 0.11** (0.02 – 0.57) | 0.09*** (0.02 – 0.33) |
| Disclosed status | 18.41*** (5.81 – 57.64) | 8.78* (1.38 – 55.66) | 19.45*** (5.70 – 66.35) | 12.41*** (4.02 – 38.29) |
| Mother age (years) | 1.00 (0.83 – 1.21) | 1.00 (0.74 – 1.36) | 1.16* (1.02 – 1.33) | 1.08 (0.98 – 1.20) |
| Infant age (months) | 1.01 (0.84 – 1.22) | 0.92 (0.66 – 1.28) | 0.97 (0.78 – 1.20) | 0.96 (0.82 – 1.13) |
| Parity | 0.72 (0.93 – 3.10) | 0.80 (0.43 – 1.50) | 0.65* (0.43 – 0.99) | 0.96 (0.66 – 1.36) |
| PMTCT knowledge | 1.70 (0.89 – 2.89) | 1.19 (0.54 – 2.64) | 1.24 (0.71 – 2.16) | 1.16 (0.76 – 1.78) |
| Number of household assets | 1.04 (0.90 – 1.20) | 0.95 (0.74 – 1.20) | 1.16 (0.97 – 1.37) | 1.05 (0.92 – 1.19) |
| Completed primary education | 0.42 (0.14 – 1.27) | 0.24 (0.03 – 2.18) | 1.21 (0.40 – 3.62) | 1.36 (0.58 – 3.17) |
| Mother on treatment | 2.90* (1.12 – 7.53) | N/A | 2.26 (0.79 – 6.43) | 3.05** (1.36 – 6.83) |
Notes. Logistic regression results are presented as adjusted odds ratios and 95% confidence intervals (adjusted for covariates: mother’s age, infant’s age, education, parity, knowledge of PMTCT, number of household assets, PMTCT regimen, and HIV status disclosure to husband/partner). Abbr. cART=Combination antiretroviral therapy. IVP=Intimate partner violence. NVP=Nevirapine. PMTCT=Prevention of mother-to-child transmission. sdNVP=Single dose NVP.
Different sample size because women were offered medication differentially for each protocol.
p<0.05
p<0.01
p<0.001 level of significant difference.
Different types of IPV affected adherence to PMTCT protocols differentially (see Table 3). Sexual violence was associated with non-adherence to the greatest amount of PMTCT protocols compared to physical and emotional violence. Women who experienced sexual violence in their current relationship had a 68% reduced odds of adherence to drugs during pregnancy (p<0.05); an 83% reduced odds of taking sdNVP during labor/delivery (p<0.05); a 74% reduced odds of adherence during postpartum (p<0.01); and a 66% reduced odds of adherence to giving the infant prophylaxis (p<0.01).
Table 3.
Logistic regression results for the odds of adhering to prevention of mother-to-child transmission (PMTCT) protocols by type and severity of intimate partner violence.
| Factor: | cART adherence >80% during pregnancy (n=271) 1 | Took sdNVP during childbirth (n=131) 1 | cART adherence >80% during postpartum (n=285) 1 | Infant NVP prophylaxis adherence >80% during postpartum (n=303) 1 |
|---|---|---|---|---|
|
|
|
|||
| Emotional IPV | 0.43 (0.17 – 1.06) | 0.37 (0.08 – 1.82) | 0.10*** (0.03 – 0.36) | 0.10*** (0.04 – 0.26) |
| Physical IPV | 0.90 (0.36 – 2.27) | 0.95 (0.22 – 4.18) | 0.65 (0.24 – 1.72) | 0.38* (0.18 – 0.80) |
| Sexual IPV | 0.32* (0.13 – 0.80) | 0.17* (0.03 – 0.92) | 0.26** (0.10 – 0.72) | 0.34** (0.16 – 0.71) |
| Mean number of IPV events | 0.80* (0.66 – 0.98) | 0.80 (0.58 – 1.13) | 0.69** (0.56 – 0.86) | 0.68*** (0.57 – 0.81) |
| Injuries from IPV (n=157) 3 | 0.78 (0.22 – 2.74) | 0.56 (0.08 – 4.12) | 0.62 (0.15 – 2.55 | 0.28* (0.09 – 0.85) |
Notes. Logistic regression results are presented as adjusted odds ratios and 95% confidence intervals; Adjusted for mother’s age, infant’s age, education, parity, knowledge of PMTCT, number of household assets, PMTCT regimen, and HIV status disclosure to husband/partner. Abbr. cART=Combination antiretroviral therapy. IVP=Intimate partner violence. NVP=Nevirapine. PMTCT=Prevention of mother-to-child transmission. sdNVP=Single dose NVP.
