Abstract
Here we show that the interplay between exercise training and dietary fat regulates myelinogenesis in the adult central nervous system. Mice consuming high fat with coordinate voluntary running wheel exercise for 7 weeks showed increases in the abundance of the major myelin membrane proteins, proteolipid (PLP) and myelin basic protein (MBP), in the lumbosacral spinal cord. Expression of MBP and PLP RNA, as well that for Myrf1, a transcription factor driving oligodendrocyte differentiation were also differentially increased under each condition. Furthermore, expression of IGF-1 and its receptor IGF-1R, known to promote myelinogenesis, were also increased in the spinal cord in response to high dietary fat or exercise training. Parallel increases in AKT signaling, a pro-myelination signaling intermediate activated by IGF-1, were also observed in the spinal cord of mice consuming high fat alone or in combination with exercise. Despite the pro-myelinogenic effects of high dietary fat in the context of exercise, high fat consumption in the setting of a sedentary lifestyle reduced OPCs and mature oligodendroglia. Whereas 7 weeks of exercise training alone did not alter OPC or oligodendrocyte numbers, it did reverse reductions seen with high fat. Evidence is presented suggesting that the interplay between exercise and high dietary fat increase SIRT1, PGC-1α and antioxidant enzymes which may permit oligodendroglia to take advantage of diet and exercise-related increases in mitochondrial activity to yield increases in myelination despite higher levels of reactive oxygen species.
Graphical abstract

Introduction
Myelin loss is a key pathophysiological component of neurological injury and disease, including multiple sclerosis, traumatic brain and spinal cord injury, stroke and certain neuropsychiatric disorders (Almad et al. 2011; Armstrong et al. 2015; Franklin and Goldman 2015; Itoh et al. 2015). The loss of myelin is also a recognized part of normal ageing and a risk factor in obesity contributing to cognitive and sensorimotor decline (Kullmann et al. 2015). The physiological significance of oligodendrocytes relates not only to the ability of the myelin sheath to electrically insulate axons thereby increasing the capacity for information processing, but also to their ability to support axon metabolism (Funfschilling et al. 2012; Hirrlinger and Nave 2014). Thus the health of the oligodendrocyte is paramount to the metabolic function of axons (Kang et al. 2013). Identifying factors that impact the function of oligodendrocytes, their progenitors and myelin homeostasis therefore holds important potential for the development of new approaches to promote CNS health and to optimize nervous system function.
Diet is an intrinsic aspect of everyday life and is emerging as a major regulator of brain function and plasticity. In particular, the increasing consumption of saturated fats and sugars is considered detrimental for CNS function (Molteni et al. 2002); however, based on the high content of lipids in brain, how to manage consumption of dietary fats for optimal CNS health is controversial. Myelin membranes have a very high lipid-to-protein ratio, in which lipids account for at least 70% of the dry weight. Myelin assembly therefore requires an extraordinary amount of lipids, especially lipids such as cholesterol, galactolipids, plasmalogen and fatty acids that are enriched in myelin. Indeed, the myelin membrane contains at least 26% cholesterol by weight (Norton and Poduslo 1973), with cholesterol availability rate limiting for myelin formation (Saher et al. 2011). Myelin is particularly enriched in saturated very long chain fatty acids (Chrast et al. 2011), and this high degree of saturation provides a thick permeability barrier for ions and contributes to axon electrical insulation. New studies suggest the role of lipids in myelinating glial cells goes far beyond structural considerations, to include actions in glial development and function, myelin protein trafficking, myelin compaction, and axon metabolic support (Bansal et al. 1999; Hirahara et al. 2004; Nave 2010). Alterations in myelin membrane lipids impact protein packing and consequently can result in disturbances in insulation, myelin compaction, lipid raft signaling and normal interactions between the oligodendrocyte and axon essential for metabolic and trophic support (Wake et al. 2011).
Exercise training promotes beneficial effects on nervous system function developmentally and in adulthood. Increasing evidence indicates that exercise can modulate the action of diet on the CNS since there is a strong metabolic coupling between diet and exercise (Gomez-Pinilla and Tyagi 2013). Given the metabolic partnership between axons and oligodendrocytes, myelin plasticity may play a central role in meeting the increased energy requirements of highly active axons, and in turn modulate neuronal function. For example, new myelin formation in the brain was recently shown to be required for mice to learn to run on a complex wheel (McKenzie et al. 2014). Also, piano playing (Bengtsson et al. 2005), abacus training (Hu et al. 2011) and juggling (Scholz et al. 2009) all result in structural enhancements in white matter tracts within the human brain. Even more subtle environmental changes can impact myelin with rats raised with increased social and cognitive stimulation showing increased myelination of the corpus callosum (Juraska and Kopcik 1988) and oligodendroglia in the occipital cortex (Sirevaag and Greenough 1987; Szeligo and Leblond 1977).
The current study was undertaken to determine the interaction between high fat consumption and exercise training on myelin and myelin forming cells in the adult spinal cord. The potential for oligodendroglial metabolic support of axons is a particularly important consideration for the long axon tracts of the spinal cord (Nave 2010). Results suggest that consumption of high levels of saturated fat in conjunction with a sedentary lifestyle can lead to a loss of myelin forming cells, but that exercise training has the capacity to reverse these adverse effects and promote increased levels of myelinogenesis likely necessary to meet the increased energy demands of exercise.
Materials and Methods
Dietary and exercise interventions
To investigate the impact of high dietary fat or exercise training alone or in combination on myelin in the adult spinal cord, 9 week old male C57BL6J mice (Charles River Laboratories International, Inc., Wilmington, MA) were randomized in individual cages across 4 experimental conditions. Experimental groups included those with a sedentary lifestyle and free access to either a regular diet (SRD) or a high fat diet (SHF). Two additional groups were also created in which mice were placed on either diet, but also provided free access to an exercise running wheel (ERD, EHF). The high fat diet (D12079B) was obtained from Research Diets, (New Brunswick, NJ) and the regular diet (5001) from LabDiet (St. Louis, MO). Mice were housed under environmentally controlled conditions (22–24 C) with a 12 h light/dark cycle. The high fat diet contained 21% fat with 62% of that from saturated fats (D12079B). For comparison, the regular diet (5001) contained 4.5% total fat with 35% from saturated fat. In each case the source of saturated fat was from milk and corn oil fat. The high fat diet contained 2.1% cholesterol and the regular diet contained 0.021%. The high fat diet contained 34% sucrose and the regular diet 3.8% sucrose. The high fat and regular diets contained 4.7 kCal/g (41% from fat) and 4.07 kCal/g (13% from fat) respectively.
Standard polyethylene cages were used for housing and equipped with running wheels (Mini MItter, Bend, Oregon) permitting the mice to run ad libitum. Animal weights and the weight of foot consumed were measured at baseline and weekly for 7 weeks. Running revolutions were recorded using VitalViewer software (Mini Mitter) (n=10 per group). At the end of the 7 week intervention, mice were given a overdose of sodium pentobarbital and perfused with 4% paraformaldehyde with the spinal cord retrieved for histopathology (n = 6 per group). Alternatively, the lumbosacral spinal cord was retrieved unfixed, sectioned sagittally and snap frozen for either protein or RNA isolation (n = 10 mice per group). All animal experiments were carried out with adherence to NIH Guidelines for animal care and safety and were approved by the Mayo Clinic and the University of California, Los Angeles Institutional Animal Care and Use Committees.
Quantification of myelin related proteins
To determine the impact of the dietary and exercise interventions on myelin and the potential mechanism of action, myelin and signaling proteins were quantified by Western blot. One half of the lumbosacral spinal cord harvested unfixed from each mouse was homogenized in radio-immunoprecipitation assay buffer and 35 μg of protein resolved on sodium dodecyl sulfate-polyacrylamide gels (Bio-Rad Laboratories, Hercules, CA). In all cases, electroblotted membranes across groups were processed in unison. Membranes in each case were sequentially probed for antigens of interest, including myelin proteins proteolipid protein (PLP, Ab28486, Abcam, Cambridge, MA), myelin basic protein (MBP, MAB386, Chemicon, Billerica, MA), or the phosphorylated or total protein forms of select signaling proteins, protein kinase B (AKT, 4058L, 9272S, Cell signaling, Boston, MA), or extracellular signal-regulated kinase 1/2 (ERK1/2, 9101S, 9102S, Cell signaling). By careful cutting of membrane strips containing proteins of known molecular weight, we were able to determine each of these proteins across experimental conditions on the same electroblotted membrane. An additional Western blotted membrane per treatment condition was created to determine any impact of the interventions examined on the energy sensing molecules, silent mating type information regulation 2 homolog (SIRT1, Ab121193, Abcam), or peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α, Ab54481, Abcam). Mitochondrial abundance was estimated on the same blots using an anti-mitochondria specific antibody (MTC02, Ab3298, Abcam). Evidence of lipid peroxidation was assessed on additional blotted membranes using 4-HNE (Ab46545, Abcam). In all cases, each unique membrane was also re-probed for β-actin (NB600-501, Novus Biological, Littleton, CO, USA) to further control for loading. Western blotting of proteins isolated from the spinal cord of mice in each of the animal groups was performed in parallel and exposed to film in parallel. Films were scanned and the ROD of bands in each case determined using Image Lab 2.0 software (Bio-Rad Laboratories). The relative optical density (ROD) of each protein of interest was normalized to the ROD of Actin, or in the case of pAKT or pERK1/2, to total AKT or ERK1/2, respectively. The mean and standard error (s.e.) of ROD readings across at least 3 independent Westerns for each antigen of interest was used for statistical comparisons (Yoon et al. 2013).
