Abstract
Objectives:
The interferon-gamma–induced chemokine CXCL9 is expressed in a wide range of inflammatory conditions including those affecting the female genital tract. CXCL9 promotes immune cell recruitment, activation, and proliferation. The role of CXCL9 in modulating HIV-1 infection of cervicovaginal tissues, a main portal of viral entry, however, has not been established. We report a link between CXCL9 and HIV-1 replication in human cervical tissues and propose CXCL9 as a potential target to enhance the anti–HIV-1 activity of prophylactic antiretrovirals.
Design:
Using ex vivo infection of human cervical tissues as a model of mucosal HIV-1 acquisition, we described the effect of CXCL9 neutralization on HIV-1 gene expression and mucosal CD4+ T-cell activation. The anti-HIV-1 activity of tenofovir, the leading mucosal pre-exposure prophylactic microbicide, alone or in combination with CXCL9 neutralization was also studied.
Methods:
HIV-1 replication was evaluated by p24 ELISA. HIV-1 DNA and RNA, and CD4, CCR5, and CD38 transcription were evaluated by quantitative real-time polymerase chain reaction. Frequency of activated cervical CD4+ T cells was quantified using fluorescence-activated cell sorting.
Results:
Antibody blocking of CXCL9 reduced HIV-1 replication by decreasing mucosal CD4+ T-cell activation. CXCL9 neutralization in combination with suboptimal concentrations of tenofovir, possibly present in the cervicovaginal tissues of women using the drug inconsistently, demonstrated an earlier and greater decrease in HIV-1 replication compared with tissues treated with tenofovir alone.
Conclusions:
CXCL9 neutralization reduces HIV-1 replication and may be an effective target to enhance the efficacy of prophylactic antiretrovirals.
Key Words: CXCL9, HIV-1 replication, cervical tissues, prophylactic microbicides
INTRODUCTION
Immune cell activation by HIV-1 is essential to mount an effective host immune response. This process however also provides an immunological microenvironment that drives viral replication and disease progression in HIV-1–infected individuals.1–3
HIV-1 replication is regulated by a complex network of immune factors produced by a variety of hematopoietic and nonhematopoietic cells. Although immune factors with stimulatory and inhibitory effects on HIV-1 replication in peripheral blood mononuclear cells (PBMCs) are expressed at mucosal sites of HIV-1 exposure, for example, the female genital tract (FGT), knowledge about their contribution to HIV-1 replication is limited.4–7
The genital mucosa is populated with T cells, macrophages, and dendritic cells expressing the HIV-1 receptor CD4, and coreceptors CCR5 and CXCR4.8,9 Each of these cell populations is a potential target for infection; yet, recent findings identified CD4+ T cells as the main infected cell population in cervicovaginal tissues.10 Moreover, activated compared with resting CD4+ T cells are more susceptible to HIV-1.7,11–13 These findings suggest that HIV-1 induces inflammatory cytokine production in mucosal CD4+ T cells, leading to immune cell activation, proliferation, and HIV-1 receptor expression,2,14–16 thereby enhancing HIV-1 infection.7
The relevance of inflammatory cytokines in driving HIV-1 replication in the FGT is underscored by our previous work demonstrating that HIV-1 enhances interleukin (IL)-6 expression in cervical tissues (CTs).17 Higher IL-6 production is associated with increased HIV-1 transcription. Evaluating the impact of a woman's reproductive phase on HIV-1 replication, we demonstrated greater expression of pro-inflammatory cytokines and chemokines and enhanced HIV-1 replication in CTs from postmenopausal women compared with premenopausal women.7 Regarding the impact of sexually transmitted pathogens on HIV-1 infection, specifically herpes simplex virus type 2 (HSV-2), we reported enhanced viral replication in HIV-1/HSV-2 coinfected CTs compared with HIV-1–infected CTs.18 Greater HIV-1 replication was associated with higher CD4, CCR5, and CD38 expression and frequency of HIV-1–infected cells.18 These findings underscore the role of mucosal inflammation and sex hormones in regulating HIV-1 replication.19–22
The identification of factors driving immune cell activation and HIV-1 replication by HSV-2 is incomplete. Productive HSV-2 infection of cervical epithelial cells stimulates C-X-C motif chemokine 9 (CXCL9) expression, which controls viral infection.23–25 CXCL9 triggers CD4+ T-cell migration, activation, and proliferation26; yet, the extent to which CXCL9 impacts HIV-1 replication in the genital mucosa is unknown.
Increased CXCL9 expression has been reported in blood, semen, gut mucosa, and colostrum samples of HIV-infected individuals.27–29 Furthermore, low CXCL9 expression in the genital mucosa of HIV-exposed seronegative commercial sex workers was associated with HIV-1 protection,30 suggesting that CXCL9 may be a target to decrease HIV-1 replication.
We report on the scope and mechanism of CXCL9 modulation of HIV-1 replication. HIV-1 enhanced CXCL9 expression and blocking CXCL9 decreased HIV-1 replication in CTs. CXCL9 induction by HIV-1 and decreased HIV-1 replication by CXCL9 neutralization were also observed in PBMCs, suggesting that immune cells may contribute to CXCL9 expression and signaling in CTs. CXCL9 neutralization decreased mucosal immune cell activation, thereby lowering the number of activated CD4+ T cells.
CXCL9 neutralization enhanced the efficacy of suboptimal concentrations of tenofovir (TFV), the leading mucosal pre-exposure prophylactic microbicide, especially early during the infection. When used in combination with TFV at suboptimal concentrations possibly present in poor adherers, CXCL9 neutralization could further decrease HIV-1 replication in CTs, a main portal of viral entry.
METHODS
Tissue Samples
CTs were obtained from HIV-1 seronegative women undergoing hysterectomy at Dartmouth–Hitchcock Medical Center for benign medical conditions after an institutional review board–approved protocol. Nonpolarized CTs were established as described.17,31–33 Using our culture conditions, tissues can be maintained for until 21 days without significant decrease in viability, as determined by lactate dehydrogenase viability assay (Cytotoxicity Detection Kit; Roche, Indianapolis, IN).
