Abstract
Hormones present in hair provide summative information about endocrine activity while the hair was growing. Therefore, it can be collected from an infant after birth and still provide retrospective information about hormone exposure during prenatal development. We employed this approach to determine whether a delimited period of maternal stress during pregnancy affected the concentrations of glucocorticoids and gonadal hormones in the hair of neonatal rhesus monkeys. Hair from 22 infant monkeys exposed to 5 weeks of gestational disturbance was compared to specimens from 13 infants from undisturbed control pregnancies. Using an LC/MS/MS based technique, which permitted seven steroid hormones to be quantified simultaneously, we found 2 hormones were significantly different in infants from disturbed pregnancies. Cortisol and testosterone levels were lower in the hair of both male and female neonates. Maternal hair hormone levels collected on the same day after delivery no longer showed effects of the disturbance earlier during pregnancy. This study documents that a period of acute stress, lasting for 20% of gestation, has sustained effects on the hormones to which a developing fetus is exposed.
Keywords: hair, hormones, stress, prenatal, cortisol, programming, monkey
1. Introduction
It is well established that stress during pregnancy can impact the developing fetus. Both physical and psychological insults have been associated with lasting effects, including altered neuroendocrine and immune function, emotional and attentional problems, and a greater risk for obesity in adulthood (Davis et al., 2011; Entringer, 2013; Laplante et al., 2004; O’Connor et al., 2002; O’Connor et al., 2013; Pluess et al., 2010). One of the primary processes underlying these relationships is a re-programming of the fetal hypothalamic-pituitary-adrenal (HPA) axis by the placental transfer of maternal glucocorticoids (Coe and Lubach, 2005; Glover et al., 2009; Jensen Pena et al., 2012; Sarkar et al., 2008; Walsh et al., 1979). A plethora of studies in humans and animal models have provided support for this view that maternal hormone responses can act on the regulatory set points of the fetal endocrine system (Baibazarova et al., 2013; Buss et al., 2012; Coe et al., 2002; Kapoor and Matthews, 2005; La Marca-Ghaemmaghami et al., 2015), but it has been difficult to directly assess the extent of change in the fetal compartment. The capacity to measure the hormone content of infant hair, which had grown during fetal period, now provides that opportunity.
The use of hair to analyze hormones is a novel, non-invasive method for obtaining information about the endocrine system over an extended period of time while the hair had been growing. The hypothesized way that hormone is incorporated into hair is largely through passive diffusion from systemic circulation during the formation of the hair shaft, although some have argued that the follicle itself can synthesize and regulate hormones, and secretions from sweat and sebum may play a role (Davenport et al., 2006; Grass et al., 2015; Sauve et al., 2007; Stalder and Kirschbaum, 2012). Therefore, in contrast to point measurements, such as from serum, saliva and urine, hair provides information about the cumulative exposure to hormones representative of weeks to months, depending on the length of the hair.
We have previously shown that hair from newborn rhesus monkeys can be used to measure steroid hormones, which reflects their prior fetal experiences since the hair started growing approximately two months before term (Kapoor et al., 2014; Schultz, 1937). Using this technique, we found that parity affected hormone levels, with higher glucocorticoids, testosterone, and estrogen in the hair obtained from infants of primiparous mothers. It seemed reasonable, therefore, to investigate whether a similar analysis of neonatal hair would permit one to detect the influence of maternal stress during pregnancy.
The majority of studies that have analyzed hair hormone levels, both in human and animal research, have measured only cortisol. We developed innovative mass spectrometry methods permitting the simultaneous determination of seven hormones (Kapoor et al., 2014). This refinement is significant for two reasons. First, it is often informative to assess cortisone in addition to cortisol when assessing hair (Ito et al., 2005; Keckeis et al., 2012). Second, a comprehensive panel is especially important when investigating prenatal stress, because it is known that other hormones synthesized by the adrenal and gonads can be affected (DiPietro et al., 2011; Kapoor and Matthews, 2008, 2011; Kim et al., 2015; Pepe and Albrecht, 1995; Sharp et al., 2014). For example, prenatal stress in some animal species has been shown to affect sexual differentiation by influencing the organizational actions of androgens and estrogens.
The stress condition in the present study involved acute manipulations of the gravid female on a daily basis for 20% of pregnancy. The disturbance was then stopped before the final weeks of pregnancy to determine if there were persistent effects on fetal endocrine activity. We employed a brief acoustical startle paradigm known to elevate maternal cortisol levels for several hours. Previous studies showed that prenatal manipulations had a number of persistent effects on infant physiology and brain development after birth (Coe et al., 2003). Its advantages are that: 1) it is reliable, eliciting a cortisol response in all pregnant females, 2) both the stressor and reaction are delimited; a moderate elevation in cortisol is induced by a brief 10-minute event, and 3) it is temporally defined and finite, with the stressful period resolving immediately at the end of 5 weeks of daily manipulation. During the first week after delivery, hair samples were collected from the mother and the newborn infant to assay the hormone panel, which included glucocorticoids, androgens, estrogens, and progesterone.
2. Methods
2.1. Animals
Mother and infant rhesus monkeys (Macaca mulatta) were evaluated from a large, long-established breeding colony at the Harlow Primate Laboratory. All were descendants of monkeys originally imported from India, >14 generations earlier in the 1950s and 1960s (Price and Coe, 2000). The adult females were laboratory-reared, multiparous adults, 5.8 to 15.4 years of age. Each was bred with one male for 4–7 days to verify paternity and date of conception. Pregnancy was confirmed by implantation bleeding and the cessation of menstruation. Females were housed under standardized conditions in similar size, stainless steel cages until the birth of their infants (Coe and Shirtcliff, 2004). The monkeys were continuously in visual and auditory communication with other animals, either in pairs or partitioned with an open mesh panel from one other female. The diet is based on a commercial biscuit with known nutritional constituents (PMI International, 5LFD) plus fruits and vegetables provided as part of an enrichment husbandry program to give additional stimulation, along with foraging devices and other manipulanda. All 35 infants, 13 controls (4 female and 9 male) and 22 prenatally stressed (12 female, 10 male), were born naturally at term. To determine typical level of glucocorticoids in the hair of a non-pregnant female for comparison, hair specimens were obtained from 7 females and analyzed with the same assay methods. All experimental and husbandry procedures were approved by the Institutional Care and Animal Use Committee at UW-Madison.
