Abstract
Ultraviolet B (UVB) radiation is the major environmental risk factor for developing skin cancer, the most common cancer worldwide, which is characterized by a berrant activation of Akt/mTOR (mammalian target of rapamycin). Importantly, the link between UV irradiation and mTOR signaling has not been fully established. Apigenin is a naturally occurring flavonoid that has been shown to inhibit UV-induced skin cancer. Previously, we have demonstrated that apigenin activates AMP-activated protein kinase (AMPK), which leads to suppression of basal mTOR activity in cultured keratinocytes. Here, we demonstrated that apigenin inhibited UVB-induced mTOR activation, cell proliferation and cell cycle progression in mouse skin and in mouse epidermal keratinocytes. Interestingly, UVB induced mTOR signaling via PI3K/Akt pathway, however, the inhibition of UVB-induced mTOR signaling by apigenin was not Akt-dependent. Instead, it was driven by AMPK activation. In addition, mTOR inhibition by apigenin in keratinocytes enhanced autophagy, which was responsible, at least in part, for the decreased proliferation in keratinocytes. In contrast, apigenin did not alter UVB-induced apoptosis. Taken together, our results indicate the important role of mTOR inhibition in UVB protection by apigenin, and provide a new target and strategy for better prevention of UV-induced skin cancer.
Keywords: mTOR, Akt, AMPK, UVB, apigenin, autophagy
1. Introduction
Non-melanoma skin cancer (NMSC), including basal cell carcinomas (BCC) and squamous cell carcinomas (SCC), is the most common form of cancer worldwide, with more than two million new cases diagnosed yearly in the United State alone. Extensive epidemiologic, clinical and biological studies have proven that ultraviolet B (UVB) radiation is the major carcinogen for NMSC[1-3]. While UV exposure can be reduced by application of sunscreens, a substantial part of the population continues to receive significant doses of UV due to occupational and recreational activities, and the number of NMSC cases continues to rise.
Apigenin (5,7,4’-trihydroxyflavone) is a bioflavonoid present in a wide variety of food sources including tea, onions, parsley, thyme, celery and sweet red pepper[4]. Topical application of apigenin to mouse skin has been shown to reduce the incidence and the size of tumors in mouse models of skin carcinogenesis using both chemical carcinogens and UV exposure[5, 6]. Apigenin has multiple properties that make it a promising chemopreventive agent[7]. It can inhibit angiogenesis[8, 9], stabilize and enhance the expression of tumor suppressive p53[10, 11], inhibit pro-inflammatory COX-2 expression[12, 13], and cause cell cycle arrest [14, 15] and apoptosis[16, 17] through multiple mechanisms. However, the full scope of apigenin's chemopreventive function is not known. We reported recently that apigenin can activate AMP-activated protein kinase (AMPK) in keratinocytes (both human keratinocyte cell lines and primary normal human epidermal keratinocytes) and the activation of AMPK results in the inhibition of baseline activity of the mammalian target of rapamycin (mTOR) signaling pathway[18].
In mammalian cells, mTOR is the key component of two distinct functional complexes, mTORC1 and mTORC2. mTORC1 contains the regulatory-associated protein of mTOR (Raptor), mLST8, and proline-rich Akt substrate 40 kDa (Pras40). The mTORC1 is sensitive to rapamycin and regulates cell growth by controlling ribosome biogenesis, protein and lipid synthesis. The two best-known substrates of mTORC1 are the ribosomal protein S6 kinase (p70S6K) and the eukaryotic initiation factor 4E binding protein (4E-BP1)[19]. mTORC2 comprises mLST8, mSIN1, and the rapamycin-insensitive companion of TOR protein (Rictor). mTORC2 can activate Akt by phosphorylation on S473 and is generally thought to be rapamycin-insensitive although prolonged exposure to high rapamycin concentrations may prevent its assembly and function[19, 20].
Multiple lines of evidence indicate a crucial role of mTOR in the etiology of NMSC. UV-induced NMSC is a major problem in organ transplant recipients, and the use of mTOR inhibitor as a post-transplantation immunosuppressive significantly reduces NMSC occurrence[21]. More directly, Chen et al examined many cases of malignant SCC, precancerous actinic keratosis and normal skin samples by immunohistochemistry, and found that mTOR itself, as well as its downstream targets p70S6K, 4E-BP1 and Akt, are phosphorylated at much higher levels in SCC than actinic keratosis and normal skin[22]. Protein microarray analysis of SCC, actinic keratosis and normal skin similarly revealed abnormally activated mTOR signaling in SCC[23]. More recently, Carr et al reported unique but complementary roles of mTORC1 and mTORC2 in controlling proliferation and apoptosis in the skin, whereby inhibition of mTOR suppresses UVB-induced keratinocyte proliferation and survival[24].
Here, we investigated mTOR activation by UVB and whether the blockade of UVB-induced mTOR activity by apigenin are transduced via Akt or AMPK. Results from both in vitro and in vivo analyses demonstrate simultaneous inhibition of UVB-induced keratinocyte proliferation, cell cycle progression, and mTOR activation by apigenin. Despite the fact that UVB-induced mTOR activation is driven by PI3K/Akt signaling and apigenin is capable of blocking Akt phosphorylation/activation, apigenin's inhibition of UVB-induced mTOR signaling was mainly due to its activation of AMPK rather than suppression of Akt activation. Finally, consistent with mTOR's role as a hub between proliferation and autophagy, apigenin treatment of UVB-irradiated keratinocytes results in elevated autophagy levels, but not apoptosis. Thus mTOR inhibition and increase in autophagy likely contribute to the reduction in the UVB-induced keratinocyte proliferation by apigenin. Our results indicate the importance of mTOR blockade for apigenin's chemopreventive function and point to a new target and strategy to improve prevention of skin cancer.
2. Materials and Methods
2.1. Cell culture, UVB irradiation and drug treatment
The mouse 308 keratinocyte cell line derived from Balb/c mouse skin initiated with dimethylbenz[a]anthracene contains a point-mutated H-ras gene and wild-type p53 gene[25]. 308 cells were maintained in Suspension Minimum Essential Medium (United States Biologicals) supplemented with 8% chelexed (Bio-Rad Laboratories) fetal bovine serum, 0.02 mM Ca2+, 0.1 mM non-essential amino acids, 2 mM L-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin (Life Technologies). Cells were used at 80-90% confluence. For UVB exposure, the culture medium was removed and saved, the cells were rinsed with phosphate-buffered saline (PBS), irradiated, and the medium replaced. We used FS40T12 lamps (National Biological) with emission peak at 313 nm, in combination with Kodacel filter (Eastman Kodak) to eliminate UVC (< 295 nM). Apigenin, wortmannin (Sigma-Aldrich), Compound C (EMD Chemicals), MK-2206 and A-769662 (Selleckchem) stock solutions were prepared in dimethyl sulfoxide (DMSO). Chloroquine was dissolved in water. Apigenin, wortmannin and MK-2206 were added 1 h prior to UVB irradiation, and Compound Cand chloroquine were added 1 h before UVB or apigenin + UVB treatment. The concentration of DMSO in cell cultures was less than 0.1%.