HIV-1 Infection
HIV-1 stocks were generated in human PBMCs. Tissues were infected with 104 cell-free R5-tropic HIV-1BaL, a 50% tissue culture infectious dose (TCID50)/mL. After overnight incubation at 37°C, tissues were washed to remove residual input virus (day 0) and cultured for 21 days in Leibovitz L-15 medium (Gibco, Grand Island, NY) as described.7,17,18 Day 11 and 21 supernatants were evaluated for HIV-1 p24 antigen by enzyme-linked immunosorbent assay (ELISA) (PerkinElmer, Boston, MA).
PBMC Isolation and Infection
PBMCs were isolated by Ficoll-Paque Plus (Amersham, Piscataway, NJ)34 and incubated overnight at 37°C in complete RPMI-1640 (GIBCO). Next day, cells were resuspended at 2 × 106 cells per milliliter and infected with one 50 TCID50/mL of HIV-1BaL. After overnight infection, PBMCs were washed, resuspended in medium, and cultured for 7 days.
Neutralizing Antibody and TFV Treatment
Of note, 1 μg/mL of either antigen affinity-purified polyclonal goat neutralizing CXCL9 or isotype control antibodies (abs; R&D systems, Minneapolis, MN) was added to CTs or PBMCs on day 0, replenished every 3 days, and maintained in the cultures throughout the experiment. No ab cytotoxic effects were observed. Tissues were treated with TFV at either 100 or 10 μg/mL for 6 hours before HIV-1 infection. When indicated, CXCL9 neutralizing or isotype control abs was added to designated wells on day 0. TFV was kindly provided by CONRAD (Arlington, VA).
Nucleic Acid Isolation
Genomic DNA and RNA were isolated as previously described.7,17,18,35
HIV-1 Reverse Transcription and Integration
HIV-1 total DNA and integration were detected by a 2-step quantitative real-time polymerase chain reaction amplification as described.35–37
Gene Transcription
HIV-1 RNA and HIV-1 receptor, coreceptor, and immune cell activation markers' expression was detected as described.18
Fluorescence–Activated Cell Sorting Experiments
CTs (8–10 pieces) were digested with collagenase D at 5 mg/mL in complete L15 for 1 and half hour at 37°C (Roche Diagnostics, Indianapolis, IN). Single cell suspensions were fixed and stained as described.38 Data were acquired on a BD FCASCanto II (BD Biosciences, San Jose, CA) using FACSDiva software (BD Biosciences) and analyzed with FlowJo version 10.0.7 (Ashland, OR). CD4+ T cells were defined as CD3+ and CD8−.10
Statistical Analysis
Our experimental design fits a hierarchical model (dependent observations) to account for repeated measurements, each triplicated, within tissues. We included a random intercept for tissue and triplicates within tissue, in addition to fixed effects for the day and treatment. Analysis of datasets for HIV-1 reverse transcription, integration, and p24 containing 2 groups (Isotype and CXCL9 neutralizing ab)-treated tissues on days 11 and 21 after infection as well as CD4, CCR5, and CD38 RNA expression on days 3 and 5 after infection was performed by paired Student t test after logarithmic transformation to achieve normality. Nontransformed data of CXCL9 expression were expressed as arithmetic mean values and compared by paired Student t test. P values of <0.05 were considered significant.
RESULTS
HIV-1 Enhances CXCL9 Expression and Blocking CXCL9 Decreases HIV-1 Replication in CTs
CXCL9 expression is enhanced in the peripheral blood, semen, and gut mucosa of HIV-1–infected individuals27,29; yet, modulation of CXCL9 expression by HIV-1 in the FGT remains to be evaluated. To address whether HIV-1 enhances CXCL9 expression at primary sites of viral exposure, we evaluated CXCL9 protein levels in uninfected and HIV-1–infected CTs. CXCL9 levels were expressed as fold increase in HIV-1–infected tissues compared with uninfected controls set to 1. CXCL9 expression was significantly enhanced by HIV-1 on day 7 (Fig. 1A, P = 0.04) compared with day 4 (P = 0.128) after infection.
FIGURE 1.
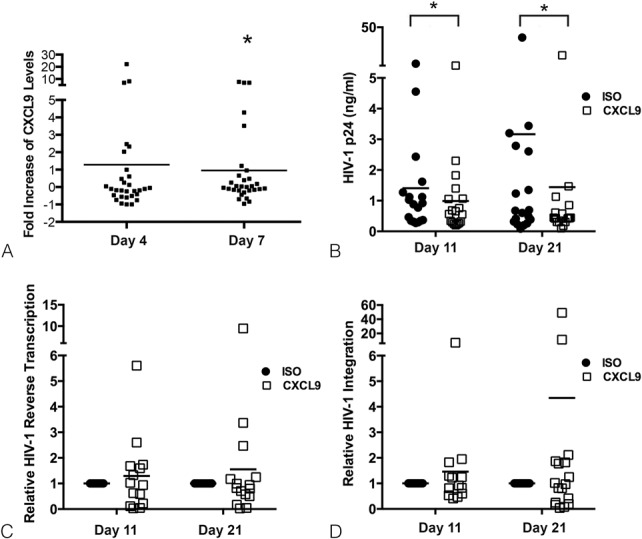
HIV-1 induces CXCL9 expression and blocking CXCL9 decreases HIV-1 replication in ex vivo cervical tissues. CXCL9 levels (pg/mL) (A) in culture supernatants from HIV-1–infected cervical tissues were evaluated by ELISA on days 4 and 7 after infection. The results from 15 individual donors assessed in duplicate are shown. For each donor, CXCL9 levels were expressed as fold increase in HIV-1–infected tissues compared with uninfected controls set to 1. *P < 0.05 for HIV-infected vs. uninfected cervical tissues. HIV-1 p24 levels (ng/mL) (B), viral reverse transcription (C), and integration (D) in HIV-1–infected cervical tissues treated with CXCL9 neutralizing (CXCL9) or isotype control (ISO) abs were measured by p24 ELISA (B) or real-time polymerase chain reaction (C and D) on days 11 and 21 after infection. Data from 18 (B), 14 (C), and 16 (D) individual donors are shown with each condition evaluated in triplicates. For HIV-1 reverse transcription and integration, all data were normalized to human β actin. Day 11 values in ISO-treated tissues were set to 1. Day 11 values in CXCL9 neutralizing ab treated tissues or day 21 values in ISO and CXCL9 neutralizing ab treated tissues were normalized to 1. *P < 0.05 for CXCL9 neutralizing vs. ISO abs.