2.2. Prenatal Stress
The prenatal stress condition involved a controlled disturbance induced with an acoustical startle protocol (Coe et al., 2003; Shirtcliff et al., 2013). Briefly, 5 days a week for 5 weeks, or 20% of the 24-week pregnancy, the gravid female was relocated to a darkened room where a computer program randomly broadcast 3 loud noises (1 sec, 110 dB) during a 10-min period. This protocol induces a transient activation of the maternal HPA axis (Clarke and Schneider, 1993; Rendina et al., in press). The stress induction occurred during one of three 5-week periods during pregnancy, either early (Weeks 7–11, n=6), mid (Weeks 12–16, n=9) or late in pregnancy (Weeks 17–21, n=7). For the current analyses, hair hormone data from the 3 periods are considered together because there was not a significant effect of gestational stage on either the dam or infant. The stress manipulations were stopped 3–13 weeks before the end of gestation, ensuring that hormone differences in infant hair reflected hormonal changes that continued after the gestational disturbance. Maternal age was similar between the females assigned to the Control and Stress conditions (11.38 ± 4.7 years and 12.95 ± 3.5 years, respectively). To verify that the disturbance protocol elicited HPA activation, small blood samples (1 mL) were obtained at 1, 3 and 5 weeks after the startle protocol. Blood samples were obtained from the Control females at the same time of day and same 2-week intervals in order to determine basal levels of cortisol and to control for the handling procedures required for the blood sampling. No sedation was employed, and samples were collected via saphenous venipuncture in a specially designed apparatus for blood collection. Cortisol levels were determined for 28 of the females from both Control and Stress conditions via radioimmunoassay (MP Biomedicals).
2.3. Hair Collection
Hair specimens were collected 2–4 days after delivery. Adult female monkeys at the HPL are trained to leave their cages and transfer into a smaller apparatus for brief immobilization, where the infants could be manually removed for approximately 15 min between 0900h–1100h. The mothers were not sedated for this procedure, nor were they sedated during pregnancy for the blood collection. Hair from the mother was shaved with commercial grooming clippers from the upper back region; infant hair was obtained from the posterior region of the neck, with some from the back of the head and the upper shoulders. The mother and infant were then reunited, and returned to their cage. Hair samples were placed into small aluminum foil pouches and stored at room temperature until ground and assayed.
2.4. Analytical Method
Hair washing, steroid extraction and LC/MS/MS method were conducted as previously described (Kapoor et al., 2014). Briefly, hair was washed twice with 2-propanol and dried. Hair was ground to a fine powder using a Retsch ball mill (Verder Scientific, Newtown, PA) and carefully weighed into 50 mg aliquots in culture tubes. For steroid extraction, methanol, Sorenson buffer and internal standard mixture were added to the ground hair and the tubes were incubated at 30 °C for 16 h. After incubation, tubes were centrifuged and the supernatant was run through solid-phase extraction followed by liquid-liquid extraction with ethyl acetate. Samples were re-suspended in 40 μL of 20:80 methanol/water for liquid-chromatography/tandem mass spectrometry.
All samples were analyzed on a QTRAP 5500 quadrupole linear ion trap mass spectrometer (AB Sciex, Concord, ON, Canada) equipped with an atmospheric pressure chemical ionization source. The system included two Shimadzu LC20ADXR pumps and a Shimadzu SIL20ACXR autosampler. The chromatographic separation was performed on Phenomenex Kinetex 2.6u C18 100A, 150 × 4.6 mm column (Phenomenex, Torrance, CA).
Quantitative results were recorded as multiple reaction monitoring (MRM) area counts after determination for the response factor for each compound and internal standard. Internal standard pairs were as follows: d5-estradiol with estradiol and estrone, d5-testosterone with testosterone and DHEA, d9-progesterone with progesterone, d4-cortisol with cortisol and cortisone. All data were acquired and processed with Analyst software, Ver 1.5.1 (AB-Sciex). Eight-point standard calibration curves were obtained by using 1 mg of melanin. The final concentrations of the calibration curves were: 0.0016, 0.0031, 0.0063, 0.013, 0.025, 0.05, 0.1 and 0.2 ng/tube. The linearity for all was r>0.9990 and the curve fit was linear through zero with 1/x weighting. None of the compounds of interest were detected in zero or blank samples. The recovery ranged from 77–103%, with the glucocorticoids displaying the highest recovery and progesterone the lowest. The intra-assay coefficient of variance (CV) ranged from 2–9%. The inter-assay CV ranged from 8–17%.
2.5. Statistics
Independent sample t-tests were used to test the significance of differences in hair hormone levels between infants from Undisturbed Control mothers and those exposed to the pregnancy stress manipulation. Two-way ANOVAs (Pregnancy Condition x Infant Sex) were used to test for a differential influence of prenatal stress on male and female infants. We also conducted two-way ANOVAs (Pregnancy Condition x Gestation Age), but found no effect of gestation age at the time of the manipulation on any hair analytes. Therefore this variable was not considered in subsequent analyses. The Spearman test was employed to examine the association between hormone concentrations in maternal and infant hair. Normality of distribution was assessed for all hair analytes before statistical analysis and if needed, data were log-transformed. Outliers were detected using the extreme studentized deviate. All statistical analyses were completed using SPSS (IBM, Armonk, NY). Statistical significance was defined as p<.05 for all tests.
