Abstract
Background
It remains unclear if methamphetamine is merely associated with high risk behavior or if methamphetamine use causes high risk behavior. Determining this would require a randomized controlled trial, which is clearly not ethical. A possible surrogate would be to investigate individuals before and after starting the use of methamphetamine.
Methods
We performed a cohort study to analyze recent self-reported methamphetamine use and sexual risk behavior among 8,905 MSM receiving the “Early Test”, a community-based, HIV screening program in San Diego, California, between April 2008 and July 2014 (total 17,272 testing encounters). Sexual risk behavior was evaluated using a previously published risk behavior score (San Diego Early Test [SDET] score) that predicts risk of HIV acquisition.
Results
Methamphetamine use during the last 12 months (hereafter, recent-meth) was reported by 754/8,905 unique MSM (8.5%). SDET scores were significantly higher in the 754 MSM with recent-meth use compared to the 5,922MSM who reported that they have never used methamphetamine (p<0.001). Eighty-two repeat testers initiated methamphetamine between testing encounter, with significantly higher SDET scores after starting methamphetamine (median 5 [IQR 2–7] at recent-meth versus median 3 [IQR 0–5] at never-meth; p<0.001, respectively).
Conclusions
Given the ethical impossibility of conducting a randomized, controlled trial, the results presented here provide the strongest evidence yet that initiation of methamphetamine use increases sexual risk behavior among HIV-uninfected MSM. Until more effective prevention or treatment interventions are available for methamphetamine users, HIV-uninfected MSM who use methamphetamine may represent ideal candidates for alternative effective prevention interventions (i.e., pre-exposure prophylaxis).
Keywords: MSM, risk behavior, methamphetamine, SDET score, recreational drugs, stimulants
Introduction
Use of illicit substances such as methamphetamine, poppers (amyl nitrites), gamma hydroxybutyrate, cocaine or ecstasy is more prevalent in men who have sex with men(MSM) than in the general population1–3. In the US National HIV Behavioral Surveillance survey, 42% of the HIV uninfected MSM reported illicit substance use in the past year with 74% of these MSM reporting substance use before or during sex4.Substance use has been associated with HIV transmission and acquisition in MSM1, 2, 5–8. A dose-response has been described between the variety and frequency of substance use and sexual risk behaviors among MSM9, 10. While most studies have focused on the effect of substance use on risk behavior, adherence and resistance in HIV positive individuals1, 11–17, fewer studies have examined the interaction of substance use and sexual risk behavior in HIV uninfected individuals5, 18,19.
Over the past two decades, methamphetamine use has become increasingly used among MSM in the United States, with the prevalence of its use among MSM in the previous year as high as 18.8%4, 20. Methamphetamine is a central nervous system stimulant with a high potential for abuse and for psychological or physical dependence. In relation to sex, methamphetamine can increase libido and sexual pleasure, prolong sexual performance, and make receptive anal intercourse less painful21; thus, its use may be an important predictor for HIV risk2, 12,19. It remains unclear, however, if methamphetamine is merely associated with high risk behavior or if methamphetamine use causes high risk behavior. Determining this would require a randomized control trial, which is clearly not ethical. A possible surrogate would be to investigate individuals before and after starting the use of methamphetamine.
To this end, this study characterized the temporal relationship between methamphetamine use and sexual risk behavior among HIV uninfected MSM undergoing community-based screening for acute and early HIV infection (AEH) in San Diego, California22, 23.
Material and Methods
We performed a retrospective analysis of illicit substance use and sexual risk behavior data among individuals enrolled to the San Diego “Early Test” HIV screening program between April 2008 and July 2014. The “Early Test” is a community-based, confidential AEH screening program that provides point of care rapid HIV serologic testing followed by routine reflex to HIV NAT in all antibody (Ab) negative persons22, 23. The UCSD Human Research Protections Program approved the study protocol, consent and all study related procedures. All study participants provided voluntary, written informed consent before any study procedures were undertaken.