To determine if there was a causal relationship between CXCL9 and HIV-1 replication, we blocked CXCL9 signaling with a specific neutralizing ab and infected CTs with HIV-1.
We detected a significant decrease in HIV-1 p24 levels in supernatants from HIV-1–infected CTs treated with CXCL9 neutralizing ab compared with isotype control–treated tissues on both days 11 (P = 0.009) and 21 (P = 0.027) after infection (Fig. 1B). Day 11 is one of the earliest time points where we consistently detect new viral release and HIV-1 DNA expression. Day 21 is the day when we terminated our experiments.7,18 To ascertain the step in the virus life cycle, we next tested whether CXCL9 neutralization decreased early events, that is, viral reverse transcription and integration. We detected no differences in HIV-1 reverse transcription between tissues treated with CXCL9 neutralizing or isotype control abs on either day 11 (P = 0.215) or 21 (P = 0.569) after infection (Fig. 1C). Likewise, CXCL9 neutralization did not alter HIV-1 integration at either time point (Fig. 1D; P = 0.824 and P = 0.698 on days 11 and 21 after infection, respectively).
Blocking CXCL9 Signaling Decreases HIV-1 Receptor and Immune Activation Markers in CTs
The lack of effect of CXCL9 neutralization on HIV-1 reverse transcription and integration suggests that CXCL9 decreases HIV-1 replication by targeting postintegration events. CXCL9 stimulates proliferation and activation of CD4+ T cells.26,39 Thus, CXCL9 may down-regulate HIV-1 replication by decreasing the activation phenotype of HIV-1 target cells. This hypothesis is supported by our published data demonstrating enhanced HIV-1 replication in CTs, which is associated with increased immune cell activation marker expression.18 Furthermore, decreasing mucosal inflammation by depressing NFκB signaling lowered HIV-1 replication.7
To test whether CXCL9 neutralization reduces mucosal immune cell activation, we evaluated the impact of blocking CXCL9 on CD4, CCR5, and CD38 RNA expression by reverse transcriptase polymerase chain reaction on days 3 and 5 after infection. Selection of these time points allowed evaluation of gene transcription after immune cell activation before T-cell depletion by HIV-1.18,40,41
On day 3, we detected a transient increase in CD4 (P = 0.0002), CCR5 (P = 0.0018), and CD38 (P = 0.03) RNA expression in CXCL9 neutralizing ab compared with isotype control–treated tissues (Figs. 2A–C), which was associated with similar levels of HIV-1 transcription (Fig. 2D). On day 5, CD4 (P = 0.0003), CCR5 (P = 0.0005), and CD38 (P = 0.006) RNA expression was decreased in HIV-1–infected tissues treated with CXCL9 neutralizing ab compared with isotype control–treated tissues (Figs. 2A–C). Down-regulation of receptor RNA expression correlated with decreased HIV-1 RNA production (P = 0.02, Fig. 2D).
FIGURE 2.

Blocking CXCL9 decreases CD4, CCR5, CD38, and HIV-1 RNA expression in ex vivo cervical tissues. CD4 (A), CCR5 (B), CD38 (C), and HIV-1 (D) transcription in HIV-1–infected cervical tissues treated with CXCL9 neutralizing or isotype control (ISO) abs was measured on days 3 and 5 after infection. All data were normalized to GAPDH. For each gene, day 3 values in ISO-treated tissues were set to 1. Day 3 values in CXCL9 neutralizing ab treated tissues or day 5 values in ISO and CXCL9 neutralizing ab treated tissues were normalized to 1. Data are shown as mean ± SD of 8 donors with each condition tested in triplicate. *P < 0.05 for CXCL9 neutralizing vs. ISO abs.
HIV-1 Induces CXCL9 Expression, and CXCL9 Neutralization Decreases HIV-1 Replication in PBMCs
CD4+ immune cells are the primary HIV-1 targets in mucosal tissues.10 To assess the potential of immune cells as CXCL9 producers, we evaluated CXCL9 expression in supernatants from nonactivated HIV-1–infected PBMCs, which were used as a surrogate of mucosal immune cells. Supernatants were harvested on days 2 and 5 after infection and evaluated for CXCL9 levels by ELISA. CXCL9 expression was significantly enhanced in supernatants from HIV-1–infected compared with uninfected PBMCs. We detected a 5-fold and a 1.5-fold increase on days 2 (P = 0.001) and 5 (P = 0.02) after infection, respectively (Fig. 3A).
FIGURE 3.