3. Results
All infants were born after normal term pregnancies and unassisted vaginal deliveries. Gestation length was typical for the species and was not significantly affected by prenatal condition (Undisturbed Control: 167.5 ± 3, Stress: 167.2 ± 5 days, (F1,33=0.059, p>.05)). The prenatal stress manipulation did not affect maternal weight gain from pre-pregnancy to delivery (Undisturbed Control: 1.68 ± 0.18 kg, Stress: 1.21 ± 0.16 kg; (F1,31=4.08, p=0.052), nor was there an effect of maternal stress on infant birth weights (Undisturbed Control: 461.4 ± 80.8 g, Stress: 497.6 ± 57.5 g (F1,33=0.840, p>.05)).
Assessment of cortisol levels in the pregnant females via blood samples at the end of 1, 3 and 5 weeks of the disturbance protocol verified that it effectively activated the HPA axis. As compared to a baseline blood obtained prior to the disturbance period, there was a significant increase from 28.3±0.95 μg/dL to 41.87±1.46 μg/dL, and these higher disturbance-induced levels were also significantly above cortisol values in the blood of Control females sampled at the same time of day (28.78±1.13) (F1,26=22.23, p<.01).
Two-way ANOVA also revealed a significant effect of prenatal stress on cortisol levels in infant hair (Figure 1A). Hair cortisol was lower in infant monkeys gestated by stressed mothers (F1,31=9.24, p<.05). However, there was not an effect of prenatal stress on cortisone levels in the infants’ hair (Table 1). Neither cortisol nor cortisone levels were differentially affected by the sex of the infant.
Figure 1.
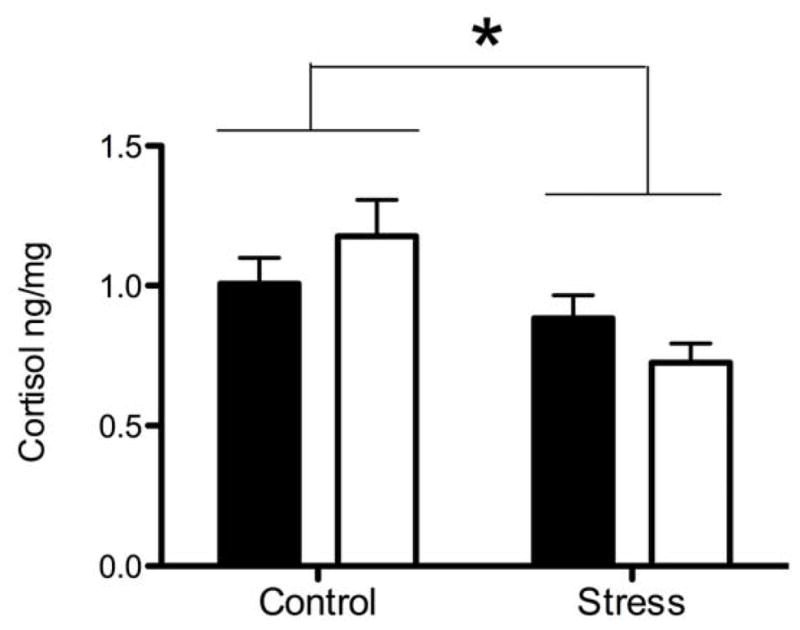
Mean (± SEM) hair cortisol in male (black bar) and female (white bar) infant monkeys born to mothers from Undisturbed Control or Stressed pregnancies. *indicates p<.05 between prenatal condition.
Table 1.
Mean (± S.D) concentrations of steroid hormones in the hair of monkey neonates from Undisturbed Control or Prenatally Stressed pregnancies. Bolded values are statistically significant at p<0.05.
| Analyte (ng/mg) | Control | Stress | P |
|---|---|---|---|
| Cortisol | 1.04 ± 0.3 | 0.82 ± 0.3 | .02 |
| Cortisone | 1.25 ± 1.2 | 1.09 ± 1.1 | >.05 |
| DHEA | 1.06 ± 0.7 | 0.87 ± 0.7 | >.05 |
| Testosterone | 0.0031 ± 0.002 | 0.0029 ± 0.002 | .01 |
| Estrone | 0.55 ± 0.5 | 0.71 ± 0.5 | >.05 |
| Estradiol | 0.002 ± 0.002 | 0.004 ± 0.006 | <.06 |
| Progesterone | 0.048 ± 0.01 | 0.052 ± 0.02 | >.05 |
There was a significant effect of prenatal stress on gonadal hormones in the infants’ hair. Infants from the gestational stress condition had significantly lower hair testosterone levels than did the Undisturbed Control infants (F1,31=7.32, p<.02; Figure 2A) and the higher estradiol levels approached statistical significance (F1,31=4.03, p=.051; Figure 2B). This bidirectional shift was also reflected in the testosterone-to-estradiol ratio, with infant monkeys from the stressed pregnancies having a significantly lower T/E2 ratio (F1,31=8.68, p<.01; Figure 2C). There was no effect of the prenatal stress condition on DHEA, progesterone, or estrone levels in the infants’ hair (Table 1). Gonadal hormone concentrations in the hair did not differ significantly between male and female infants.
Figure 2.