Sexual risk behavior was evaluated by calculating San Diego Early Test (SDET) scores for every single testing encounter24. The SDET score was recently developed to better estimate incident HIV infection risk in MSM, as assessed by a score ranging from 0 to 10, with scores above 5 associated with a 6-fold increased risk of HIV acquisition. The score is based on key risk variables that predict risk of HIV acquisition among MSM: condom less receptive anal intercourse (CRAI),number of male partners, and self-reported bacterial sexually transmitted infection (STI).
Study inclusion criteria were limited to men, age ≥13, who reported sexual contact with one or more male partners during the previous 12 months and who had at least one testing encounter during the study period.
Methamphetamine use and sexual risk behavior
Methamphetamine use was determined by response to four survey questions assessed before each HIV testing encounter. Early Test clients who responded yes to “injected/non-injected methamphetamine use in previous 12 months” questions were considered recent-methamphetamine users (hereafter, recent-meth) and those who responded no to both,“injected/non-injected methamphetamine use in previous 12 months” AND “injected/non-injected methamphetamine use ever” questions were considered lifelong non-methamphetamine users (hereafter, never-meth). Information on “methamphetamine use ever” was not available in 1811 MSM who tested before the introduction of this question into the Early Test questionnaire in August 2010. These individuals were therefore excluded from the respective analyses. We compared risk behavior variables (i.e., components of SDET score), and SDET score sat most recent testing encounters (i.e. one testing encounter per individual) between MSM who reported recent-meth use compared to MSM who reported never-meth use using Chi squared and Mann Whitney U test.
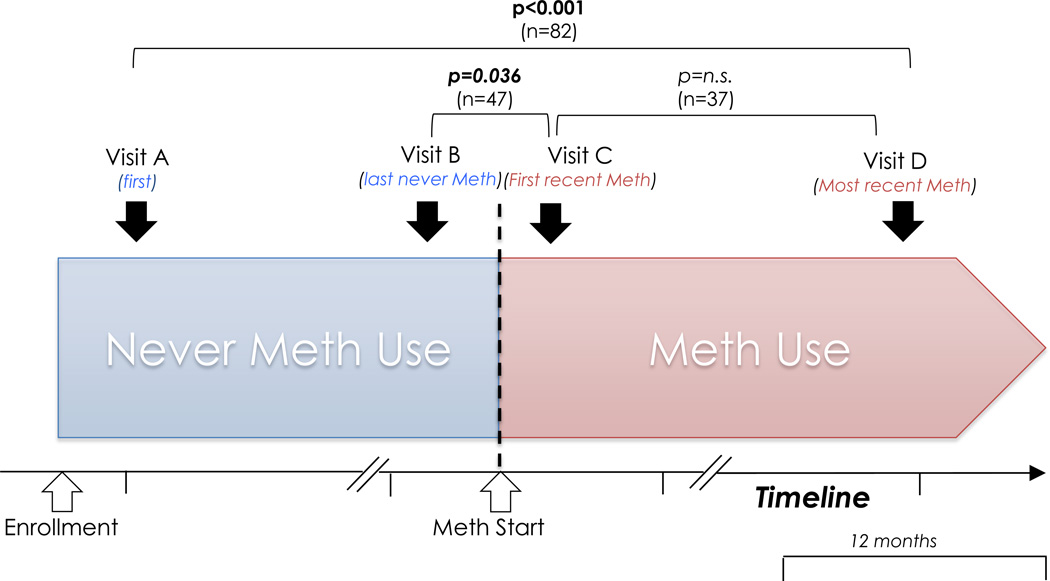
We also performed analyses on methamphetamine use in repeat testers (analyses comparing repeat testers and single testers have been published previously25). Eligible participants were assigned to one of four groups based on their reported methamphetamine use at the first and most recent repeat HIV testing encounter: i) Group 1: started using meth (i.e. never-meth to recent-meth), ii) Group 2: stopped using meth(i.e. recent-meth to no recent-meth), iii) Group 3: continued meth (i.e. recent-meth to recent-meth), and iv) Group 4: does not use meth (i.e. never-meth to never-meth). In all four groups, we compared changes in sexual risk behavior and SDET scores between first and most recent non-overlapping (i.e. >12 months apart, as the risk behavior was always reported for the prior 12 months) repeat testing encounter(Figure 1) using McNemar test, Wilcoxon’s signed rank test and a repeated measures analysis of variance (ANOVA; F values displayed).