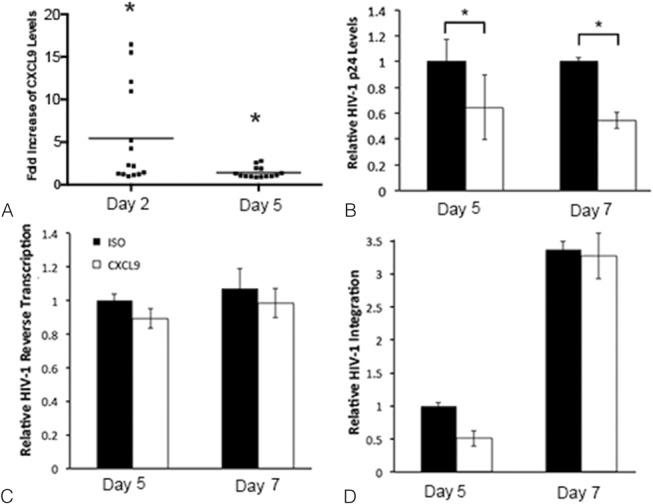
HIV-1 induces CXCL9 expression and blocking CXCL9 decreases HIV-1 replication in PBMCs. CXCL9 levels in HIV-1–infected PBMCs were measured on days 2 and 5 after infection (A). The results from 7 individual experiments evaluated in duplicate are shown. For each experiment, CXCL9 levels were expressed as fold increase in HIV-1–infected cells compared with uninfected controls set to 1. *P < 0.05 for HIV-1–infected vs. uninfected PBMCs. HIV-1 p24 (B) expression, HIV-1 reverse transcription (C) and viral integration (D) were measured in HIV-infected PBMCs treated with CXCL9 neutralizing (CXCL9) or isotype control (ISO) abs on days 5 and 7 after infection. At both time points, HIV-1 p24 levels in CXCL9 neutralizing ab treated tissues were expressed as fold decrease compared with ISO-treated PBMCs set to 1. For HIV-1 reverse transcription and integration, all data were normalized to human β actin. Day 5 values in ISO-treated tissues were set to 1. Day 5 values in CXCL9 neutralizing ab treated tissues or day 7 values in ISO or CXCL9 neutralizing abs treated tissues were normalized to 1. Data are shown as the mean ± SD of 3 experiments with each condition tested in triplicate. *P < 0.05 for CXCL9 neutralizing vs. ISO abs.
CXCL9 neutralization decreased HIV-1 p24 levels on both days 5 (P = 0.05) and 7 (P = 0.006) after infection in PBMCs (Fig. 3B). Blocking CXCL9 signaling had no direct effect on either HIV-1 reverse transcription (Fig. 3C) or viral integration (Fig. 3D).
Blocking CXCL9 Signaling Decreased the Number of Activated Cervical CD4+ T Cells
Our data suggest that blocking CXCL9 signaling reduces HIV-1 replication in both CTs and PBMCs (Figs. 1B, 3B). Given that our transcription experiments in CTs indicated a correlation between decreased HIV-1 transcription and immune cell activation marker expression (Fig. 2), we defined the impact of blocking CXCL9 on the activation phenotype of cervical CD4+ T cells.
Consistent with the results from our transcription experiments (Figs. 2A–C above), CXCL9 neutralization decreased the number of CD4+ T cells expressing CCR5 and CD38 on day 7 after infection (P = 0.019; Fig. 4B). On day 5, we detected a transient yet not significant increase in CCR5+ and CD38+ expression on CD4+ T cells in CXCL9 neutralizing compared with isotype control–treated tissues (P = 0.17; Fig. 4A).
FIGURE 4.

Blocking CXCL9 decreases the activation phenotype of CD4+ T cells in ex vivo cervical tissues. Fluorescence–activated cell sorting analysis of single cell suspensions from HIV-1–infected cervical tissues treated with CXCL9 neutralizing or isotype control abs. Cells were stained for CD3, CD8, CCR5, and CD38. CD4+ T cells were defined as CD3+ CD8−. CCR5 and CD38 double positive CD4+ T cells on days 5 (A) and 7 (B) after infection are shown. For each panel in (A and B), the percentage of CCR5+ and CD38+ double positive CD4+ T cells is displayed in the upper right quadrant. This result was consistent among 7 experiments.
Blocking CXCL9 Signaling is an Effective Target to Reduce HIV-1 Replication and Improve the Anti-HIV-1 Activity of TFV in CTs
To explore the translational/clinical relevance of our findings, we tested the effect of CXCL9 neutralization as a means to enhance the efficacy of TFV at suboptimal concentrations. In these experiments, CTs were left untreated or treated with TFV at either suboptimal concentrations (10 μg/mL) possibly found in women with incomplete/inadequate drug use42 or optimal prophylactic concentrations (100 μg/mL) in our experimental system,18 before infection with HIV-1. Compared with untreated controls, tissues treated with TFV at 100 µg/mL decreased HIV-1 p24 levels by 53% (P = 0.003) and 77% (P = 0.00003) on days 11 and 21 after infection, respectively (Fig. 5A). As expected, tissues treated with this TFV concentration displayed higher reduction in HIV-1 p24 levels than tissues treated with TFV at 10 µg/mL or those exposed to CXCL9 neutralizing ab (Figs. 5B, C). By comparing tissues treated with suboptimal concentrations of TFV and those subjected to CXCL9 neutralization, we found that TFV at 10 µg/mL lowered HIV-1 p24 levels by 17% (P = 0.01) on day 11 (Fig. 5B), whereas tissues treated with CXCL9 neutralizing ab reduced HIV-1 p24 levels by 42% (Fig. 5C; P = 0.009). On day 21, both treatments decreased HIV-1 p24 release by 52% (Figs. 5B, C; P = 0.0007 and P = 0.027 for TFV and CXCL9 neutralization, respectively).
FIGURE 5.