Different sample size because women were offered medication differentially for each protocol
Adjusting for mother’s age, infant’s age, education, parity, knowledge of PMTCT, number of household assets, PMTCT regimen, and HIV status disclosure to husband/partner
Of the women who reported experiencing physical/sexual violence
p<0.05
p<0.01
p<0.001 level of significant difference
Emotional violence was also associated with non-adherence to several PMTCT protocols. During postpartum, women who experienced emotional violence had a 90% reduced odds of adherence to their medication (p<0.001), as well as a 90% reduced odds of giving the infant prophylaxis (p<0.001). Physical violence, interestingly, was only associated with decreased odds of adherence to giving infant prophylaxis: Women who experienced physical IPV had a 62% reduced odds of adherence to giving infant prophylaxis (p<0.05).
In addition, there appears to be a dose-response relationship between IPV and non-adherence to PMTCT (see Table 3). For each additional violent event a woman experienced, she had a 20% reduced odds of adherence to drugs during pregnancy (p<0.05); a 31% reduced odds of adherence to drugs postpartum (p<0.01); and a 32% reduced odds of adherence to giving the infant prophylaxis (p<0.001). Lastly, severity of physical/sexual violence was associated with non-adherence to giving the infant prophylaxis. Of the women who experienced physical/sexual IPV (n=157), those who experienced injuries had a 72% reduced odds of adherence to giving the infant prophylaxis (p<0.05).
PMTCT and Covariates
In addition to experiencing IPV, three covariates were also significantly associated with non-adherence to various PMTCT medication protocols in the adjusted models (see Table 2). First, disclosure of an HIV positive status to the husband/partner was associated with increased adherence to all PMTCT medication protocols. This is not a new finding, but does provide support from the Zambian context for what other studies have reported (Auvinen, Suominen, & Valimaki, 2010; Jasseron et al., 2011; Theuring et al., 2009). Second, parity was negatively associated with PMTCT medication, but only for maternal medication postpartum. More research is needed to unpack the reasons why having more children could result in worse medication adherence. Lastly, the type of PMTCT regimen the woman was taking (i.e., either lifelong ART or short course prophylaxis) was significantly associated with adherence to PMTCT during pregnancy and to giving the infant prophylaxis. This finding has been reported elsewhere in greater detail (Hampanda, 2015).
Discussion
To the author’s knowledge, this study is the first to report a quantitative relationship between IPV and non-adherence to PMTCT in sub-Saharan Africa (see Hatcher et al., 2015, for a systematic review on IPV and women’s engagement in HIV care and treatment). These findings are supported by recent qualitative research from South Africa that found HIV-positive women report fear of violence from a male partner as a barrier to PMTCT (Hatcher et al., 2014; Mepham et al., 2011). The present study’s findings, however, run contrary to what Kiarie et al. (2006) found in Kenya. They reported no association between IPV and uptake of PMTCT; however, PMTCT was limited to sdNVP during childbirth at the time of the Kiarie et al. study. In the present study’s analysis, sdNVP was also not significantly associated with any experiences with IPV. Yet, IPV was associated with non-adherence to all other PMTCT protocols in the cascade of care.
PMTCT Medication across the Cascade of Care
There are several reasons why sdNVP may not be as vulnerable to non-adherence as other PMTCT medication protocols. First, sdNVP during labor/delivery is easier to conceal from a husband/partner who does not know the woman’s HIV status because men are generally not present in the delivery room. Second, if HIV-positive women come to deliver in a health center, they should receive this dose of medication, whereas the other protocols require constant refills of ARV prescriptions and daily consumption. This requires some travel and other potential expenses, which may be challenging if women have limited access or decision-making power over how financial resources are spent. Although the current PMTCT regimens are more effective than only sdNVP during childbirth (WHO, 2010a), the extended duration of taking cART may be more difficult for HIV-positive mothers to follow without the knowledge, permission, and/or support of their male partners.