Figure 1.
Graphical illustration of temporal relationship between methamphetamine use and sexual risk behavior among repeat testing men who have sex with men (MSM) who started methamphetamine between their testing encounters.
Temporal Relationship: Meth Start and Sexual Risk Behavior (Group 1 Sub analyses)
To better characterize the temporal relationship between start of methamphetamine use and risk behavior, we also compared risk behaviors between overlapping (<12 months apart) last never-meth and first recent-meth testing encounters (Figure 1). To characterize whether there is a stepwise increase in risk behavior after starting meth use we also compared risk behavior variables and SDET scores between first recent-meth encounter versus the next non-overlapping later testing encounter (i.e. also recent-meth; Figure 1), among MSM who had two or more non-overlapping testing encounters after starting methamphetamine.
Model on Substance use and high SDET risk scores
To identify other recreational substances that were associated with high SDET scores and evaluate whether methamphetamine or other drugs showed the strongest associations with high risk behavior in our settings, we used a binary logistic regression model that included the one most recent testing encounter in all individuals. Drug use reported for the previous 12 months were used as predictors and SDET scores >5 were used as the outcome. A SDET score cutoff of >5 was chosen, as it was previously associated with a positive predictive value of 10% for incident HIV infection24.In the first step, univariate analyses were performed, and variables with p < 0.20 were included in the multivariable model. Variables in the final model were selected with a forward stepwise procedure. Odds ratios (OR) including 95% confidence intervals (CI) were displayed. Model discrimination was assessed by the goodness-of-fit Hosmer-Lemeshow statistic and its predictive potential was assessed by using receiver operating characteristic (ROC) curve analysis. For these statistical analyses, SPSS 21 (SPSS Inc., Chicago, IL, USA) was used.
Results
A total of 14,612unique persons underwent HIV screening through the “Early Test” program between April 2008 and July 2014, including 8,905 (61%) unique individual MSM, who participated in17,272voluntary HIV tests. Overall,20% of the MSM (1,788/8,905) were repeat testers with first and most recent testing encounters more than 12 months apart (i.e. non-overlapping).
Methamphetamine use and sexual risk behavior
Use of methamphetamine was reported during6.3% testing encounters (1,105/17,272) by 8.5%of MSM testers (754/8,905). While non-injected methamphetamine use was reported in all of these testing encounters, injected use was also reported in 175 of the 1,105 testing encounters. On an individual basis (demographics displayed in Table 1), SDET scores were significantly higher in the 754MSM who reported recent-meth use compared to the 5,922 MSM who reported never-meth use (median 5, IQR 2–7 versus median 0, IQR 0–3; p<0.001; 54% of recent-meth users had SDET scores ≥5 versus 6% of never-meth users). Similar results were found for number of male partners and each of the 4 variables of the SDET score (all p<0.001; Table 2). Overall 418 MSM reported that they had used meth before, but not during the previous 12 months (SDET scores median 2, IQR 0–5).
Table 1.
Demographic characteristics among men who have sex with men who reported methamphetamine use (repeat testers on the right), and those that never used methamphetamine.