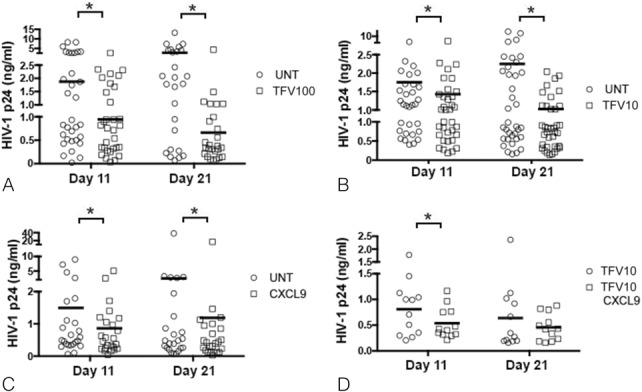
Anti–HIV-1 activity of TFV and CXCL9 neutralization alone or in combination in ex vivo cervical tissues. HIV-1 p24 levels (ng/mL) in supernatants from HIV-1–infected cervical tissues left untreated or treated with TFV at either 100 (A) or 10 (B) µg/mL, or with CXCL9 neutralizing or isotype control abs (C) were measured by ELISA on days 11 and 21 after infection. Mean values of triplicates from 32 (A), 26 (B), and 34 (C) individual donors are shown. *P < 0.05 for untreated vs. TFV-treated tissues and for CXCL9 neutralizing ab vs. isotype control–treated tissues. HIV-1 p24 levels (ng/mL) in supernatants from HIV-1–infected cervical tissues treated with TFV at 10 µg/mL in combination with either CXCL9 neutralizing or isotype control abs (D) were measured by ELISA on days 11 and 21 after infection. Mean values of triplicates from 12 individual donors are shown. *P < 0.05 for TFV isotype vs. TFV CXCL9 neutralizing abs. Each panel represents a different set of donors. Within each panel, the bars represent the average of the mean values from all donors.
Given that on day 11, CXCL9 neutralization decreased HIV-1 replication to a greater extent than TFV at 10 µg/mL, we next tested whether CXCL9 neutralization improved the anti–HIV-1 activity of suboptimal concentrations of TFV. On day 11, tissues treated with TFV in combination with CXCL9 neutralizing ab displayed a significant decrease (23%, P = 0.02) in HIV-1 replication compared with those treated with TFV alone (Fig. 5D). This effect was lost on day 21 (P = 0.309), given that treatment with either TFV at 10 μg/mL or CXCL9 neutralizing ab displayed similar levels of protection at this time point (Figs. 5B, C).
DISCUSSION
Mucosal immune responses modulate HIV-1 replication. Understanding mechanisms of HIV-1 regulation of mucosal immune responses and identifying novel targets that control HIV-1 replication in the FGT, the main portal of viral entry in women after vaginal intercourse43 is critical in the development of approaches to decrease viral replication and prevent systemic HIV-1 dissemination. We provide novel insights into the scope and mechanism of CXCL9 regulation of HIV-1 replication in CTs, a relevant model of mucosal HIV-1 transmission. HIV-1 enhanced CXCL9 expression, and blocking either constitutive or HIV-1–induced CXCL9 levels decreased HIV-1 replication in CTs. Reduced HIV-1 p24 release was the result of lower immune cell activation, that is, CCR5 and CD38 expression on cervical CD4+ T cells. Data consistency between CTs and PBMCs underscores the role of immune cells on CXCL9 production in CTs and highlights the relevance of CXCL9 signaling on enhancing HIV-1 replication. When evaluating the anti–HIV-1 activity of CXCL9 neutralization in combination with suboptimal concentrations of TFV possibly present in poor adherers in clinical trials,44,45 we detected a greater decrease in HIV-1 replication in tissues cotreated with TFV and CXCL9 neutralizing ab compared with those treated with TFV alone at earlier stages of infection. Thus, targeting mucosal innate immune responses by blocking CXCL9 signaling may be a relevant therapeutic approach to down-regulate HIV-1 replication and potentially reduce viral genital shedding or dissemination to the periphery when suboptimal concentrations of microbicides such as TFV are present in the cervicovaginal mucosa.
HIV-1 Infection Enhances CXCL9 Expression
CXCL9 is produced in a wide range of inflammatory conditions.46,47 In line with epidemiological studies describing increased CXCL9 levels in the blood, semen, and gut mucosa of HIV-1–infected treatment-naive patients,27,29 we demonstrated enhanced CXCL9 expression by HIV-1 in CTs (Fig. 1A) and PBMCs (Fig. 3A). Although we acknowledge variations in phenotype between PBMCs and cervical leukocytes, our findings suggest that immune cells may contribute to CXCL9 expression in CTs.
Blocking CXCL9 Signaling Decreases HIV-1 Replication
Blocking CXCL9 signaling significantly decreased HIV-1 p24 release in CTs (Fig. 1B) and PBMCs (Fig. 3B), supporting the hypothesis that CXCL9 promotes HIV-1 replication in immune cells of the FGT. This conclusion is consistent with studies demonstrating greater genital mucosa CXCL9 levels in HIV-1–infected compared with uninfected female sex workers (FSWs) or uninfected control women, suggesting that CXCL9 expression induces a mucosal microenvironment that favors HIV-1 replication.48 Furthermore, HIV-1-exposed seronegative FSWs displayed significant lower CXCL9 expression compared with HIV-1-seropositive FSWs,30 implying a potential role of CXCL9 in the establishment of the infection.