Quantification of myelin related gene expression
Quantitative real time PCR was used to evaluate the impact of the dietary and exercise interventions on the expression of myelin-related genes (PLP, MBP, 2′,3′-cyclic-nucleotide 3′-phosphodiesterase (CNPase) and myelin regulatory factor (Myrf), insulin like growth factor 1 (IGF-1) or insulin like growth factor 1 receptor (IGF-1R), or the antioxidant enzymes glutathione peroxidase (GPx1) and superoxide dismutase 2 (SOD2). RNA was isolated from that half of the unfixed lumbosacral spinal cord not used for protein extraction using RNA STAT-60 (Tel-Test, Friendswood, TX). The relative amount of RNA in each case was determined in 0.10 μg of RNA using an iCycler iQ5 system (BioRad) and the probes (Thermo Fisher Scientific, Waltham, MA) and primers (Integrated DNA Technologies, Coralville, IA) described in Table 1 (Burda et al. 2013). The relative amount of RNA in each case was normalized to the constitutively expressed gene Rn18S. Mean expression levels under each condition were expressed as a percent of that seen in the spinal cord of mice under SRD conditions.
Table 1. Probes and primers used for quantitative real-time PCR.
Probe sets were obtained from Thermo Fisher Scientific (Assay ID provided) and primers (Forward/Reverse) from Integrated DNA Technologies. (2′,3′-Cyclic-nucleotide 3′-phosphodiesterase (CNPase); glutathione peroxidase (Gpx1); insulin like growth factor 1 (IGF-1); insulin like growth factor 1-receptor (IGF-1R); myelin basic protein (MBP); myelin regulatory factor (Myrf); proteolipid protein (PLP)); 18S ribosomal RNA Rn18S; superoxide dismutase 2 (SOD2).
| Gene | Accession number | Probe and Primer Sets |
|---|---|---|
| CNPase | NM_001146318 | CAAATTCTGTGACTACGGGGGCCTTGCCATACGA |
| Gpx1 | NM_008160.6 | Mm04230607_s1 |
| IGF-1 | NM_010512.4 | Mm00439560_m1 |
| IGF-1R | NM_010513 | Mm00802831_m1 |
| MBP | NM_001025251 | CCAGTAGTCCATTTCTTCAAGAACAT/GCCGATTTATAGTCGGAAGCTC |
| Myrf | NM_001033481.1 | Mm01194959_m1 |
| PLP | NM_011123.2 | TCTTTGGCGACTACAAGACCAC/CACAAACTTGTCGGGATGTCCTA |
| Rn18S | NR_003278.3 | Mm03928990_g1 |
| SOD2 | NM_013671.3 | Mm01313000_m1 |
Quantification of oligodendrocyte and OPC number
To evaluate whether the dietary and exercise interventions affected myelinating cells within the spinal cord, we used immunohistochemical techniques to identify and enumerate OPCs or mature oligodendrocytes. The 4% paraformaldehyde fixed lumbosacral spinal cord retrieved from mice in each condition was blocked into four 1 mm segments with the first and third segments embedded together in paraffin, and the second and fourth segments cryoprotected in sucrose, embedded together in OCT and frozen for cryosectioning. 5 μm paraffin sections were immunostained for oligodendrocyte lineage transcription factor 2 (Olig2) (AB9610, Millipore), or CC-1/APC 1 (adenomatous polyposis coli, Ab16794, Abcam). Olig2 is a basic helix-loop-helix transcription factor expressed by OPCs and oligodendroglia at the early stages of differentiation, whereas CC-1 is associated only with differentiated oligodendrocytes (Funfschilling et al. 2012; Kuhlmann et al. 2008; Ligon et al. 2006). 5 μm frozen sections were immunostained for Nkx2.2 (74.5A5, Developmental Studies Hybridoma Bank, University of Iowa, Iowa city, IA), or for anti-neural glial antigen-2 (NG2, AB5320, Millipore). Nkx2.2 is a homeodomain transcription factor expressed by OPCs that regulates differentiation (Cai et al. 2010; Qi et al. 2001; Zhu et al. 2014). NG2 is a chondroitin sulfate proteoglycan that recognizes OPCs in the intact nervous system (Nishiyama et al. 2009). Immune localization of each antigen was visualized using standard immunoperoxidase techniques (Yoon et al. 2015). Sections immunostained for CC-1 or NG2 were additionally counterstained with methyl green (Vector, Burlingame, CA) to visualize nuclei. In all cases, immunohistochemistry across experimental groups was carried out in parallel. Immunoperoxidase stained sections were cover slipped and the dorsal column and ventrolateral white matter of each section imaged digitally with a 10X objective (Olympus BX51 microscope, Olympus, Center Valley, PA). Counts were made from the digital images of immunopositive cells with a clearly visible nucleus by two independent investigators (HY, AK) without knowledge of the treatment groups. Cell counts made in the dorsal column and ventrolateral white matter were combined for the final analysis. All counts were expressed per unit area with area measurements in each case made using NIH IMAGE J (Bethesda, MD).
Statistical Comparisons
All data were expressed as mean ± s.e.. Comparisons between multiple groups were made using a One-Way Analysis of Variance (ANOVA) and the Newman Keuls (NK) post-hoc test. In the histograms shown, a line is used to show the comparisons being made which begins with the group to which the other groups are being compared and any significance shown using asterisk(s). When data for multiple comparisons was not normally distributed, the Kruskal-Wallis ANOVA on Ranks was applied with Dunn’s method. Pearson correlation analysis was performed to determine potential associations between the changes in protein expression observed in individual samples. Statistical significance was set at P < 0.05.
Results
Weight, food intake and running distance
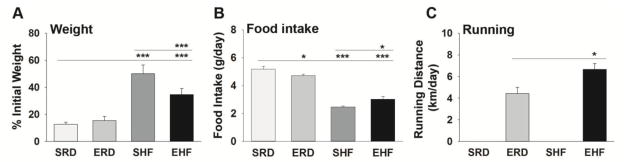
At the conclusion of the 7 week period of dietary and exercise intervention the sedentary regular diet (SRD) mice gained 12.7% ± 1.5% of their initial body weight and the exercise regular diet (ERD) mice gained 15.3% ± 3.0% (Fig. 1). As expected, mice provided a sedentary lifestyle and free access to a high fat diet (SHF) gained a significantly higher percentage of their initial body weight (50.1% ± 6.5%) compared to mice provided regular chow. Mice provided access to a high fat diet and free wheel running (EHF) also showed significant gains over their initial body weight (34.7% ± 4.4%) relative to the SRD and ERD mice, but this gain was significantly less than that seen in mice provided a high fat diet and sedentary lifestyle (SHF) (P < 0.001). Relative to mice in the SRD group in which mean food intake (g/day) over the 7 week period of study was 5.2 ± 0.2 (SRD); food intake was lower in ERD (4.7 ± 0.1, P = 0.03), SHF (2.5 ± 0.1, P < 0.001), and EHF (2.0 ± 0.2, P < 0.001) mice. The mean running distance was 4.4 ± 0.6 km/day for the ERD mice and significantly higher at 6.7 ± 0.5 km/day for the EHF mice (P < 0.05, NK). Despite significant differences in food intake and running distance, kCal/day consumed was nearly identical across the groups (SRD, 71.3 ± 0.8; ERD, 71.9 ± 0.4; SHF, 71.3 ± 0.4; EHF, 72.9 ± 0.9, n=10 per group). This finding is consistent with evidence that food intake is strictly controlled by neuronal circuits to achieve cellular energy homeostasis (Schwartz et al. 2000).
Figure 1. Impact of varying levels of dietary fat and exercise on weight, food intake and running distance.
Histograms show changes in (A) weight (g) from baseline, (B) food intake (g/day), and (C) running distance (km/day) of adult mice provided a sedentary lifestyle and regular diet (SRD), free access to a running wheel and a regular diet (ERD), a sedentary lifestyle and a high fat diet (SHF), or free access to a running wheel and a high fat diet (EHF) for 7 weeks. (n = 10 in each group, *P < 0.05, **P ≤ 0.01, ***P ≤ 0.001, NK).