|
Demographic characteristics |
MSM reporting never-meth (n=5922)* |
MSM reporting recent-meth (n=754)# |
P value§ |
Repeat Testers with ≥1 year between first and most recent test | |||
|---|---|---|---|---|---|---|---|
| Group 1 started meth (n=82) |
Group 2 stopped meth (n=55) |
Group 3 always meth (n=48) |
Group 4 Never Meth (n=1603) |
||||
| Age (years; median, IQR) | 32 (26–43) | 32 (27–41) | n.s. | 31 (26–37) | 32 (26–43) | 36 (26–42). | 32 (26–42) |
| Race/Ethnicity | <0.001 | ||||||
| Hispanic Ethnicity | 1615(27.3%) | 213 (28.2%) | n.s. | 15 (18.3%) | 13 (23.6%) | 12 (25.0%) | 450 (28.1%) |
| White Race | 3171(53.5%) | 404 (53.6%) | n.s. | 57 (69.5%) | 33 (60.0%) | 29 (60.4%) | 892 (55.6%) |
| Black Race | 288 (4.9%) | 38 (5.0%) | n.s. | 3 (3.7%) | 3 (5.5%) | 3 (6.3%) | 65 (4.1%) |
| Asian Race | 442(7.5%) | 16 (2.1%) | <0.001. | 3 (3.7%) | 0 | 0 | 96 (6.0%) |
| Pacific Islander Race | 107 (1.8%) | 3 (0.4%) | 0.002 | 0 | 0 | 0 | 33 (2.1%) |
| Native American | 15 (0.3%) | 9 (1.2%) | <0.001 | 0 | 1 (1.8%) | 0 | 5 (0.3%) |
| Race | |||||||
| Other Race | 264 (4.5%) | 71 (9.4%) | <0.001 | 4 (4.9%) | 5 (9.1%) | 4 (8.3%) | 62 (3.9%) |
| Duration between first and most recent testing encounter (days; median, IQR) | - | - | 901(670–1307) | 953 (568–1567) | 732 (518–1123) | 886 (599–1337) | |
Abbreviations:; IQR, inter-quartile range; MSM, men who have sex with men; n.s.; not significant; TSX, transgender
Always the first testing encounter use was considered
Always the first testing encounter where individuals reported methamphetamine use was considered
Calculated using Chi squared and Mann Whitney U test
Table 2.
Risk behaviour variables of the SDET score and number of male partners at first and most recent testing encounter among repeat testers who reported methamphetamine use (on the right). On the left risk behaviours in those who reported methamphetamine use, and those that never used methamphetamine.
|
Variables of the SDET Score (reported for previous 12 months) |
MSM reporting never- meth (n=5922) * |
MSM reporting recent- meth (n=754)# |
P value§ |
Repeat Testers reporting Methamphetamine use with ≥1 year between first and most recent test (n=185) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group 1 started meth (n=82) | Group 2 stopped meth (n=55) |
Group 3 always meth (n=48) | ||||||||||
| First test | Most recent test |
P value¥ |
First test |
Most recent test |
P value¥ |
First test | Most recent test |
P value¥ |
||||
| CRAI with a HIV positive | 335 (5.7%) | 222 (29.4%) | <0.001 | 6 (7.3%) | 18 (22.0%) | 0.007 | 11 (20.0%) | 9 (16.4%) | n.s. | 19 (39.6%) | 17 (35.4%) | n.s. |
| Combination CRAI plus 5 or more male partners | 1765 (29.8%) | 423 (56.1%) | <0.001 | 44 (53.7%) | 56 (68.3%) | 0.065 | 31 (56.4%) | 32 (58.2%) | n.s. | 30 (62.5%) | 31 (64.6%) | n.s. |
| 10 or more male partners | 1858 (31.4%) | 450 (59.7%) | <0.001 | 36 (43.9%) | 59 (72.0%) | <0.001 | 37 (67.3%) | 29 (52.7%) | 0.096 | 34 (70.8%) | 31 (64.6%) | n.s. |
| Bacterial STI | 580 (9.8%) | 129 (17.1%) | <0.001 | 13 (15.9%) | 16 (19.5%) | n.s. | 13 (23.6%) | 11 (20%) | n.s. | 7 (14.6%) | 13 (27.1%) | n.s. |
| Number male partners | 5 (3–10) | 11 (5–30) | <0.001 | 7 (3–20) | 12 (7–40) | <0.001 | 15 (8–33) | 10 (4–20) | 0.004 | 15 (8–29) | 13 (6–20) | n.s. |
| SDET scores (median, IQR) | 0 (0–3) | 5 (2–7) | <0.001 | 3 (0–5) | 5 (2–7) | <0.001 | 4 (2–7) | 3 (0–5) | n.s. | 5 (3–8) | 5 (2–8) | n.s. |
Abbreviations: CRAI, condom less receptive anal intercourse; IQR, inter-quartile range; MSM, men who have sex with men; n.s.; not significant; SDET, San Diego Early Test; STI, sexually transmitted infection.