2.2. Treatment of animals
All studies involving mice were performed in accordance with the Guide for the Care and Use of Laboratory Animals, and all the procedures approved by Northwestern University Animal Care and Use Committee. Adult (6-8 week old) female SKH-1 hairless mice (Charles River Laboratories) were assigned to 4 groups (control, apigenin alone, UVB, and apigenin + UVB, n=5). Apigenin was applied at 5 μmol in 0.2 ml DMSO/ acetone (1:9) vehicle mix. Mice were topically treated with apigenin or vehicle daily for 5 days, 1 hr prior to UVB irradiation (1000 J/m2 daily) where applies. At 24 h after final UVB exposure mice were sacrificed to harvest dorsal skin. Skin tissue was formalin-fixed and paraffin-embedded for further analysis or frozen for biochemical studies.
2.3. Western blotting
For in vitro experiments, cells were harvested in the lysis buffer (20 mM Tris HCl, pH 7.5, 150 mM NaCl, 2 mM EDTA, 10% glycerol, 1% Triton X-100, 1 mM PMSF, protease and phosphatase inhibitor cocktail). Protein concentration was measured with BCA reagent (Pierce). Equal amounts of protein lysates were loaded and resolved by SDS PAGE followed by eletrophoretic transfer onto nitrocellulose membranes. The membranes were blocked for 90 min at room temperature in 5% dry milk in Tris-buffered saline (TBS) with 0.1% Tween 20 (TBS-T) and incubated at 4 °C overnight with primary antibodies. Following incubation with appropriate horse radish peroxidase-conjugated secondary antibodies, signals were detected using Enhanced Chemiluminescence System(Thermo Scientific). For in vivo experiments, dorsal skin was harvested and underlying fat and blood vessels were removed. Epidermal tissues were collected by scraping the surface of epidermis with a razor blade and placed into ice-cold RIPA buffer. The lysates were sonicated on ice for 10 sec, centrifuged at 16,000 × g for 20 min at 4°C, and supernatants were collected. Western blotting was performed as described above. The following antibodies were used: AMPK, p-AMPK (T172), Akt, p-Akt (S473), p70S6K, p-p70S6K (T389), 4E-BP1, p-4E-BP1 (S65), LC3, p44/42 MAPK (Cell Signaling Technology) and CDK2, Actin (Santa Cruz Biotechnology). Protease and phosphatase inhibitor cocktail was from Sigma-Aldrich.
2.4. Histology and immunohistochemistry
Dorsal skins from at least 3 mice per condition were immediately fixed in 10% neutral buffered formalin for 24 h, embedded in paraffin and 5 μm sections were cut. H&E staining and immunohistochemistry for Ki-67, CDK2 and p-mTOR (Ser2448) were conducted at Northwestern University Mouse Histology and Phenotyping Core. Measurement of epidermal thickness and count of Ki-67 positive cells were performed as described previously[9].
2.5. Immunofluorescence
Cells were grown on Lab-Tek Chamber Slides (Nalgene Nunc International), treated as desired, rinsed in PBS and fixed for 15 min in 4% paraformaldehyde in PBS at room temperature. The cells were further washed with PBS and treated for 10 min with 0.3% Triton X-100 in PBS. After 1 h blocking (0.1% Tween 20, 5% goat serum in PBS) the slides were incubated with LC3 antibody in 5% goat serum for 48 h at 4°C, followed by incubating with goat anti-rabbit IgG conjugated with Alexa Fluor 488 (Life Technologies) at room temperature for 2h. After final wash with PBS, the slides were mounted in Vectashield with DAPI (Vector Laboratories) and visualized with a fluorescence microscope.
2.6. Transient Transfection
Cells were transiently transfected using Lipofectamine 3000 reagent (Life Technologies) following the manufacturer's instructions. Briefly, cells were plated in 6-well plates and grown to 70-80% confluence in antibiotic-free medium, 2 μg plasmid DNA in 100 μl of Opti-MEM medium was incubated with 4 μl of P3000 reagent at room temperature for 5 min, followed by 5 μl of Lipofectamine 3000 in an additional 100 μl of Opti-MEM medium. The mixture was incubated for 20 min and layered onto the cells. After 6 h incubation, the transfection medium was replaced with fresh growth medium, and the cells incubated overnight for gene expression.
2.7. RNA Interference
siRNA duplexes targeting mouse AMPKα1/2, Akt1/2, Akt3 and non-targeting control siRNA were purchased from Santa Cruz Biotechnology. The transfection of siRNA into 308 cells was performed by using Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer's instructions. Forty eight h (for AMPK) or 72 h (for Akt) after transfection, cell density was adjusted so cell confluence would be 90%, then the transfected cells were treated with apigenin and UVB.
2.8. Flow cytometry
For cell cycle analysis, cells were serum-starved overnight and treated as indicated, then serum was added back to the medium. Cells were harvested 24 h after UVB exposure, fixed in ice-cold methanol for 30 min, washed twice with PBS and incubated with propidium iodide (PI) solution (50 μg/ml PI, 0.1% Triton X-100 and 0.2 mg/ml RNase A) at 37 °C for 20 minutes. Cells were further incubated for 1 h at 4 °C and proceed to analysis using a BD LSR Fortessa Cell Analyzer. For apoptosis analysis, cells were cultured in normal medium with serum and harvested at 24 h after UVB irradiation. Apoptosis was measured by using the Annexin V Apoptosis Detection Kit (BD Biosciences) according to the manufacturer's protocol.
2.9. Statistical analyses
Statistical analyses were carried out using Prism 6 (Graph Pad software). Data were expressed as the mean of at least three independent experiments and error bars indicated standard deviation of the mean (SD). Data were analyzed by one-way or two-way ANOVA and Tukey's multiple comparisons test. P values below 0.05 were set as significant.
