Abstract
Importance
Time to surgery (TTS) is of concern to patients and clinicians, but controversy surrounds its impact on breast cancer survival. There remains little national data evaluating the association.
Objective
To investigate the relationship between the time from diagnosis to breast cancer surgery and survival, using separate analyses of two of the largest cancer databases in the United States.
Design
Two independent population-based studies of prospectively-collected national data utilizing the Surveillance Epidemiology and End Results (SEER)-Medicare-linked database (SMDB), and the National Cancer Database (NCDB).
Setting
The SMDB cohort included Medicare patients >65 years of age, and the NCDB cohort included patients cared for at Commission on Cancer-accredited facilities throughout the United States. Each analysis assessed overall survival as a function of time between diagnosis and surgery by evaluating intervals encompassing ≤30, 31–60, 61–90, 91–120, and 121–180 days in length, and disease-specific survival at 60-day intervals.
Participants
All patients were diagnosed with noninflammatory, nonmetastatic, invasive breast cancer and underwent surgery as initial treatment.
Main Outcomes and Measures
Overall and disease-specific survival as a function of time between diagnosis and surgery, after adjusting for patient, demographic and tumor-related factors.
Results
The SMDB cohort had 94,544 patients ≥66 years old, diagnosed 1992 – 2009. With each interval delay increase, overall survival was lower overall (hazard ratio [HR] 1.09, p<0.001), and in stage I (HR 1.13, p<0.001) and II (HR 1.06, p=0.010) patients. Breast cancer-specific mortality increased with each 60-d interval (subhazard ratio [sHR] 1.26, p= 0.03). The NCDB study evaluated 115,790 patients ≥18 years old, diagnosed 2003 – 2005. The overall mortality HR was 1.10 (p<0.001) for each increasing interval, significant in stages I (HR 1.16, p<0.001) and II (1.09, p<0.001) only, adjusting for demographic, tumor and treatment factors.
Conclusions and Relevance
Greater TTS confers lower overall and disease-specific survival, and a shortened delay is associated with benefits comparable to some standard therapies. Although time is required for preoperative evaluation and consideration of some options such as reconstruction, efforts to reduce TTS should be pursued where possible to enhance survival.
INTRODUCTION
Delays in the treatment of breast cancer have been feared for decades, as even William Halsted proclaimed in 1907 that “we no longer need the proof [that]…the slightest delay is dangerous…in the early stage of breast cancer.”1 There is little doubt that waiting for treatment causes anxiety, but the published medical literature has not provided a consistent answer as to whether any specific preoperative time to surgery (TTS) is associated with an effect on overall or disease-specific survival.
There has been a movement to include TTS as a breast cancer quality measure,2–4 but only recently has this preoperative interval and the relationship of patient evaluation components to delay been comprehensively evaluated in Medicare patients.5 We have found that while the interval between presentation and surgery in Medicare patients is short, that time interval has been rising, from 21 days in 1992 to 32 days in 2005.5
This report details two separate studies undertaken to evaluate the relationship between TTS and survival, using two of the largest datasets in existence for the United States population: the Surveillance Epidemiology End Results (SEER)-Medicare linked database (SMDB), and the National Cancer Database (NCDB). If breast cancer survival is a function of the time between diagnosis and surgery, efforts to expedite care may be of value because of the outcome benefit that occurs.
METHODS
The SMDB and NCDB analyses were separately IRB-approved and permission was obtained, respectively, from the National Cancer Institute and American College of Surgeons. The data and analyses were kept separate and no attempts were made to compare data between cohorts, nor to determine whether patients overlapped, for privacy reasons and to comply with NCI requirements. Both analyses are presented here because of the representativeness of each cohort, and the consistent findings. No statistical analysis between the cohorts has been attempted, nor is warranted because the populations, variable definitions, and ranges differ.
Time intervals between diagnosis and surgery were set at 30-day increments, with the last two intervals combined due to smaller numbers. Intervals to assess overall survival (OS) were thus categorized as ≤30, 31–60, 61–90, 91–120, and 121–180 days, while disease-specific survival (DSS) was characterized as ≤60, 61–120, and 121–180 days because of the lower rate of cancer-specific events, and in order to minimize estimator variance. Time from diagnosis was used for OS and DSS so that patients would have a uniform starting time.
Race/ethnicity was included in the analysis to make the results more generalizable to the US population. Propensity score-based weighting, to adjust for confounding, was used to adjust for covariate differences in the time interval groups.6 We used multinomial logistic regression to estimate the propensity scores, stabilized them to improve covariate balance,7 and used restricted cubic splines for continuous covariates.8 We created adjusted OS curves and adjusted cause-specific cumulative incidence functions using the inverse probability weight method.9 Cox proportional hazards regression with propensity score-based weights were used to estimate the hazard ratio (HR) associated with the time interval groupings and overall survival. Fine and Gray10 proportional hazards regression with propensity score-based weights was used to estimate the subdistribution hazard ratio (sHR) associated with the interval length and breast-cancer specific mortality. We used bootstrap standard errors for hypothesis testing and 95% confidence intervals; the bootstrap method accounted for propensity score estimation. Differences in the effect of preoperative time interval by AJCC stage were examined via propensity score-based weighted regressions in which we included main effect terms for stage (two dummy indicators), the preoperative time interval variable (ordinal variable), and interactions of AJCC stage indicators with that interval length.
SEER-Medicare Database
Patients were diagnosed between 1992 and 2009 with invasive, noninflammatory, nonmetastatic breast cancer, having surgery as first therapy, and a definitive surgery date in Medicare claims ≤180 days after diagnosis. Exclusions included those having missing covariate data and those aged <66 to permit comorbidity assessment 12 months prior to diagnosis. Although patients were restricted to their first breast cancer occurrence, a history of other malignancies was permitted. While substage (i.e. II-A, II-B) migration between AJCC editions can occur in nearly 20% of patients, stage migration occurs in <0.2%,11 so substages were collapsed and not differentiated by edition. The diagnosis date, used as the preoperative interval start date, was determined by using SEER clinical diagnosis date (which only consists of a month and year) and searching for the first biopsy date during that month or the subsequent month. Patients were excluded who had no such discernable biopsy date.
As procedure codes for excisional biopsy and segmental mastectomy are sometimes used interchangeably in billing, inference of therapeutic intent was achieved by defining a patient’s definitive surgery as the first date on which claims for both ≥1 breast excision or mastectomy and a lymph node procedure were performed (eTable 1).
