Summary
Objective
Given the association between maternal caregiving behavior and heightened neural reward activity in experimental animal studies, the present study examined whether motherhood in humans positively modulates reward-processing neural circuits, even among mothers exposed to various life stressors and depression.
Methods
Subjects were 77 first-time mothers and 126 nulliparous young women from the Pittsburgh Girls Study, a longitudinal study beginning in childhood. Subjects underwent a monetary reward task during functional magnetic resonance imaging in addition to assessment of current depressive symptoms. Life stress was measured by averaging data collected between ages 8–15 years. Using a region-of-interest approach, we conducted hierarchical regression to examine the relationship of psychosocial factors (life stress and current depression) and motherhood with extracted ventral striatal (VST) response to reward anticipation. Whole-brain regression analyses were performed post-hoc to explore non-striatal regions associated with reward anticipation in mothers vs nulliparous women.
Results
Anticipation of monetary reward was associated with increased neural activity in expected regions including caudate, orbitofrontal, occipital, superior and middle frontal cortices. There was no main effect of motherhood nor motherhood-by-psychosocial factor interaction effect on VST response during reward anticipation. Depressive symptoms were associated with increased VST activity across the entire sample. In exploratory whole brain analysis, motherhood was associated with increased somatosensory cortex activity to reward (FWE cluster forming threshold p<0.001).
Conclusions
These findings indicate that motherhood is not associated with reward anticipation-related VST activity nor does motherhood modulate the impact of depression or life stress on VST activity. Future studies are needed to evaluate whether earlier postpartum assessment of reward function, inclusion of mothers with more severe depressive symptoms, and use of reward tasks specific for social reward might reveal an impact of motherhood on reward system activity.
Keywords: Motherhood, depression, reward, life stress, ventral striatum
1. Introduction
Postpartum depression is highly prevalent in low income, young mothers and thus poses a serious public health concern(Gaynes et al., 2005; O’Hara and Swain, 1996). Maternal mood impairment is often associated with deficits in maternal caregiving and compromised cognitive, behavioral and emotional development of the offspring. There is currently a disappointingly low rate of postpartum depression-related treatment remission of only 30–50%,(Wisner et al., 2006) akin to that in the broader mood disorder clinical population. Increased understanding of the neurobiological features of postpartum depression offers the promise of designing specific treatments that can more directly improve maternal mood and caregiving deficits.
The dopaminergic reward system is a promising candidate circuit for neurobiological mechanism investigations of postpartum depression by virtue of known dopaminergic/reward changes which occur after childbirth(Moses-Kolko et al., 2008) and also because of convergent findings of fronto-striatal reward dysfunction in depression and anhedonia.(Dunlop and Nemeroff, 2007; Nestler and Carlezon, 2006; Zhang et al., 2013) Recent studies have described brain molecular and functional activity-related disturbances in postpartum depressed women including lower [11C]raclopride-positron emission tomography (PET) measures of dopamine D2/3 receptor binding (DRD2)(Moses-Kolko et al., 2012) as well as less sustained or lower ventral striatal responses to monetary reward(Moses-Kolko et al., 2011) and positive words(Silverman et al., 2007) relative to healthy mothers. Corroborating this work are findings of reduced cortico-striatal responses to positive infant stimuli in mothers with attachment disturbances, intrusive parenting and depressive symptoms.(Atzil et al., 2011; Barrett et al., 2011; Strathearn et al., 2009)
While the above studies suggest reward dysfunction in postpartum depression, it remains unknown whether dopaminergic reward processes are generally altered by motherhood, thus conferring a distinct neurobiology for depression in postpartum compared to non-postpartum women. Studies of maternal behavior in rodents highlight the amplification of mesolimbic dopamine system signals necessary to stimulate the appetitive aspects of maternal behavior (Fleming et al., 2008; Numan, 2015). Specifically, hypothalamic medial preoptic area (MPOA) and paraventricular nucleus stimulate ventral tegmental area (VTA) dopamine release into ventral striatum (VST), which disinhibits ventral pallidum to generate maternal behaviors such as grooming, pup retrieval, and nest building(Numan and Stolzenberg, 2009). Because new mothers have heightened hormonal and psychosocial drive for approach behaviors and also because heightened DA system function in new mothers facilitates positive affect and states of well-being,(Fleming et al., 2008) it is possible that the dopamine reward function operates at a different set-point in postpartum women. To our knowledge, there has been no direct comparison of neural reward system function between mothers and non-mothers
Another missing link pertains to the notion that the combined physiological and psychosocial changes inherent in the perinatal period, and particularly the new attachment relationship between mother and infant and increased social support, can ameliorate adverse neural effects of early life experiences.(Barrett and Fleming, 2011; Kinsley and Lambert, 2008; McEwen, 2003) In a study of rodent dams reared in isolated, artificial neonatal environments, mesolimbic dopamine function varied as a function of the presence of hormonal or tactile inputs in early life or adulthood (Afonso et al., 2011). While a broadening literature describes that early childhood adversity(Dillon et al., 2009; Guyer et al., 2006) and prior major depression (Dichter et al., 2012; Hasler et al., 2009) confer significant ongoing signs of vulnerability in behavioral and neural responses to reward, whether motherhood can mitigate against the impact of psychosocial factors on reward system functioning has not yet been tested.
We examined the association between motherhood, neural reward function, depression and life stress in young women, varied for motherhood. The young women had undergone the same fMRI reward task in separate research studies, but, were also all participants of a large-scale prospective study of psychopathology, substance use and life stress that began in childhood (Keenan et al., 2010). This is an advantageous cohort because sampling was based on community demographic parameters as opposed to maternal caregiving experiences. In addition, data on exposure to life stress were collected prospectively, depression symptoms were measured concurrently with the assessment of neural activity, and we had nicotine use data in the entire cohort to control for confounding effects on neural reward function (Bühler et al., 2010). We tested the hypotheses that 1) VST reward system activity would be enhanced in motherhood and 2) motherhood would moderate the relationship between both depression and life stress and VST reward-related activity, such that VST reward activity would be reduced in depression and in the context of high life stress, but only for non-mothers.