Specific Experiences with Intimate Partner Violence
Importantly, not all IPV is equal when it comes to PMTCT adherence. In this study, physical violence had a less pronounced association on women’s adherence to the various PMTCT drugs than emotional, and especially sexual violence. Often, emotional violence is not evaluated in studies examining IPV (Abramsky et al., 2011; Harling, Msisha, & Subramanian, 2010; Pallitto et al., 2013). This study underscores the importance of examining IPV as a multi-dimensional phenomenon. Indeed, emotional violence was the most commonly reported form of IPV among women in this study and was associated with non-adherence to many of the PMTCT protocols. Sexual violence, to an even greater extent, was associated with non-adherence to PMTCT. It is feasible that physical and emotional violence are more normative in Zambia and that the presence of sexual violence represents a more severe IPV situation. Greater research is needed; however, into why sexual violence in particular has a greater negative impact on PMTCT adherence.
There appears to be a dose-response relationship between IPV and non-adherence to PMTCT. The women in this study who experienced more violent events in their current relationship had reduced odds of adherence to all PMTCT medication protocols except sdNVP during childbirth. Interestingly, severity of IPV, measured by any injuries experienced as a result of physical/sexual IPV, was only associated with non-adherence to giving the infant NVP prophylaxis. Indeed, infant prophylaxis was the protocol with the worst reported adherence and the protocol most consistently negatively associated with the various IPV measures in this study.
Reasons Why IPV May Hinder PMTCT Adherence
IPV likely hinders PMTCT adherence through several social mechanisms. First, fear of IPV decreases HIV status disclosure to husbands/partners (Medley, Garcia-Moreno, McGill, & Maman, 2004), which in turn decreases women’s adherence to PMTCT (Jasseron et al., 2011). However, IPV was independently associated with non-adherence to PMTCT after controlling for status disclosure in this study.
A second plausible explanation is that IPV may result in poor spousal communication (Blanc, 2001), which is an important factor in women’s PMTCT-related behavior (Sarker, Sanou, Snow, Ganame, & Gondos, 2007). Third, abusive male partners may be less supportive of women’s health and less likely to participate in PMTCT services (Auvinen et al., 2010; Kiarie et al., 2006), such as accompanying women to visits or picking up medication, thus making adherence more arduous on the woman. Lastly, women who experience IPV may suffer from negative mental health. IPV increases the risk of emotional distress, depression, and post-traumatic stress disorder (PTSD), which are known barriers to medical adherence in general (Ellsberg, Jansen, Heise, Watts, & Garcia-Moreno, 2008). For example, Hatcher et al. (2014) reported that women in South Africa described feeling depressed and anxious due to IPV, which in turn negatively impacted their PMTCT adherence.
Implications
IPV is a large health concern in its own right. The current study indicates, moreover, that IPV may also play a significant role in pediatric HIV through maternal non-adherence to PMTCT. Interventions at the individual, relationship, facility, and structural levels are urgently needed to address IPV against women. Many of the risk factors associated with IPV are symptoms of persistent gender inequity. For example, women’s low education and acceptance of violence are risk factors for victimization at the individual-level (WHO, 2012), which could be addressed through greater efforts to empower girls and young women. At the relationship level, male dominance, which is related to the structure of cathexis (i.e., unequal gender norms), is a risk factor associated with IPV perpetration (WHO/LSHTM, 2010). Not only is women’s empowerment essential, but working with men to promote nonviolence and gender equality will also be a key intervention, starting with young school aged boys to evolve the cultural acceptance of IPV.
In addition, at the facility-level, PMTCT counseling should attempt to evaluate women’s social circumstances and determine who may be at risk for low compliance due to factors such as IPV. Questions regarding the home environment and whether IPV occurs should be standard in PMTCT counseling. An appropriate referral system between PMTCT services and mental health/victim support services should also be prioritized at health clinics. Another potential intervention that has shown improved PMTCT adherence in sub-Saharan Africa is support groups and “mentor mothers” for HIV-positive pregnant and breastfeeding women (Besser, 2010), although more research is needed into whether such mentorship programs can counter the effects of IPV. At the structural level, although much harder to implement and evaluate, a systems approach should include creating equitable gender norms; reducing poverty; improving the low social and economic status of women; reforming legal frameworks; establishing community sanctions against IPV; and progressing women’s civil rights (WHO, 2012).