Adjustments were made for age, sex, race, marital status, income, education, size of metropolitan area, geographical region, year of diagnosis, sequence of breast cancer (within a history of other cancers), Charlson12 and Elixhauser13 comorbidity scores, histology, grade, tumor size, number of lymph nodes examined, number of lymph nodes positive, AJCC stage, surgery type, chemotherapy use, and radiotherapy use, via propensity score-based weighting. Patients receiving neoadjuvant chemotherapy were excluded, and chemotherapy and radiotherapy use were defined as being administered if given ≤1 year after surgery. Race was determined from the Medicare enrollment database variable, while comorbidity, surgery, chemotherapy, and radiotherapy, came from Medicare claims. Missing covariate data is listed in eTable 2.
National Cancer Database
The National Cancer Database14 cohort included those having noninflammatory, invasive, nonmetastatic breast cancer, having surgical treatment as their first modality ≤6 months after their diagnosis date. Patients were included if breast cancer was their first and only malignancy, and if diagnosis and treatment (all or part) was at the reporting facility. Patients without lymph node surgery or whose staging, diagnosis method, or treatment order was unknown were excluded. The NCDB does not provide a diagnosis date, but after 2002 recorded the length of the interval between diagnosis and surgery. This interval length was present for cases diagnosed from 2003 onward. The NCDB requires follow-up of >5 years, so the cohort only included cases from 2003–2005 with follow-up through 2010.
The NCDB contains the most extensive surgery (e.g. a lumpectomy followed by mastectomy lists the patient as having a mastectomy). The NCDB also contains interval lengths from diagnosis to first surgery and from diagnosis to definitive surgery, to determine if the patient underwent >1 procedure. We excluded patients with >1 breast surgery to ensure capture of therapeutic surgery and to eliminate possible confounding excisional biopsies, ensuring that the analysis evaluated the time to therapeutic surgery. Patients receiving neoadjuvant chemotherapy were excluded, and chemotherapy and radiotherapy use were defined as being administered if given ≤1 year after surgery. Missing covariate data is listed in eTable 2.
Adjustments were made for age, sex, race, income, education, size of metropolitan area, geographical region, year of diagnosis, Charlson-Deyo comorbidity score, histology, grade, tumor size, surgical margins, number of nodes examined, number of nodes positive, AJCC stage, surgery type, chemotherapy, radiotherapy, endocrine therapy, facility type, distance to facility, class of case, and insurance type, via propensity score based weighting.
RESULTS
SEER-Medicare Database
There were 94,544 patients analyzed, after all exclusions (eFigure 1). Mean age (± standard deviation [SD]) was 75.2 (± 6.2) years and 99% were women. Individuals having ≤30, 31–60, 61–90, 91–120, and 121–180 days between diagnosis and surgery comprised 77.7%, 18.3%, 2.7%, 0.7%, and 0.5% of the patients, respectively; patient and tumor characteristics of these groups are listed in Table 1, demonstrating greater similarity among the groups after adjustment. Black race and Hispanic ethnicity, lobular histology, fewer node examined, large metropolitan region, higher Charlson and Elixhouser comorbidity scores, tumor size, the proportion of stage III tumors, the percentage of patients undergoing mastectomy, and a lack of chemotherapy use, increased steadily in the unadjusted data with an increase in the delay interval (Table 1).
TABLE 1.
Adjusted/weighted and unadjusted/unweighted patient and tumor characteristics of the SEER-Medicare database study, by delay interval.
| Characteristic | Adjusted % (Unadjusted %)* unless otherwise specified | ||||
|---|---|---|---|---|---|
| ≤30 days | 31–60 days | 61–90 days | 91–120 days | 121–180 days | |
| Total Patients | 73,491 | 17,345 | 2,586 | 686 | 436 |
| Mean age in years | 75.2 (75.1) | 75.2 (75.3) | 75.1 (75.7) | 75.5 (75.9) | 75.3 (77.2) |
| Gender | |||||
| Female | 99.2 (99.1) | 99.1 (99.3) | ** | ** | ** |
| Male | 1.0 (0.9) | 1.0 (0.7) | ** | ** | ** |
| Race/Ethnicity | |||||
| White | 89.3 (90.2) | 89.1 (86.9) | 89.1 (83.3) | 88.3 (77.1) | 87.9 (75.9) |
| Black | 6.3 (5.5) | 6.5 (8.2) | 6.8 (11.3) | 5.9 (16.3) | 7.6 (18.1) |
| Asian | 1.9 (1.8) | 1.8 (2.0) | 1.8 (2.3) | 2.3 (2.0) | 1.2 (2.3) |
| Hispanic | 1.0 (0.9) | 1.0 (1.2) | 1.0 (1.7) | 1.3 (1.9) | 0.9 (2.3) |
| Other/Unknown | 1.6 (1.5) | 1.6 (1.7) | 1.2 (1.4) | 2.6 (1.8) | 1.4 (2.1) |