High dietary fat in combination with exercise training positively modulate myelin protein expression
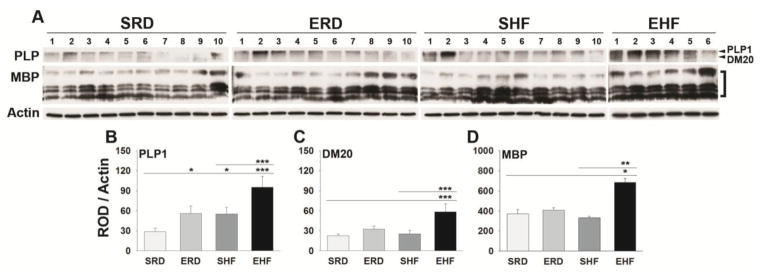
To determine the impact of high dietary fat consumption and exercise training alone or in combination on myelin abundance in the adult lumbosacral spinal cord, the level of the major myelin proteins, PLP and MBP were quantified by Western blot (Fig. 2). PLP has two distinct developmentally regulated isoforms, PLP1 (30 kDa) and DM20 (26 kDa) (Nave et al. 1987), and these were quantified separately. MBP has 4 isoforms (21.5, 20.2, 18.5, and 17.2 kDa) which are generally quantified in collectively (Mi et al. 2011). The combination of high fat consumption and exercise training increased the amount of PLP1 and DM20 protein by 3.4-fold and 2.6-fold respectively, relative to that in the SRD condition (P ≤ 0.004, NK). Exercise training or high fat consumption alone also increased PLP1 by 1.4-fold and 1.1-fold, respectively (P ≤ 0.04, NK).
Figure 2. High dietary fat together with exercise training increases PLP and MBP protein in the adult spinal cord.
Western blots and histograms show that 7 weeks of consumption of a high fat diet in combination with exercise training (EHF) increase the levels of the major myelin proteins, PLP (PLP1 and DM20 isoforms) and MBP in the lumbosacral spinal cord of adult mice. Exercise alone (ERD) or high fat (SHF) also promoted significant increases in PLP1. The two major isoforms of PLP, PLP1 and DM20 were quantified separately while all MBP isoforms were by convention quantified collectively. (*P < 0.05, **P ≤ 0.01, ***P ≤ 0.001, NK). All of the proteins shown in Figs. 2 and 6 were probed on the same membrane and hence one corresponding Actin blot is shown across the Figures. (sedentary regular diet (SRD); exercise regular diet (ERD); sedentary high fat (SHF). (n = 10 per group, except, EHF n = 6)
Levels of MBP were increased by 2.1-fold in mice with access to a high fat diet and exercise training (EHF) relative to the SRD condition (P = 0.02, NK). No significant changes were seen in MBP with exercise (ERD) or high fat consumption (SHF) alone. We point out that myelin proteins (Fig. 2) and the total and activated forms of AKT and ERK1/2 (Fig. 6) were detected on the same membranes.
Figure 6. High fat consumption promotes AKT signaling in the spinal cord.
(A) Western blots and histograms show that 7 weeks of consumption of a high fat diet alone (SHF), or a high fat diet plus exercise training (EHF), each elicit robust increases in AKT signaling (B–D). High fat consumption also promotes a significant increase in total ERK (E–G). The relative optical density (ROD) of the activated form of AKT (pAKT), or ERK1/2 (pERK1/2), was normalized to total AKT or ERK respectively, or to Actin as a loading control (*P < 0.05, **P ≤ 0.01, ***P ≤ 0.001, NK). The proteins shown in Fig. 6 were probed on the same membranes shown in Fig. 2 and hence the corresponding Actin loading control is shown in each Figure. (sedentary regular diet (SRD); exercise regular diet (ERD)).
Differential regulation of oligodendrocyte and OPC-related RNA expression by exercise training and high fat consumption
Changes in the abundance of myelin-related proteins in the spinal cord elicited by exercise training and high dietary fat consumption were further investigated using quantitative real time PCR (Fig. 3). Relative to mice with a sedentary lifestyle (SRD), PLP RNA expression levels were increased by 1.2-fold in response to 7 weeks of exercise training and by 1.4-fold by a parallel period high fat consumption alone, or in combination with exercise training (P < 0.001). Elevations in PLP RNA induced by high fat were significantly greater than those observed by exercise training alone (P = 0.006). Paralleling the increases in PLP expression seen with exercise or high fat consumption, expression of Myrf, a transcription factor mediating oligodendrocyte differentiation (Bujalka et al. 2013), was increased by approximately 1.4-fold (P < 0.001). MBP expression was elevated by exercise training (1.3-fold relative to sedentary mice, P = 0.019, NK), while levels of CNPase were unaffected under any of the conditions examined (data not shown).
Figure 3. Differential effect of exercise training and high fat consumption on the expression of myelin-associated genes.
(A–C) Histograms show changes in myelin-related RNA expression detected by real time PCR in samples isolated from the lumbosacral spinal cord of mice under sedentary conditions (SRD), following 7 weeks of exercise training (ERD) or consumption of a high fat diet (SHF) alone, or in combination (EHF). Expression of myelin proteins (PLP and MBP) and a transcription factor essential for oligodendrocyte differentiation (Myrf1), were all differentially regulated by the high fat and exercise interventions examined. (*P < 0.05, **P ≤ 0.01, ***P ≤ 0.001, NK)). (n = 10 per group)
Exercise training protects from high dietary fat-induced reductions in OPC and oligodendrocyte numbers
Whether the dynamic changes observed in myelin proteins and RNA in response to exercise training and high fat consumption alone or in combination occur at a cellular level was addressed by making counts of OPCs (NG2, Nkx2.2, Olig2), or mature oligodendrocytes (CC-1, Olig2), in the dorsal column and ventrolateral white matter of the lumbosacral spinal cord of mice in each condition (Fig. 4). High fat consumption in the context of a sedentary lifestyle resulted in a 30 to 50% reduction in the number of NG2, Nkx2.2, CC-1 or Olig2-immunopositive cells (P < 0.05). While exercise training alone did not significantly alter OPC or oligodendrocyte numbers, exercise training in combination with high fat consumption, prevented the loss of OPCs and oligodendrocytes seen in the context of high fat consumption alone (P < 0.05, NK).
Figure 4. Exercise training protects against a loss of OPCs and mature oligodendrocytes induced by high fat consumption.
Histograms show counts of NG2 (A), Nkx2.2 (C), CC-1 (E), or Olig2-immunopositive cells (G) in the white matter of the lumbosacral spinal cord of adult mice housed under sedentary regular diet (SRD), exercise regular diet (ERD), sedentary high fat diet (SHF), or exercise high fat (EHF) conditions for 7 weeks. Counts shown represent the mean number per mm2 counted across the dorsal columns and ventrolateral funiculi and are expressed as a percent of that observed in SRD mice. Representative images of NG2 (B), Nkx2.2 (D), CC-1 (F) and Olig2 (H) staining across the different experimental groups are shown. Mice in the SHF group showed reduced numbers of NG2, Nkx2.2, CC-1 and Olig2-immunoreactive cells, an effect completely prevented by co-ordinate exercise training (EHF). (*P < 0.05, **P ≤ 0.01, ***P ≤ 0.001, NK) Scale bar = 50 μm. (n = 6 per group)
Differential regulation of IGF-1 in the spinal cord by dietary fat and exercise training
Given significant increases in the expression of myelin-related proteins and genes occurring in under the influence of high fat consumption and/or exercise, we sought to determine the potential involvement of IGF-1. IGF-1 was selected for initial analysis since it is known to play prominent roles in OPC proliferation, survival and differentiation (Flores et al. 2008; Tyler et al. 2009) by way of its high affinity receptor IGF-1R (D’Ercole et al. 2002; Zeger et al. 2007) and to be regulated by exercise and dietary factors (Ding et al. 2006; Mattson et al. 2004). We used real time PCR to determine expression levels of IGF-1 or IGF-1R in the lumbosacral spinal cord of mice in each experimental condition (Fig. 5). IGF-1 RNA was elevated by 1.4 to 1.6-fold by exercise training or high fat alone and in combination (P < 0.05, NK). IGF-1R RNA was elevated by 1.5-fold by high dietary fat consumption alone or in combination with exercise.
Figure 5. Exercise training and high fat consumption positively modulate expression of IGF-1 in the spinal cord.
Histograms show the differential impact of 7 weeks of high dietary fat consumption and exercise training on the expression of IGF-1 (A) and IGF-1R (B) detected by real time PCR in RNA samples isolated from the lumbosacral spinal cord. Spinal cord and liver samples analyzed were isolated from mice under sedentary conditions (SRD), following 7 weeks of exercise training (ERD) or consumption of a high fat diet (SHF) alone, or in combination (EHF). (*P < 0.05, **P ≤ 0.01, ***P ≤ 0.001, NK).