Always the first testing encounter use was considered
Always the first testing encounter where individuals reported methamphetamine use was considered
Calculated using Chi squared and Mann Whitney U test
Calculated using McNemar and Wilcoxon signed rank test
Of 1,788 MSM repeat testers with non-overlapping first and last test, 185 (10.3%) reported recent-meth use at their first and/or most recent non-overlapping testing encounter. Of those 185, 82 started using methamphetamine between their testing encounters with Early Test (i.e. Group 1), 55 reported that they stopped using methamphetamine during follow-up (i.e. Group 2), while 48 reported continued methamphetamine use (i.e. Group 3).Demographics of the groups as well as time between first and most recent testing encounter are depicted in Table 1.
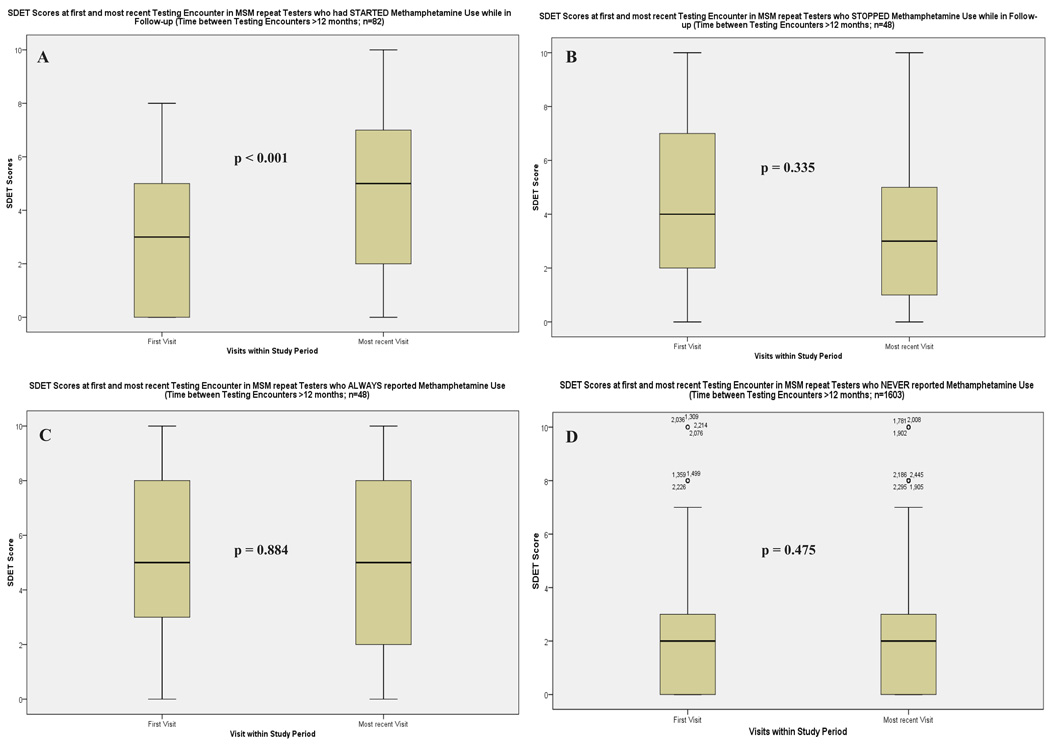
Box plots of SDET scores at first and most recent testing encounter in these groups are depicted in Figure 2. In Group 1 (i.e. 83 repeat testers who started using methamphetamine) significantly higher SDET scores were documented at their most recent testing encounter (recent-meth, Visit D in Figure 1),compared to their first testing encounter (never-meth, visit A; median 5, IQR 2–7 versus median 3, IQR 0–5; F=25.649, both p<0.001; Table 2, Figure 1). In Group 2 (i.e. 55 participants who stopped meth), SDET scores did not change significantly (median 4, IQR 2–7 at first visit; median 3, IQR 0–5 at most recent visit, p=0.335; F=0.740, p=0.393). While number of male partners decreased significantly between the first and most recent testing encounter, no change was observed for other risk variables (Table 2). In Group 3 (i.e. 48 participants who reported recent-meth at their first and last visit), SDET scores remained unchanged (median 5, IQR 3–8 at first visit; median 5, IQR 2–8 at most recent visit; F=1.614, p=0.209). SDET scores remained also unchanged in Group 4 (i.e. never-meth at their first and most recent testing encounter, n=1603; median 2, IQR 0–3 in both; F=0.415, p=0.520).