| Mean Charlson Comorbidity | 0.5 (0.5) | 0.5 (0.6) | 0.5 (0.7) | 0.5 (0.8) | 0.5 (0.8) |
| Setting§ | |||||
| Large metropolitan | 54.6 (51.5) | 54.5 (64.6) | 53.2 (67.1) | 50.4 (67.5) | 56.1 (69.0) |
| Metropolitan | 29.1 (30.5) | 28.7 (24.3) | 28.6 (22.9) | 33.2 (21.8) | 26.5 (23.2) |
| Urban | 6.0 (6.6) | 6.3 (4.4) | 7.1 (4.3) | 6.2 (3.8) | 3.7 (2.3) |
| Less Urban/Rural | 10.3 (11.4) | 10.5 (6.7) | 11.1 (5.8) | 10.3 (7.0) | 13.8 (5.5) |
| Region¶ | |||||
| Northeast | 17.4 (16.0) | 17.7 (22.2) | 17.3 (22.2) | 18.0 (23.3) | 18.9 (22.9) |
| South | 20.8 (22.4) | 20.4 (15.2) | 21.0 (13.5) | 19.2 (13.6) | 18.4 (18.4) |
| Midwest | 18.5 (19.3) | 19.0 (15.8) | 19.1 (14.2) | 19.5 (14.6) | 14.9 (12.4) |
| West | 43.3 (42.3) | 42.9 (46.8) | 42.6 (50.1) | 43.3 (48.5) | 47.9 (46.3) |
| Invasive Histology | |||||
| Ductal | 86.6 (86.9) | 86.4 (85.9) | 86.1 (85.6) | 85.8 (85.6) | 87.1 (82.3) |
| Lobular | 10.9 (10.6) | 11.0 (11.9) | 10.7 (11.9) | 11.5 (12.0) | 11.6 (14.5) |
| Other/unknown | 2.5 (2.6) | 2.6 (2.3) | 3.3 (2.5) | 2.7 (2.5) | 1.3 (3.2) |
| Grade | |||||
| Well differentiated | 22.2 (21.8) | 22.5 (24.1) | 22.2 (23.0) | 21.3 (20.4) | 17.3 (20.2) |
| Moderately differentiated | 41.8 (41.3) | 42.1 (43.2) | 40.4 (43.2) | 43.6 (43.6) | 44.5 (42.4) |
| Poorly differentiated | 25.2 (25.6) | 24.6 (23.7) | 26.0 (24.3) | 26.0 (24.8) | 28.7 (24.5) |
| Undifferentiated/Anaplastic | 1.3 (1.4) | 1.2 (1.0) | 1.3 (1.0) | 1.0 (1.8) | 1.6 (2.3) |
| Unknown | 9.5 (9.9) | 9.7 (8.0) | 10.2 (8.6) | 8.1 (9.5) | 7.9 (10.6) |
| Mean tumor size in mm | 19.1 (19.2) | 18.9 (18.3) | 19.5 (20.1) | 20.0 (21.6) | 20.4 (26.3) |
| AJCC Stage‡ | |||||
| I | 57.9 (57.8) | 58.4 (59.3) | 56.8 (55.2) | 50.9 (55.3) | 56.2 (44.0) |
| II | 35.9 (36.2) | 35.6 (34.5) | 37.2 (36.2) | 42.6 (33.8) | 37.4 (39.5) |
| III | 6.2 (6.0) | 6.1 (6.2) | 6.0 (8.6) | 6.4 (10.9) | 6.5 (16.5) |
| Median Income (Census Tract, $) | 50,363 (49,728) | 50,312 (52,882) | 49,735 (51,649) | 49,990 (26,805) | 49,549 (21,076) |
| % of Persons 25+ Years Old Having < High School (12 years) Education by Census Tract | |||||
| 17.6 (17.6) | 17.6 (17.2) | 18.0 (18.4) | 17.5 (20.4) | 18.5 (19.5) | |
| Mean Year of Diagnosis | 2002.3 (2001.8) | 2002.3 (2004.0) | 2002.4 (2004.5) | 2002.3 (2004.2) | 2002.2 (2004.3) |
| Marital Status | |||||
| Married | 45.5 (46.7) | 45.0 (42.9) | 46.6 (36.9) | 46.7 (33.5) | 41.6 (31.0) |
| Not Married | 54.5 (53.3) | 55.0 (57.1) | 53.4 (63.1) | 53.3 (66.5) | 58.4 (69.0) |
| Mean Breast Cancer Sequence** | |||||
| 1.1 (1.1) | 1.1 (1.1) | 1.1 (1.1) | 1.1 (1.1) | 1.1 (1.1) | |
| Mean Elixhauser Score | 1.1 (1.0) | 1.1 (1.1) | 1.1 (1.5) | 1.5 (1.7) | 1.0 (1.6) |
| Mean # Nodes Positive | 1.0 (1.0) | 1.0 (0.9) | 0.9 (1.1) | 1.0 (1.2) | 1.1 (1.5) |
| Mean # Nodes Examined | 8.9 (9.3) | 8.8 (7.6) | 8.7 (7.3) | 8.7 (7.3) | 8.0 (7.1) |
| Surgery Type | |||||
| Breast Conservation | 72.7 (73.6) | 73.3 (69.9) | 73.2 (68.7) | 74.6 (64.4) | 72.2 (63.5) |
| Mastectomy | 27.3 (26.4) | 26.7 (30.1) | 26.8 (31.3) | 25.4 (35.6) | 27.8 (36.5) |
| Chemotherapy Use | |||||
| Yes | 22.5 (23.0) | 21.9 (21.6) | 24.7 (18.2) | 21.1 (18.5) | 26.5 (15.6) |
| No | 77.5 (77.0) | 78.0 (78.4) | 75.3 (81.8) | 78.9 (81.5) | 73.5 (84.4) |
| Radiotherapy Use | |||||
| Yes | 50.9 (50.9) | 51.0 (52.7) | 51.8 (44.8) | 55.4 (38.5) | 49.0 (41.7) |
| No | 49.1 (49.1) | 49.0 (47.3) | 46.2 (55.2) | 44.6 (61.5) | 51.0 (58.3) |
Totals may vary from 100% due to rounding.
Cells have been deleted as per SEER Medicare requirements to censor cells containing <11 individuals, or other cells that make such cells calculable.
Setting definitions: Large metropolitan = counties of metropolitan areas of ≥1,000,000; metropolitan = counties in metropolitan areas of <250,000–1,000,000; urban = urban population of ≥20,000 adjacent or nonadjacent to a metropolitan area; less urban = urban population of 2,500–19,999 adjacent or nonadjacent to a metropolitan area; rural = completely rural or <2,500 urban population, adjacent or nonadjacent to a metropolitan area.
Region groupings: Northeast = Connecticut and New Jersey registries; South = Atlanta, Kentucky, and Louisiana registries; Midwest = Detroit and Iowa registries; West = Hawaii, New Mexico, Seattle, Utah, and California registries. Registries were adjusted individually, but are grouped for reporting purposes.
AJCC Stage: 3rd and 6th editions were combined. Substages (e.g. IIA, IIB) were collapsed to minimize differences between these AJCC Editions.
The number of the cancer, when a patient has one or more distinct primary cancers during their lifetime.