3. Results
3.1. Apigenin suppresses UVB-induced keratinocyte proliferation and cell cycle progression
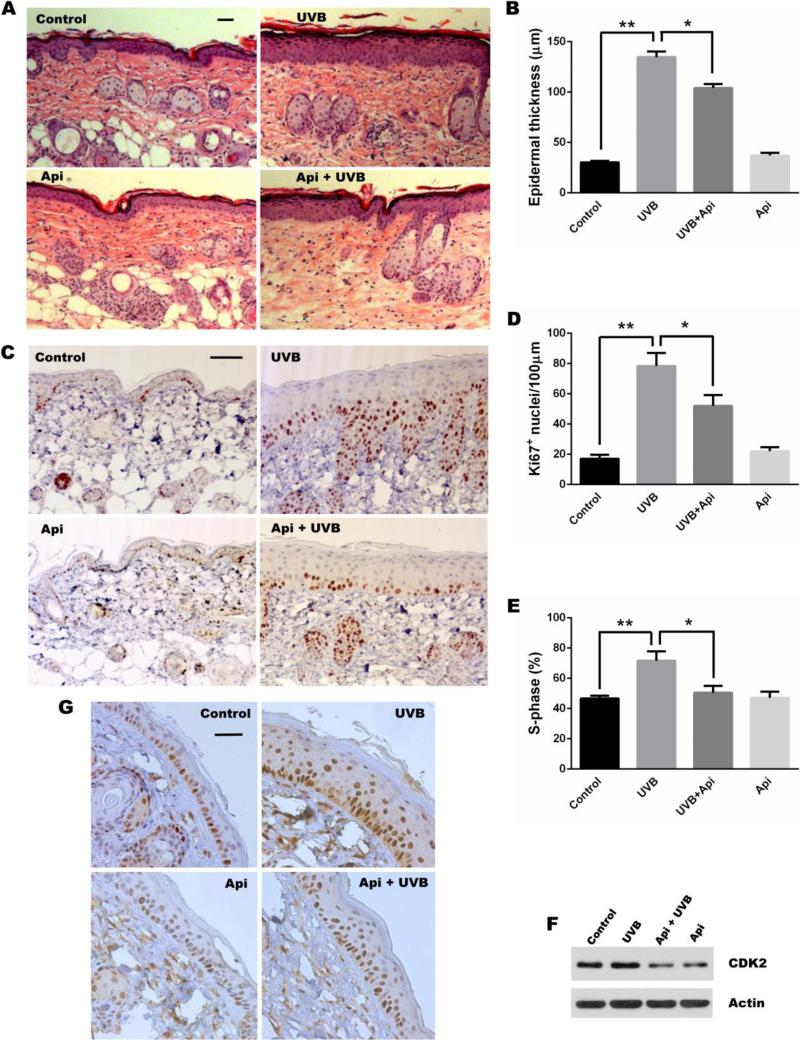
During UVB-induced NMSC, exacerbated proliferation of keratinocytes is a key feature responsible for epidermal hyperplasia and tumor promotion. To determine whether apigenin inhibits this aspect of UVB-induced skin carcinogenesis in vivo, we compared skins of SKH-1 mice subjected to UVB irradiation in the absence and in the presence of topical apigenin. Substantial thickening of the epidermis in UVB-irradiated mice compared to sham-irradiated control animals was indicative of proliferation and apigenin significantly attenuated the UVB-induced thickening (Fig. 1A, B). Importantly, apigenin alone did not cause any thinning of the epidermis in sham-irradiated mice (Fig. 1A, B). Direct assessment of cellular proliferation by Ki-67 labeling showed similar pattern, whereby UVB induced an approximately 4-fold increase in Ki-67-positive nuclei at 24 hours post UVB irradiation and this increase was significantly reduced by apigenin (Fig. 1C, D). Again, apigenin had no effect on basal proliferation in sham-irradiated epidermis (Figure 1C, D).
Fig. 1. Apigenin inhibits UVB-induced proliferation and cell cycle progression in mouse keratinocytes.
(A-D) SKH-1 mice were subjected to UVB radiation (1000 J/m2 daily, 5 days), or topical apigenin (Api, 5 μmol, in 200 μl vehicle, DMSO: acetone 1:9) was applied 1 h prior to each UVB exposure. Mice were also sham-irradiated and treated with apigenin, and the control group of mice was subjected to sham irradiation and vehicle. 24 h after the final UVB exposure, the mice were euthanized, dorsal skin was harvested, fixed in formalin and paraffin-embedded. (A) Representative Hematoxylin and Eosin (H&E) staining of skin sections (scale bar, 100 μm). (B) Quantitation of epidermal thickness (mean ± SD), *, P < 0.001; **, P< 0.0001. Three sections per mouse and 3 mice per group were evaluated. (C) Immunohistochemistry (IHC) for proliferation marker Ki-67 (scale bar, 100 μm). (D) Quantification of Ki-67 staining (mean ± SD), *, P < 0.01; **, P < 0.0001. Three fields from 3 independent sections/group were examined, Ki-67 positive nuclei were calculated per linear 100 μm of epidermis and hair follicles were not included in analysis. (E) Cell cycle analysis of mouse 308 keratinocytes: determination of cells in S-phase (mean ± SD), *, P < 0.01; **, P < 0.001. 308 cells at 80-90% confluence were synchronized by nutrient withdrawal overnight, pre-treated for 1 hr with apigenin (25 μM), and irradiated with UVB (500 J/m2), as indicated. Cells were harvested at 24 h post irradiation and analyzed by flow cytometry. Data are from 3 independent experiments. (F) 308 cells were treated as above and CDK2 expression was analyzed by Western blot. (G) IHC for CDK2. The mice were treated as in (A-D).
Cell proliferation can also be measured by G1-S transition of the cell cycle. In agreement with the results of epidermal thickening and Ki-67 positive nuclei, UVB irradiation increased the number of 308 mouse keratinocytes in S-phase, apigenin treatment abolished this increase, when cells were grown in serum-free medium to synchronize them in G0 phase and then subjected to treatments (Fig. 1E) Because G1 – S transition is dependent on cyclin-dependent kinase 2 (CDK2) binding to Cyclin E, we assessed CDK2 levels in 308 cells and in mouse skin. In cultured cells, UVB caused a moderate increase in CDK2 protein and apigenin strongly reduced both basal and UVB-induced CDK2 expression (Fig. 1F). Similarly, CDK2 expression in the skin of SKH-1 mice underwent similar changes, with moderate nuclear staining in control animals, which was visibly increased after UVB irradiation and considerably reduced by apigenin (Fig. 1G). Collectively, these results demonstrate that apigenin dramatically inhibits UVB-induced keratinocyte proliferation and cell cycle progression both in vivo and in vitro.
3.2. Apigenin inhibits UVB-mediated mTOR activation
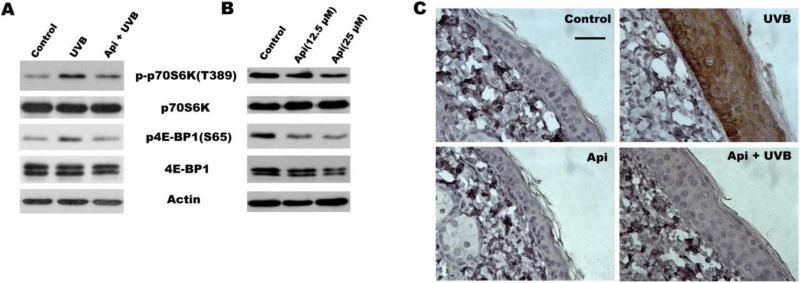
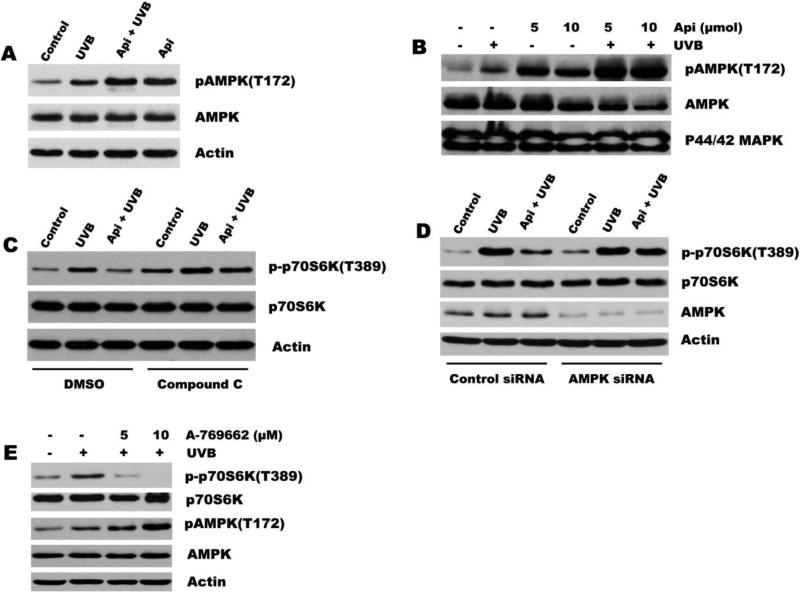
Recent studies indicate that UV irradiation induces mTOR activation and inhibition of mTOR signaling suppresses UVB-induced keratinocyte proliferation[24]. We have demonstrated previously that apigenin can inhibit mTOR signaling at baseline in cultured keratinocytes[18]. We therefore hypothesized that it may also block mTOR activation induced by UVB. To assess mTOR activity, we measured the phosphorylation of p70S6 kinase (p70S6K) and eIF4E binding protein (4E-BP1), two immediate downstream targets of mTOR. As shown in Fig. 2A, UVB irradiation increased the phosphorylation of p70S6K and 4E-BP1 in 308 cells. Importantly, apigenin inhibited UVB-induced phosphorylation of both mTOR targets. Our previous study has demonstrated that apigenin inhibits base level of mTOR activity in human keratinocytes[18]. Here, we also found apigenin treatment suppressed the phosphorylation of p70S6K and 4E-BP1 in normal culture of mouse keratinocytes (Fig. 2B). In addition, we detected mTOR activation directly, by immunostaining of mouse skin with antibodies for active, phosphorylated mTOR (Fig. 2C) and showed a dramatic increase in epidermal mTOR due to UVB irradiation, which was completely abolished by apigenin (Fig. 2C).