High dietary fat promotes AKT signaling in the lumbosacral spinal cord
To further investigate the potential mechanism by which dietary fat and exercise training may affect levels of spinal cord myelin, we investigated the AKT and ERK1/2 signaling pathways known to be points of convergence for multiple growth factors playing essential roles in myelin development, including IGF-1 (Bibollet-Bahena and Almazan 2009; McMorris and Dubois-Dalcq 1988) (Fig. 6). Mice consuming a high a fat diet increased levels of the activated form of AKT by approximately 2-fold (P < 0.001, NK). Exercise training alone did not impact AKT and did not significantly alter the elevated levels of AKT signaling resulting from high fat consumption. High dietary fat consumption did increase levels of total ERK1/2 by approximately 1.2-fold. Exercise training alone did not impact ERK1/2 signaling, but in combination with high fat, returned ERK1/2 levels to baseline. Overall levels of the activated form of ERK were not significantly impacted by the dietary and exercise training interventions examined. The data reported for AKT and ERK1/2 were determined on the same membrane as that used to demonstrate changes in PLP and MBP (Fig. 2).
Dietary fat and exercise regulate energy homeostasis in the spinal cord
Whether consumption of high dietary fat and exercise may converge on mitochondrial biogenesis and energy metabolism within the spinal cord was examined by quantifying SIRT1 and PGC-1α, in addition to MTC02 a mitochondrial protein reflective of mitochondrial abundance (Fig. 7). SIRT1 is an energy-sensing molecule that regulates PGC-1α, a transcriptional co-activator playing critical roles in regulating cellular energy metabolism (Ng et al. 2015) and myelin formation (Rafalski et al. 2013). SIRT1 protein was elevated by 1.2-fold by the combined effects of high fat consumption and exercise (P = 0.006), but not by either intervention alone. PGC-1α levels were increased by approximately 1.6-fold by high fat consumption or exercise, alone or in combination (P = 0.006). The abundance of the mitochondrial protein MTC02 was elevated by 2.0-fold under the influence of high dietary fat (P = 0.014), an effect reversed by exercise training (P = 0.009, NK).
Figure 7. High dietary fat and exercise converge on SIRT1 and PGC-1α to modulate mitochondrial abundance in the adult spinal cord.
Western blots (A) and histograms show that 7 weeks of high fat consumption in combination with exercise training (EHF) promotes increases in SIRT1 (B). High fat consumption (SHF) also promotes a significant increase in MTC02 (C), a mitochondrial protein used as measure of abundance. Relative to sedentary conditions (SRD), exercise (ERD) or high fat alone, or in combination, each increased PGC-1α (D). (E) MTC02 levels were positively correlated with PLP in mice with free access to exercise training. (F) PGC-1α levels were positively correlated with those for MBP in mice consuming high fat in conjunction with exercise. The relative optical density (ROD) of each protein was normalized to Actin as a loading control. All of the proteins shown were probed on the same Western blot membrane and hence one corresponding Actin loading control is shown. (*P < 0.05, ** P ≤ 0.01, ***P ≤ 0.001, NK).
To determine the potential for diet and exercise induced changes in SIRT1, PGC-1α and mitochondrial abundance to impact the expression of myelin proteins the percent change in the level of each protein relative to SRD conditions was examined for correlations using Pearson product-moment correlation coefficient (Fig. 7E–F). Mitochondrial abundance, as measured by MTC02, was positively correlated with PLP protein levels under ERD conditions (Fig. 7E, P = 0.04). Spinal cord PGC-1α was positively correlated with MBP levels under EHF conditions (Fig. 7F, P = 0.05, NK).
High dietary fat and exercise promote lipid peroxidation and coordinate changes in antioxidant enzymes
Reactive oxygen species (ROS) are a byproduct of the mitochondrial electron transport chain and fatty acid oxidation and promote oxidative damage by increasing peroxidation of unsaturated fatty acids. Given the elevations in mitochondrial markers seen with the consumption of high fat, we examined the effects of exercise or high fat consumption alone, or in combination, on the levels of 4-hydroxynonenan (4-HNE), a lipid degradation product that is elevated during oxidative stress. Across the groups examined three major protein bands with molecular weights of approximately 39, 50 and 70 kDa showed strong immunoreactivity for 4-HNE modifications in the spinal cord (Fig. 8A–E). Exercise training reduced 4-HNE modification of the 50 kDa protein by 4-fold (P < 0.001). Significant increases in 4-HNE modifications to the 39 kDa protein were seen with high fat (3.8-fold), effects that were magnified by exercise (8.9-fold elevation relative to SRD mice) (P < 0.001). High fat in combination with exercise also increased 4-HNE modifications to the 70 kDa protein (1.4-fold over SRD, P = 0.01, NK). These data suggest that while exercise reduces ROS, high fat alone or in combination with exercise have the potential to increases ROS in the adult spinal cord.
Figure 8. Differential impact of exercise and high fat consumption on spinal cord 4-HNE.
Western blots (A) and histograms (B–E) demonstrate that while 7 weeks of exercise training alone (ERD) results in reductions in 4-HNE modified proteins, consumption of a high fat diet alone (SHF, B), or in combination with exercise (EHF, BE) results in significant increases relative to sedentary mice consuming regular chow (SRD). The relative optical density (ROD) of each 4-HNE modified protein (39 kDa, 50 kDa or 70 kDa) was examined alone or in combination and in each case was normalized to Actin as a loading control. Expression of antioxidant enzymes (F) GPx1 and (G) SOD2 were increased by either exercise or high fat alone or in combination. (H) The overall levels of expression of these ROS defense genes were higher in EHF relative to SHF conditions (*P < 0.05, ** P ≤ 0.01, ***P ≤ 0.001, NK).
GPx1 and SOD2 are among the most abundant antioxidant enzymes and their levels of expression in the spinal cord were examined to consider how they might impact the dynamic changes in ROS observed (Fig. 8F–H). The expression of each enzyme was increased in the spinal cord by approximately 20 to 35% by exercise or high fat alone, or in combination (P ≤ 0.01, NK). Since it is the overall levels of these ROS defense genes that would impact lipid peroxidation, we examined their levels in combination and found these to be significantly increased in EHF mice relative to those consuming high fat alone. These results point to a potentially positive impact of exercise on the levels of antioxidant enzymes, even in the context of high fat consumption.
Discussion
We assessed the effects of exercise training alone or in the context of high dietary fat on parameters of myelin formation and oligodendrogenesis in the adult spinal cord. Findings suggest that consumption of a diet high in saturated fat in the setting of a sedentary lifestyle leads to reductions in myelinating cells and that these deleterious effects can be prevented by coordinate exercise training. In addition results point to an important interplay between high dietary fat and exercise training that increases the predominant myelin proteins PLP and MBP. Exercise in conjunction with high fat consumption also boosted spinal cord IGF-1, its high affinity receptor (IGF-1R), and activated AKT, a key signaling partner known to promote myelinogenesis. Coordinate elevations in SIRT1, PGC-1α, 4-HNE, GPx1 and SOD2 suggest the dietary- and exercise-mediated changes in spinal cord myelinogenesis observed may be linked to changes in mitochondrial function, energy metabolism, lipid peroxidation and levels of antioxidant enzymes. Altogether, results suggest that the beneficial effects of exercise on spinal cord function seen with exercise training (Roy et al. 2012) may be mediated in part by regulating energy metabolism, reducing oxidative stress and stimulating myelinogenesis, and importantly that the availability of dietary saturated fat can play an important role in this process.
The interplay between exercise training and high dietary fat results in increased PLP and MBP production
The combination of exercise training and high dietary fat consumption elicited significant increases in the major myelin proteins, PLP and MBP. The myelin membrane has a very high lipid-to-protein ratio, with lipids accounting for at least 70% of its dry weight (Saher et al. 2011). PLP is a transmembrane protein holding the myelin wraps together with abnormalities resulting in axonopathy in Pelizaeus-Merbacker disease (Werner et al. 1998) and spastic paraplegia type 2 (Garbern et al. 1997; Inoue 2005). Exercise or high fat consumption each drove production of PLP RNA and PLP1 protein, but their interactive properties had the greatest capacity to increase protein levels, including both the PLP1 and DM20 isoforms. MBP also contributes to maintenance of myelin membrane structure by interacting with lipids (Aggarwal et al. 2013). While exercise training resulted in significant increases in MBP RNA, elevations at a protein level were limited to mice also consuming a high fat diet. The overlapping changes in PLP RNA and protein imply that elevations were due at least in part to new protein synthesis rather than changes in degradation or stability. Since OPC and oligodendrocyte numbers were not higher than baseline under any condition examined, elevations in PLP and MBP under the influence of exercise and high fat likely occur as a result of increased protein production per cell. Even in the context of high fat alone, in which OPCs and oligodendrocyte numbers were reduced, increases in PLP RNA and protein were observed, suggesting that the remaining cells increased levels of expression in a compensatory manner. Also, since no effects of the interventions examined occurred in the expression of another myelin-associated gene CNPase, the effects on myelin dynamics observed may occur in part through differential gene expression.