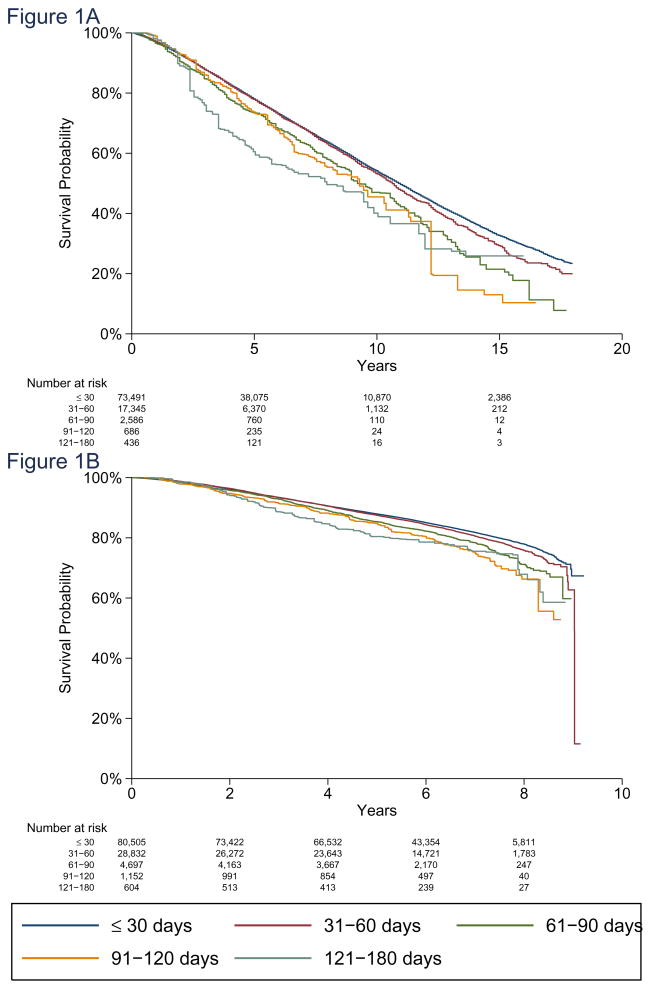
The increase in mortality in all stages for all patients and from all causes was 9% (HR 1.09, 95% CI 1.06–1.13, p<0.001) for each preoperative interval category increase (Figure 1A). TTS was statistically significant with respect to OS in stage I (HR 1.13, p<0.001, 95% CI 1.08–1.18) and stage II (HR 1.06, p=0.010, 95% CI 1.01–1.11), but not in stage III (HR 1.06, p=0.17, 95% CI 0.97–1.16, eFigure 2). The HR interaction p for stages I vs II was p=0.048, stages I vs III was p=0.21, and stages II vs III was p=0.95.
Figure 1. Adjusted Overall Survival.
A. Adjusted overall survival for SEER-Medicare Database patients for preoperative delay intervals of ≤30, 31–60, 61–90, 91–120, and 121–180 days. Hazard ratio for each increasing delay interval = 1.09 (95%CI 1.06–1.13, p<0.001).
B. Adjusted overall survival for National Cancer Database patients for preoperative delay intervals of ≤30, 31–60, 61–80, 81–120, and 121–180 days. Hazard ratio for each increasing delay interval = 1.10 (95%CI 1.07–1.13, p<0.001).
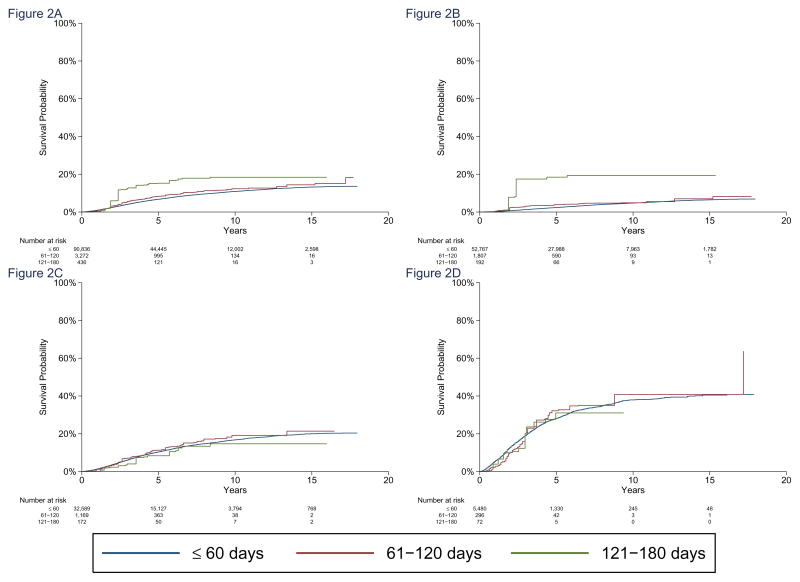
Added risk of death due to breast cancer for each 60-day increase in TTS had a subhazard ratio [sHR] of 1.26 (95% CI 1.02–1.54, p=0.03). The association with disease-specific mortality was significant for stage I patients (subHazard ratio [sHR] 1.84; 95% CI 1.10–3.07, p=0.02), but not for stage II or stage III patients. Interaction p’s for sHR were 0.042 for stage I vs. II; 0.059 for stage I vs III. Adjusted five-year OS is listed in Table 3A, and 62.6% of patients were diagnosed before 2005, allowing for at least 5 years of mortality follow-up. Hazard and subHazard ratios from the Cox and Fine and Gray models are listed in eTable 3. Cardiac and cerebrovascular disease, along with chronic obstructive pulmonary disease were the most frequent nononcologic specified causes of death (eTable 4).
TABLE 3.
Point estimates for adjusted overall survival for each study, by interval delay. Table values showing adjusted overall survival are in percent.