2. Method
2.1. Participants and recruitment
Subjects were participants in the Pittsburgh Girls Study (PGS; n=2450) (Hipwell et al., 2002; Keenan et al., 2010), a longitudinal community cohort study of the development of psychopathology and substance use. The PGS sample was established in 1999 based upon enumeration of 100% low-income environments and 50% of remaining neighborhoods in the City of Pittsburgh. Young women were recruited into four age cohorts (ages 5, 6, 7 and 8 years in wave 1) and have been assessed annually into late adolescence/early adulthood. Separate interviews of the child and her caregivers have been conducted annually, in the home, for the past 16 years, covering multiple domains related to individual, family, school, peer and neighborhood. Mean participant retention in the PGS is over 90%, and 86.3% of the original sample of girls was interviewed in wave 15.
Two distinct PGS substudies have examined neural response to monetary reward. The Mother-Baby Substudy (PGS-MB) has recruited all PGS participants, ages 18–22, who were pregnant or postpartum for the first time. These women were interviewed in the home and in the lab and completed the monetary reward fMRI task (see below) at approximately 16 weeks postpartum. At the time of this analysis 97 mothers were enrolled in the study of whom 77 (79.4%) had useable fMRI and psychosocial data. The Learning about Girls Emotions Substudy (PGS-E) recruited 232 participants from the youngest age cohort of the PGS who either screened high on measures of depression symptoms by self- or maternal report at age eight years (n=135), or who were included in a random selection from the remaining girls, matched to the screen high group on race (n=136) [see (Keenan et al., 2009) for details]. At the time of this analysis, 194 (71.6%) of the PGS-E sample had been retained in the study, and 169 completed the monetary reward fMRI task. Motherhood was not exclusionary for participation in the PGS-E study and 11 women (7.2%) had a live birth prior to their scan. We excluded these women to maintain distinct groupings with respect to motherhood. The final subgroup was comprised of nulliparous, late adolescents ages 15–18 years, (n=158), of whom 126 (79.7%) had useable fMRI and psychosocial data.
Subjects provided written informed consent as approved by the University of Pittsburgh Human Research Protection Office. Two prior analyses in the nulliparous cohort have examined the mediating role of neural reward responses between psychosocial stressors and depression.(Casement et al., 2014; Romens et al., 2015)
2.2. Psychological measures
Depressive symptoms at the time of the fMRI scan were assessed with the Hamilton Rating Scale for Depression (HAM-D) in mothers (PGS-MB study) and the Kiddie-Schedule for Affective Disorders and Schizophrenia-Present and Life version (KSADS-PL) for non-mothers (PGS-E study). To use a common depression variable for analyses within the full study sample (mothers and non-mothers), we derived a 12-item depression severity score from the KSADS-PL scale which directly overlapped 12-items of the HAM-D. Applying a 17-item HAM-D severity grading system (Hamilton, 1960) to the KSADS, as we did here, was initially described by Endicott et al (Endicott et al., 1981) for the Schedule for Affective Disorders and Schizophrenia and later for the KSADS by Williamson et al (Williamson et al., 1992). The 12-items for which there was direct overlap between the depression module of the KSADS and the 17-item HAM-D included depressed mood, participation in work and activities, loss of appetite, weight loss, insomnia (early, middle, late), fatigue, guilt, suicidal ideation, and psychomotor retardation and agitation. Because reversed neurovegetative symptoms of depression included in the 25-item HAM-D (ie: fatigue, hypersomnia, hyperphagia) can be overrepresented in motherhood, we intentionally excluded these symptoms so as not to bias the severity of depression in the group of mothers compared to non-mothers. The 12-item depression severity score did not include worry, tension, or somatic anxiety due to missing KSADS anxiety module data in a subset of non-mothers (n=35). An approximate threshold score for a diagnosis of major depression on this 12-item common scale is 9, based upon the designation of 13 as a cutoff for major depression on the 17-item HAM-D (Bagby et al., 2004).
In this analysis, we created a composite measure of life stress using key environmental variables associated with neurobiological function.(McEwen and Gianaros, 2011) Life stress variables included receipt of public assistance (parent report), single parent status (parent report), sexual abuse (parent and child report), non-sexual trauma (child report), and whether the subject was in the top quartile for ratings on the Difficult Life Circumstances measure (parent report) (Barnard, 1994), which included questions about regular arguments with family members, partner absence or incarceration, long-term debt/credit problems, lack of privacy, crowding in the home, long-term family illness, abuse of self or children in the home, and substance abuse. The five life stress variables were each coded as 1 (present) or 0 (absent) and then averaged to obtain the annual life stress score. We computed an average of these annual life stress scores based upon data acquired during the PGS annual interviews from ages 8 through 15 (8 years). Subjects without these data for at least 6 of the 8 years of interest were considered to have missing data for this variable. A maximum score for this 8-year life stress average variable was 1. Across the full PGS cohort (n=2227 with complete data), the average life stress measure from ages 8–15 ranged from 0–0.74, with a mean of 0.27, and standard deviation of 0.19. Nicotine use, a covariate included in the analysis based upon its association with reward function,(Bühler et al., 2010) was defined as the frequency of smoking cigarettes in the past year. This data was collected as part of the annually-assessed questionnaire of nicotine, alcohol and drug use derived from the Computerized Diagnostic Interview for Psychiatry (CIDI).