Supporting the concept that increases in either exercise or dietary fat have the potential to promote new myelin synthesis, we observed that both interventions stimulated the expression of Myrf1. Myrf1 is a transcription factor essential for initiating and maintaining the oligodendrocyte differentiation program, including the expression of MBP and PLP (Bujalka et al. 2013; Emery et al. 2009). Highlighting the need for active renewal of myelin membranes, a loss of Myrf1 in adulthood results in demyelination (Koenning et al. 2012) and affects motor skill learning in mice (McKenzie et al. 2014). The current studies provide new rationale to examine the link between high fat consumption, exercise and Myrf1.
IGF-1-AKT signaling in diet and exercise induced myelin plasticity
AKT signaling was increased in the spinal cord of mice consuming high fat under either sedentary or exercise conditions. AKT is a key signaling intermediate established to play essential roles in oligodendrocyte development and myelination (Czopka et al. 2010; Flores et al. 2000; Goebbels et al. 2010; Harrington et al. 2010; Narayanan et al. 2009; Sherman et al. 2012; Tyler et al. 2009). The actions of AKT signaling in myelin growth extend to the development of morphological complexity, myelin protein expression, lipid synthesis, and cytoskeletal rearrangement (Narayanan et al. 2009; Tyler et al. 2009; Tyler et al. 2011; Wahl et al. 2014). High dietary fat also elicited a small but significant increase in total ERK1/2, another signaling pathway with pro-myelination effects (Fyffe-Maricich et al. 2011; Fyffe-Maricich et al. 2013; Guardiola-Diaz et al. 2012; Ishii et al. 2013; Ishii et al. 2012). While additional studies regarding the contributory role of AKT in the pro-myelination effects observed with high dietary fat and exercise will be needed, a potential link is supported by the co-ordinate increases occurring in the expression of IGF-1 under either condition alone or in combination. IGF-1 drives the PI3K-AKT-mTOR signaling pathway and is among the key growth factors regulating oligodendrocyte physiology and myelin production (Cao et al. 2003; Carson et al. 1993; Lin et al. 2005). Notably, the high affinity receptor for IGF-1 was also positively modulated by high fat alone or with exercise, an effect that could serve to potentiate the actions of IGF. Together, these findings suggest that lifestyle interventions can positively modulate growth factor signaling mechanisms that are likely to improve myelin dynamics in the adult spinal cord.
High fat consumption in sedentary mice reduces spinal cord oligodendrocytes and their progenitors: an effect prevented by exercise training
We sought to determine whether changes in myelin and pro-myelination signaling systems observed at a molecular level were reflected in the abundance of oligodendrocytes or their progenitors. Rather unexpectedly based on the results discussed above, consumption of high fat in the setting of a sedentary lifestyle resulted in a loss of NG2+ or Nkx2.2+ OPCs and mature CC-1+ oligodendrocytes in spinal cord white matter. Parallel changes in the number of Olig2+ cells, a transcription factor present in both OPCs and young oligodendrocytes, supports the conclusion that the negative effects of high fat consumption in sedentary mice extends to both oligodendrocytes and their progenitors. Of considerable therapeutic interest, findings suggest that the deleterious effects of a high fat diet on the abundance of myelinating cells in the adult spinal cord can be completely reversed by coordinate exercise. While 7 weeks of exercise training by itself did not alter OPC or oligodendrocyte numbers, it completely prevented the loss of myelinating cells observed in sedentary mice consuming high fat. Whether preservation of oligodendrocytes and their progenitors under conditions of high fat and exercise occurred by preventing loss or cell replacement will require further investigation. Together the data presented suggest that rehabilitative exercise programs are likely of fundamental importance to the maintenance of spinal cord myelin health, particularly in individuals consuming a high fat diet. Therefore, levels of exercise and dietary fat are both key considerations for the development of treatment plans for neurological conditions in which myelin integrity and repair are primary concerns.
Role of cell metabolic signals driven by diet and exercise in myelinogenesis
PGC-1α, a transcriptional co-activator known to enhance mitochondrial biogenesis, fatty acid oxidation and oxidative metabolism (Johri et al. 2013; Liang and Ward 2006) and to play key roles in myelinogenesis (Camacho et al. 2013; Kiebish et al. 2012; Xiang et al. 2011), was increased in the spinal cord by exercise or high fat, alone and in combination. In relation to cellular energy metabolism, MTC02 protein levels, a marker of mitochondrial abundance, were also increased in the spinal cord of mice consuming high fat. Prior studies provide strong links between PGC-1α and myelin health, with PGC-1α knockout mice having pronounced white matter abnormalities in the brain that may be connected to its involvement in expression of MBP and cholesterol synthesis intermediates such as SREBP and LXR (Kiebish et al. 2012; Leone et al. 2005; Xiang et al. 2011). The current studies strengthen the link between PGC-1α and myelin by demonstrating that under the influence of high dietary fat and exercise; PGC-1α levels were positively correlated with those for MBP. Together these findings support the concept that increasing dietary fat in conjunction with exercise training has the greatest capacity to promote myelinogenesis and that this may be facilitated in part by increases in PGC-1α.
Elevations in spinal cord SIRT1, an energy-sensing molecule controlling mitochondrial energy metabolism, were only observed under the influence of high dietary fat in combination with exercise. SIRTs have emerged as key metabolic sensors that directly link environmental signals to metabolic homeostasis and stress responses. SIRT1 is a histone/protein deacetylase that regulates the activity of a variety of transcription factors and co-regulators, including PGC-1α to increase mitochondrial function, energy metabolism and gluconeogenesis (Ng et al. 2015). Increases in spinal cord PLP and MBP protein under the influence of high dietary fat in combination with exercise occurred in conjunction with increases in SIRT1, suggesting a possible link between the events that warrants further study.
Potential involvement of reactive oxygen species and lipid peroxidation in spinal cord myelinogenesis
Based on prior research demonstrating that high dietary saturated fat and high levels of mitochondrial activity promote the formation of free radicals, we sought to determine whether reactive oxygen species (ROS) were altered in the spinal cord of mice consuming high fat since this could account for the reductions in myelinating cells observed. Oxygen free radicals are a byproduct of ATP production with 1–4% of oxygen consumed converted to O2-. ROS disrupt the integrity of cellular and intracellular membrane polyunsaturated fatty acids compromising essential membrane functions. Notably, OPCs and oligodendrocytes are particularly vulnerable to the effects of ROS since they express low levels of enzymes that counteract oxygen free radicals, including GPx1 and SOD2 (Husain and Juurlink 1995; Yeo et al. 2012). In addition, oligodendrocytes have a high iron content that can play a key role in the initiation and propagation of lipid peroxidation (Hall et al. 2010). Lipid peroxidation generates fragmented peroxidized polyunsaturated fatty acids and aldehydic end products including 4-HNE. 4-HNE in turn can bind to key mitochondrial proteins, impairing their function (Hall et al. 2010; Vaishnav et al. 2010) and resulting in neurotoxicity (Raza and John 2006; Uchida 2003). Supporting the involvement of ROS in the loss of OPCs and oligodendrocytes, we document elevated levels of 4-HNE modified proteins in the spinal cord of mice consuming high fat. Supporting the beneficial roles of exercise in regulating oxidative stress, 4-HNE levels were reduced in the spinal cord of mice afforded exercise training alone.
Even though exercise reduced 4-HNE levels when provided under the influence of a regular diet, in the setting of high fat consumption, 4-HNE byproducts were observed at even higher levels. Higher levels of lipid peroxidation end products observed in the spinal cord of EHF mice may relate to the additive effects of ROS generated from cellular respiration, likely the largest source, and fatty acid oxidation (Rosca et al. 2012). Despite the higher levels of ROS, no overall reduction in OPCs or oligodendrocytes was observed in the spinal cord white matter of mice consuming high fat in the setting of exercise. A possible explanation for the protective effect of exercise on myelinating cell numbers in the context of high fat consumption relates to the coordinate increases observed in SIRT1. There is strong evidence that SIRT1 plays key roles in managing oxidative stress responses by deacetylating several transcription factors that regulate antioxidant genes including PGC-1α examined here, in addition to FOXO family transcription factors (Brunet et al. 2004). For example, PGC-1α up regulates expression of oxidative stress genes, including GPx1, catalase, and SOD2 (Pardo and Boriek 2012; Pardo et al. 2011; St-Pierre et al. 2006). Higher levels of GPx1 and SOD2 expression were observed in the spinal cord of mice consuming high fat under the influence of exercise, which may have protected myelinating cells from the higher levels of ROS. It will be important in future studies to determine the extent to which the ability of exercise training to mitigate high fat-mediated loss of myelinating cells can be attributed to SIRT1-PCG-1α driven antioxidant mechanisms. Prior studies demonstrate that SIRT1 activation improves outcomes in EAE, while SIRT1 inhibition blocks the neuroprotective effects (Shindler et al. 2010; Shindler et al. 2007). The feasibility of targeting SIRT1 activity for myelin protection in health and disease will be an important line of future investigation.