CXCL9 Signaling Decreases the Activation Phenotype of Mucosal CD4+ T Cells
Knowing that each infected cell harbors 1 copy of integrated viral DNA,49 and using HIV-1 integration as a surrogate of the number of infected cells, we saw no effect of CXCL9 neutralization on HIV-1 integration in either CTs (Fig. 1D) or PBMCs (Fig. 3D). No difference in the frequency of HIV-1–infected cells, however, was associated with decreased HIV-1 p24 release from tissues or cells treated with CXCL9 neutralizing compared with isotype control abs (Figs. 1B, 3B), suggesting that, under the settings of CXCL9 neutralization, HIV-1–infected cells produced fewer viral particles. HIV-1 replication depends on immune cell activation,50–52 and CXCL9 induces activation and proliferation of CD4+ T cells.53 Thus, we evaluated CD4 and immune activation markers' CCR5 and CD38 RNA expression in CTs. Early during the infection, CXCL9 neutralization enhanced CD4, CCR5, and CD38 RNA levels (Figs. 2A–C), suggesting an increase in immune cell activation marker expression likely by additional chemokines such as CXCL10 or CXCL11. This transient increase in receptor expression was associated with no differences in HIV-1 transcription (Fig. 2D). In contrast, at later time points, we detected a significant decrease in CD4, CCR5, and CD38 transcription in tissues treated with a CXCL9 neutralizing ab compared with isotype control–treated tissues (Figs. 2A–C) that was associated with decreased HIV-1 RNA expression. These findings imply that CXCL9 neutralization down-regulates HIV-1 replication in CTs by reducing CD4+ T-cell activation. Supporting our results, it has been demonstrated that compared with resting T lymphocytes activated CD4+ T cells are more susceptible to HIV-1 infection.11–13 Findings from our fluorescence–activated cell sorting experiments demonstrated a lower frequency of cervical CCR5+ CD38+ CD4+ T cells in tissues treated with CXCL9 neutralizing compared with isotype control abs on day 7 after infection (Fig. 4B), indicating consistency yet different kinetics of RNA and protein expression of HIV-1 receptor and immune activation marker expression in CTs.
Our results suggest that CXCL9 down-regulates HIV-1 replication by decreasing immune cell activation in CTs. Supporting this hypothesis, we have previously demonstrated that HSV-2, a virus that also induces CXCL9 expression,23 enhances HIV-1 replication by up-regulating CCR5 and CD38 expression in CTs.18 These findings underscore a positive correlation between immune cell activation and HIV-1 production at the site of primary infection.
One limitation of the tissue explant model is that we cannot evaluate cell recruitment and influx of new cells.31,33 Because CXCL9 induces immune cell recruitment, we are potentially underestimating the effect of CXCL9 neutralization on HIV-1 replication in our model.
Blocking CXCL9 Signaling Improves the Anti-HIV-1 Activity of Suboptimal Concentrations of TFV in CTs
Pre-exposure prophylactic regimens containing nucleoside reverse transcriptase inhibitors have shown discrepant results in men who have sex with men, serodiscordant couples, and women.43,54–58 Although biological factors cannot be ruled out, these inconsistencies are most likely attributable to poor adherence resulting in inadequate antiretrovirals' tissue concentrations and protective effects. This concept has also been cited to explain the discrepant results of TFV 1% vaginal gel showing 39%, 14%, and 0% effectiveness under intent to treat analysis in CAPRISA 004, MTN-003, and FACTS 001 trials, respectively.59–61 Case–cohort analysis based on returned applicators and/or drug levels, however, demonstrated protection in 50%–60% of women who used the gel consistently per protocol.44 These results underscore the role of adherence in decreasing the efficacy of TFV.54 Additional data, however, suggest that partial protection may also have been due to biological factors such as subclinical inflammation triggered by vaginal infections known to enhance mucosal susceptibility to HIV-1.7,18 Thus, alternative and multi target treatments are likely to provide more efficient preexposure prophylaxis to women.
Given that blocking CXCL9 signaling significantly reduced HIV-1 replication, we explored the anti-HIV-1 efficacy of CXCL9 neutralization in combination with TFV at suboptimal concentrations. When TFV concentrations were suboptimal, CXCL9 neutralization decreased HIV-1 replication to a higher degree than TFV alone early during the infection (Figs. 5B, C). Indeed a combined multitarget treatment of TFV at suboptimal concentrations and CXCL9 neutralizing ab demonstrated an additional protection compared with the treatment with TFV alone (Fig. 5D). We have previously demonstrated that TFV at suboptimal concentrations enhances the frequency of CD4+ T cells early during the infection.38 Thus, CXCL9 reduced HIV-1 replication, likely by decreasing the immune activation of CD4+ T cells. This additive effect provides a proof of concept that understanding the pathogenesis of the initial HIV-1 mucosal infection and designing specific interventions may be a potential approach to enhance the activity of topical or systemic prophylactic antiretrovirals. Thus, by decreasing activation of mucosal CD4+ T cells, CXCL9 neutralization could delay viral spread to the periphery or reduce HIV-1 genital shedding complementing the antiviral effects of topical antiretrovirals.
ACKNOWLEDGMENTS
The authors thank Anatomic Pathology, DHMC, Lebanon, NH, for tissue procurement.
Footnotes
Supported by the United States Agency for International Development (AID-OAA-A-14-00010) (G.F.D.), the Department of Veterans Affairs, Merit Review Program (S.N.A.), and by the Campbell Foundation (C.R.).
The authors have no conflicts of interest to disclose.
S.N.A. and C.R. contributed equally to this study.
C.R., S.N.A., and G.F.D. conceived and designed the study; S.L.M., C.R., M.J.L., and S.N.A. performed experiments; J.G. performed statistical analysis; G.F.D. contributed reagents; G.F.D. and S.L.M. contributed editorial comments; and C.R. and S.N.A. wrote the article.