2.3. Neuroimaging acquisition and analysis
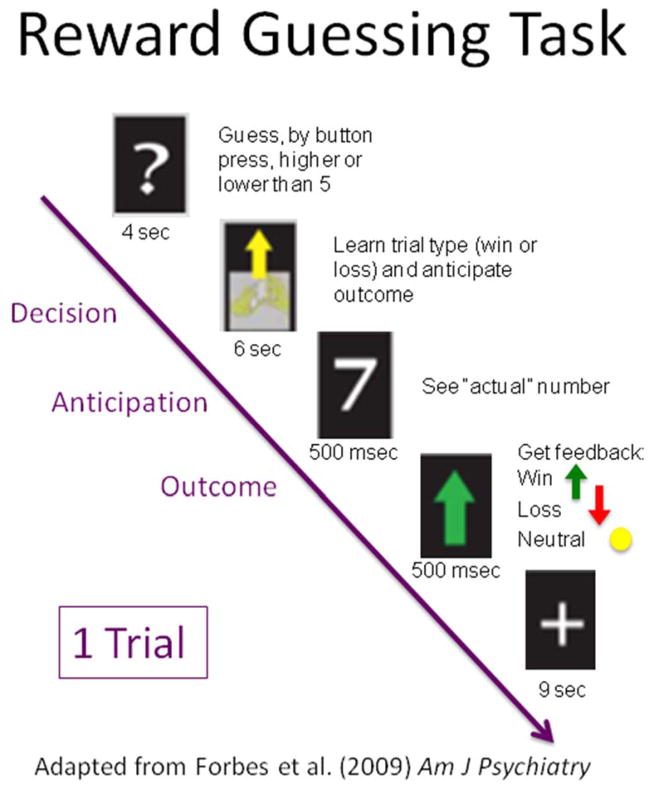
A Siemens 3.0 TIM Trio scanner was used to acquire a high-resolution anatomical image in the sagittal plane(192, 1mm thick, TR=2300msec, TE=3.31msec, FOV=25 cm, matrix=256×208). BOLD functional images were acquired with a gradient echo planar imaging sequence in the axial plane (39 slices, 3.1mm thick, TR=2000msec, TE=28msec, FOV=20cm, matrix=64×64) during which participants completed an 8-min, computer-based, card-guessing monetary reward task that consisted of 24, 20-second trials(Forbes et al., 2009). This slow event-related fMRI task has both anticipatory and feedback components. In the paradigm (Figure 1), participants are told that a hidden card has a value from 1 to 9 (the number 5 is excluded). They are instructed to guess (via button press) whether they think the value of the card is greater than 5 or less than 5. When a card is presented, subjects have 4 seconds to make their guess. Following that interval, a shuffling cards image appears with an arrow cue that indicates the trial type (gain or loss). An up-facing green arrow means a “reward” trial; a down-facing red arrow means a “loss” trial. The participants are told that a correct guess in a gain trial will earn them $1, while an incorrect guess in a gain trial results in no change in earnings. A correct guess in a loss trial earns them no money, but an incorrect guess in a loss trial will cause them to lose $0.50. The trial type cue is presented for a 6 second anticipation period that is then followed by the outcome period of the trial. In the outcome period, the actual value of the card is flashed for 500 msec, followed by an outcome cue for 500 msec. An up-facing green arrow means a correct guess for a “win” trial (money won) a down-facing red arrow means an incorrect guess for a “lose” trial (money lost) and a yellow circle means no change (this occurs either for the incorrect guess on a gain trial or the correct guess on a loss trial). Following the outcome period of the trial, there is a 9 second inter-trial-interval (ITI) that serves as the “baseline” period for contrasts performed at the first level, within-subject regression analysis. Although participants were told that their winnings depended on their correct guesses, trials were in fact presented in a pseudorandom order that ensured a balance of trial types and outcomes trial (12 gain trials and 12 loss trials; each trial type had 6 correct guesses and 6 neutral outcomes). Therefore, each subject received the predetermined amount of $3. This task, and tasks very similar to it, have been shown to reliably recruit brain regions that support the processing of feedback and reward-based learning (Delgado et al., 2003; Forbes et al., 2009; Tricomi et al., 2006)
Figure 1.
Single trial of the reward task. For interpretation of the reference to color, the reader is referred to the web version of the article.
Pre-processing and data analysis were conducted with Statistical Parametric Mapping (SPM8; http://www.fil.ion.ucl.ac.uk/spm) to include motion correction, spatial normalization (Montreal Neurological Institute space), and smoothing with a Gaussian filter (6mm full-width half-maximum). A final sample of 203 (77 mothers; 126 non-mothers) subjects remained after exclusions due to: IQ < 70 or unknown (n=9), missing scan or behavioral data (n=2), >25% missed trials (n=10), MRI acquisition quality fail (artifact, <80% VST coverage, >4mm of head movement) (n=26), and incomplete psychosocial data (n=5).
Single subject scans were analyzed using a first-level model with regressors for response (4 sec), anticipation (6 sec), outcome (1 sec), baseline (the last 3 sec during fixation), omission errors (17 sec), and head movement vectors derived from preprocessing. Serial autocorrelations were modeled using an AR(1) process. Average BOLD activity from the first-level T-contrast of reward anticipation relative to baseline was extracted from separate, right and left ventral striatal masks for analysis in IBM SPSS 22. These masks were based upon published findings of reward-related functional striatal activity in a separate sample (Chase et al., 2013), with peak coordinates 13,15,-4 and -8,13,-3. We chose VST activity to reward anticipation as the dependent variable of greatest interest based upon the appetitive nature of maternal caregiving (Numan, 2015) and prior results from a subset of this cohort in which depression was associated with increased neural response to reward anticipation (Casement et al., 2014). We used hierarchical multiple regression to examine the effects of race and nicotine use (Step 1), age and motherhood status (Step 2), depression and life stress (Step 3), and interactions of step 2 and 3 variables (Step 4) on VST BOLD response during reward anticipation in separate models for right and left VST. We conducted several post-hoc tests for the full sample and for the mother and nulliparous groups separately to examine robustness of the results. Finally, we conducted a whole-brain exploratory analysis to examine effects of motherhood on reward-related activity in non-VST brain regions.