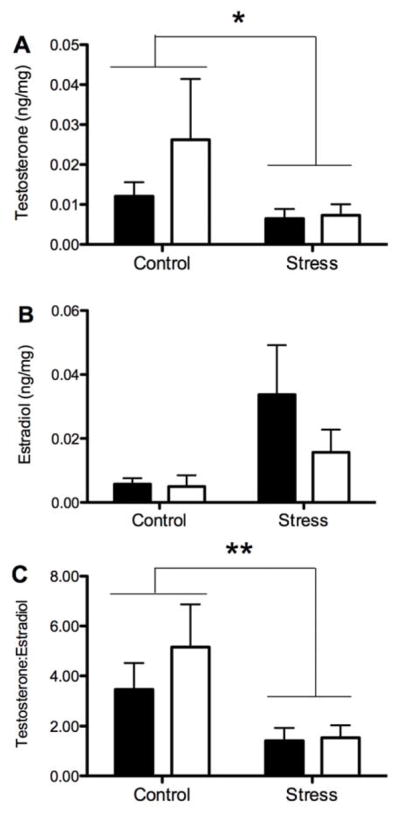
Mean (± SEM) hair testosterone (A) estradiol, (B), and testosterone:estradiol ratio (C) in male (black bar) and female (white bar) infant monkeys from Undisturbed Control or Stressed pregnancies. *indicates p<.05 and ** indicates p<.01 between prenatal condition.
The gestational disturbance manipulation did not affect hormone levels in maternal hair obtained after delivery (Table 2). However, cortisol levels in the hair obtained postpartum was elevated as compared to comparison hair collected from 7 non-pregnant females (mean =102.79±5.58 as compared to 73.53±6.01 pg/mg, respectively). Similarly, the hair cortisone concentrations in the hair of these new mothers were also high as compared to a non-pregnant female (336.52±52.5 versus 140.0±1.30 pg/mg, respectively). For two of the measured hormones, the levels in maternal hair did appear to contribute to the concentrations in infant hair. There was a significant correlation between hormone levels in maternal and infant hair for cortisone (r=0.55, p<.001) and estradiol (r=0.43, p<.01). One other notable finding was that hormone levels in infant hair were nearly 10-fold higher than the levels in maternal hair.
Table 2.
Mean (±SD) steroid hormone concentrations in the hair of rhesus monkey mothers after delivery following Undisturbed Control or Stressed pregnancies. There was no effect of prenatal condition on any hair analytes in the parturient females.
| Analyte (ng/mg) | Control | Stress |
|---|---|---|
| Glucocorticoids | ||
| Cortisol | 0.11 ± 0.04 | 0.097 ± 0.03 |
| Cortisone | 0.31 ± 0.3 | 0.35 ± 0.3 |
| Androgens | ||
| Testosterone | 0.0031 ± 0.002 | 0.0029 ± 0.002 |
| DHEA | 0.085 ± 0.06 | 0.085 ± 0.09 |
| Progestins | ||
| Progesterone | 0.013 ± 0.01 | 0.009 ± 0.005 |
| Estrogens | ||
| Estrone | 0.046 ± 0.03 | 0.047 ± 0.03 |
| Estradiol | 0.0019 ± 0.002 | 0.0040 ± 0.006 |
4. Discussion
The current study demonstrates significant and persistent effects of prenatal stress on infant hair hormone levels, which were indicative of their prenatal conditions. Specifically, we found that hair cortisol levels were lower in infant monkeys born to mothers exposed to a delimited period of stress during pregnancy. This finding differs from many published papers, which have generally reported increased glucocorticoid levels after stress, both with amniotic fluid sampling in humans (Baibazarova et al., 2013; O’Donnell et al., 2012; Sarkar et al., 2008) and fetal blood sampling in rodents (Takahashi et al., 1998). One explanation for the difference is our use of hair to measure cortisol release, which we can assume grew after the 5-week period of disturbance had ended and therefore represents a subsequent and lasting shift in the regulation of the HPA axis. Hair provides a summative view of endocrine changes, as opposed to the point measurement at a moment in time with other body fluids, including blood and saliva.
It is possible that there had an been an initial up-regulation in glucocorticoids in the fetal compartment during the period of maternal stress, which was then followed by a compensatory down-regulation of HPA axis activity. Using blood samples obtained right at the end of the disturbance manipulation, we documented that cortisol levels in the gravid female were elevated, both as compared to their prior basal levels and also with respect to levels in the Control females sampled at the same time of day. Thus, during the 5 weeks of the stress manipulations, elevated placental transfer of maternal cortisol may have exerted an inhibitory effect on fetal HPA activity. That change in the amount of cortisol reaching the fetal compartment may then have induced a compensatory and sustained reduction in fetal cortisol release, which was reflected in lower hair cortisol concentrations after delivery. When dexamethasone is administered to a pregnant monkey, it is known that this glucocorticoid drug will cross the placenta and suppresses fetal cortisol in a more prolonged manner than in the mother (Coe and Lubach, 2005). Our use of hair analyses, which provided a summative look at endocrine activity over an extended period of time, appears to have captured this type of longer-lasting change.
It is also important to mention that a previous study using the same gestational stress paradigm documented long-lasting postnatal changes in the regulatory set points for the HPA axis still evident in older, juvenile monkeys. An overnight Dexamethasone Suppression Test conducted when monkeys were 2 years of age indicated that the offspring from stress-exposed pregnancies had a greater propensity to break through the glucocorticoid negative feedback (Coe et al., 2003). This evidence of sustained maturational changes in endocrine activity after prenatal insults concurs with many studies in rodent models, including shifts that remain after puberty (Sominsky et al., 2012). Moreover, we know from analyses of other infant monkeys, that there can be effects of this prenatal manipulation on their behavioral and emotional reactivity, which can be sustained after birth (Rendina et al., in press).
It is noteworthy that the lingering hormonal effects were evident only in infant hair, and not found in the mothers’ hair at this time point right after delivery, even though we had documented with blood sampling that the disturbance had acutely increased cortisol levels during the 5-week stress period. Fetal hair, which begins to grow during the last 1–2 months of gestation, may thus provide a sensitive biomarker of endocrine changes occurring within the fetal compartment even after the precipitating events have ended. In addition, it was striking that the steroid hormone concentrations in the infants’ hair were tenfold higher than found in maternal hair. Newborn monkeys have distinctive, fine hair, commonly described as a natal coat, which may be particularly prone to absorbing hormones circulating in the amniotic fluid and blood stream. The results from this study concur with existing literature on hair hormone studies in monkeys which show that infant hair cortisol levels are high compared to juveniles and adults (Dettmer et al., 2014; Dettmer et al., 2012).