Fig. 2. Apigenin suppresses mTOR activationby UVB.
(A) Western blot analysis of mTOR downstream targets p-p70S6K and p4E-BP1 in 308 cells exposed to UVB (500 J/m2) and/or pre-treated with apigenin (25 μM) for 1 h where indicated, and harvested at 24 h post irradiation. (B) Dose-dependent effect of apigenin on baseline mTOR activation. 308 cells were treated with apigenin or vehicle for 24 h and harvested for immunoblotting. (C) Inhibition of mTOR activation in vivo: Control and UVB-irradiated SKH-1 mice (see Fig.1 for details) were treated topically with apigenin or vehicle 1 hr prior to irradiation. IHC was performed on dorsal epidermis sections using antibodies for active phosphorylated mTOR (p-mTOR, Ser 2448).
3.3. Activation of mTOR by UVB irradiation is dependent on PI3K/Akt signaling
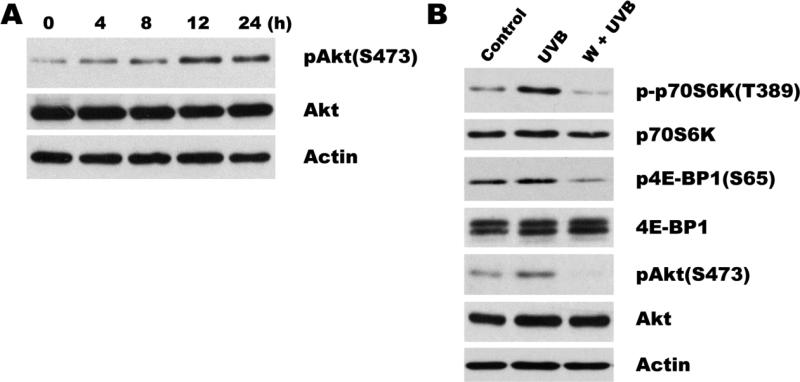
The PI3K/Akt and the mTOR signals form interdependent pathways that play critical roles in the regulation of cell growth, proliferation, and survival. Several studies have demonstrated that Akt can induce mTOR activity in multiple ways[26]. In agreement, we found that in 308 cells UVB irradiation induced Akt activation (phosphorylation on Ser 473) that reached its maximum by 12 h and lasted for at least 24 h following UVB exposure (Fig. 3A). To determine whether UVB-induced mTOR activation is dependent on PI3K/Akt pathway, we used wortmannin, a specific and potent inhibitor of PI3K. Pretreatment with wortmannin completely abolished UVB-induced increase in Akt phosphorylation as well as UVB-induced phosphorylation of p70S6K and 4E-BP1, indicating that mTOR activation by UVB is a downstream event following PI3K/Akt activation.
Fig. 3. mTOR activation by UVB involves PI3K/Akt signaling.
(A) Time course of Akt activation in UVB irradiated 308 cells. Cells were irradiated (500 J/m2) for indicated time periods, and phospho-Akt assessed by immunoblotting. Total Akt and Actin were used to as certain equal loading. (B) PI3K inhibitor Wortmannin attenuates UVB-induced mTOR activation. 308 keratinocytes were pre-treated with 5 μM wortmannin for 1 h, where indicated, irradiated and harvested 12 h (for Akt analysis) or 24 h (for p70S6K and 4E-BP1) following UVB irradiation. Representative Western blots are shown.
3.4. Apigenin inhibits UVB-induced mTOR signaling through activation of AMPK
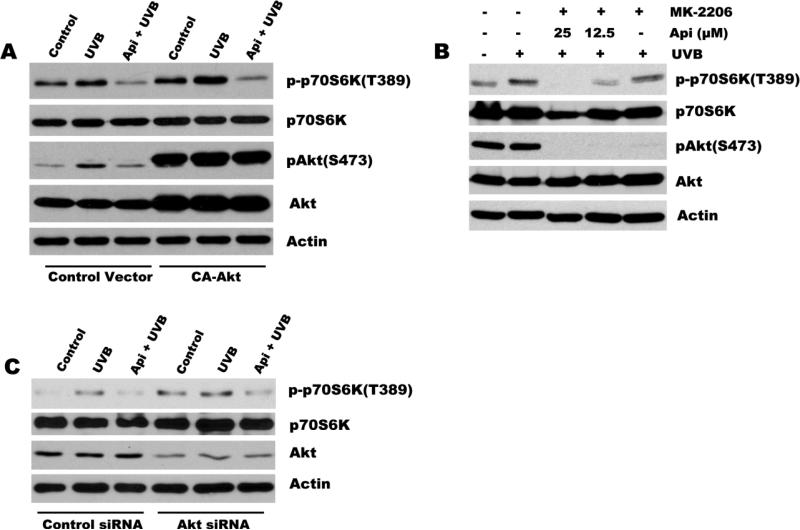
Previously, we have reported that apigenin inhibits Akt activation in many different cell types, including normal keratinocytes, prostate and pancreatic cancer cells[27-29]. Because UVB-induced mTOR activation was dependent on PI3K/Akt pathway, we sought to determine whether apigenin blocks UVB-induced mTOR activation by Akt interference. For that purpose we have generated 308 cells expressing constitutively active Akt (CA-Akt) (Fig. 4A). CA-Akt overexpression in 308 cells caused a significant increase in activation of mTOR pathway at baseline and in response to UVB irradiation, as was evidenced by increased phosphorylation of p70S6K(Fig. 4A). However, apigenin still effectively blocked mTOR activation regardless of CA-Akt presence (Fig. 4A). In order to further confirm that the inhibition of UVB-induced mTOR activation is not dependent on Akt, another two approaches were used: First, 308 cells were pretreated with either MK-2206 (an Akt specific inhibitor), or a combination of MK-2206 with different doses of apigenin prior to UVB irradiation. As illustrated in Fig. 4B, MK-2206 completely suppressed Akt activity but failed to inhibit UVB-induced phosphorylation of p70S6K. In addition, dose-dependent inhibition of mTOR activation by apigenin was not affected by MK-2206 (Fig. 4B). Second, cells were transfected with Akt siRNA to knock down Akt expression, and the knockdown of Akt was confirmed by immunoblotting as shown in Fig. 4C. Although transfection of 308 cells with the Akt siRNA decreased the Akt protein level dramatically compared with the non-targeting control siRNA, apigenin still inhibited UVB-induced phosphorylation of p70S6K (Fig. 4C). Together, these studies demonstrate that suppression of Akt does not contribute to apigenin-mediated mTOR inhibition.