3. Results
3.1. Age and psychosocial characteristics
Subjects ranged in age from late adolescence through early adulthood with mothers being older than non-mothers (Mean±SD) (19.95±1.04 vs 17.04±0.61 yrs, p<0.001). The entire sample was largely of minority race, without a significant difference between mothers and non-mothers (81.5% vs. 70.1%, p=ns). Behavioral performance on the fMRI task, measured as missed trials and reaction time, did not differ between groups. In general, participants had low levels of depressive symptoms on the 12-item depression scale in both PGS subsamples. Within this non-clinical range, mothers had higher scores than non-mothers (4.2 ± 3.54 vs 2.75 ± 2.8; p=0.001). The life stress measure, a composite of several variables, did not differ between groups (0.34±0.17 vs 0.30±0.20, p=0.12) with mean scores which corresponded to the 54th through 62nd percentile of life stress scores in the full PGS cohort. Life stress data and depressive symptoms measured at the time of scanning were only weakly correlated (R=0.18, p=0.01).
3.2. Neural responses to reward
Our card-guessing, monetary reward task elicited the intended distribution of neural activity. The first-level T-contrast of reward anticipation relative to baseline across the entire sample, controlling for demographic (age, race) and psychosocial factors (nicotine use, depression, life stress), revealed large clusters of significant activation in right caudate, orbitofrontal, occipital, inferior parietal, superior frontal, middle frontal, middle temporal, angular, and fusiform cortices (cluster-forming threshold FWE p<0.001; peak-level significance FWE p<0.001; Table 1).
Table 1. Reward anticipation-related positive neural activity clusters across the entire sample.
Cluster-forming threshold FWE p<0.05; k=30
| Region | Hemisphere | Coordinates | Cluster size | t | ||
|---|---|---|---|---|---|---|
| Orbitofrontal cortex | Left | −44 | 46 | −4 | 1230 | 14.12 |
| Orbitofrontal cortex | Right | 38 | 56 | −2 | 90 | 9.39 |
| Occipital cortex | Left | −22 | −94 | 0 | 305 | 12.60 |
| Occipital cortex | right | 30 | −90 | 4 | 257 | 11.08 |
| Inferior parietal lobe | Left | −50 | −60 | 44 | 441 | 11.94 |
| Superior frontal gyrus | Right | 8 | 42 | 44 | 630 | 9.83 |
| Middle frontal gyrus | Right | 44 | 20 | 48 | 75 | 8.70 |
| Middle temporal gyrus | Left | −60 | −44 | −8 | 173 | 8.08 |
| Middle temporal gyrus | Right | 60 | −40 | −8 | 49 | 6.68 |
| Angular gyrus | Right | 36 | −72 | 46 | 353 | 7.19 |
| Fusiform gyrus | Left | −32 | −50 | 2 | 47 | 6.46 |
| Caudate | Right | 6 | 8 | 18 | 54 | 6.42 |
| Caudate | Right | 6 | 18 | 10 | 38 | 6.29 |
For the specific tests of our hypotheses, results of the ROI-based hierarchical multiple regression analyses of VST BOLD response during reward anticipation for right and left VST are shown in Table 2. There were no significant effects of minority race, nicotine use, age or motherhood. Depressive symptoms (β=.19, p=0.011) were associated with increased right VST activity to reward anticipation compared to baseline (ΔR2=0.044, p=0.011) and there was a trend toward significance for the left VST (ΔR2=0.027, p=0.06). The robustness of the depression association with increased right VST activity in this model was confirmed by repeating the regression without life stress in the model (β=.18, p=0.01).
Table 2.
Full sample - Hierarchical regression of right VST reward anticipation activity
| All predictors included | Age included (Motherhood status excluded) | Motherhood included (Age excluded) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| B | SE B | β | B | SE B | β | B | SE B | β | |
| Step 1 | |||||||||
| Constant | −0.135 | 0.047** | −0.135 | 0.047** | −0.135 | 0.047** | |||
| Minority race | −0.005 | 0.053 | −.006 | −0.005 | 0.053 | −.006 | −0.005 | 0.053 | −.006 |
| Nicotine use | −0.004 | 0.011 | −.023 | −0.004 | 0.011 | −.023 | −0.004 | 0.011 | −.023 |
| R2(2,200)=0.001 | R2(2,200)=0.001 | R2(2,200)=0.001 | |||||||
| Step 2 | |||||||||
| Constant | 0.779 | 0.491 | 0.308 | 0.260 | −0.124 | 0.048* | |||
| Minority race | −0.006 | 0.053 | −.008 | 0.002 | 0.052 | .002 | 0.002 | 0.053 | .002 |
| Nicotine use | 0.000 | 0.011 | −.003 | 0.000 | 0.011 | −.002 | −0.002 | 0.011 | −.013 |
| Age | −0.053 | 0.029 | −.263 | −0.025 | 0.014 | −.124 | --- | --- | --- |
| Motherhood | 0.109 | 0.096 | .162 | --- | --- | --- | −0.045 | 0.048 | −.067 |
| R2(4,198)=0.022 | R2(3,199)=0.015 | R2(3,199)=0.005 | |||||||
| Step 3 | |||||||||
| Constant | 0.718 | 0.483 | 0.370 | 0.258 | −0.138 | 0.052** | |||
| Minority race | 0.031 | 0.058 | .041 | 0.034 | 0.058 | .046 | 0.035 | 0.058 | .047 |
| Nicotine use | −0.003 | 0.011 | −.020 | −0.003 | 0.011 | −.021 | −0.005 | 0.011 | −.031 |
| Age | −0.05 | 0.028 | −.251 | −0.029 | 0.014 | −.147* | --- | --- | --- |
| Motherhood | 0.082 | 0.096 | .121 | --- | --- | --- | −0.066 | 0.049 | −.098 |
| Depression | 0.019 | 0.007 | .187* | 0.020 | 0.007 | .198* | 0.020 | 0.007 | .198** |
| Life stress | −0.256 | 0.135 | −.148 | −0.248 | 0.135 | −.143 | −0.242 | 0.136 | −.140 |
| R2(6,196)=0.065* | R2(5,197)=0.038** | R2(5,197)=0.050** | |||||||
| Step 4 | |||||||||
| Constant | 0.548 | 0.504 | 0.338 | 0.264 | −0.137 | 0.053 | |||
| Minority race | 0.049 | 0.060 | .066 | 0.034 | 0.058 | .046 | 0.035 | 0.059 | |
| Nicotine use | −0.003 | 0.012 | −.018 | −0.004 | 0.012 | −.025 | −0.005 | 0.011 | |
| Age | −0.041 | 0.029 | −.206 | −0.028 | 0.014 | −.138 | --- | --- | --- |
| Motherhood | 0.056 | 0.099 | .084 | --- | --- | --- | −0.066 | 0.049 | −.097 |
| Depression | 0.019 | 0.017 | .183 | 0.020 | 0.008 | .194* | 0.023 | 0.011 | .227* |
| Life stress | −0.537 | 0.246 | −.310* | −0.257 | .137 | −.149 | −0.264 | .171 | −.152 |