In contrast, the hair of the adult monkey is both denser and longer. Stressful events that had occurred during early and mid-gestation may be reflected only in a specific segment of the maternal hair further away at a point 2–4 cm away from the scalp. Analyses of the hair of pregnant women have indicated that the more distal hair reflects hormone activity from earlier in pregnancy, whereas hair near the scalp is more representative of the third trimester (Kirschbaum et al., 2009). Our hair grinding protocol included the entire shaft and thus may not have been sensitive enough to capture a prior, discrete history of elevated cortisol levels in the stressed female monkeys. However, our decision to use the whole shaft may not have been a factor given that others could not distinguish a difference in hair cortisol levels between the proximal and distal sections of monkey hair, which is typically shorter than in humans (Davenport et al., 2006).
Cortisol and cortisone levels were also much higher in the hair of these new mothers, as compared to non-pregnant females, and the elevated endocrine secretion during pregnancy may have obscured the saliency of an acute stress increase. Higher glucocorticoid hormone levels in the hair of pregnant monkeys replicates what has been found in women (D’Anna-Hernandez et al., 2011). It is also the case that not all studies have been able to document that maternal hair captures a history of gestational disturbance in women, at least in the case of previously anxious and depressed women at delivery (Braig et al., 2015).
An additional, and perhaps complementary, explanation for our finding of lower cortisol in the hair of infants from stressed pregnancies may be a change in local hormone synthesis within the hair follicle. A study using radiolabelled hormone in guinea pigs demonstrated that the majority of cortisol in hair may actually arise from in situ synthesis by the hair follicle (Ito et al., 2005; Keckeis et al., 2012). Moreover, analyses of cultured human skin cells support the idea of a functional and responsive endocrine axis in the hair follicle (Ito et al., 2005). If also true for primates, maternal stress may affected this intrinsic hormone production during the growth of the hair shaft, in addition to affecting cortisol circulating in the amniotic fluid and fetal blood stream.
We had demonstrated previously that hair hormones at birth can also reflect parity in the rhesus monkey (Kapoor et al., 2014) and this finding has now has also been reported in monkeys by other investigators (Dettmer et al., 2015). In our previous study, cortisone levels were found to be higher both in the hair of primiparous mothers and their infants as compared to multiparous dyads. For that reason, all of the dams used in the current project were multiparous. The range of cortisol and cortisone concentrations in the prior and current experiments was similar, indicating that any difference in hormone results was not due to shift in assay methods. A possible parsimonious explanation for a greater sensitivity of cortisone in the prior paper on parity is that hair cortisone may better reflect persistent changes across the full span of pregnancy, whereas the decreased cortisol found now in the hair of stressed infants was due more to a shift that occurred during the last month of gestation. We need to reiterate that the stress manipulation lasted for only 5 weeks, was moderate, and stopped during the final month of pregnancy. When thinking about stressful life events in humans, one might expect a different endocrine outcome following severe disturbance, such as interpersonal violence or environmental disasters, which might induce sustained stress throughout pregnancy. Even in the current project, we found a high correlation between the levels of cortisone in maternal and infant hair, and thus any life event that resulted in a more prolonged increase in glucocorticoid synthesis and metabolism might result in elevated hair cortisone levels at birth.
Considering the delimited period of disturbance, it is striking that we also found a significant effect on sex steroid hormones in the hair. Lower testosterone and lower testosterone:estradiol levels were present in the hair of both male and female infants from the prenatal stress condition. It is important to acknowledge that the actual difference in levels of hair testosterone was small, but the functional impact of this type of sustained change is not yet clear until we learn more about how androgens become incorporated into hair. Androgen in the fetus is derived from both the fetal gonads and adrenal glands (Resko and Roselli, 1997). If differences in infant hair testosterone levels were attributable primarily to gonadal hormone synthesis, we would have expected to see a greater or exclusive shift in male infants. Because sex differences were not observed, the prenatal stress effects may be due to a change in adrenal steroid hormone precursors or the adrenal enzymes involved in this steroidogenic pathway. One pathway that could result in decreased infant hair testosterone and increased hair estradiol levels would be differential activity of 17β-hydroxysteroid dehydrogenase, which converts androstenedione to testosterone, and/or altered aromatase activity, which converts testosterone to estradiol (Ellinwood et al., 1989). Previous studies have demonstrated that both hypoxia and expression of increased corticotropin releasing hormone levels can affect placental aromatase expression (Imperatore et al., 2009; Jiang et al., 2000). Further research to more specifically delineate how stress affects enzyme activity in these steroidal pathways will help to address this question.
Even though the prenatal stress exposure affected testosterone levels, we did not find a clear sex difference in overall hormone levels. Sexual differentiation in the fetal monkey begins during the first trimester, and sex differences in fetal hormone levels occur only at specific points during gestation (Barry et al., 2011; Resko et al., 1980). Thus, it may be more challenging to use hair hormone levels at birth to capture these sex-specific hormone differences, especially when fetal hair first starts to grow during the final 2 months of gestation. Further, it should be acknowledged that there was a relatively small number of male and female infants in each pregnancy condition, which may have limited our ability to discern sex differences. In other analyses, we were able to detect a differential effect of prenatal stress on the growth rates of male and female fetuses, but it required many more infants of each sex (Rendina et al., in press).