Limitations
This study has a several limitations. First, it is cross-sectional and cannot establish causality or the timing of events. The results are also based on self-reporting, which is vulnerable to recall and social desirability biases. In addition, the sample is non-representative, limiting the generalizability of findings. Lastly, due to the relatively small sample size, there may have been inadequate statistical power to detect all significant relationships in the case of some IPV measures.
Conclusions
This is the first study from sub-Saharan Africa to establish a quantitative relationship between IPV and non-adherence to PMTCT. The HIV-positive women in this cohort who experienced IPV, especially sexual and emotional IPV, had reduced odds of cART adherence across the PMTCT cascade from pregnancy to the postpartum period. In addition, there appears to be a dose-response relationship between IPV and non-adherence: Women who experienced more violent events, had reduced odds of PMTCT adherence. This study provides compelling evidence that IPV against women deserves increased attention within the global PMTCT effort.
Research Highlights.
Spousal violence decreases the odds of PMTCT adherence during and after pregnancy.
Sexual and emotional violence have the strongest association with non-adherence.
There is a dose-response relationship between violence and non-adherence to PMTCT.
Acknowledgments
Research reported in this publication was funded by the National Institute of Mental Health of the National Institutes of Health (Award Number F31MH107348) and the Center for Global Health at the University of Colorado Denver. A tremendous thank you goes to Dr. Sara Yeatman for her guidance and support, as well as to Dr. Monica Onyango and Dr. Lisa Messersmith for their help preparing this manuscript. Additionally, I would like to thank Dr. Yusuf Ahmed for his mentorship and the Zambian District Health Office for their support. Lastly, this research would not have been possible without the help and commitment of my wonderful Zambian research team, Christine Chewe Sakala, Grace Lungeani Phiri, Franklin Munsanje, and Bibi Lambert Manda.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abramsky T, Watts CH, Garcia-Moreno C, Devries K, Kiss L, Ellsberg M, … Heise L. What factors are associated with recent intimate partner violence? findings from the WHO multi-country study on women’s health and domestic violence. BMC Public Health. 2011;11:109. doi: 10.1186/1471-2458-11-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alio AP, Nana PN, Salihu HM. Spousal violence and potentially preventable single and recurrent spontaneous fetal loss in an African setting: cross-sectional study. Lancet. 2009;373(9660):318–324. doi: 10.1016/S0140-6736(09)60096-9. [DOI] [PubMed] [Google Scholar]
- Auvinen J, Suominen T, Valimaki M. Male participation and prevention of human immunodeficiency virus (HIV) mother-to-child transmission in Africa. Psychology, Health & Medicine. 2010;15(3):288–313. doi: 10.1080/13548501003615290. [DOI] [PubMed] [Google Scholar]
- Awiti Ujiji O, Ekstrom AM, Ilako F, Indalo D, Wamalwa D, Rubenson B. Reasoning and deciding PMTCT-adherence during pregnancy among women living with HIV in Kenya. Cult Health Sex. 2011;13(7):829–840. doi: 10.1080/13691058.2011.583682. [DOI] [PubMed] [Google Scholar]
- Besser M. Preventing mother-to-child HIV transmission in Africa using new paradigms in health care delivery. 2010 Retrieved from http://www.m2m.org/media/publications.html.