REFERENCES
- 1.Gupta P, Collins KB, Ratner D, et al. Memory CD4+ T cells are the earliest detectable human immunodeficiency virus type 1 (HIV-1)-infected cells in the female genital mucosal tissue during HIV-1 transmission in an organ culture system. J Virol. 2002;76:9868–9876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawn SD, Butera S, Folks TS. Contribution of immune activation to the pathogenesis and transmission of human immunodeficiency virus type 1 infection. Clin Microbiol Rev. 2001;14:753–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mogensen TH, Melchjorsen J, Larsen CS, et al. Innate immune recognition and activation during HIV infection. Retrovirology. 2010;7:2–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cummins JE, Christensen L, Lennox JL, et al. Mucosal innate immune factors in the female genital tract are associated with vaginal HIV-1 shedding independent of plasma viral load. AIDS Res Hum Retroviruses. 2006;22:788–795. [DOI] [PubMed] [Google Scholar]
- 5.Pudney J, Quayle AJ, Anderson DJ. Immunological microenvironments in the human Vagina and Cervix: Mediators of Cellular immunity are Concentrated in the cervical transformation Zone. Biol Reprod. 2005;73:1253–1263. [DOI] [PubMed] [Google Scholar]
- 6.Arici A, Head JR, MacDonald PC, et al. Regulation of interleukin-8 gene expression in human endometrial cells in culture. Mol Cell Endocrinol. 1993;94:195–204. [DOI] [PubMed] [Google Scholar]
- 7.Rollenhagen C, Asin SN. Enhanced HIV-1 replication in ex vivo ectocervical tissues from post-menopausal women correlates with increased inflammatory responses. Mucosal Immunol. 2011;4:671–681. [DOI] [PubMed] [Google Scholar]
- 8.Hladik F, McElrath MJ. Setting the stage: host invasion by HIV. Nat Rev Immunol. 2008;8:447–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shacklett BL. Cell-mediated immunity to HIV in the female reproductive tract. J Reprod Immunol. 2009;83:190–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saba E, Grivel JC, Vanpouille C, et al. HIV-1 sexual transmission: early events of HIV-1 infection of human cervico-vaginal tissue in an optimized ex vivo model. Mucosal Immunol. 2010;3:280–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang ZQ, Wietgrefe SW, Li Q, et al. Roles of substrate availability and infection of resting and activated CD4+ T cells in transmission and acute simian immunodeficiency virus infection. Proc Natl Acad Sci U S A. 2004;101:5640–5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brass AL, Dykxhooorm D, Benita Y, et al. Identification of host proteins required for HIV infection through a functional genomic screen. Science. 2008;319:921–926. [DOI] [PubMed] [Google Scholar]
- 13.König R, Zhou Y, Elleder D, et al. Global analysis of host-pathogen interactions that regulate early-stage HIV-1 replication. Cell. 2008;135:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen R, Richter H, Clements RH, et al. Macrophages in vaginal but not intestinal mucosa are monocyte-like and permissive to human immunodeficiency virus type 1 infection. J Virol. 2009;83:3258–3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moreira C, Venkatesh KK, DeLong A, et al. Effect of treatment of asymptomatic bacterial vaginosis on HIV-1 shedding in the genital tract among women on antiretroviral therapy: a pilot study. Clin Infect Dis. 2009;49:991–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson BL, Wang CC, Delong AK, et al. Genital tract leukocytes and shedding of genital HIV type 1 RNA. Clin Infect Dis. 2008;47:1216–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asin SN, Eszterhas S, Rollenhagen C, et al. HIV-1 in women: increased transcription of HIV-1 in ectocervical tissue explants. J Infect Dis. 2009;200:965–972. [DOI] [PubMed] [Google Scholar]
- 18.Rollenhagen C, Lathrop MJ, Macura SL, et al. Herpes simplex virus type-2 stimulates HIV-1 replication in cervical tissues: implications for HIV-1 transmission and efficacy of anti-HIV-1 microbicides. Mucosal Immunol. 2014;7:1165–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yao XD, Omange RW, Henrick BM, et al. Acting locally: innate mucosal immunity in resistance to HIV-1 infection in Kenyan commercial sex workers. Mucosal Immunol. 2014;7:268–279. [DOI] [PubMed] [Google Scholar]
- 20.Li L, Ben Y, Zhu Z, et al. Minocycline down-regulates topical mucosal inflammation during the application of microbicide candidates. PLoS One. 2012;7:e43211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKinnon LR, Nyanga B, Kim CJ, et al. Early HIV-1 infection is associated with reduced frequencies of cervical Th17 cells. J Acquir Immune Defic Syndr. 2015;68:6–12. [DOI] [PubMed] [Google Scholar]
- 22.Card CM, Ball TB, Fowke KR. Immune quiescence: a model of protection against HIV infection. Retrovirology. 2013;10:2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang W, Hu K, Luo S, et al. Herpes simplex virus type 2 infection of human epithelial cells induces CXCL9 expression and CD4+ T cell migration via activation of p38-CCAAT/enhancer-binding protein-β pathway. J Immunol. 2012;188:6247–6257. [DOI] [PubMed] [Google Scholar]
- 24.Thapa M, Carr DJ. Chemokines, Chemokine Receptors Critical to host resistance following genital herpes simplex virus type 2 (HSV-2). Infect Open Immunol J. 2008;1:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thapa M, Welner RS, Pelayo R, et al. CXCL9 and CXCL10 expression are critical for control of genital herpes simplex virus type 2 infection through mobilization of HSV-specific CTL and NK cells to the nervous system. J Immunol. 2008;180:1098–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hertenstein A, Schumacher T, Litzenburger U, et al. Suppression of human CD4+ T cell activation by 3,4-dimethoxycinnamonyl-anthranilic acid (tranilast) is mediated by CXCL9 and CXCL10. Biochem Pharmacol. 2011;82:632–641. [DOI] [PubMed] [Google Scholar]
- 27.Allers K, Fehr M, Conrad K, et al. Macrophages accumulate in the gut mucosa of untreated HIV-infected patients. J Infect Dis. 2014;209:739–748. [DOI] [PubMed] [Google Scholar]