Fig. 4. Inhibition of UVB-induced mTOR by apigenin is independent of its suppression of Akt.
(A) 308 cells were transiently transfected with an expression vector encoding constitutively active Akt (CA-Akt) or a control vector, incubated overnight. Apigenin (25 μM) was added 1 h prior to UVB irradiation (500 J/m2) and the cells were harvested 24 after UVB irradiation. mTOR activation (phosphorylation of its target p70S6K) and Akt activation/phosphorylation were assessed by Western blot. (B) 308 cells were pre-treated with Akt inhibitor MK-2206 (1 μM) or in combination with different doses of apigenin for 1 h before subjecting to UVB irradiation (500 J/m2), or (C) 308 cells were transfected with siRNA against Akt or non-targeting control siRNA, and 72 h later cells were exposed to apigenin and UVB irradiation as described above. All samples were collected 24 post irradiation. Western blotting was performed to measure phosphorylated p70S6K/Akt and total p70S6K/Akt.
We have previously shown that apigenin activates AMPK, an mTOR inhibitor, in human keratinocytes and thus causes decreased mTOR activation at baseline[18]. Therefore it was possible that in UVB-irradiated cells AMPK is similarly involved in mTOR regulation by apigenin as well. To address this possibility, we examined AMPK activation in 308 cells. Interestingly, UVB increased AMPK phosphorylation over baseline(sham-irradiated cells). However, apigenin increased AMPK phosphorylation with much greater potency, both in control and irradiated mouse keratinocytes (Fig. 5A). In agreement, Western blot of mouse epidermis showed modest upregulation of phosphorylated-AMPK by UVB and dramatic, > 10-fold upregulation by apigenin in sham-irradiated controls and UVB-irradiated skin (Fig. 5B). Importantly, pre-treatment of 308 keratinocytes with AMPK inhibitor Compound C prior to UVB and/ or apigenin treatment, completely abolished the inhibition effect of apigenin on p70S6K phosphorylation (Fig. 5C). We further confirmed the critical role of AMPK in apigenin's inhibition of UVB-induced mTOR activation by siRNA approach. As shown in Fig. 5D, AMPK protein level was dramatically reduced by transfection cells with AMPK siRNA, and apigenin lost the ability to block UVB-induced mTOR activation. Instead of apigenin, we also found that A-769662, an activator of AMPK, can inhibit UVB-induced mTOR signaling through the activation of AMPK (Fig. 5E), providing another piece of evidence to support the important role of AMPK in inhibition of UVB-induced mTOR activation. Taken together, our results demonstrate that apigenin-mediated AMPK activation, but not Akt interference, is necessary and sufficient for suppression of UVB-induced mTOR by apigenin.
Fig. 5. AMPK is essential for the blockade of UVB-induced mTOR pathway by apigenin.
(A) Apigenin activates AMPK at baseline and in UVB-irradiated cells. 308 cells were treated as indicated. Apigenin (25 μM) was added 1 hr prior to irradiation. The cells were harvested at 24 h after irradiation. (B) Apigenin activates AMPK in mouse skin. SKH-1 mice were irradiated as described in Fig.1. Apigenin was given topically 1 hr prior to irradiation at 5 and 10 μmol. Whole-skin protein extracts were immunoblotted for phosphorylated and total AMPK and p44/42 MAPK was used as loading control. (C) 308 cells were pre-treated with 5 μM compound C or vehicle (DMSO) for 1 h, then 25 μM apigenin was added to the media for another 1 h and followed by UVB exposure (500 J/m2). (D) 308 cells were transfected with AMPK siRNA or non-targeting control siRNA, and 48 h later cells were exposed to apigenin and UVB irradiation as described above. (E) 308 cells were pre-treated with different doses of AMPK activator A-769662 for 1 h before subjecting to UVB irradiation. All samples in (C-D) were harvested 24 h after irradiation and phosphorylated and total p70S6K/AMPK were assessed by Western blot.
3.5. Apigenin enhances UVB-induced autophagy, but not apoptosis, in mouse keratinocytes
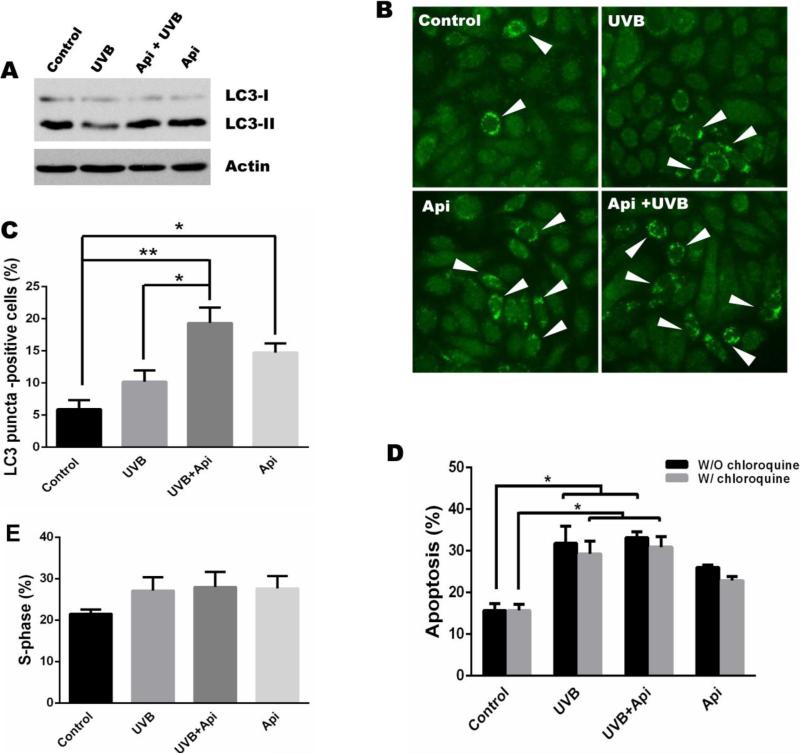
mTOR is an important signaling hub, which balances proliferation and autophagy, where by low mTOR activity results in metabolic distress and autophagy. In autophagic cells, the cytosolic form of microtubule-associated protein 1A/1B-light chain 3 (LC3-I) is converted to the low molecular weight form, LC3-II, a common indicator of autophagy. Western blot analysis of 308 cell lysates showed decreased levels of LC3-II upon UVB irradiation, suggesting decreased autophagy. In contrast, apigenin increased LC3-II (LC3-I cleavage), both in untreated and in UVB-treated cells (Fig. 6A). This was confirmed by immunofluorescence staining of 308 cells for LC3 (Fig. 6B). Using the number of cells with LC3-positive puncta (autophagosomes) as the autophagy index, we observed a modest increase in autophagy in UVB-irradiated keratinocytes, which did not reach statistical significance (Fig. 6B, C). Importantly, apigenin caused statistically significant increases in autophagy both in control and in UVB-treated keratinocytes (Fig. 6B, C). Recently Carr et al reported on distinct roles of mTORC1 and mTORC2 in control of cutaneous proliferation and apoptosis in the skin: Selective mTORC1 inhibition by rapamycin suppressed UVB-induced proliferation but had no effect on cell death; in contrast, mTOR deletion or mTORC2 disruption sensitized keratinocytes to UVB-induced apoptosis[24]. In this study we showed that apigenin inhibited the activation/phosphorylation of downstream targets of both mTORC1 (p70S6K and 4E-BP1, Fig. 2A) and mTORC2 (Akt (S473), Fig. 4A). To assess the contribution of the two mTOR complexes to apigenin effects on cell survival, we measured apoptosis in UVB-irradiated 308 keratinocytes in the absence and in the presence of apigenin. Apigenin caused an increase in the baseline apoptosis of cultured keratinocytes. However, apigenin did not augment UVB-induced apoptosis (Fig. 6D), suggesting that apigenin acts in this case via mTORC1. Since apigenin enhances autophagy through inhibition of mTOR, we further determined whether autophagy is involved in the induction of apoptosis by UVB or apigenin. Treatment of cells with chloroquine, an inhibitor of autophagy, didn't change the pattern of apoptosis induced by UVB or apigenin (Fig. 6D). However, compared to the result that UVB irradiation increases the number of cells in S-phase and apigenin treatment abolishes this increase (Fig. 1E), inhibition of autophagy blocked cells from entering S-phase (the percentage of S-phase cells dropped from 40% to 20% in control cells), and UVB couldn't increase S-phase cells and apigenin lost its ability to suppress cells to enter S-phase (Fig. 6E), indicating apigenin inhibits UVB-induced cell proliferation through enhancing autophagy.