- Blanc AK. The effect of power in sexual relationships on sexual and reproductive health: An examination of the evidence. Stud Fam Plann. 2001;32(3):189–213. doi: 10.1111/j.1728-4465.2001.00189.x. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11677692. [DOI] [PubMed] [Google Scholar]
- Boy A, Salihu HM. Intimate partner violence and birth outcomes: A systematic review. Int J Fertil Womens Med. 2004;49(4):159–164. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15481481. [PubMed] [Google Scholar]
- Campbell J, Baty M, Ghandour R, Stockman J, Francisco L, Wagman J. The intersection of intimate partner violence against women and HIV/AIS: A review. Int J Inj Contr Saf Promot. 2008;15(4):221–231. doi: 10.1080/17457300802423224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J, Jones AS, Dienemann J, Kub J, Schollenberger J, O’Campo P, … Wynne C. Intimate partner violence and physical health consequences. Arch Intern Med. 2002;162(10):1157–1163. doi: 10.1001/archinte.162.10.1157. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12020187. [DOI] [PubMed] [Google Scholar]
- Campbell JC. Health consequences of intimate partner violence. Lancet. 2002;359(9314):1331–1336. doi: 10.1016/S0140-6736(02)08336-8. [DOI] [PubMed] [Google Scholar]
- Conkling M, Shutes EL, Karita E, Chomba E, Tichacek A, Sinkala M, … Allen SA. Couples’ voluntary counselling and testing and nevirapine use in antenatal clinics in two African capitals: A prospective cohort study. J Int AIDS Soc. 2010;13:10. doi: 10.1186/1758-2652-13-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CSO. Zambia demographic and health survey 2007. Central Statistics Office; Calverton, Maryland, USA: 2009. [Google Scholar]
- CSO. Zambia demographic and health survey 2013–14. Central Statistics Office; Rockville, Maryland, USA: 2014. [Google Scholar]
- Ellsberg M, Jansen HA, Heise L, Watts CH, Garcia-Moreno C. Intimate partner violence and women’s physical and mental health in the WHO multi-country study on women’s health and domestic violence: an observational study. Lancet. 2008;371(9619):1165–1172. doi: 10.1016/S0140-6736(08)60522-X. [DOI] [PubMed] [Google Scholar]
- Emenike E, Lawoko S, Dalal K. Intimate partner violence and reproductive health of women in Kenya. Int Nurs Rev. 2008;55(1):97–102. doi: 10.1111/j.1466-7657.2007.00580.x. [DOI] [PubMed] [Google Scholar]
- Fabbiani M, Di Giambenedetto S, Cingolani A, Fanti I, Colafigli M, Tamburrini E, … Murri R. Relationship between self-reported adherence, antiretroviral drug concentration measurement and self-reported symptoms in patients treated for HIV-1 infection. Infect Dis (Lond) 2015:1–8. doi: 10.3109/23744235.2015.1082034. [DOI] [PubMed] [Google Scholar]
- Fletcher CV, Testa MA, Brundage RC, Chesney MA, Haubrich R, Acosta EP, … Gulick RM. Four measures of antiretroviral medication adherence and virologic response in AIDS clinical trials group study 359. J Acquir Immune Defic Syndr. 2005;40(3):301–306. doi: 10.1097/01.qai.0000180078.53321.6a. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/16249704. [DOI] [PubMed] [Google Scholar]
- Futterman D, Shea J, Besser M, Stafford S, Desmond K, Comulada WS, Greco E. Mamekhaya: a pilot study combining a cognitive-behavioral intervention and mentor mothers with PMTCT services in South Africa. AIDS Care. 2010;22(9):1093–1100. doi: 10.1080/09540121003600352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon LL, Gharibian D, Chong K, Chun H. Comparison of HIV virologic failure rates between patients with variable adherence to three antiretroviral regimen types. AIDS Patient Care STDS. 2015;29(7):384–388. doi: 10.1089/apc.2014.0165. [DOI] [PubMed] [Google Scholar]
- Hampanda K. A Comparison of Antiretroviral Therapy and Prophylaxis Adherence for the Prevention of Mother-to-Child Transmission in an Urban Zambian Health Center. Paper presented at the International Conference on HIV Treatment & Prevention Adherence; Miami, FL. 2015. [Google Scholar]
- Harling G, Msisha W, Subramanian SV. No association between HIV and intimate partner violence in 10 developing countries. PLoS One. 2010;5(12):1–8. doi: 10.1371/journal.pone.0014257. doi:10.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey A, Garcia-Moreno C, Butchart A. Primary prevention of intimate-partner violence and sexual violence: Background paper for WHO expert meeting; May 2–3, 2007.2007. [Google Scholar]
- Hatcher AM, Smout EM, Turan JM, Christofides N, Stockl H. Intimate partner violence and engagement in HIV care and treatment among women: a systematic review and meta-analysis. AIDS. 2015 doi: 10.1097/qad.0000000000000842. [DOI] [PubMed] [Google Scholar]
- Hatcher AM, Woollett N, Pallitto CC, Mokoatle K, Stockl H, MacPhail C, … Garcia-Moreno C. Bidirectional links between HIV and intimate partner violence in pregnancy: implications for prevention of mother-to-child transmission. J Int AIDS Soc. 2014;17:19233. doi: 10.7448/ias.17.1.19233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Human Rights Watch. Hidden in the mealie meal: Gender-based abuses and women’s HIV treatment in Zambia. 2007 Retrieved from Retrieved from http://www.unhcr.org/refworld/docid/4768e6292.html.