The hormone content in the infant’s hair also reflected some contribution of maternal endocrine activity. For both estradiol and cortisone, there was a significant correlation between maternal and infant hair levels. Given that the primary source of estradiol is the placenta, this common source may account for the similarity of estradiol in the maternal and fetal compartments and hair concentrations. A correlation between maternal-fetal glucocorticoid levels has also been found in other types of biological specimens. In humans it has been demonstrated that higher maternal salivary and plasma cortisol levels were positively associated with amniotic fluid levels of cortisol and cortisone (Baibazarova et al., 2013). In monkeys, it is known that the majority of glucocorticoid in the fetal compartment derives from the mother up through mid-gestation, while the fetal adrenal is still small, but that the fetal adrenal gradually increases its own contributions by the end of gestation (Kittinger, 1974; Mitchell et al., 1981; Seron-Ferre et al., 1978).
In conclusion, we have shown that a moderate prenatal stressor can induce persistent effects on hair cortisol and gonadal steroids in neonatal monkeys. Further studies to understand the mechanisms underlying how hormones are incorporated into hair are now in progress. We used a similar methodology to our previously published research on the effects of maternal parity (Kapoor et al., 2014), and now extend the conclusions about infant hair to being able to reflect prenatal conditions associated with maternal stress. This innovative technique provides a noninvasive approach to better understand hormone activity during this critical developmental phase, which is more difficult to investigate with other methods.
Highlights.
Hormones in hair reflect cumulative endocrine activity while the hair was growing.
Hair hormones measured in neonates can be employed to assess the prenatal hormone environment.
LC/MS/MS methods permitted seven hormones to be determined in hair.
Prenatal stress impacted the levels of cortisol and gonadal hormones in infant hair.
Acknowledgments
Role of the funding source
This research was supported by the NICHD, and enabled by the Assay Services at the WNPRC. Neither exerted a controlling influence on the study design, analysis and interpretation of data or the decision to submit a paper to this journal.
Funding was provided by NICHD (HD057064 and HD057064-A2S1) and the assays facilitated by resources from the Wisconsin National Primate Research Center (P51OD011106).
Footnotes
Contributors
Amita Kapoor contributed to acquisition of data, analysis and interpretation of data, drafting of manuscript and critical revision.
Gabriele Lubach contributed to study conception and design, acquisition of data and critical revision.
Toni Ziegler contributed to acquisition of data and critical revision.
Christopher Coe contributed to study conception and design, analysis and interpretation of data, drafting of manuscript and critical revision.
Conflict of interest
The authors do not have any conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Gabriele R. Lubach, Email: grlubach@wisc.edu.
Toni E. Ziegler, Email: ziegler@primate.wisc.edu.
Christopher L. Coe, Email: ccoe@wisc.edu.
References
- Baibazarova E, van de Beek C, Cohen-Kettenis PT, Buitelaar J, Shelton KH, van Goozen SH. Influence of prenatal maternal stress, maternal plasma cortisol and cortisol in the amniotic fluid on birth outcomes and child temperament at 3 months. Psychoneuroendocrinology. 2013;38:907–915. doi: 10.1016/j.psyneuen.2012.09.015. [DOI] [PubMed] [Google Scholar]
- Barry JA, Hardiman PJ, Siddiqui MR, Thomas M. Meta-analysis of sex difference in testosterone levels in umbilical cord blood. J Obstet Gynaecol. 2011;31:697–702. doi: 10.3109/01443615.2011.614971. [DOI] [PubMed] [Google Scholar]
- Buss C, Davis EP, Shahbaba B, Pruessner JC, Head K, Sandman CA. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proc Natl Acad Sci U S A. 2012;109:E1312–1319. doi: 10.1073/pnas.1201295109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke AS, Schneider ML. Prenatal stress has long-term effects on behavioral responses to stress in juvenile rhesus monkeys. Dev Psychobiol. 1993;26:293–304. doi: 10.1002/dev.420260506. [DOI] [PubMed] [Google Scholar]
- Coe CL, Kramer M, Czeh B, Gould E, Reeves AJ, Kirschbaum C, Fuchs E. Prenatal stress diminishes neurogenesis in the dentate gyrus of juvenile rhesus monkeys. Biol Psychiatry. 2003;54:1025–1034. doi: 10.1016/s0006-3223(03)00698-x. [DOI] [PubMed] [Google Scholar]
- Coe CL, Kramer M, Kirschbaum C, Netter P, Fuchs E. Prenatal stress diminishes the cytokine response of leukocytes to endotoxin stimulation in juvenile rhesus monkeys. J Clin Endocrinol Metab. 2002;87:675–681. doi: 10.1210/jcem.87.2.8233. [DOI] [PubMed] [Google Scholar]
- Coe CL, Lubach GR. Neurosci Biobehav Rev. United States: 2005. Developmental consequences of antenatal dexamethasone treatment in nonhuman primates; pp. 227–235. [DOI] [PubMed] [Google Scholar]