| Table 3A: SEER-Medicare Database study | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤30 days | 31–60 days | 61–90 days | 91–120 days | 121–180 days | |||||||||||
| Years | N at risk | Adjust. OS |
95% CI | N at risk | Adjust. OS |
95% CI | N at risk | Adjust. OS |
95% CI | N at risk | Adjust. OS |
95% CI | N at risk | Adjust. OS |
95% CI |
| 5 | 38,075 | 78.1 | 77.7–78.4 | 6,370 | 77.9 | 77.0–78.8 | 760 | 73.5 | 70.4–76.7 | 235 | 73.5 | 66.4–80.5 | 121 | 60.9 | 50.5–71.3 |
| 10 | 10,870 | 54.2 | 53.7–54.7 | 1,132 | 53.2 | 51.7–54.7 | 110 | 47.1 | 41.3–52.9 | 24 | 45.0 | 33.7–56.3 | 16 | 40.2 | 27.7–52.7 |
| 15 | 2,386 | 32.7 | 32.0–33.4 | 212 | 29.3 | 26.7–31.9 | 12 | 21.7 | 13.7–29.7 | <11 | 14.9 | 2.1–27.7 | <11 | 26.0 | 9.0–43.1 |
| Table 3B: National Cancer Database study | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤30 days | 31–60 days | 61–90 days | 91–120 days | 121–180 days | |||||||||||
| Years | N at risk | Adjust. OS |
95% CI | N at risk | Adjust. OS |
95% CI | N at risk | Adjust. OS |
95% CI | N at risk | Adjust. OS |
95% CI | N at risk | Adjust. OS |
95% CI |
| 5 | 60,909 | 88.0 | 87.7–88.2 | 21,464 | 87.5 | 87.1–87.9 | 3,269 | 85.4 | 84.1–86.7 | 746 | 84.9 | 81.9–87.9 | 359 | 80.4 | 75.4–85.5 |
N at risk = number at risk; Small N’s of less than 11 are not reported, per NCI SEER-Medicare guidelines
Adjust. OS = adjusted overall survival estimate
95% CI = 95% confidence interval for adjusted OS
National Cancer Database
There were 115,790 patients analyzed, after all exclusions (eFigure 1). NCDB cohort characteristics are shown with adjusted and unadjusted data by preoperative interval group in Table 2 and eTable 5, demonstrating greater similarity among the groups after adjustment. Mean age was 60.3 years (± 13.4), ranging from 18 to 90 years old, and nearly all were women. Patients who had intervals of ≤30, 31–60, 61–90, 91–120, and 121–180 days accounted for 69.5%, 24.9%, 4.1%, 1.0%, and 0.5% of the patients, respectively. Unadjusted prevalence of Black and Asian race, higher Charlson comorbidity score, large metropolitan setting, pacific region of the US, unknown grade/differentiation, stage III tumors, income <$30,000, zip codes with the highest levels of education, the proportion of patients undergoing mastectomy, lack of chemotherapy, radiotherapy, and endocrine therapy use, and a lower proportion of private insurance increased steadily in the unadjusted data with an increase in the delay interval (Table 2).
TABLE 2.
Adjusted/weighted and unadjusted/unweighted patient and tumor characteristics of the National Cancer Database study, by delay interval. Variables also included in the analysis, but not displayed below, are region, income, education, year of diagnosis, facility/cancer program type, mean distance to facility, and analytic case type. These are delineated in eTable 5 for space reasons.
| Characteristic | Adjusted % (Unadjusted %)* unless otherwise specified | ||||
|---|---|---|---|---|---|
| ≤30 days | 31–60 days | 61–90 days | 91–120 days | 121–180 days | |
| Total Patients | 80,505 | 28,832 | 4,697 | 1,152 | 604 |
| Mean age in years | 60.4 (60.1) | 60.5 (60.8) | 60.9 (60.8) | 60.6 (60.7) | 60.3 (61.3) |
| Gender | |||||
| Female | 99.1 (99.0) | 99.1 (99.3) | 98.7 (99.3) | ** | ** |
| Male | 0.9 (1.0) | 0.9 (0.7) | 1.3 (0.7) | ** | ** |
| Race | |||||
| White | 82.8 (85.4) | 82.9 (79.6) | 82.4 (71.0) | 80.7 (58.5) | 85.0 (57.0) |
| Black | 9.2 (7.6) | 9.1 (11.2) | 9.2 (16.7) | 9.6 (23.1) | 8.5 (26.0) |
| Asian | 2.1 (2.0) | 2.2 (2.3) | 2.3 (2.4) | 2.2 (3.0) | 1.7 (3.8) |
| Hispanic | 4.1 (3.2) | 4.0 (5.1) | 4.1 (8.2) | 5.0 (12.5) | 3.5 (10.8) |
| Other/Unknown | 1.8 (1.8) | 1.8 (1.8) | 1.9 (1.8) | 2.5 (2.9) | 1.4 (2.5) |
| Charlson Comorbidity Score† | |||||
| 0 | 88.1 (88.8) | 88.1 (87.4) | 87.9 (85.6) | 86.9 (84.2) | 88.0 (83.6) |
| 1 | 10.0 (9.6) | 10.1 (10.5) | 10.0 (11.4) | 10.9 (11.9) | 10.0 (12.4) |
| ≥2 | 1.8 (1.7) | 1.9 (2.1) | 2.1 (3.0) | 2.3 (3.9) | 2.0 (4.0) |
| Setting§ | |||||
| Large metropolitan | 51.3 (47.9) | 51.2 (57.9) | 51.3 (63.1) | 52.9 (67.5) | 46.9 (68.9) |
| Metropolitan | 32.6 (34.5) | 32.7 (29.1) | 32.5 (25.8) | 30.9 (23.0) | 35.2 (21.5) |
| Urban | 6.5 (7.0) | 6.5 (5.6) | 6.3 (4.9) | 6.3 (3.8) | 7.6 (4.3) |
| Less Urban/Rural | 9.6 (10.7) | 9.6 (7.5) | 9.9 (6.2) | 9.9 (5.6) | 10.3 (5.3) |
| Invasive Histology | |||||
| Ductal | 89.2 (89.5) | 89.3 (88.7) | 88.9 (88.6) | 87.4 (87.7) | 87.9 (87.3) |
| Lobular | 8.1 (7.7) | 8.1 (8.7) | 8.0 (8.6) | 9.5 (9.6) | 9.7 (8.9) |
| Other/unknown | 2.7 (2.8) | 2.7 (2.6) | 3.2 (2.8) | 3.1 (2.8) | 2.4 (3.8) |
| Grade | |||||
| Well differentiated | 21.0 (20.4) | 21.0 (22.5) | 20.9 (22.5) | 21.4 (18.4) | 19.7 (17.7) |
| Moderately differentiated | 39.7 (39.4) | 39.8 (40.3) | 40.1 (40.5) | 42.9 (43.0) | 40.9 (35.6) |