| Age * depression | −0.002 | 0.010 | −.032 | −0.001 | 0.004 | −.016 | --- | --- | --- |
| Age * life stress | −0.228 | 0.157 | −.209 | −0.050 | 0.079 | −.045 | --- | --- | --- |
| Motherhood * depression | 0.004 | 0.033 | .027 | --- | --- | --- | −0.006 | 0.015 | −.038 |
| Motherhood * life stress | 0.727 | 0.537 | .236 | --- | --- | --- | 0.032 | 0.272 | .010 |
| R2(10,192)=0.076 | R2(7,195)=0.064 | R2(7,195)=0.051 | |||||||
p < 0.05
p < 0.01
Motherhood group and age were highly correlated (R=0.87, p<0.001) and both had large variance proportions distributed on the same small eigenvalues (1.0 and 0.74, respectively). Notably, the average VIF, 2.19, was not substantially greater than 1, and the average tolerance was only 0.65. To evaluate the impact that multicollinearity could have on the stability of the β estimates in the model, we repeated the hierarchical regression twice for right VST, alternately excluding motherhood and age (Table 2; columns 2 and 3). With age (and not motherhood status) included in the model, increasing age was significantly associated with reduced reward activity (β=−.15, p<0.05). With motherhood status (and not age) included in the model, there was still no significant relationship between motherhood and right VST activity. In both of these follow-up regressions, the relationship between depression and increased right VST activity remained significant. We found no significant motherhood-by-psychosocial factor or age-by-psychosocial factor interactions nor were there any significant group-specific effects for age-by-psychosocial factor interactions (supplemental table 2).
In order to explore whether the lack of a significant association between life stress and VST reward activity was related to the timing of life stress, we tested exposure to life stress separately for childhood (ages 8–12) and adolescence (ages 13–15). We observed a significant inverse relationship between VST reward activity and exposure to life stress during adolescence (β= −0.19; p=0.02) but not during childhood (β= −0.12; p=ns). We found no age-by-psychosocial factor nor motherhood-by-psychosocial factor interactions effects in right VST activity to reward (Table 3).
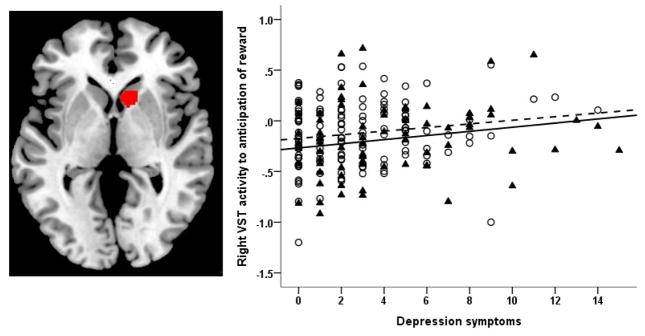
Finally, we conducted a post-hoc, whole-brain regression analysis (Figure 2) with the anticipation of reward vs baseline contrast as the dependent measure to examine associations with motherhood, depression symptoms, life stress and their interactions (controlling race, and nicotine use) to confirm ROI analyses and to examine reward anticipation-related BOLD response in non-VST brain regions. Using a small volume correction for VST, we confirmed the positive association between VST activity and depression symptoms, and the lack of a significant association between motherhood and VST activity. Motherhood was associated with increased reward-related activity, at a whole brain corrected threshold, in a cluster of the right postcentral gyrus (peak voxel 44 −28 52, k=92, cluster forming threshold p<0.001; FWE corrected p=0.05).
Figure 2.
Panel A shows the right VST region of interest mask (shown in red) used to extract reward anticipation BOLD activity. Panel B shows the association between depressive symptoms (Hamilton scale-12 item) and increased right VST activity during anticipation of reward. Mothers (depicted with ▲ and solid fit line) did not differ from non-mothers (depicted with ○ and dashed line) in this brain-behavior relationship. For interpretation of the reference to color, the reader is referred to the web version of the article.
4. Discussion
Based upon rodent and human maternal neuroscience studies, which revealed amplification of mesolimbic dopamine system responses to reward in motherhood, we hypothesized that mothers versus nulliparous women would have heighted VST responses to the anticipation of monetary reward. This is the only study, to our knowledge, that has directly compared neural reward system function between mothers and non-mothers. The results showed that motherhood was not associated with reward anticipation-related VST response, and therefore refuted our hypothesis. Our confidence in this null result is increased by the relatively large sample size of mothers and non-mothers who were all drawn from the same large-scale, prospective study in which sampling was based on community demographic parameters as opposed to motherhood. Furthermore, we controlled for a number of potential confounding variables including depressive symptoms at the time of the scan, life stress, and nicotine use, data which were collected in a uniform manner within the context of the prospective study.
An important limitation of our analyses was the significant age difference between mothers and non-mothers, with mothers being 3 years older than non-mothers on average (Mean±SD) (19.95±1.04 vs 17.04±0.61 yrs, p<0.001). To address multicollinearity between motherhood status and age in the models, therefore, we repeated the analyses with and without age and arrived at the same null association between motherhood and VST reward activity. Importantly, age was not associated with VST reward activity when mothers and non-mothers were analyzed separately; however, there was a negative association between age and VST reward activity when the entire cohort was examined without control for motherhood status. Our findings are consistent with prior research that has shown heighted behavioral and VST response to reward–predictive cues in late adolescence (ages 15–18), which becomes dampened with maturation of prefrontal cortical systems in young adulthood (age 19–22) (Bjork et al., 2010).