- Davenport MD, Tiefenbacher S, Lutz CK, Novak MA, Meyer JS. Analysis of endogenous cortisol concentrations in the hair of rhesus macaques. Gen Comp Endocrinol. 2006;147:255–261. doi: 10.1016/j.ygcen.2006.01.005. Epub 2006 Feb 2017. [DOI] [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Waffarn F, Sandman CA. Prenatal maternal stress programs infant stress regulation. J Child Psychol Psychiatry. 2011;52:119–129. doi: 10.1111/j.1469-7610.2010.02314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer A, Novak M, Meyer J, Suomi S. Population density-dependent hair cortisol concentrations in rhesus monkeys (Macaca mulatta) Psychoneuroendocrinology. 2014;42:59–67. doi: 10.1016/j.psyneuen.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer AM, Novak MA, Suomi SJ, Meyer JS. Physiological and behavioral adaptation to relocation stress in differentially reared rhesus monkeys: hair cortisol as a biomarker for anxiety-related responses. Psychoneuroendocrinology. 2012;37:191–199. doi: 10.1016/j.psyneuen.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer AM, Rosenberg KL, Suomi SJ, Meyer JS, Novak MA. Associations between parity, hair hormone profiles during pregnancy and lactation, and infant development in rhesus monkeys (Macaca mulatta) PLoS One. 2015;10:e0131692. doi: 10.1371/journal.pone.0131692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPietro JA, Costigan KA, Kivlighan KT, Chen P, Laudenslager ML. Maternal salivary cortisol differs by fetal sex during the second half of pregnancy. Psychoneuroendocrinology. 2011;36:588–591. doi: 10.1016/j.psyneuen.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellinwood WE, Stanczyk FZ, Lazur JJ, Novy MJ. Dynamics of steroid biosynthesis during the luteal-placental shift in rhesus monkeys. J Clin Endocrinol Metab. 1989;69:348–355. doi: 10.1210/jcem-69-2-348. [DOI] [PubMed] [Google Scholar]
- Entringer S. Impact of stress and stress physiology during pregnancy on child metabolic function and obesity risk. Curr Opin Clin Nutr Metab Care. 2013;16:320–327. doi: 10.1097/MCO.0b013e32835e8d80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover V, Bergman K, Sarkar P, O’Connor TG. Association between maternal and amniotic fluid cortisol is moderated by maternal anxiety. Psychoneuroendocrinology. 2009;34:430–435. doi: 10.1016/j.psyneuen.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Grass J, Kirschbaum C, Miller R, Gao W, Steudte-Schmiedgen S, Stalder T. Sweat-inducing physiological challenges do not result in acute changes in hair cortisol concentrations. Psychoneuroendocrinology. 2015;53:108–116. doi: 10.1016/j.psyneuen.2014.12.023. [DOI] [PubMed] [Google Scholar]
- Imperatore A, Li W, Petraglia F, Challis JR. Reprod Sci. United States: 2009. Urocortin 2 stimulates estradiol secretion from cultured human placental cells: an effect mediated by the type 2 corticotrophin-releasing hormone (CRH) receptor; pp. 551–558. [DOI] [PubMed] [Google Scholar]
- Ito N, Ito T, Kromminga A, Bettermann A, Takigawa M, Kees F, Straub RH, Paus R. Human hair follicles display a functional equivalent of the hypothalamic-pituitary-adrenal axis and synthesize cortisol. Faseb j. 2005;19:1332–1334. doi: 10.1096/fj.04-1968fje. [DOI] [PubMed] [Google Scholar]
- Jensen Pena C, Monk C, Champagne FA. Epigenetic effects of prenatal stress on 11beta-hydroxysteroid dehydrogenase-2 in the placenta and fetal brain. PLoS One. 2012;7:e39791. doi: 10.1371/journal.pone.0039791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Kamat A, Mendelson CR. Hypoxia prevents induction of aromatase expression in human trophoblast cells in culture: potential inhibitory role of the hypoxia-inducible transcription factor Mash-2 (mammalian achaete-scute homologous protein-2) Mol Endocrinol. 2000;14:1661–1673. doi: 10.1210/mend.14.10.0539. [DOI] [PubMed] [Google Scholar]
- Kapoor A, Lubach G, Hedman C, Ziegler TE, Coe CL. Hormones in infant rhesus monkeys’ (Macaca mulatta) hair at birth provide a window into the fetal environment. Pediatr Res. 2014;75:476–481. doi: 10.1038/pr.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor A, Matthews SG. Short periods of prenatal stress affect growth, behaviour and hypothalamo-pituitary-adrenal axis activity in male guinea pig offspring. J Physiol. 2005;566:967–977. doi: 10.1113/jphysiol.2005.090191. Epub 2005 Jun 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor A, Matthews SG. Prenatal stress modifies behavior and hypothalamic-pituitary-adrenal function in female guinea pig offspring: effects of timing of prenatal stress and stage of reproductive cycle. Endocrinology. 2008;149:6406–6415. doi: 10.1210/en.2008-0347. Epub 2008 Aug 6428. [DOI] [PubMed] [Google Scholar]
- Kapoor A, Matthews SG. Testosterone is involved in mediating the effects of prenatal stress in male guinea pig offspring. J Physiol. 2011;589:755–766. doi: 10.1113/jphysiol.2010.200543. Epub 2010 Dec 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keckeis K, Lepschy M, Schopper H, Moser L, Troxler J, Palme R. Hair cortisol: a parameter of chronic stress? Insights from a radiometabolism study in guinea pigs. J Comp Physiol B. 2012;182:985–996. doi: 10.1007/s00360-012-0674-7. Epub 2012 May 2017. [DOI] [PubMed] [Google Scholar]
- Kim DR, Bale TL, Epperson CN. Prenatal programming of mental illness: current understanding of relationship and mechanisms. Curr Psychiatry Rep. 2015;17:546. doi: 10.1007/s11920-014-0546-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittinger GW. Feto-maternal production and transfer of cortisol in the rhesus (Macaca mulatta) Steroids. 1974;23:229–243. doi: 10.1016/0039-128x(74)90154-8. [DOI] [PubMed] [Google Scholar]
- La Marca-Ghaemmaghami P, Dainese SM, La Marca R, Zimmermann R, Ehlert U. The acute autonomic stress response and amniotic fluid glucocorticoids in second-trimester pregnant women. Psychosom Med. 2015;77:41–49. doi: 10.1097/PSY.0000000000000130. [DOI] [PubMed] [Google Scholar]