Conclusion
Data presented suggest that the spinal cord is capable of adapting to the demands of a high-energy diet when afforded ample exercise, by increasing IGF-1 signaling, SIRT1, PGC-1α and free radical scavengers (Fig. 9). The powerful impact of exercise on promoting myelin production in the presence of high dietary fat is of particular interest in the context of emerging evidence that myelinogenesis can be positively modulated by neural activity (Bengtsson et al. 2005; McKenzie et al. 2014; Sanchez et al. 1998; Scholz et al. 2009; Sirevaag and Greenough 1987; Szeligo and Leblond 1977; Teicher et al. 2004). These key changes in critical regulators of metabolism may provide protection to OPCs and oligodendrocytes and result in increases in the production of myelin proteins. In the absence of exercise however, evidence presented suggests that a high fat diet results in increases in ROS without coordinate elevations in SIRT1 and this could account for the deleterious impact of excess high fat on myelinating cells observed. These findings have important implications for the design of rehabilitative programs to enhance functional capacity by promoting myelin repair with both dietary fat content and exercise being essential considerations.
Figure 9. Hypothetical model by which dietary fat and exercise influence myelin dynamics in the adult spinal cord.
Taken with evidence from prior studies, we highlight 3 possible interactive mechanisms by which dietary fat and exercise may promote myelin homeostasis. (1) Dietary fat may serve as a source of myelin membrane precursors, including fatty acids and cholesterol (Chrast et al. 2011). (2) Data presented suggest that high fat consumption on its own, or in combination with exercise, can increase IGF-1 and pro-myelinogenic signaling pathways such as AKT. (3) The interactive actions of exercise and high fat may also serve to regulate pathways associated with energy homeostasis involving mitochondrial function. Our data show that exercise and diet increased key regulators of energy homeostasis including SIRT1 and PGC-1α, mitochondria, and the balance between reactive oxygen species (ROS) and antioxidant enzymes. We suggest that increases in SIRT1 under conditions of high fat consumption with exercise drive PGC-1α and increased levels of antioxidant enzymes (see Fig. 8) that may serve to protect OPCs and mature oligodendrocytes from the damaging effects of ROS. Future studies will be needed to verify different parts of this model and determine whether any or all of the components depicted are necessary or sufficient to promote adaptive myelination.
Highlights.
High dietary fat alone promotes loss of spinal cord oligodendrocytes and their progenitors.
Exercise training attenuates the negative effects of high dietary fat on myelin.
Exercise appears as driving force to re-establish myelin homeostasis.
Acknowledgments
Studies were supported by the National Institutes of Health R01NS052741 (IAS) and NIH R01NS050465-11 (FGP), Pilot Project PP2009 and a RG4958 from the National Multiple Sclerosis Society (IAS), and an Accelerated Regenerative Medicine Award from the Mayo Clinic Center for Regenerative Medicine (IAS).
Footnotes
Conflicts of Interest: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggarwal S, Snaidero N, Pahler G, Frey S, Sanchez P, Zweckstetter M, Janshoff A, Schneider A, Weil MT, Schaap IA, et al. Myelin membrane assembly is driven by a phase transition of myelin basic proteins into a cohesive protein meshwork. PLoS Biol. 2013;11:e1001577. doi: 10.1371/journal.pbio.1001577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almad A, Sahinkaya FR, McTigue DM. Oligodendrocyte fate after spinal cord injury. Neurotherapeutics. 2011;8:262–73. doi: 10.1007/s13311-011-0033-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong RC, Mierzwa AJ, Sullivan GM, Sanchez MA. Myelin and oligodendrocyte lineage cells in white matter pathology and plasticity after traumatic brain injury. Neuropharmacology. 2015 doi: 10.1016/j.neuropharm.2015.04.029. [DOI] [PubMed] [Google Scholar]
- Bansal R, Winkler S, Bheddah S. Negative regulation of oligodendrocyte differentiation by galactosphingolipids. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:7913–24. doi: 10.1523/JNEUROSCI.19-18-07913.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, Ullen F. Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci. 2005;8:1148–50. doi: 10.1038/nn1516. [DOI] [PubMed] [Google Scholar]
- Bibollet-Bahena O, Almazan G. IGF-1-stimulated protein synthesis in oligodendrocyte progenitors requires PI3K/mTOR/Akt and MEK/ERK pathways. J Neurochem. 2009;109:1440–51. doi: 10.1111/j.1471-4159.2009.06071.x. [DOI] [PubMed] [Google Scholar]
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–5. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- Bujalka H, Koenning M, Jackson S, Perreau VM, Pope B, Hay CM, Mitew S, Hill AF, Lu QR, Wegner M, et al. MYRF is a membrane-associated transcription factor that autoproteolytically cleaves to directly activate myelin genes. PLoS biology. 2013;11:e1001625. doi: 10.1371/journal.pbio.1001625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burda JE, Radulovic M, Yoon H, Scarisbrick IA. Critical role for PAR1 in kallikrein 6-mediated oligodendrogliopathy. Glia. 2013;61:1456–70. doi: 10.1002/glia.22534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Zhu Q, Zheng K, Li H, Qi Y, Cao Q, Qiu M. Co-localization of Nkx6.2 and Nkx2.2 homeodomain proteins in differentiated myelinating oligodendrocytes. Glia. 2010;58:458–68. doi: 10.1002/glia.20937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho A, Huang JK, Delint-Ramirez I, Yew Tan C, Fuller M, Lelliott CJ, Vidal-Puig A, Franklin RJ. Peroxisome proliferator-activated receptor gamma-coactivator-1 alpha coordinates sphingolipid metabolism, lipid raft composition and myelin protein synthesis. The European journal of neuroscience. 2013;38:2672–83. doi: 10.1111/ejn.12281. [DOI] [PubMed] [Google Scholar]
- Cao Y, Gunn AJ, Bennet L, Wu D, George S, Gluckman PD, Shao XM, Guan J. Insulin-like growth factor (IGF)-1 suppresses oligodendrocyte caspase-3 activation and increases glial proliferation after ischemia in near-term fetal sheep. J Cereb Blood Flow Metab. 2003;23:739–47. doi: 10.1097/01.WCB.0000067720.12805.6F. [DOI] [PubMed] [Google Scholar]
- Carson MJ, Behringer RR, Brinster RL, McMorris FA. Insulin-like growth factor I increases brain growth and central nervous system myelination in transgenic mice. Neuron. 1993;10:729–40. doi: 10.1016/0896-6273(93)90173-o. [DOI] [PubMed] [Google Scholar]
- Chrast R, Saher G, Nave KA, Verheijen MH. Lipid metabolism in myelinating glial cells: lessons from human inherited disorders and mouse models. J Lipid Res. 2011;52:419–34. doi: 10.1194/jlr.R009761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czopka T, von Holst A, ffrench-Constant C, Faissner A. Regulatory mechanisms that mediate tenascin C-dependent inhibition of oligodendrocyte precursor differentiation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:12310–22. doi: 10.1523/JNEUROSCI.4957-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ercole AJ, Ye P, O’Kusky JR. Mutant mouse models of insulin-like growth factor actions in the central nervous system. Neuropeptides. 2002;36:209–20. doi: 10.1054/npep.2002.0893. [DOI] [PubMed] [Google Scholar]
- Ding Q, Vaynman S, Akhavan M, Ying Z, Gomez-Pinilla F. Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience. 2006;140:823–33. doi: 10.1016/j.neuroscience.2006.02.084. [DOI] [PubMed] [Google Scholar]
- Emery B, Agalliu D, Cahoy JD, Watkins TA, Dugas JC, Mulinyawe SB, Ibrahim A, Ligon KL, Rowitch DH, Barres BA. Myelin gene regulatory factor is a critical transcriptional regulator required for CNS myelination. Cell. 2009;138:172–85. doi: 10.1016/j.cell.2009.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores AI, Mallon BS, Matsui T, Ogawa W, Rosenzweig A, Okamoto T, Macklin WB. Akt-mediated survival of oligodendrocytes induced by neuregulins. J Neurosci. 2000;20:7622–30. doi: 10.1523/JNEUROSCI.20-20-07622.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores AI, Narayanan SP, Morse EN, Shick HE, Yin X, Kidd G, Avila RL, Kirschner DA, Macklin WB. Constitutively active Akt induces enhanced myelination in the CNS. J Neurosci. 2008;28:7174–83. doi: 10.1523/JNEUROSCI.0150-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin RJ, Goldman SA. Glia Disease and Repair-Remyelination. Cold Spring Harb Perspect Biol. 2015;7 doi: 10.1101/cshperspect.a020594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J, Brinkmann BG, Kassmann CM, Tzvetanova ID, Mobius W, et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature. 2012;485:517–21. doi: 10.1038/nature11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyffe-Maricich SL, Karlo JC, Landreth GE, Miller RH. The ERK2 mitogen-activated protein kinase regulates the timing of oligodendrocyte differentiation. J Neurosci. 2011;31:843–50. doi: 10.1523/JNEUROSCI.3239-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyffe-Maricich SL, Schott A, Karl M, Krasno J, Miller RH. Signaling through ERK1/2 controls myelin thickness during myelin repair in the adult central nervous system. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:18402–8. doi: 10.1523/JNEUROSCI.2381-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbern JY, Cambi F, Tang XM, Sima AA, Vallat JM, Bosch EP, Lewis R, Shy M, Sohi J, Kraft G, et al. Proteolipid protein is necessary in peripheral as well as central myelin. Neuron. 1997;19:205–18. doi: 10.1016/s0896-6273(00)80360-8. [DOI] [PubMed] [Google Scholar]