| Poorly differentiated | 32.6 (33.8) | 32.5 (30.2) | 31.9 (28.9) | 29.0 (29.9) | 32.8 (33.8) |
| Undifferentiated/Anaplastic | 0.9 (0.9) | 0.8 (0.8) | 0.9 (1.0) | 0.6 (1.7) | 0.5 (2.3) |
| Unknown | 5.8 (5.5) | 5.9 (6.2) | 6.3 (7.1) | 6.1 (7.0) | 6.1 (10.6) |
| Mean tumor size in cm | 2.1 (2.1) | 2.0 (2.0) | 2.1 (2.1) | 2.1 (2.1) | 2.2 (3.4) |
| AJCC Stage‡ | |||||
| I | 52.1 (51.6) | 52.2 (53.6) | 51.9 (52.1) | 50.2 (50.4) | 48.0 (41.6) |
| II | 37.2 (37.6) | 37.0 (36.3) | 37.2 (35.6) | 39.3 (34.9) | 40.4 (38.9) |
| III | 10.8 (10.7) | 10.8 (10.1) | 11.0 (12.4) | 10.5 (14.7) | 11.7 (19.5) |
| Surgical Margins: Residual Tumor | |||||
| None | 94.6 (94.8) | 94.6 (94.4) | 94.3 (93.6) | 94.6 (91.1) | 95.6 (92.4) |
| Residual | 4.3 (4.2) | 4.3 (4.3) | 4.7 (4.6) | 4.2 (6.4) | 3.3 (4.8) |
| Other/Unknown | 1.1 (1) | 1.1 (1.3) | 1.1 (1.8) | 1.2 (2.5) | 1 (2.8) |
| Mean # Nodes Examined | 7.8 (7.8) | 7.8 (7.7) | 7.8 (8.2) | 7.8 (8.7) | 8.5 (9.4) |
| Mean # Nodes Positive | 1.2 (1.2) | 1.2 (1.1) | 1.2 (1.3) | 1.2 (1.4) | 1.4 (1.9) |
| Surgery Type | |||||
| Breast Conservation | 62.9 (65.4) | 63 (58.9) | 63.7 (51.9) | 60.3 (48.2) | 64.4 (45.5) |
| Mastectomy | 37.1 (34.6) | 37 (41.1) | 36.3 (48.1) | 39.7 (51.8) | 35.6 (54.5) |
| Chemotherapy Use | |||||
| Yes | 37.1 (34.6) | 37 (41.1) | 36.3 (48.1) | 39.7 (51.8) | 35.6 (54.5) |
| No | 62.9 (65.4) | 63 (58.9) | 63.7 (51.9) | 60.3 (48.2) | 64.4 (45.5) |
| Radiotherapy Use | |||||
| Yes | 62.8 (65.5) | 62.7 (58.7) | 63.3 (50.6) | 60.4 (44.8) | 65.4 (40.9) |
| No | 37.2 (34.5) | 37.3 (41.3) | 36.7 (49.4) | 39.6 (55.2) | 34.6 (59.1) |
| Endocrine Therapy Use | |||||
| Yes | 52.5 (53.1) | 52.5 (52.8) | 51.5 (48) | 51.7 (43.1) | 53.5 (36.6) |
| No | 47.5 (46.9) | 47.5 (47.2) | 48.5 (52) | 48.3 (56.9) | 46.5 (63.4) |
| Insurance Type | |||||
| None | 2.0 (1.8) | 2.0 (2.2) | 2.0 (3.3) | 1.9 (5.1) | 1.9 (5.5) |
| Private | 16.5 (17.8) | 16.5 (13.9) | 16.6 (12.1) | 17.2 (10) | 17.6 (9.6) |
| Managed Care | 39.2 (39.2) | 39 (40.6) | 37.1 (36.5) | 38.3 (33.9) | 36.4 (30.1) |
| Medicaid | 4.3 (3.5) | 4.3 (5.2) | 4.6 (8.2) | 4.8 (11.1) | 4.5 (10.9) |
| Medicare | 35 (34.6) | 35.3 (35.4) | 37.2 (35.7) | 35.5 (33.5) | 38.1 (36.9) |
| Other Government/Unknown | 3.0 (3) | 2.9 (2.7) | 2.4 (4.2) | 2.4 (6.3) | 1.6 (7.0) |
Totals may vary from 100% due to rounding.
Cells have been deleted as per NCDB requirements to censor cells containing <11 individuals, or other cells that make such cells calculable.
Charlson scores of ≥2 are collapsed together into one group and so mean Charlson scores for NCDB data cannot be calculated.
Setting definitions: Large metropolitan = counties of metropolitan areas of ≥1,000,000; metropolitan = counties in metropolitan areas of <250,000–1,000,000; urban = urban population of ≥20,000 adjacent or nonadjacent to a metropolitan area; less urban = urban population of 2,500–19,999 adjacent or nonadjacent to a metropolitan area; rural = completely rural or <2,500 urban population, adjacent or nonadjacent to a metropolitan area.
AJCC Stage: 5rd and 6th editions were combined. Substages (e.g. IIA, IIB) were collapsed to minimize differences between these AJCC Editions.
The added risk of death from all causes for each interval increase in TTS was 10.0% (HR 1.10, 95% CI 1.07–1.13, p<0.001, Figure 1B) for the entire cohort. TTS was associated with OS for stage I (HR 1.16, p<0.001, 95% CI 1.12–1.21) and stage II (HR 1.09, p<0.001, 95% CI 1.05–1.13), but not in stage III (HR 1.01, p=0.640, 95% CI 0.96–1.07, eFigure 3). Interaction p’s for sHR were 0.028 for stage I vs. II, <0.001 for stage I vs. III, and 0.039 for stage II vs. III. Hazard and subHazard ratios are listed in eTable 3. Cause-specific mortality is not available for the NCDB dataset. Mean follow-up among those who did not die was 6.00 years (SD 1.80 years). Subgroup point estimates for five-year OS are listed in Table 3B.
DISCUSSION
Although the relationship between the TTS and breast cancer outcomes might be assumed to be a modern healthcare concern, admonition about breast cancer treatment delays first occurred over 100 years ago1 with TTS at that time measured in months rather than days or weeks.15 Until recently, there have been little data about waiting times in the United States5,16 and there remains little consensus about the relationship between delays and survival.
Although no dataset can determine every cause of delay, especially those on the part of the patient, we have noted that some factors increase in prevalence as preoperative delays increase. We have previously found that multiple factors correlate with a longer time to breast cancer surgery,5 but irrespective of the cause, when adjusting for these and numerous other demographic, tumor and treatment factors, delays still independently correlated with a slightly lower survival rate in both the SMDB and NCDB cohorts.