- Jasseron C, Mandelbrot L, Dollfus C, Trocme N, Tubiana R, Teglas JP, Warszawski J. Non-disclosure of a pregnant woman’s HIV status to her partner is associated with non-optimal prevention of mother-to-child transmission. AIDS Behav. 2011 doi: 10.1007/s10461-011-0084-y. [DOI] [PubMed] [Google Scholar]
- Johnson MP. A typology of domestic violence : Intimate terrorism, violent resistance, and situational couple violence. Boston, MA; Hanover, NH: Northeastern University Press; Published by University Press of New England; 2008. [Google Scholar]
- Kiarie JN, Farquhar C, Richardson BA, Kabura MN, John FN, Nduati RW, John-Stewart GC. Domestic violence and prevention of mother-to-child transmission of HIV-1. AIDS. 2006;20(13):1763–1769. doi: 10.1097/01.aids.0000242823.51754.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishor S, Johnson K. Profiling Domestic Violence: A Multi-Coutry Study. Calverton, Maryland: 2004. [Google Scholar]
- Kobin AB, Sheth NU. Levels of adherence required for virologic suppression among newer antiretroviral medications. Ann Pharmacother. 2011;45(3):372–379. doi: 10.1345/aph.1P587. [DOI] [PubMed] [Google Scholar]
- Lawoko S, Dalal K, Jiayou L, Jansson B. Social inequalities in intimate partner violence: A study of women in Kenya. Violence Vict. 2007;22(6):773–784. doi: 10.1891/088667007782793101. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/18225388. [DOI] [PubMed] [Google Scholar]
- Li Y, Marshall CM, Rees HC, Nunez A, Ezeanolue EE, Ehiri JE. Intimate partner violence and HIV infection among women: A systematic review and meta-analysis. J Int AIDS Soc. 2014;17:18845. doi: 10.7448/IAS.17.1.18845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQuarrie KL, Winter R, Kishor S. Spousal violence in Sub-Saharan Africa: Structure, forms, and levels. Paper presented at the Population Association of America Annual Meeting; Boston, MA. 2014. [Google Scholar]
- Medley A, Garcia-Moreno C, McGill S, Maman S. Rates, barriers and outcomes of HIV serostatus disclosure among women in developing countries: Implications for prevention of mother-to-child transmission programmes. Bull World Health Organ. 2004;82(4):299–307. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15259260. [PMC free article] [PubMed] [Google Scholar]
- Megazzini KM, Sinkala M, Vermund SH, Redden DT, Krebs DW, Acosta EP, … Stringer JS. A cluster-randomized trial of enhanced labor ward-based PMTCT services to increase nevirapine coverage in Lusaka, Zambia. AIDS. 2010;24(3):447–455. doi: 10.1097/QAD.0b013e328334b285. [DOI] [PubMed] [Google Scholar]
- Mepham S, Zondi Z, Mbuyazi A, Mkhwanazi N, Newell ML. Challenges in PMTCT antiretroviral adherence in northern KwaZulu-Natal, South Africa. AIDS Care. 2011;23(6):741–747. doi: 10.1080/09540121.2010.516341. [DOI] [PubMed] [Google Scholar]
- Mulrenan C, Colombini M, Howard N, Kikuvi J, Mayhew SH, Integra I. Exploring risk of experiencing intimate partner violence after HIV infection: A qualitative study among women with HIV attending postnatal services in Swaziland. BMJ Open. 2015;5(5):e006907. doi: 10.1136/bmjopen-2014-006907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray LK, Haworth A, Semrau K, Singh M, Aldrovandi GM, Sinkala M, … Bolton PA. Violence and abuse among HIV-infected women and their children in Zambia: A qualitative study. J Nerv Ment Dis. 2006;194(8):610–615. doi: 10.1097/01.nmd.0000230662.01953.bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murri R, Ammassari A, Gallicano K, De Luca A, Cingolani A, Jacobson D, … Antinori A. Patient-reported nonadherence to HAART is related to protease inhibitor levels. J Acquir Immune Defic Syndr. 2000;24(2):123–128. doi: 10.1097/00126334-200006010-00006. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10935687. [DOI] [PubMed] [Google Scholar]