- Goebbels S, Oltrogge JH, Kemper R, Heilmann I, Bormuth I, Wolfer S, Wichert SP, Mobius W, Liu X, Lappe-Siefke C, et al. Elevated phosphatidylinositol 3,4,5-trisphosphate in glia triggers cell-autonomous membrane wrapping and myelination. J Neurosci. 2010;30:8953–64. doi: 10.1523/JNEUROSCI.0219-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Tyagi E. Diet and cognition: interplay between cell metabolism and neuronal plasticity. Curr Opin Clin Nutr Metab Care. 2013;16:726–33. doi: 10.1097/MCO.0b013e328365aae3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardiola-Diaz HM, Ishii A, Bansal R. Erk1/2 MAPK and mTOR signaling sequentially regulates progression through distinct stages of oligodendrocyte differentiation. Glia. 2012;60:476–86. doi: 10.1002/glia.22281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall ED, Vaishnav RA, Mustafa AG. Antioxidant therapies for traumatic brain injury. Neurotherapeutics. 2010;7:51–61. doi: 10.1016/j.nurt.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington EP, Zhao C, Fancy SP, Kaing S, Franklin RJ, Rowitch DH. Oligodendrocyte PTEN is required for myelin and axonal integrity, not remyelination. Annals of neurology. 2010;68:703–16. doi: 10.1002/ana.22090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirahara Y, Bansal R, Honke K, Ikenaka K, Wada Y. Sulfatide is a negative regulator of oligodendrocyte differentiation: development in sulfatide-null mice. Glia. 2004;45:269–77. doi: 10.1002/glia.10327. [DOI] [PubMed] [Google Scholar]
- Hirrlinger J, Nave KA. Adapting brain metabolism to myelination and long-range signal transduction. Glia. 2014;62:1749–61. doi: 10.1002/glia.22737. [DOI] [PubMed] [Google Scholar]
- Hu Y, Geng F, Tao L, Hu N, Du F, Fu K, Chen F. Enhanced white matter tracts integrity in children with abacus training. Hum Brain Mapp. 2011;32:10–21. doi: 10.1002/hbm.20996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain J, Juurlink BH. Oligodendroglial precursor cell susceptibility to hypoxia is related to poor ability to cope with reactive oxygen species. Brain Res. 1995;698:86–94. doi: 10.1016/0006-8993(95)00832-b. [DOI] [PubMed] [Google Scholar]
- Inoue K. PLP1-related inherited dysmyelinating disorders: Pelizaeus-Merzbacher disease and spastic paraplegia type 2. Neurogenetics. 2005;6:1–16. doi: 10.1007/s10048-004-0207-y. [DOI] [PubMed] [Google Scholar]
- Ishii A, Furusho M, Bansal R. Sustained activation of ERK1/2 MAPK in oligodendrocytes and schwann cells enhances myelin growth and stimulates oligodendrocyte progenitor expansion. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:175–86. doi: 10.1523/JNEUROSCI.4403-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii A, Fyffe-Maricich SL, Furusho M, Miller RH, Bansal R. ERK1/ERK2 MAPK signaling is required to increase myelin thickness independent of oligodendrocyte differentiation and initiation of myelination. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:8855–64. doi: 10.1523/JNEUROSCI.0137-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Maki T, Lok J, Arai K. Mechanisms of cell-cell interaction in oligodendrogenesis and remyelination after stroke. Brain Res. 2015 doi: 10.1016/j.brainres.2015.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johri A, Chandra A, Beal MF. PGC-1alpha, mitochondrial dysfunction, and Huntington’s disease. Free Radic Biol Med. 2013;62:37–46. doi: 10.1016/j.freeradbiomed.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juraska JM, Kopcik JR. Sex and environmental influences on the size and ultrastructure of the rat corpus callosum. Brain Res. 1988;450:1–8. doi: 10.1016/0006-8993(88)91538-7. [DOI] [PubMed] [Google Scholar]
- Kang SH, Li Y, Fukaya M, Lorenzini I, Cleveland DW, Ostrow LW, Rothstein JD, Bergles DE. Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis. Nat Neurosci. 2013;16:571–9. doi: 10.1038/nn.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiebish MA, Young DM, Lehman JJ, Han X. Chronic caloric restriction attenuates a loss of sulfatide content in PGC-1alpha−/− mouse cortex: a potential lipidomic role of PGC-1alpha in neurodegeneration. J Lipid Res. 2012;53:273–81. doi: 10.1194/jlr.M020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenning M, Jackson S, Hay CM, Faux C, Kilpatrick TJ, Willingham M, Emery B. Myelin gene regulatory factor is required for maintenance of myelin and mature oligodendrocyte identity in the adult CNS. J Neurosci. 2012;32:12528–42. doi: 10.1523/JNEUROSCI.1069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann T, Miron V, Cui Q, Wegner C, Antel J, Bruck W. Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain. 2008;131:1749–58. doi: 10.1093/brain/awn096. [DOI] [PubMed] [Google Scholar]
- Kullmann S, Schweizer F, Veit R, Fritsche A, Preissl H. Compromised white matter integrity in obesity. Obes Rev. 2015;16:273–81. doi: 10.1111/obr.12248. [DOI] [PubMed] [Google Scholar]
- Leone TC, Lehman JJ, Finck BN, Schaeffer PJ, Wende AR, Boudina S, Courtois M, Wozniak DF, Sambandam N, Bernal-Mizrachi C, et al. PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol. 2005;3:e101. doi: 10.1371/journal.pbio.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, Ward WF. PGC-1alpha: a key regulator of energy metabolism. Adv Physiol Educ. 2006;30:145–51. doi: 10.1152/advan.00052.2006. [DOI] [PubMed] [Google Scholar]
- Ligon KL, Fancy SP, Franklin RJ, Rowitch DH. Olig gene function in CNS development and disease. Glia. 2006;54:1–10. doi: 10.1002/glia.20273. [DOI] [PubMed] [Google Scholar]
- Lin S, Fan LW, Pang Y, Rhodes PG, Mitchell HJ, Cai Z. IGF-1 protects oligodendrocyte progenitor cells and improves neurological functions following cerebral hypoxia-ischemia in the neonatal rat. Brain Res. 2005;1063:15–26. doi: 10.1016/j.brainres.2005.09.042. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Maudsley S, Martin B. A neural signaling triumvirate that influences ageing and age-related disease: insulin/IGF-1, BDNF and serotonin. Ageing Res Rev. 2004;3:445–64. doi: 10.1016/j.arr.2004.08.001. [DOI] [PubMed] [Google Scholar]
- McKenzie IA, Ohayon D, Li H, de Faria JP, Emery B, Tohyama K, Richardson WD. Motor skill learning requires active central myelination. Science. 2014;346:318–22. doi: 10.1126/science.1254960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMorris FA, Dubois-Dalcq M. Insulin-like growth factor I promotes cell proliferation and oligodendroglial commitment in rat glial progenitor cells developing in vitro. J Neurosci Res. 1988;21:199–209. doi: 10.1002/jnr.490210212. [DOI] [PubMed] [Google Scholar]
- Mi S, Lee X, Hu Y, Ji B, Shao Z, Yang W, Huang G, Walus L, Rhodes K, Gong BJ, et al. Death receptor 6 negatively regulates oligodendrocyte survival, maturation and myelination. Nature medicine. 2011;17:816–21. doi: 10.1038/nm.2373. [DOI] [PubMed] [Google Scholar]
- Molteni R, Ying Z, Gomez-Pinilla F. Differential effects of acute and chronic exercise on plasticity-related genes in the rat hippocampus revealed by microarray. Eur J Neurosci. 2002;16:1107–16. doi: 10.1046/j.1460-9568.2002.02158.x. [DOI] [PubMed] [Google Scholar]
- Narayanan SP, Flores AI, Wang F, Macklin WB. Akt signals through the mammalian target of rapamycin pathway to regulate CNS myelination. J Neurosci. 2009;29:6860–70. doi: 10.1523/JNEUROSCI.0232-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nave KA. Myelination and the trophic support of long axons. Nat Rev Neurosci. 2010;11:275–83. doi: 10.1038/nrn2797. [DOI] [PubMed] [Google Scholar]