We have found that OS declines when the TTS increases, with OS affected in stage I and II but not stage III patients. The data for DSS are similar, with cancer-specific mortality data only available in the SMDB, where stage I patients exhibited lower survival as TTS increased. This observation that only stage I patients’ DSS and stage I and stage II patients’ OS are affected by preoperative delays could be due to lower numbers of higher stage patients, but we believe that breast cancer survivability in its earliest stage is more influenced by the TTS than later stages, because baseline mortality is smaller relative to the effect imposed by a delay in treatment. In both cohorts, OS and disease-specific survival for stage III disease were not influenced by TTS, suggesting either partial biologic predestination of outcome or a mortality risk that overshadows any small effect of improving delay by a matter of months. This effect may also be attenuated with age due to competing mortality risks. Because of this and because final stage is only available postoperatively, we believe that efforts to minimize preoperative delay for all patients is advisable.
We have adjusted for numerous variables in each study, but unmeasured confounders could still exist, as with every series, affecting survival negatively or positively. We excluded patients having neoadjuvant chemotherapy in these analyses to maintain cohort homogeneity and because we found that these patients had a markedly longer TTS because of the lengthy time imposed by the treatment itself, with lower survival related to its indications, skewing the data towards the appearance of artificially worse outcomes with longer delays. The slight differences we see in the magnitude of effect by delay for the SMDB versus NCDB cohort may reflect the complexities in the relationship between age and tumor biology,17 or age and treatment,18 that cannot be clearly defined in these datasets. It also must be recognized that the effects seen here may result from delay to surgery, delay to postoperative therapy, or both. For patients where surgery is the first treatment before systemic therapy, these possibilities are inextricable, and all underscore the need to avoid undue delay.
TTS and its effect on survival is a ubiquitous concern of cancer patients, and a question frequently posed in consultations with surgeons. Elimination of undue delay is desirable to both reduce anxiety and lower risk, and we believe that this study provides clinicians needed data to answer patients’ questions about TTS and its impact on outcome. While the absolute magnitude of the 5-year survival difference was small (4.6% and 3.1% for ≤30 days vs 91–120 days in SMDB and NCDB patients, respectively) this benefit is comparable to the addition of some standard therapies, such as the recent extension of tamoxifen therapy from 5 to 10 years19 while not having the side effects or costs found with most interventions.
Whether TTS should be revisited as a quality measure could be debated in light of practical matters that contribute to delay. Some of these are patient-driven, such as the desire for multiple opinions, limitations in the patient’s schedule, or not seeking care as instructed. Some may be system-driven such as a lack of available operating room time, appointment times, insurance issues and barriers to care. Yet others may be physician-related, such as schedule limitations, or excessive use of imaging or other testing. The National Quality Forum, National Comprehensive Cancer Network, and American Society of Clinical Oncology have already ratified at least three time-dependent breast cancer measures.20 These include receipt of tamoxifen or an aromatase inhibitor within 1 year of diagnosis, initiation of breast radiotherapy within 1 year of diagnosis, and receipt of adjuvant chemotherapy within 4 months of diagnosis.
Questions remain as to whether time-dependent measures improve the quality of care,21 but there has already been consideration of TTS as a quality measure.2–4 The previous lack of clear data has weakened the need for such a standard, but our findings here suggest that consideration of a reasonable delay threshold for surgery might be appropriate, as it has been for medical oncology and radiation oncology. Because only 1.2% and 1.5% of the SMDB and NCDB patients, respectively, had a TTS that was over 90 days, providing these few breast cancer patients the 3–5% survival benefit associated with reduced delay also seems achievable.
Unfortunately, prior studies on survival and delay have been inconclusive. While some suggest that these factors are linked,22–24 others have found no correlation.25–27 Many select an arbitrary single interval cutoff23,24,26 at which delay is defined. In our two series, the cohort sizes provide power beyond that achieved by prior analyses, and allow for multiple delay groups of varying lengths, while adjusting for numerous confounders to allow the relationship to become clearer. The similar results between separate analyses of these two large national datasets, having different characteristics, is also compelling and suggests that the effect of delay on survival is a true phenomenon and not one specific to a particular cohort.
Although this report describes two population-based series, a prospective study randomizing patients to varying degrees of delay is unlikely to occur because of both ethical considerations and aversion to delays in treatment. For this reason, we believe that these analyses of two of the largest prospectively-collected datasets in existence for the United States provide the most definitive demonstration possible. The 15-year estimates and the 120–180 day estimates do show a larger benefit of minimizing delay, but these subgroups also have very few individuals at risk, limiting the power of even these large analyses.
In conclusion, survival outcomes in early stage breast cancer patients are affected by the length of the interval between diagnosis and surgery, and efforts to minimize that interval are appropriate. Although the effect on both overall and disease-specific survival remains small, consideration should be given to establishing reasonable and attainable goals for the timing of surgical interventions, to afford this population a finite, but clinically relevant, survival benefit.
Supplementary Material
Figure 2. Adjusted Breast Cancer-Specific Mortality.
Adjusted breast cancer-specific mortality for SEER-Medicare patients, for preoperative delay intervals of ≤60, 61–120, and 121–180 days. Panels show (A) all stages combined, (B) Stage I, (C) Stage II, and (D) Stage III patients. SubHazard ratio for all stages was 1.26 (95% CI: 1.02–1.54, p=0.03), and subHazard ratios were 1.84 for Stage I (95% CI: 1.10–3.07, p=0.020), 1.03 (95% CI: 0.83–1.28, p=0.80) for Stage II, and 1.04 (95%CI: 0.82–1.33, p=0.74) for Stage III. p-values for comparing the sHR in Stage I to the sHRs in Stage II and III were p=0.042 and p=0.059, respectively.
A. All stages combined.
B. Stage I patients.
C. Stage II patients.
D. Stage III patients.
Acknowledgments
This work was supported by United States Public Health Services grant P30 CA006927 for analysis of the data via support of our biostatistics facility, by an internal American Cancer Society grant #IRG-92-027-17 that supported preliminary analysis of data, and by generous private donor support, for analysis and interpretation of the data.
Richard J. Bleicher, MD, and Brian L. Egleston, PhD, had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Brian L. Egleston, PhD, Karen Ruth, MS, and Richard J. Bleicher, MD, all of Fox Chase Cancer Center, conducted and are responsible for the data analysis.