- Nachega JB, Hislop M, Dowdy DW, Chaisson RE, Regensberg L, Maartens G. Adherence to nonnucleoside reverse transcriptase inhibitor-based HIV therapy and virologic outcomes. Ann Intern Med. 2007;146(8):564–573. doi: 10.7326/0003-4819-146-8-200704170-00007. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/17438315. [DOI] [PubMed] [Google Scholar]
- Nachega JB, Uthman OA, Anderson J, Peltzer K, Wampold S, Cotton MF, … Mofenson LM. Adherence to antiretroviral therapy during and after pregnancy in low-income, middle-income, and high-income countries: A systematic review and meta-analysis. AIDS. 2012;26(16):2039–2052. doi: 10.1097/QAD.0b013e328359590f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassali M, Nakanjako D, Kyabayinze D, Beyeza J, Okoth A, Mutyaba T. Access to HIV/AIDS care for mothers and children in sub-Saharan Africa: Adherence to the postnatal PMTCT program. AIDS Care. 2009;21(9):1124–1131. doi: 10.1080/09540120802707467. [DOI] [PubMed] [Google Scholar]
- National Statistical Office, & ICF Macro. Malawi Demographic and Health Survey 2010. Zomba, Malawi, and Calverton, Maryland, USA: 2011. [Google Scholar]
- Ngoma MS, Misir A, Mutale W, Rampakakis E, Sampalis JS, Elong A, … Silverman MS. Efficacy of WHO recommendation for continued breastfeeding and maternal cART for prevention of perinatal and postnatal HIV transmission in Zambia. J Int AIDS Soc. 2015;18:19352. doi: 10.7448/IAS.18.1.19352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyasulu JY, Naysulu P. Barrier to the Uptake of Prevention of Mother to Child Transmission (PMTCT) Service in Rural Blantyre and Balaka Districts, Malawi. Journal of Rural and Tropical Public Health. 2011;10:48–52. [Google Scholar]
- Onono M, Owuor K, Turan J, Bukusi EA, Gray GE, Cohen CR. The role of maternal, health system, and psychosocial factors in prevention of mother-to-child transmission failure in the era of programmatic scale up in western Kenya: A case control study. AIDS Patient Care STDS. 2015;29(4):204–211. doi: 10.1089/apc.2014.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallitto CC, Garcia-Moreno C, Jansen HA, Heise L, Ellsberg M, Watts C, … Domestic V. Intimate partner violence, abortion, and unintended pregnancy: Results from the WHO Multi-country Study on Women’s Health and Domestic Violence. Int J Gynaecol Obstet. 2013;120(1):3–9. doi: 10.1016/j.ijgo.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Raghunathan TE, Lepkowski JM, Hoewyk JV, Solenberger P. A multivariate technique for multiply imputing missing values using a sequence of regression models. Survey Methodology. 2001;27:85–95. [Google Scholar]
- Sarker M, Sanou A, Snow R, Ganame J, Gondos A. Determinants of HIV counselling and testing participation in a prevention of mother-to-child transmission programme in rural Burkina Faso. Trop Med Int Health. 2007;12(12):1475–1483. doi: 10.1111/j.1365-3156.2007.01956.x. [DOI] [PubMed] [Google Scholar]
- Siemieniuk RA, Krentz HB, Miller P, Woodman K, Ko K, Gill MJ. The clinical implications of high rates of intimate partner violence against HIV-positive women. J Acquir Immune Defic Syndr. 2013;64(1):32–38. doi: 10.1097/QAI.0b013e31829bb007. [DOI] [PubMed] [Google Scholar]