- Nave KA, Lai C, Bloom FE, Milner RJ. Splice site selection in the proteolipid protein (PLP) gene transcript and primary structure of the DM-20 protein of central nervous system myelin. Proc Natl Acad Sci U S A. 1987;84:5665–9. doi: 10.1073/pnas.84.16.5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng F, Wijaya L, Tang BL. SIRT1 in the brain-connections with aging-associated disorders and lifespan. Front Cell Neurosci. 2015;9:64. doi: 10.3389/fncel.2015.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A, Komitova M, Suzuki R, Zhu X. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci. 2009;10:9–22. doi: 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]
- Norton WT, Poduslo SE. Myelination in rat brain: method of myelin isolation. 1973;21(4):749–757. doi: 10.1111/j.1471-4159.1973.tb07519.x. [DOI] [PubMed] [Google Scholar]
- Pardo PS, Boriek AM. An autoregulatory loop reverts the mechanosensitive Sirt1 induction by EGR1 in skeletal muscle cells. Aging (Albany NY) 2012;4:456–61. doi: 10.18632/aging.100470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo PS, Mohamed JS, Lopez MA, Boriek AM. Induction of Sirt1 by mechanical stretch of skeletal muscle through the early response factor EGR1 triggers an antioxidative response. J Biol Chem. 2011;286:2559–66. doi: 10.1074/jbc.M110.149153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y, Cai J, Wu Y, Wu R, Lee J, Fu H, Rao M, Sussel L, Rubenstein J, Qiu M. Control of oligodendrocyte differentiation by the Nkx2.2 homeodomain transcription factor. Development. 2001;128:2723–33. doi: 10.1242/dev.128.14.2723. [DOI] [PubMed] [Google Scholar]
- Rafalski VA, Ho PP, Brett JO, Ucar D, Dugas JC, Pollina EA, Chow LM, Ibrahim A, Baker SJ, Barres BA, et al. Expansion of oligodendrocyte progenitor cells following SIRT1 inactivation in the adult brain. Nature cell biology. 2013;15:614–24. doi: 10.1038/ncb2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza H, John A. 4-hydroxynonenal induces mitochondrial oxidative stress, apoptosis and expression of glutathione S-transferase A4–4 and cytochrome P450 2E1 in PC12 cells. Toxicol Appl Pharmacol. 2006;216:309–18. doi: 10.1016/j.taap.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Rosca MG, Vazquez EJ, Chen Q, Kerner J, Kern TS, Hoppel CL. Oxidation of fatty acids is the source of increased mitochondrial reactive oxygen species production in kidney cortical tubules in early diabetes. Diabetes. 2012;61:2074–83. doi: 10.2337/db11-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy RR, Harkema SJ, Edgerton VR. Basic concepts of activity-based interventions for improved recovery of motor function after spinal cord injury. Arch Phys Med Rehabil. 2012;93:1487–97. doi: 10.1016/j.apmr.2012.04.034. [DOI] [PubMed] [Google Scholar]
- Saher G, Quintes S, Nave KA. Cholesterol: a novel regulatory role in myelin formation. Neuroscientist. 2011;17:79–93. doi: 10.1177/1073858410373835. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Hearn EF, Do D, Rilling JK, Herndon JG. Differential rearing affects corpus callosum size and cognitive function of rhesus monkeys. Brain Res. 1998;812:38–49. doi: 10.1016/s0006-8993(98)00857-9. [DOI] [PubMed] [Google Scholar]
- Scholz J, Klein MC, Behrens TE, Johansen-Berg H. Training induces changes in white-matter architecture. Nat Neurosci. 2009;12:1370–1. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–71. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- Sherman DL, Krols M, Wu LM, Grove M, Nave KA, Gangloff YG, Brophy PJ. Arrest of myelination and reduced axon growth when Schwann cells lack mTOR. J Neurosci. 2012;32:1817–25. doi: 10.1523/JNEUROSCI.4814-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindler KS, Ventura E, Dutt M, Elliott P, Fitzgerald DC, Rostami A. Oral resveratrol reduces neuronal damage in a model of multiple sclerosis. J Neuroophthalmol. 2010;30:328–39. doi: 10.1097/WNO.0b013e3181f7f833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindler KS, Ventura E, Rex TS, Elliott P, Rostami A. SIRT1 activation confers neuroprotection in experimental optic neuritis. Invest Ophthalmol Vis Sci. 2007;48:3602–9. doi: 10.1167/iovs.07-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirevaag AM, Greenough WT. Differential rearing effects on rat visual cortex synapses. III. Neuronal and glial nuclei, boutons, dendrites, and capillaries. Brain Res. 1987;424:320–32. doi: 10.1016/0006-8993(87)91477-6. [DOI] [PubMed] [Google Scholar]
- St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, Handschin C, Zheng K, Lin J, Yang W, et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Szeligo F, Leblond CP. Response of the three main types of glial cells of cortex and corpus callosum in rats handled during suckling or exposed to enriched, control and impoverished environments following weaning. J Comp Neurol. 1977;172:247–63. doi: 10.1002/cne.901720205. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Dumont NL, Ito Y, Vaituzis C, Giedd JN, Andersen SL. Childhood neglect is associated with reduced corpus callosum area. Biol Psychiatry. 2004;56:80–5. doi: 10.1016/j.biopsych.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Tyler WA, Gangoli N, Gokina P, Kim HA, Covey M, Levison SW, Wood TL. Activation of the mammalian target of rapamycin (mTOR) is essential for oligodendrocyte differentiation. J Neurosci. 2009;29:6367–78. doi: 10.1523/JNEUROSCI.0234-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler WA, Jain MR, Cifelli SE, Li Q, Ku L, Feng Y, Li H, Wood TL. Proteomic identification of novel targets regulated by the mammalian target of rapamycin pathway during oligodendrocyte differentiation. Glia. 2011;59:1754–69. doi: 10.1002/glia.21221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida K. 4-Hydroxy-2-nonenal: a product and mediator of oxidative stress. Prog Lipid Res. 2003;42:318–43. doi: 10.1016/s0163-7827(03)00014-6. [DOI] [PubMed] [Google Scholar]
- Vaishnav RA, Singh IN, Miller DM, Hall ED. Lipid peroxidation-derived reactive aldehydes directly and differentially impair spinal cord and brain mitochondrial function. J Neurotrauma. 2010;27:1311–20. doi: 10.1089/neu.2009.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl SE, McLane LE, Bercury KK, Macklin WB, Wood TL. Mammalian target of rapamycin promotes oligodendrocyte differentiation, initiation and extent of CNS myelination. J Neurosci. 2014;34:4453–65. doi: 10.1523/JNEUROSCI.4311-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake H, Lee PR, Fields RD. Control of local protein synthesis and initial events in myelination by action potentials. Science. 2011;333:1647–51. doi: 10.1126/science.1206998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner H, Jung M, Klugmann M, Sereda M, Griffiths IR, Nave KA. Mouse models of myelin diseases. Brain Pathol. 1998;8:771–93. doi: 10.1111/j.1750-3639.1998.tb00200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Z, Valenza M, Cui L, Leoni V, Jeong HK, Brilli E, Zhang J, Peng Q, Duan W, Reeves SA, et al. Peroxisome-proliferator-activated receptor gamma coactivator 1 alpha contributes to dysmyelination in experimental models of Huntington’s disease. J Neurosci. 2011;31:9544–53. doi: 10.1523/JNEUROSCI.1291-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo YA, Martinez Gomez JM, Croxford JL, Gasser S, Ling EA, Schwarz H. CD137 ligand activated microglia induces oligodendrocyte apoptosis via reactive oxygen species. J Neuroinflammation. 2012;9:173. doi: 10.1186/1742-2094-9-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon H, Radulovic M, Drucker KL, Wu J, Scarisbrick IA. The thrombin receptor is a critical extracellular switch controlling myelination. Glia. 2015;63:846–859. doi: 10.1002/glia.22788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon H, Radulovic M, Wu J, Blaber SI, Blaber M, Fehlings MG, Scarisbrick IA. Kallikrein 6 signals through PAR1 and PAR2 to promote neuron injury and exacerbate glutamate neurotoxicity. Journal of neurochemistry. 2013;127:283–298. doi: 10.1111/jnc.12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeger M, Popken G, Zhang J, Xuan S, Lu QR, Schwab MH, Nave KA, Rowitch D, D’Ercole AJ, Ye P. Insulin-like growth factor type 1 receptor signaling in the cells of oligodendrocyte lineage is required for normal in vivo oligodendrocyte development and myelination. Glia. 2007;55:400–11. doi: 10.1002/glia.20469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, Zhao X, Zheng K, Li H, Huang H, Zhang Z, Mastracci T, Wegner M, Chen Y, Sussel L, et al. Genetic evidence that Nkx2.2 and Pdgfra are major determinants of the timing of oligodendrocyte differentiation in the developing CNS. Development. 2014;141:548–55. doi: 10.1242/dev.095323. [DOI] [PMC free article] [PubMed] [Google Scholar]