- 28.Pereira NZ, Cardoso E, Oliveira LM, et al. Upregulation of innate antiviral restricting factor expression in the cord blood and decidual tissue of HIV-infected mothers. PLoS One. 2013;8:e84917 eCollection 0082013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lisco A, Introini A, Munawwar A, et al. HIV-1 imposes rigidity on blood and semen cytokine networks. Am J Reprod Immunol. 2012;68:515–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lajoie J, Juno J, Burgener A, et al. A distinct cytokine and chemokine profile at the genital mucosa is associated with HIV-1 protection among HIV-exposed seronegative commercial sex workers. Mucosal Immunol. 2012;5:277–287. [DOI] [PubMed] [Google Scholar]
- 31.Grivel JC, Margoli L. Use of human tissue explants to study human infectious agents. Nat Protoc. 2009;4:256–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greenhead P, Hayes P, Watts PS, et al. Parameters of human immunodeficiency virus infection of human cervical tissue and inhibition by vaginal virucides. J Virol. 2000;74:5577–5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Merbah M, Introini A, Fitzgerald W, et al. Cervico-vaginal tissue ex vivo as a model to study early events in HIV-1 infection. Am J Reprod Immunol. 2011;65:268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen L, Guyre P, Anderson CL, et al. Heteroantibody-mediated cytotoxicity: antibody to the high affinity Fc receptor for IgG mediates cytotoxicity by human monocytes that is enhanced by interferon-gamma and is not blocked by human IgG. J Immunol. 1986;137:3378–3382. [PubMed] [Google Scholar]
- 35.Rollenhagen C, Asin SN. IL-8 decreases HIV-1 transcription in peripheral blood lymphocytes and ectocervical tissue explants. J Acquir Immune Defic Syndr. 2010;54:463–469. [DOI] [PubMed] [Google Scholar]
- 36.Brussel A, Delelis O, Sonigo P. Alu-LTR real -time nested PCR assay for quantifying integrated HIV-1 DNA. Methods Mol Biol. 2005;304:139–154. [DOI] [PubMed] [Google Scholar]
- 37.Brooks D, Hammer DH, Arlen PA, et al. Molecular Characterization, Reactivation and depletion of Latent HIV. Immunity. 2003;19:413–423. [DOI] [PubMed] [Google Scholar]
- 38.Rollenhagen C, Macura SL, Lathrop MJ, et al. Enhancing Interferon Regulatory Factor 7 mediated antiviral responses and decreasing nuclear factor kappa B expression limit HIV-1 replication in cervical tissues. PLos One. 2015;6:e0131919 eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karin N, Wildbaum G. The role of chemokines in adjusting the balance between CD4+ effector T cell subsets and FOXp3-negative regulatory T cells. Int Immunopharmacol. 2015;28:829–35. 2015. 03. 037. [DOI] [PubMed] [Google Scholar]
- 40.Brenchley JM, Schaker T, Ruff LE, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;2000:749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mehandru S, Poles MA, Tenner-Racz K, et al. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med. 2004;200:761–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andrei G, Lisco A, Vanpouille C, et al. Topical tenofovir, a microbicide effective against HIV, inhibits herpes simplex virus-2 replication. Cell Host Microbe. 2011;10:379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hankins CA, Dybul MR. The promise of pre-exposure prophylaxis with antiretroviral drugs to prevent HIV transmission: a review. Curr Opin HIV AIDS. 2013;8:50–58. [DOI] [PubMed] [Google Scholar]
- 44.Mansoor LE, Karim QA, Werner L, et al. Impact of an adherence intervention on the effectiveness of tenofovir gel in the CAPRISA 004 trial. AIDS Behav. 2014;18:841–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roberts L, Liebenberg L, Barnabas S, et al. Vaginal microbicides to prevent human immunodeficiency virus infection in women: perspectives on the female genital tract, sexual maturity and mucosal inflammation. Best Pract Res Clin Obstet Gynaecol. 2012;26:441–449. [DOI] [PubMed] [Google Scholar]
- 46.Groom JR, Luster AD. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol Cell Biol. 2011;89:207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Raemdonck K, Van den Steen PE, Liekens S, et al. CXCR3 ligands in disease and therapy. Cytokine Growth Factor Rev. 2015;26:311–327. [DOI] [PubMed] [Google Scholar]
- 48.Lajoie J, Poudrier J, Massinga Loembe M, et al. Chemokine expression patterns in the systemic and genital tract compartments are associated with HIV-1 infection in women from Benin. J Clin Immunol. 2010;30:90–98. [DOI] [PubMed] [Google Scholar]
- 49.Josefsson L, Palmer S, Faria NR, et al. Single cell analysis of lymph node tissue from HIV-1 infected patients reveals that the majority of CD4+ T-cells contain one HIV-1 DNA molecule. Plos Pathog. 2013;9:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Phetsouphanh C, Xu Y, Zaunders J. CD4 T Cells Mediate both positive and negative regulation of the immune response to HIV infection: complex Role of T Follicular Helper cells and regulatory t cells in pathogenesis. Front Immunol. 2014;5:1–14. eCollection 02014. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jakobsen MR, Olagnier D, Hiscott J. Innate immune sensing of HIV-1 infection. Curr Opin HIV AIDS. 2015;10:96–102. [DOI] [PubMed] [Google Scholar]
- 52.Chakrabarti LA, Boucherie C, Bugault F, et al. Biomarkers of CD4+ T-cell activation as risk factors for tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS. 2014;28:10368–10380. [DOI] [PubMed] [Google Scholar]
- 53.Whiting D, Hsieh G, Yun JJ, et al. Chemokine monokine induced by IFN-gamma/CXC chemokine ligand 9 stimulates T lymphocyte proliferation and effector cytokine production. J Immunol. 2004;172:7417–7424. [DOI] [PubMed] [Google Scholar]
- 54.Baeten J, Celum C. Oral antiretroviral chemoprophylaxis: current status. Curr Opin HIV AIDS. 2012;7:514–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McEnery R. Oral tenofovir arm of VOICE trial discontinued early. IAVI Rep. 2011;15:21. [PubMed] [Google Scholar]
- 56.Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367:411–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baeten JM, Donnel D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abdool Karim Q, Abdool Karim S, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372:509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rees H, Delany-Moretlwe S, Lombard C, et al. FACTS 001: a multi-centred phase III randomised, double-blind, placebo-controlled tiral of periocoital tenofovir 1% gel for HIV prevention in Women. Presented at Conference on Retroviruses and Opportunistic Infections 2015, Seattle, Washigton, USA.