We suspect that the late adolescent/young adult age span of our sample also played a role in the unexpected positive association between depressive symptoms and VST reward activity in the combined sample. The majority of studies of reward in depression highlight an inverse association between depression and VST reward activity both in adults as well as younger cohorts (Dunlop and Nemeroff, 2007; Forbes et al., 2009; Nestler and Carlezon, 2006; Zhang et al., 2013). This association of depressive symptoms with increased reward-related VST activity parallels that of a smaller subsample of this cohort (Casement et al., 2014). Additionally, when we examined these associations within maternal and nulliparous subgroups separately, the depression effect was significant only for the nulliparous, younger women (supplemental table 1). This could mean that the nulliparous subgroup was better powered to detect a significant depression effect on VST reward activity, but it could also suggest that reward systems of late adolescents raised in low-income environments are uniquely characterized by a distinctive pattern of reward activity in association with depression. It will be important to determine whether future research with larger cohorts replicate this result. Because depressive symptoms were also significantly associated with increased VST activity across the combined sample, we have some indication that there is a common neurophysiology of depression related to processing monetary reward in the late adolescent through young adult PGS cohort, regardless of motherhood status.
Whereas depression was associated with reward-related VST activity, there was only a trend for an association between “life stress” averaged between age 8 and 15 and reduced reward-related VST activity (β= −.148, p=0.06). Post-hoc exploratory analyses indicated that the effect became significant when life stress exposure occurred during the adolescent period between ages 13–15. The latter finding replicates that of prior studies in which high levels of life stress, including maltreatment, were associated with depressive and anhedonic symptoms and also diminished VST responses to reward cues (Dillon et al., 2009). While life stress is an important risk factor for depression, in this sample, prospectively-collected life stress data and depressive symptoms measured at the time of scanning were only weakly correlated, suggesting distinct neurobiological processes in women with extremes in either dimension in this cohort. Notably the depression effect remained even when life stress was removed from the model.
In this study, we also sought to examine moderating effects of motherhood on the relationship between several psychosocial constructs (depression and stress) and reward-related VST activity, given the enriched environment introduced by motherhood for some women, including the new attachment relationship between mother and infant and the mother’s increased social support system. Several tests of this hypothesis disproved this notion. Instead, we confirmed prior studies which found that early childhood adversity (Dillon et al., 2009; Guyer et al., 2006) and prior major depression (Dichter et al., 2012; Hasler et al., 2009) confer significant ongoing signs of vulnerability in behavioral and neural responses to reward. Follow-up studies are needed to examine the relationship between motherhood and motherhood-by-psychosocial risk factor interactions upon mesolimbic neural functions supporting social reward and attachment.
In conclusion, our null findings suggest that the increased mesolimbic dopamine reward circuit function associated with caregiving function in experimental animals and responses to infant cues in mothers is not generalized to the anticipation of monetary reward in young mothers who are 4 months postpartum. Strengths of our study included the large sample size, the use of an established cohort of mothers and non-mothers for whom prospectively collected psychosocial data were available, and use of a reliable and valid fMRI paradigm for detection of reward-related striatal functional impairment in depression. Because our sample had only mild depressive symptoms and because life stress did not include high rates of maltreatment, it remains unknown whether motherhood would have a more significant, moderating effect on the relationship between more severe depression or maltreatment and VST reward-related activity. The tendency for age to have an inverse relationship with VST activity is consistent with age-related changes described previously (Volkow et al., 1996). More precise matching on age between groups in future studies would be necessary to examine age-by-motherhood status effects on reward-related VST activity, as would testing this hypothesis across a broader range of childbearing age. Our results pertain to young mothers and therefore may not be generalizable to motherhood overall.
The choice to scan mothers at 4 months postpartum allowed us to optimize the prevalence of depressive symptoms and minimize sources of confounding such as sleep, pain, and early psychosocial adjustment following delivery. It is possible, however, that motherhood-related enhancement of VST activity was not detectable at this late interval post-birth when hormone-dependent MPOA-VTA signals become less important for promoting maternal behaviors relative to infant stimulus inputs to ventral pallidum via amygdala. It also remains possible that mesolimbic functions supporting social reward and attachment, which we could not evaluate with a monetary reward task, would have been elevated in mothers relative to non-mothers. Future studies are needed to address these timing effects, social reward processes, and a broader neural network to further elucidate the relationships between neural reward systems, motherhood, life stress and mood.
Supplementary Material
Highlights.
We compared neural reward responses between postpartum and nulliparous women.
Ventral striatal response was related to depression and late adolescence stress.
Motherhood status had no impact upon ventral striatal responses.
Future study should examine motherhood effects on neural response to social reward.
Acknowledgments
Role of the funding source
This research was supported by HD067185 (Hipwell, Moses-Kolko), MH093605 (Keenan, Guyer, Forbes), and MH056630 (Loeber; Pittsburgh Girls Study).
We thank all of the research participants and their families for their time dedicated to this study. We thank the University of Pittsburgh Magnetic Resonance Research Center staff for performing the MR acquisition.
Footnotes
- Eydie L. Moses-Kolko contributed to the conception and design of the study, the acquisition of data, the analysis and interpretation of data, drafting the article and revising it critically for important intellectual content.
- Stephanie Stepp contributed to the conception and design of the study, analysis and interpretation of data and revising the manuscript critically for important intellectual content.
- David Fraser contributed to the conception and design of the study, analysis and interpretation of data, drafting the article, and revising it critically for important intellectual content.
- Henry W. Chase contributed to the analysis and interpretation of data and revising the manuscript critically for important intellectual content.
- Mary L. Phillips contributed to the conception and design of the study, interpretation of data, and revising the manuscript critically for important intellectual content.
- Kate E. Keenan contributed to the conception and design of the study, interpretation of data, and revising the manuscript critically for important intellectual content.