Fig. 6. The effect of apigenin on UVB-induced autophagy and apoptosis.
(A) Immunoblot of endogenous LC3 (LC3-I and LC3-II) in 308 cells exposed to UVB (500 J/m2) and/or pre-treated with apigenin (25 μM) for 1 h where indicated, and harvested at 24 h post irradiation. (B) 308 cell were treated as above, and the LC3 puncta (autophagosomes, white arrows) were monitored by immunofluorescence. (C) The percentage of cells with >5 LC-3 positive puncta was calculated using least 200 cells per treatment. (D) 308 cells were treated as above, or pre-treated with autophagy inhibitor chloroquine (50 μM) for 1 h before apigenin treatment and UVB irradiation, and apoptosis detected by annexin-V staining followed by flow cytometry. Mean ± SD are shown, *, P < 0.01. (E) Determination of cells in S-phase was by carried out by cell cycle analysis as described in Fig.1, except that cells were pre-treated with chloroquine as above.
4. Discussion
The mTOR signaling pathway is essential for cell growth and proliferation, whereby it holds in check the balance between proliferation and autophagy[30]. Hyperactive mTOR signaling is a hallmark of many cancers, which prompted multiple discovery programs seeking potent small molecules that, similarly to rapamycin, specifically target mTOR. To improve the modest bioavailability and efficacy of rapamycin, several rapamycin derivatives (rapalogs) have been developed. However, the clinical efficacy of the rapalogs as cancer therapy is modest, likely due to their cytostatic rather than cytotoxic effect[31]. Another possible reason for their limited efficacy could be due to multiple negative feedback loops that moderate mTOR activity, especially for mTORC1, which repress PI3K signaling. Thus mTOR inhibitors may re-activate PI3K and Akt by disrupting this negative feedback[32-34]. The work on the second generation of inhibitors with dual mTOR/PI3K action is in progress, however these novel inhibitors, in addition to improved efficacy are likely to possess higher toxicity than rapamycin[35]. In this study, we found that the bioflavanoid apigenin is a unique and distinctive naturally occurring dual-specificity inhibitor, which simultaneously and independently blocks UVB-induced Akt and mTOR. An added benefit is that apigenin has a very favorable toxicity profile, a feature that makes it especially attractive for cancer prevention and therapy.
We have shown previously that apigenin treatment causes G2/M arrest in keratinocytes, in part due to inhibition of the mitotic kinase activity of p34cd2, and perturbation of cyclin B1 levels[15]. Since UVB mainly induces G1-S cell cycle progression[36], we synchronized cells in G0 phase prior to UVB exposure. We found that apigenin not only decreased UVB-induced G1 - S transition, but also reduced CDK2 expression. This is in line with our previous findings that apigenin blocked CDK2 kinase activity[37]. Interestingly, Chen et al reported that constitutive activation of mTOR pathway, which is frequent in epidermal tumors, especially in malignant ones, is tightly correlated with CDK2 expression[22]. Our study is the first to establish the link between UVB irradiation, CDK2 and mTOR activation. Moreover, in the present study we found that apigenin is a potent inhibitor of UVB-induced mTOR activation and associated CKD2 expression in mouse skin. Our data align mTOR and CDK2 as important mediators of UVB-induced skin carcinogenesis,although the mechanism by which mTOR regulates CDK2 expression remains elusive.
UVB irradiation induces DNA damage, which may generate mutations and genomic instability, eventually leading to carcinogenesis. Failure to eliminate the damaged cells, either by DNA repair or apoptosis, results in the retention of UVB-induced mutations that can lead to aberrant regulation of cell signaling and ultimately skin cancer[38]. Through a series of elegant experiments, Carr et al established that mTORC1 and mTORC2 play unique and distinct roles in controlling proliferation and apoptosis in skin. mTORC2 inactivation sensitizes epidermal keratinocytes to UVB-induced apoptosis. In contrast, mTORC1 blockade by rapamycin reduces G1 to S cell cycle progression and blocks the proliferation of keratinocytes induced by UVB irradiation[24]. Thus, hyper-activation of both mTOR pathways is required for damaged keratinocytes to survive and proliferate with higher risk of malignant transformation. UV-induced p53 mutations frequently occur in human skin cancer, in agreement with the critical role of p53 in the elimination of severely damaged cells[39], and keratinocytes with mutated p53 are less prone to UV-induced apoptosis as is evidenced by decreased amounts of sunburn (apoptotic cells) in the epidermis[40, 41]. However, UVB can induce apoptosis in HaCaT human keratinocytes cell line, which bears p53 mutations, suggesting p53-independent apoptosis in response to UVB[17]. Our previous studies uncovered enhanced apoptosis in UVB-irradiated keratinocytes through both the intrinsic and extrinsic apoptotic pathways, and also via a p53-independent pathway[17]. Surprisingly, in 308 mouse keratinocytes cells that express wild-type p53, apigenin failed to enhance UVB-induced apoptosis, suggesting other mechanisms of cell elimination underlying chemopreventive action.