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
The collection of the California cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract N01-PC-35136 awarded to the Northern California Cancer Center, contract N01-PC-35139 awarded to the University of Southern California, and contract N02-PC-15105 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement #U55/CCR921930-02 awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Public Health the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred.
The NCDB is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The data used in the study are derived from a de-identified NCDB File. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator.
Footnotes
Presented, in part, at the 2015 Annual Meeting of the American Society of Clinical Oncology, June 1, 2015, Chicago, IL.
References
- 1.Halsted WSI. The Results of Radical Operations for the Cure of Carcinoma of the Breast. Ann Surg. 1907;46(1):1–19. doi: 10.1097/00000658-190707000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCahill LE, Privette A, James T, et al. Quality measures for breast cancer surgery: initial validation of feasibility and assessment of variation among surgeons. Arch Surg. 2009;144(5):455–462. doi: 10.1001/archsurg.2009.56. discussion 462-453. [DOI] [PubMed] [Google Scholar]
- 3.Kaufman CS, Shockney L, Rabinowitz B, et al. National Quality Measures for Breast Centers (NQMBC): a robust quality tool: breast center quality measures. Ann Surg Oncol. 2010;17(2):377–385. doi: 10.1245/s10434-009-0729-5. [DOI] [PubMed] [Google Scholar]
- 4.Del Turco MR, Ponti A, Bick U, et al. Quality indicators in breast cancer care. Eur J Cancer. 2010;46(13):2344–2356. doi: 10.1016/j.ejca.2010.06.119. [DOI] [PubMed] [Google Scholar]
- 5.Bleicher RJ, Ruth K, Sigurdson ER, et al. Preoperative Delays in the US Medicare Population With Breast Cancer. J Clin Oncol. 2012;30(36):4485–4492. doi: 10.1200/JCO.2012.41.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lunceford JK, Davidian M. Stratification and weighting via the propensity score in estimation of causal treatment effects: a comparative study. Stat Med. 2004;23(19):2937–2960. doi: 10.1002/sim.1903. [DOI] [PubMed] [Google Scholar]
- 7.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Harrell FE. Regression modeling strategies. New York: Springer; 2001. pp. 11–40. [Google Scholar]
- 9.Cole SR, Hernan MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed. 2004;75(1):45–49. doi: 10.1016/j.cmpb.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Fine J, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 11.Kim SI, Park BW, Lee KS. Comparison of stage-specific outcome of breast cancer based on 5th and 6th AJCC staging system. J Surg Oncol. 2006;93(3):221–227. doi: 10.1002/jso.20513. [DOI] [PubMed] [Google Scholar]
- 12.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 13.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Lerro CC, Robbins AS, Phillips JL, Stewart AK. Comparison of cases captured in the national cancer data base with those in population-based central cancer registries. Ann Surg Oncol. 2013;20(6):1759–1765. doi: 10.1245/s10434-013-2901-1. [DOI] [PubMed] [Google Scholar]
- 15.Potts WJ. Results of Delay in Treatment of Breast Cancer. Ann Surg. 1928;88(5):842–844. doi: 10.1097/00000658-192811000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bilimoria KY, Ko CY, Tomlinson JS, et al. Wait times for cancer surgery in the United States: trends and predictors of delays. Ann Surg. 2011;253(4):779–785. doi: 10.1097/SLA.0b013e318211cc0f. [DOI] [PubMed] [Google Scholar]
- 17.Jatoi I, Anderson WF, Rosenberg PS. Qualitative age-interactions in breast cancer: a tale of two diseases? Am J Clin Oncol. 2008;31(5):504–506. doi: 10.1097/COC.0b013e3181844d1c. [DOI] [PubMed] [Google Scholar]
- 18.Reinisch M, von Minckwitz G, Harbeck N, et al. Side effects of standard adjuvant and neoadjuvant chemotherapy regimens according to age groups in primary breast cancer. Breast care (Basel, Switzerland) 2013;8(1):60–66. doi: 10.1159/000346834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381(9869):805–816. doi: 10.1016/S0140-6736(12)61963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desch CE, McNiff KK, Schneider EC, et al. American Society of Clinical Oncology/National Comprehensive Cancer Network Quality Measures. J Clin Oncol. 2008;26(21):3631–3637. doi: 10.1200/JCO.2008.16.5068. [DOI] [PubMed] [Google Scholar]
- 21.Vandergrift JL, Niland JC, Theriault RL, et al. Time to adjuvant chemotherapy for breast cancer in National Comprehensive Cancer Network institutions. J Natl Cancer Inst. 2013;105(2):104–112. doi: 10.1093/jnci/djs506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shin DW, Cho J, Kim SY, et al. Delay to Curative Surgery Greater than 12 Weeks Is Associated with Increased Mortality in Patients with Colorectal and Breast Cancer but Not Lung or Thyroid Cancer. Ann Surg Oncol. 2013 doi: 10.1245/s10434-013-2957-y. [DOI] [PubMed] [Google Scholar]
- 23.McLaughlin JM, Anderson RT, Ferketich AK, Seiber EE, Balkrishnan R, Paskett ED. Effect on Survival of Longer Intervals Between Confirmed Diagnosis and Treatment Initiation Among Low-Income Women With Breast Cancer. J Clin Oncol. 2012;30(36):4493–4500. doi: 10.1200/JCO.2012.39.7695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richards MA, Westcombe AM, Love SB, Littlejohns P, Ramirez AJ. Influence of delay on survival in patients with breast cancer: a systematic review. Lancet. 1999;353(9159):1119–1126. doi: 10.1016/s0140-6736(99)02143-1. [DOI] [PubMed] [Google Scholar]
- 25.Comber H, Cronin DP, Deady S, Lorcain PO, Riordan P. Delays in treatment in the cancer services: impact on cancer stage and survival. Ir Med J. 2005;98(8):238–239. [PubMed] [Google Scholar]
- 26.Sainsbury R, Johnston C, Haward B. Effect on survival of delays in referral of patients with breast-cancer symptoms: a retrospective analysis. Lancet. 1999;353(9159):1132–1135. doi: 10.1016/s0140-6736(99)02374-0. [DOI] [PubMed] [Google Scholar]
- 27.Brazda A, Estroff J, Euhus D, et al. Delays in time to treatment and survival impact in breast cancer. Ann Surg Oncol. 2010;17(Suppl 3):291–296. doi: 10.1245/s10434-010-1250-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.