- Laplante DP, Barr RG, Brunet A, Galbaud du Fort G, Meaney ML, Saucier JF, Zelazo PR, King S. Stress during pregnancy affects general intellectual and language functioning in human toddlers. Pediatr Res. 2004;56:400–410. doi: 10.1203/01.PDR.0000136281.34035.44. [DOI] [PubMed] [Google Scholar]
- Mitchell BF, Seron-Ferre M, Hess DL, Jaffe RB. Cortisol production and metabolism in the late gestation rhesus monkey fetus. Endocrinology. 1981;108:916–924. doi: 10.1210/endo-108-3-916. [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Heron J, Golding J, Beveridge M, Glover V. Maternal antenatal anxiety and children’s behavioural/emotional problems at 4 years. Report from the Avon Longitudinal Study of Parents and Children. Br J Psychiatry. 2002;180:502–508. doi: 10.1192/bjp.180.6.502. [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Winter MA, Hunn J, Carnahan J, Pressman EK, Glover V, Robertson-Blackmore E, Moynihan JA, Lee FE, Caserta MT. Prenatal maternal anxiety predicts reduced adaptive immunity in infants. Brain Behav Immun. 2013;32:21–28. doi: 10.1016/j.bbi.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell KJ, Bugge Jensen A, Freeman L, Khalife N, O’Connor TG, Glover V. Maternal prenatal anxiety and downregulation of placental 11beta-HSD2. Psychoneuroendocrinology. 2012;37:818–826. doi: 10.1016/j.psyneuen.2011.09.014. Epub 2011 Oct 2015. [DOI] [PubMed] [Google Scholar]
- Pepe GJ, Albrecht ED. Actions of placental and fetal adrenal steroid hormones in primate pregnancy. Endocr Rev. 1995;16:608–648. doi: 10.1210/edrv-16-5-608. [DOI] [PubMed] [Google Scholar]
- Pluess M, Bolten M, Pirke KM, Hellhammer D. Maternal trait anxiety, emotional distress, and salivary cortisol in pregnancy. Biol Psychol. 2010;83:169–175. doi: 10.1016/j.biopsycho.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Price KC, Coe CL. Maternal constraint on fetal growth patterns in the rhesus monkey (Macaca mulatta): the intergenerational link between mothers and daughters. Hum Reprod. 2000;15:452–457. doi: 10.1093/humrep/15.2.452. [DOI] [PubMed] [Google Scholar]
- Rendina D, Lubach GR, Coe CL. Gestataional timing of prenatal disturbance and fetal sex determine the developmental outcomes. Neonatology. doi: 10.1159/000443717. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resko JA, Ellinwood WE, Pasztor LM, Huhl AE. Sex steroids in the umbilical circulation of fetal rhesus monkeys from the time of gonadal differentiation. J Clin Endocrinol Metab. 1980;50:900–905. doi: 10.1210/jcem-50-5-900. [DOI] [PubMed] [Google Scholar]
- Resko JA, Roselli CE. Prenatal hormones organize sex differences of the neuroendocrine reproductive system: observations on guinea pigs and nonhuman primates. Cell Mol Neurobiol. 1997;17:627–648. doi: 10.1023/A:1022534019718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar P, Bergman K, O’Connor TG, Glover V. Maternal antenatal anxiety and amniotic fluid cortisol and testosterone: possible implications for foetal programming. J Neuroendocrinol. 2008;20:489–496. doi: 10.1111/j.1365-2826.2008.01659.x. [DOI] [PubMed] [Google Scholar]
- Sauve B, Koren G, Walsh G, Tokmakejian S, Van Uum SH. Measurement of cortisol in human hair as a biomarker of systemic exposure. Clin Invest Med. 2007;30:E183–191. doi: 10.25011/cim.v30i5.2894. [DOI] [PubMed] [Google Scholar]
- Schultz A. Fetal growth and development of the rhesus monkey. Contributions to Embryology. 1937;155:86–87. [Google Scholar]
- Seron-Ferre M, Rose JC, Parer JT, Foster DB, Jaffe RB. In vivo regulation of the fetal rhesus monkey adrenal gland. Endocrinology. 1978;103:368–375. doi: 10.1210/endo-103-2-368. [DOI] [PubMed] [Google Scholar]
- Sharp H, Hill J, Hellier J, Pickles A. Maternal antenatal anxiety, postnatal stroking and emotional problems in children: outcomes predicted from pre- and postnatal programming hypotheses. Psychol Med. 2014:1–15. doi: 10.1017/S0033291714001342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Phan JM, Lubach GR, Crispen HR, Coe CL. Stability of parental care across siblings from undisturbed and challenged pregnancies: intrinsic maternal dispositions of female rhesus monkeys. Dev Psychol. 2013;49:2005–2016. doi: 10.1037/a0032050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sominsky L, Meehan CL, Walker AK, Bobrovskaya L, McLaughlin EA, Hodgson DM. Neonatal immune challenge alters reproductive development in the female rat. Horm Behav. 2012;62:345–355. doi: 10.1016/j.yhbeh.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Stalder T, Kirschbaum C. Analysis of cortisol in hair--state of the art and future directions. Brain Behav Immun. 2012;26:1019–1029. doi: 10.1016/j.bbi.2012.02.002. doi:1010.1016/j.bbi.2012.1002.1002. Epub 2012 Feb 1015. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Turner JG, Kalin NH. Prolonged stress-induced elevation in plasma corticosterone during pregnancy in the rat: implications for prenatal stress studies. Psychoneuroendocrinology. 1998;23:571–581. doi: 10.1016/s0306-4530(98)00024-9. [DOI] [PubMed] [Google Scholar]
- Walsh SW, Norman RL, Novy MJ. In utero regulation of rhesus monkey fetal adrenals: effects of dexamethasone, adrenocorticotropin, thyrotropin-releasing hormone, prolactin, human chorionic gonadotropin, and alpha-melanocyte-stimulating hormone on fetal and maternal plasma steroids. Endocrinology. 1979;104:1805–1813. doi: 10.1210/endo-104-6-1805. [DOI] [PubMed] [Google Scholar]