Autophagy is a common consequence of mTOR inhibition, whereby long-lived or damaged proteins and organelles are engulfed by membrane vesicles and degraded for recycling. In addition to its role as a homeostatic mechanism, studies of the past two decades revealed dual role for autophagy in different stages of carcinogenesis. Removal of the damaged organelles and proteins reduces chromosome instability, and leads to a type II (non-apoptotic) programmed cell death, which counteracts cancer initiation[42-44]. However, in established tumors, autophagy may enhance the survival of cancer cells in conditions of low nutrients and hypoxia, and serve as a cytoprotective mechanism for cancer cells to resist cancer therapy[42-44]. A pharmacological autophagy induction in normal skin may provide a new chemoprevention strategy. Here, we demonstrate that AMPK-dependent mTOR inhibition by apigenin significantly enhances autophagy in UVB-irradiated keratinocytes. This could have two consequences. First, increased autophagy could eliminate protein complexes including those caused by single and double-stranded DNA breaks, which could, at least in part, ameliorate UVB-induced mutagenesis and subsequent carcinogenesis. On the other hand, concomitant inhibition of Akt could compromise survival of keratinocytes undergoing autophagy causing resulting in non-apoptotic death, elimination and decreased carcinogenic effect.
The link between autophagy and proliferation is not well understood, however a recent report by Cianfanelli et al identified scaffold protein AMBRA1, a component of the autophagy signaling network and a downstream target of mTOR, can facilitate the interaction between c-Myc and its phosphatase PP2A. When mTOR is inhibited, AMBRA1 enhances PP2A activity, causing c-Myc dephosphorylation and degradation, which result in attenuated proliferation[45]. Previous studies also demonstrated c-Myc deactivation by apigenin[46]. Our results (Fig. 1E and 6E) also indicate that autophagy regulates proliferation, and future studies will determine whether AMBRA1 is also involved in anti-proliferative action of apigenin.
5. Conclusions
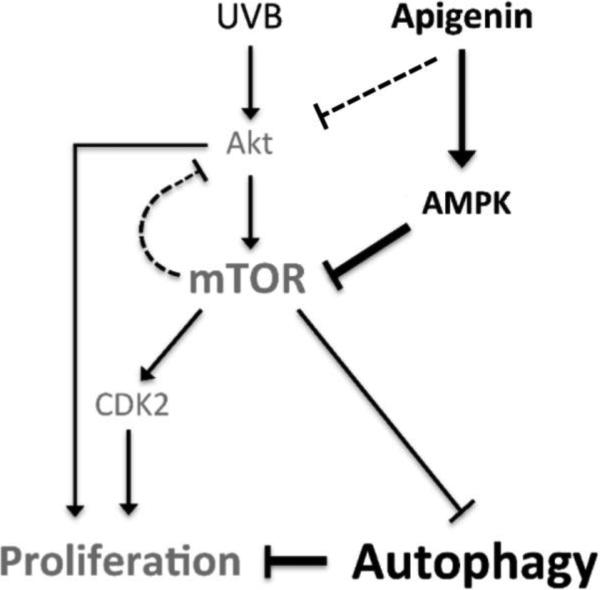
In summary, we have demonstrated that UVB activates mTOR, a critical player in skin carcinogenesis. Importantly, we show that apigenin potently and independently inhibits Akt and mTOR signaling pathways and provides a possible mechanism that links AMPK-dependent inhibition of mTOR with enhanced autophagy and decreased proliferation in UVB-exposed keratinocytes (Fig. 7). Our results identify apigenin as a naturally occurring dual specificity inhibitor of mTOR/ Akt signaling, which could replace rapalogs as an agent for prevention and treatment of skin cancer, suppresses cell proliferation and induces autophagy in keratinocytes.
Fig. 7. Signaling pathways involved in inhibition of mTOR activation by apigenin and subsequent cellular reaction in keratinocytes.
UVB activates mTOR pathway through PI3K/Akt signaling, while apigenin inhibits UVB-induced mTOR activation mainly by AMPK, further induces autophagy and suppresses cell proliferation.
Highlights.
UVB irradiation activates mTOR, a critical player in skin carcinogenesis.
Activation of mTOR by UVB is dependent on PI3K/Akt signaling.
Apigenin inhibits UVB-induced mTOR signaling through activation of AMPK.
mTOR inhibition by apigenin enhances autophagy, further reducing proliferation in keratinocytes.
Acknowledgements
This work was supported by NIH/NCI grants CA172669 (OV, JP and XT) and CA161181 (XT), and the authors thank Northwestern University Mouse Histology and Phenotyping Core for the help with immunohistochemistry staining.
Abbreviations
- UVB
ultraviolet B
- mTOR
mammalian target of rapamycin
- AMPK
AMP-activated protein kinase
- NMSC
Non-melanoma skin cancer
- SCC
squamous cell carcinomas
- p70S6K
ribosomal protein S6 kinase
- 4E-BP1
eukaryotic initiation factor 4E binding protein
- CDK2
cyclin-dependent kinase 2
- LC3
microtubule-associated protein 1A/1B-light chain 3
- PI3K
phosphoinositide 3-kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contribution
B. Bridgeman and P. Wang performed most of the experiments and analyzed data;B. Ye analyzed data and interpreted experiments with X. Tong; J. Pelling designed the study with X. Tong; O. Volpert interpreted experiments and wrote the manuscript together with X. Tong; X. Tong designed the study, planned and performed experiments, analyzed and interpreted data, and wrote manuscript.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Miller DL, Weinstock MA. J Am Acad Dermatol. 1994;30:774–778. doi: 10.1016/s0190-9622(08)81509-5. [DOI] [PubMed] [Google Scholar]
- 2.Bode AM, Dong Z. Sci STKE. 2003;2003:RE2. doi: 10.1126/stke.2003.167.re2. [DOI] [PubMed] [Google Scholar]
- 3.Bowden GT. Nat Rev Cancer. 2004;4:23–35. doi: 10.1038/nrc1253. [DOI] [PubMed] [Google Scholar]
- 4.Ross JA, Kasum CM. Annu Rev Nutr. 2002;22:19–34. doi: 10.1146/annurev.nutr.22.111401.144957. [DOI] [PubMed] [Google Scholar]
- 5.Wei H, Tye L, Bresnick E, Birt DF. Cancer Res. 1990;50:499–502. [PubMed] [Google Scholar]
- 6.Birt DF, Mitchell D, Gold B, Pour P, Pinch HC. Anticancer Res. 1997;17:85–91. [PubMed] [Google Scholar]
- 7.Patel D, Shukla S, Gupta S. Int J Oncol. 2007;30:233–245. [PubMed] [Google Scholar]
- 8.Fang J, Zhou Q, Liu LZ, Xia C, Hu X, Shi X, Jiang BH. Carcinogenesis. 2007;28:858–864. doi: 10.1093/carcin/bgl205. [DOI] [PubMed] [Google Scholar]
- 9.Tong X, Mirzoeva S, Veliceasa D, Bridgeman BB, Fitchev P, Cornwell ML, Crawford SE, Pelling JC, Volpert OV. Oncotarget. 2014;5:11413–11427. doi: 10.18632/oncotarget.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McVean M, Xiao H, Isobe K, Pelling JC. Carcinogenesis. 2000;21:633–639. doi: 10.1093/carcin/21.4.633. [DOI] [PubMed] [Google Scholar]