- Amanda E. Guyer contributed to the conception and design of the study, interpretation of data, and revising the manuscript critically for important intellectual content.
- Carlos R. Zevallos contributed to the acquisition of data, analysis of data and revising the manuscript critically for important intellectual content.
- Chaohui Guo contributed to analysis of data, interpretation of data, and revising the manuscript critically for important intellectual content.
- Alison E. Hipwell contributed to the conception and design of the study, acquisition of data, analysis and interpretation of data, and revising the manuscript for important intellectual content.
All authors have approved the submitted manuscript.
Conflict of Interest
Drs. Moses-Kolko, Forbes, Stepp, Chase, Phillips, Keenan, Guyer, Guo, and Hipwell and Mr. Zevallos report support from NIH grants during the conduct of the study; Dr. Phillips also received support from the Pittsburgh Foundation and Dr. Keenan also received support from University of Chicago Institute for Translational Medicine during the conduct of this study. Drs. Hipwell and Stepp also received support from the Office of Juvenile Justice and Delinquency Prevention and the Pennsylvania Department of Health during the conduct of this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afonso VM, King SJ, Novakov M, Burton CL, Fleming AS. Accumbal dopamine function in postpartum rats that were raised without their mothers. Horm Behav. 2011;60:632–643. doi: 10.1016/j.yhbeh.2011.08.016. [DOI] [PubMed] [Google Scholar]
- Atzil S, Hendler T, Feldman R. Specifying the neurobiological basis of human attachment: brain, hormones, and behavior in synchronous and intrusive mothers. Neuropsychopharmacology. 2011;36:2603–2615. doi: 10.1038/npp.2011.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagby RM, Ryder AG, Schuller DR, Marshall MB. The Hamilton Depression Rating Scale: has the gold standard become a lead weight? Am J Psychiatry. 2004;161:2163–2177. doi: 10.1176/appi.ajp.161.12.2163. [DOI] [PubMed] [Google Scholar]
- Barnard K. Difficult life circumstances scale (DLC) NCAST Publications; Seattle: 1994. [Google Scholar]
- Barrett J, Fleming AS. Annual Research Review: All mothers are not created equal: neural and psychobiological perspectives on mothering and the importance of individual differences. J Child Psychol Psychiatry. 2011;52:368–397. doi: 10.1111/j.1469-7610.2010.02306.x. [DOI] [PubMed] [Google Scholar]
- Barrett J, Wonch KE, Gonzalez A, Ali N, Steiner M, Hall GB, Fleming AS. Maternal affect and quality of parenting experiences are related to amygdala response to infant faces. Soc Neurosci. 2011;7:252–268. doi: 10.1080/17470919.2011.609907. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Chen G, Hommer DW. Adolescents, adults and rewards: comparing motivational neurocircuitry recruitment using fMRI. PLoS One. 2010;5:e11440. doi: 10.1371/journal.pone.0011440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bühler M, Vollstädt-Klein S, Kobiella A, Budde H, Reed LJ, Braus DF, Büchel C, Smolka MN. Nicotine Dependence Is Characterized by Disordered Reward Processing in a Network Driving Motivation. Biological Psychiatry. 2010;67:745–752. doi: 10.1016/j.biopsych.2009.10.029. [DOI] [PubMed] [Google Scholar]
- Casement MD, Guyer AE, Hipwell AE, McAloon RL, Hoffmann AM, Keenan KE, Forbes EE. Girls’ challenging social experiences in early adolescence predict neural response to rewards and depressive symptoms. Developmental Cognitive Neuroscience. 2014;8:18–27. doi: 10.1016/j.dcn.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase HW, Nusslock R, Almeida JR, Forbes EE, Labarbara EJ, Phillips ML. Dissociable patterns of abnormal frontal cortical activation during anticipation of an uncertain reward or loss in bipolar versus major depression. Bipolar Disord. 2013;15(8):839–54. doi: 10.1111/bdi.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Locke HM, Stenger VA, Fiez JA. Dorsal striatum responses to reward and punishment: effects of valence and magnitude manipulations. Cognitive, Affective & Behavioral Neuroscience. 2003;3:27–38. doi: 10.3758/cabn.3.1.27. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Kozink RV, McClernon FJ, Smoski MJ. Remitted major depression is characterized by reward network hyperactivation during reward anticipation and hypoactivation during reward outcomes. J Affect Disord. 2012;136:1126–1134. doi: 10.1016/j.jad.2011.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon DG, Holmes AJ, Birk JL, Brooks N, Lyons-Ruth K, Pizzagalli DA. Childhood adversity is associated with left basal ganglia dysfunction during reward anticipation in adulthood. Biological Psychiatry. 2009;66:206–213. doi: 10.1016/j.biopsych.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psych. 2007;64:327–337. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- Endicott J, Cohen J, Nee J, Fleiss J, Sarantakos S. Hamilton Depression Rating Scale. Extracted from Regular and Change Versions of the Schedule for Affective Disorders and Schizophrenia. Arch Gen Psychiatry. 1981;38:98–103. doi: 10.1001/archpsyc.1981.01780260100011. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Gonzalez A, Afonso VM, Lovic V. Plasticity in the maternal neural circuit: Experience, Dopamine, and Mothering. In: Bridges RS, editor. Neurobiology of the Parental Brain. Elsevier; Oxford: 2008. pp. 519–535. [Google Scholar]
- Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM, Brown SM, Ryan ND, Birmaher B, Axelson DA, Dahl RE. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. Am J Psychiatry. 2009;166:64–73. doi: 10.1176/appi.ajp.2008.07081336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynes BN, Gavin N, Meltzer-Brody S, Lohr KN, Swinson T, Gartlehner G, Brody S, Miller WC. Perinatal Depression: Prevalence, Screening Accuracy, and Screening Outcomes. Evid Rep Technol Assess. 2005;119:1–8. doi: 10.1037/e439372005-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Kaufman J, Hodgdon HB, Masten CL, Jazbec S, Pine DS, Ernst M. Behavioral alterations in reward system function: the role of childhood maltreatment and psychopathology. J Am Acad Child Adolesc Psychiatry. 2006;45:1059–1067. doi: 10.1097/01.chi.0000227882.50404.11. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, Luckenbaugh DA, Snow J, Meyers N, Waldeck T, Geraci M, Roiser J, Knutson B, Charney DS, Drevets WC. Reward processing after catecholamine depletion in unmedicated, remitted subjects with major depressive disorder. Biological Psychiatry. 2009;66:201–205. doi: 10.1016/j.biopsych.2009.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipwell AE, Loeber R, Stouthamer-Loeber M, Keenan K, White HR, Kroneman L. Characteristics of girls with early onset disruptive and antisocial behaviour. Criminal Behaviour and Mental Health. 2002;12:99–118. doi: 10.1002/cbm.489. [DOI] [PubMed] [Google Scholar]
- Keenan K, Hipwell A, Chung T, Stepp S, Stouthamer-Loeber M, Loeber R, McTigue K. The Pittsburgh Girls Study: overview and initial findings. J Clin Child Adolesc Psychol. 2010;39:506–521. doi: 10.1080/15374416.2010.486320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan K, Hipwell A, Hinze A, Babinski D. Equanimity to excess: inhibiting the expression of negative emotion is associated with depression symptoms in girls. J Abnorm Child Psychol. 2009;37:739–747. doi: 10.1007/s10802-009-9301-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsley CH, Lambert KG. Reproduction-induced neuroplasticity: natural behavioural and neuronal alterations associated with the production and care of offspring. J Neuroendocrinol. 2008;20:515–525. doi: 10.1111/j.1365-2826.2008.01667.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Early life influences on life-long patterns of behavior and health. Ment Retard Dev Disabil Res Rev. 2003;9:149–154. doi: 10.1002/mrdd.10074. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Stress- and allostasis-induced brain plasticity. Annu Rev Med. 2011;62:431–445. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses-Kolko EL, Fraser D, Wisner KL, James JA, Saul AT, Fiez JA, Phillips ML. Rapid habituation of ventral striatal response to reward receipt in postpartum depression. Biol Psychiatry. 2011;70:395–399. doi: 10.1016/j.biopsych.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses-Kolko EL, Meltzer CC, Berga SL, Wisner KL. Postpartum depression: The clinical disorder and application of PET imaging research methods. In: Bridges RS, editor. Neurobiology of the parental brain. Academic Press; Burlington: 2008. pp. 175–199. [Google Scholar]
- Moses-Kolko EL, Price JC, Wisner KL, Hanusa BH, Meltzer CC, Berga SL, Grace AA, di Scalea TL, Kaye WH, Becker C, Drevets WC. Postpartum and Depression Status are Associated With Lower [(11)C]raclopride BP(ND) in Reproductive-Age Women. Neuropsychopharmacology. 2012;37:1422–1432. doi: 10.1038/npp.2011.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA. The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Numan M. Neurobiology of Social Behavior. Elsevier; London: 2015. Parental Behavior; pp. 165–234. [Google Scholar]
- Numan M, Stolzenberg DS. Medial preoptic area interactions with dopamine neural systems in the control of the onset and maintenance of maternal behavior in rats. Frontiers in Neuroendocrinology. 2009;30:46–64. doi: 10.1016/j.yfrne.2008.10.002. [DOI] [PubMed] [Google Scholar]
- O’Hara MW, Swain AM. Rates and risks of postpartum depression - a meta-analysis. International Review of Psychiatry. 1996;8:37–54. [Google Scholar]
- Romens SE, Casement MD, McAloon R, Keenan K, Hipwell AE, Guyer AE, Forbes EE. Adolescent girls’ neural response to reward mediates the relation between childhood financial disadvantage and depression. J Child Psychol Psychiatry. 2015;56:1177–1184. doi: 10.1111/jcpp.12410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman ME, Loudon H, Safier M, Protopopescu X, Leiter G, Liu X, Goldstein M. Neural dysfunction in postpartum depression: an fMRI pilot study. CNS Spectrums. 2007;12:853–862. doi: 10.1017/s1092852900015595. [DOI] [PubMed] [Google Scholar]
- Strathearn L, Fonagy P, Amico J, Montague PR. Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacology. 2009;34:2655–2666. doi: 10.1038/npp.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricomi E, Delgado MR, McCandliss BD, McClelland JL, Fiez JA. Performance feedback drives caudate activation in a phonological learning task. Journal of Cognitive Neuroscience. 2006;18:1029–1043. doi: 10.1162/jocn.2006.18.6.1029. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, MacGregor RR, Schlyer DJ, Hitzemann R, Wolf AP. Measuring age-related changes in dopamine D2 receptors with 11C-raclopride and 18F-N-methylspiroperidol. Psychiatry Res. 1996;67:11–16. doi: 10.1016/0925-4927(96)02809-0. [DOI] [PubMed] [Google Scholar]
- Williamson DE, Ryan ND, Dahl RE, Jeannette L. Hamilton Depression Scores Can Be Extracted from the K-SADS-P in Adolescents. J Child Adolesc Psychopharmacol. 1992;2:175–181. doi: 10.1089/cap.1992.2.175. [DOI] [PubMed] [Google Scholar]
- Wisner KL, Hanusa BH, Perel JM, Peindl KS, Piontek CM, Sit DKY, Findling RL, Moses-Kolko EL. Postpartum Depression: A randomized trial of Sertraline vs. Nortriptyline. Journal of Clinical Psychopharmacology. 2006;26:353–360. doi: 10.1097/01.jcp.0000227706.56870.dd. [DOI] [PubMed] [Google Scholar]
- Zhang WN, Chang SH, Guo LY, Zhang KL, Wang J. The neural correlates of reward-related processing in major depressive disorder: a meta-analysis of functional magnetic resonance imaging studies. J Affect Disord. 2013;151:531–539. doi: 10.1016/j.jad.2013.06.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.