- Simoni JM, Kurth AE, Pearson CR, Pantalone DW, Merrill JO, Frick PA. Self-report measures of antiretroviral therapy adherence: A review with recommendations for HIV research and clinical management. AIDS Behav. 2006;10(3):227–245. doi: 10.1007/s10461-006-9078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp. Stata 11 Multiple-Imputation Reference Manual. College Station, TX: Stata Press; 2009. [Google Scholar]
- Straus MA, Hamby SL, BoneyMcCoy S, Sugarman DB. The revised Conflict Tactics Scales (CTS2) - Development and preliminary psychometric data. Journal of Family Issues. 1996;17(3):283–316. doi: 10.1177/019251396017003001. [DOI] [Google Scholar]
- Stringer EM, Ekouevi DK, Coetzee D, Tih PM, Creek TL, Stinson K, … Team PS. Coverage of nevirapine-based services to prevent mother-to-child HIV transmission in 4 African countries. JAMA. 2010;304(3):293–302. doi: 10.1001/jama.2010.990. [DOI] [PubMed] [Google Scholar]
- Theuring S, Mbezi P, Luvanda H, Jordan-Harder B, Kunz A, Harms G. Male involvement in PMTCT services in Mbeya Region, Tanzania. AIDS Behav. 2009;13(Suppl 1):92–102. doi: 10.1007/s10461-009-9543-0. [DOI] [PubMed] [Google Scholar]
- UNAIDS. Regional Fact Sheet 2012: Sub-Saharan Africa. 2012 Retrieved from http://www.unaids.org/sites/default/files/en/media/unaids/contentassets/documents/epidemiology/2012/gr2012/2012_FS_regional_ssa_en.pdf.
- UNICEF. Program Status According to PMTCT Prongs: Zambia. Preventing Mother-to-Child Transmission (PMTCT) of HIV. 2012 Retrieved from http://www.unicef.org/aids/index_preventionyoung.html.
- Varga C, Brookes H. Factors influencing teen mothers’ enrollment and participation in prevention of mother-to-child HIV transmission services in Limpopo Province, South Africa. Qual Health Res. 2008;18(6):786–802. doi: 10.1177/1049732308318449. [DOI] [PubMed] [Google Scholar]
- White JW, Smith PH, Koss MP, Figueredo AJ. Intimate Partner Aggression - What Have We Learned? Comment on Archer (2000) Pychological Bulletin. 2000;126(5):690–696. doi: 10.1037/0033-2909.126.5.690. [DOI] [PubMed] [Google Scholar]
- WHO. Putting Women First: Ethical and Safety Recommendations for Research on Domestic Violence Against Women. 2001 Retrieved from Geneva, Switerland: http://www.who.int/gender/documents/violence/who_fch_gwh_01.1/en/index.html.
- WHO. Antiretroviral Drugs for Treating Pregnant Women and Preventing HIV Infection in Infants: Recommendations for a Public Health Approach 2010 Version. Geneva, Switzerland: 2010a. Retrieved from http://apps.who.int/iris/bitstream/10665/75236/1/9789241599818_eng.pdf. [PubMed] [Google Scholar]
- WHO. Towards universal access: Scaling up priority HIV/AIDS interventions in the health sector. Geneva, Switzerland: 2010b. Retrieved from http://www.who.int/hiv/pub/2010progressreport/report/en/index.html. [Google Scholar]
- WHO. Understanding and addressing violence against women. Geneva, Switzerland: 2012. Retrieved from http://apps.who.int/iris/bitstream/10665/77432/1/WHO_RHR_12.36_eng.pdf. [Google Scholar]
- WHO. Number of women and children living with HIV. Global Health Observatory (GHO); Geneva, Switzerland: 2013. Retrieved from http://www.who.int/gho/hiv/epidemic_status/cases_adults_women_children/en/index.html. [Google Scholar]
- WHO/LSHTM. Preventing intimate partner and sexual violence against women: taking action and generating evidence. Geneva/Londons: 2010. Retrieved from http://www.who.int/violence_injury_prevention/publications/violence/9789241564007_eng.pdf. [DOI] [PubMed] [Google Scholar]