- 11.Tong X, Pelling JC. Mol Carcinog. 2009;48:118–129. doi: 10.1002/mc.20460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Dross RT, Hong X, Essengue S, Fischer SM, Pelling JC. Mol Carcinog. 2007;46:303–314. doi: 10.1002/mc.20281. [DOI] [PubMed] [Google Scholar]
- 13.Tong X, Van Dross RT, Abu-Yousif A, Morrison AR, Pelling JC. Mol Cell Biol. 2007;27:283–296. doi: 10.1128/MCB.01282-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang W, Heideman L, Chung CS, Pelling JC, Koehler KJ, Birt DF. Mol Carcinog. 2000;28:102–110. [PubMed] [Google Scholar]
- 15.McVean M, Weinberg WC, Pelling JC. Mol Carcinog. 2002;33:36–43. doi: 10.1002/mc.10016. [DOI] [PubMed] [Google Scholar]
- 16.Shukla S, Gupta S. Mol Carcinog. 2004;39:114–126. doi: 10.1002/mc.10168. [DOI] [PubMed] [Google Scholar]
- 17.Abu-Yousif AO, Smith KA, Getsios S, Green KJ, Van Dross RT, Pelling JC. Cancer Res. 2008;68:3057–3065. doi: 10.1158/0008-5472.CAN-07-2763. [DOI] [PubMed] [Google Scholar]
- 18.Tong X, Smith KA, Pelling JC. Mol Carcinog. 2012;51:268–279. doi: 10.1002/mc.20793. [DOI] [PubMed] [Google Scholar]
- 19.Guertin DA, Sabatini DM. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 21.Kauffman HM, Cherikh WS, Cheng Y, Hanto DW, Kahan BD. Transplantation. 2005;80:883–889. doi: 10.1097/01.tp.0000184006.43152.8d. [DOI] [PubMed] [Google Scholar]
- 22.Chen SJ, Nakahara T, Takahara M, Kido M, Dugu L, Uchi H, Takeuchi S, Tu YT, Moroi Y, Furue M. The British journal of dermatology. 2009;160:442–445. doi: 10.1111/j.1365-2133.2008.08903.x. [DOI] [PubMed] [Google Scholar]
- 23.Einspahr JG, Calvert V, Alberts DS, Curiel-Lewandrowski C, Warneke J, Krouse R, Stratton SP, Liotta L, Longo C, Pellacani G, Prasad A, Sagerman P, Bermudez Y, Deng J, Bowden GT, Petricoin EF., 3rd Cancer Prev Res (Phila) 2012;5:403–413. doi: 10.1158/1940-6207.CAPR-11-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carr TD, DiGiovanni J, Lynch CJ, Shantz LM. Cancer Prev Res (Phila) 2012;5:1394–1404. doi: 10.1158/1940-6207.CAPR-12-0272-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strickland JE, Greenhalgh DA, Koceva-Chyla A, Hennings H, Restrepo C, Balaschak M, Yuspa SH. Cancer Res. 1988;48:165–169. [PubMed] [Google Scholar]
- 26.Tong X, Pelling JC. Anti-cancer agents in medicinal chemistry. 2013;13:971–978. doi: 10.2174/18715206113139990119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Dross RT, Hong X, Pelling JC. Mol Carcinog. 2005;44:83–91. doi: 10.1002/mc.20123. [DOI] [PubMed] [Google Scholar]
- 28.Mirzoeva S, Kim ND, Chiu K, Franzen CA, Bergan RC, Pelling JC. Mol Carcinog. 2008;47:686–700. doi: 10.1002/mc.20421. [DOI] [PubMed] [Google Scholar]
- 29.Melstrom LG, Salabat MR, Ding XZ, Milam BM, Strouch M, Pelling JC, Bentrem DJ. Pancreas. 2008;37:426–431. doi: 10.1097/MPA.0b013e3181735ccb. [DOI] [PubMed] [Google Scholar]
- 30.Shimobayashi M, Hall MN. Nat Rev Mol Cell Biol. 2014;15:155–162. doi: 10.1038/nrm3757. [DOI] [PubMed] [Google Scholar]
- 31.Chiarini F, Evangelisti C, McCubrey JA, Martelli AM. Trends Pharmacol Sci. 2015;36:124–135. doi: 10.1016/j.tips.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 32.Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A, Egia A, Sasaki AT, Thomas G, Kozma SC, Papa A, Nardella C, Cantley LC, Baselga J, Pandolfi PP. J Clin Invest. 2008;118:3065–3074. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bertacchini J, Guida M, Accordi B, Mediani L, Martelli AM, Barozzi P, Petricoin E, 3rd, Liotta L, Milani G, Giordan M, Luppi M, Forghieri F, De Pol A, Cocco L, Basso G, Marmiroli S. Leukemia. 2014;28:2197–2205. doi: 10.1038/leu.2014.123. [DOI] [PubMed] [Google Scholar]
- 34.Huang S. Cell Cycle. 2012;11:844. doi: 10.4161/cc.11.5.19598. [DOI] [PubMed] [Google Scholar]
- 35.Willems L, Tamburini J, Chapuis N, Lacombe C, Mayeux P, Bouscary D. Curr Oncol Rep. 2012;14:129–138. doi: 10.1007/s11912-012-0227-y. [DOI] [PubMed] [Google Scholar]
- 36.Han W, He YY. Photochem Photobiol. 2009;85:997–1003. doi: 10.1111/j.1751-1097.2008.00531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lepley DM, Pelling JC. Mol Carcinog. 1997;19:74–82. doi: 10.1002/(sici)1098-2744(199707)19:2<74::aid-mc2>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 38.Strozyk E, Kulms D. Int J Mol Sci. 2013;14:15260–15285. doi: 10.3390/ijms140815260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rass K, Reichrath J. Adv Exp Med Biol. 2008;624:162–178. doi: 10.1007/978-0-387-77574-6_13. [DOI] [PubMed] [Google Scholar]
- 40.Ziegler A, Jonason AS, Leffell DJ, Simon JA, Sharma HW, Kimmelman J, Remington L, Jacks T, Brash DE. Nature. 1994;372:773–776. doi: 10.1038/372773a0. [DOI] [PubMed] [Google Scholar]
- 41.Tron VA, Trotter MJ, Tang L, Krajewska M, Reed JC, Ho VC, Li G. Am J Pathol. 1998;153:579–585. doi: 10.1016/S0002-9440(10)65600-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kondo Y, Kanzawa T, Sawaya R, Kondo S. Nat Rev Cancer. 2005;5:726–734. doi: 10.1038/nrc1692. [DOI] [PubMed] [Google Scholar]
- 43.Steeves MA, Dorsey FC, Cleveland JL. Curr Opin Cell Biol. 2010;22:218–225. doi: 10.1016/j.ceb.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maiuri MC, Tasdemir E, Criollo A, Morselli E, Vicencio JM, Carnuccio R, Kroemer G. Cell Death Differ. 2009;16:87–93. doi: 10.1038/cdd.2008.131. [DOI] [PubMed] [Google Scholar]
- 45.Cianfanelli V, Fuoco C, Lorente M, Salazar M, Quondamatteo F, Gherardini PF, De Zio D, Nazio F, Antonioli M, D'Orazio M, Skobo T, Bordi M, Rohde M, Dalla Valle L, Helmer-Citterich M, Gretzmeier C, Dengjel J, Fimia GM, Piacentini M, Di Bartolomeo S, Velasco G, Cecconi F. Nat Cell Biol. 2015;17:20–30. doi: 10.1038/ncb3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yin F, Giuliano AE, Van Herle AJ. Anticancer Res. 1999;19:4297–4303. [PubMed] [Google Scholar]