Figure 2.
SDET scores at first and most recent testing encounter in MSM repeat testers (n=1789; first and most recent test > 12 months apart): A) repeat testers that started using methamphetamine while in follow up, B) repeat testers that stopped using methamphetamine, C.) repeat testers that reported recent methamphetamine use at their first and most recent testing encounter, D) repeat testers that never used methamphetamine. P- values are displayed.
Temporal Relationship: MethStart and Sexual Risk Behavior
Of all 2,456 repeat testers (i.e. including those where first and last testing encounter were overlapping and those where first and last testing encounter were non overlapping), 224 (9.1%) MSM reported recent-meth use at their first and/or most recent testing encounter. Of those 224, 89 started using methamphetamine between their testing encounters, and significantly higher SDET scores were documented at first recent-meth versus last never-meth testing encounter (i.e. first recent-meth; median 5, IQR 2–7 for recent-meth versus median 3, IQR 0–5 for never-meth; p<0.001; time between testing encounters median 325, IQR 127–501 days; F=21.442, p<0.001).
When just focusing the analysis on the subset with overlapping testing encounters (n=47), there were also significantly higher SDET scores observed at first recent-meth (visit C, Figure 1) versus last never-meth (visit B) encounter (median 5, IQR 2–7, versus median 3, IQR 2–5; p=0.036; time between testing encounters median 165, IQR 104–278, range 34–362 days; F=4.252, p=0.045; Figure 1).
Additional analyses were performed ine37 MSM who had two or more non-overlapping testing encounters after starting methamphetamine. In those SDET scores remained unchanged between first recent-meth (visit C) versus the next non-overlapping later testing encounter (i.e. also recent-meth; SDET scores for both median 5, IQR 2–7; F=0.932, p=0.859; time between testing encounters median 499, IQR 424–723, range 365–1747 days;Figure 1), indicating that risk behavior may not increase continuously over a longer time period after starting meth.
Model on Substance use and high SDET risk scores
The most recent testing encounters of 8,905 MSM(i.e. one testing encounter per individual) were included in the binary logistic regression model for predicting SDET scores >5 (746[8.4%] of those testing encounters had SDET scores >5). Recreational drugs, number of individuals reporting recent use of each drug, as well as results of univariate and multivariable stepwise forward analysis are depicted in Table 3. Recent-meth, followed by nitrites and gamma hydroxybutyrate showed the strongest independent associations with SDET scores >5 in the multivariable analysis. The Hosmer–Lemeshow χ2 was 4.304 (p = 0.116) and the AUC was 0.679 (95% CI 0.657–0.702) for the model.
Table 3.
Univariate and multivariable binary regression model of recent recreational drugs use for predicting SDET scores >5 in most recent testing encounters of 8,905 men who have sex with men.
| Substance (reported use within 12 months prior to test) |
Univariate Binary Logistic Regression Model | Multivariable Binary Logistic Regression Model | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Drug | N Total 8905 |
Coefficient β |
OR | 95% CI | p value | Coefficient β |
OR | 95% CI | p value |
| Methamphetamine | 640 (7.2%) | 1.785 | 5.959 | 4.929–7.205 | <0.001 | 1.160 | 3.191 | 2.528–4.028 | <0.001 |
| Nitrites | 1,197 (13.4%) | 1.053 | 2.865 | 2.411–3.406 | <0.001 | 0.648 | 1.911 | 1.580–2.311 | <0.001 |
| GHB | 436 (4.9%) | 1.851 | 6.368 | 5.128–7.908 | <0.001 | 0.778 | 2.178 | 1.657–2.862 | <0.001 |
| Ecstasy | 938 (10.5%) | 1.042 | 2.834 | 2.352–3.416 | <0.001 | - | - | - | n.s. |
| Alcohol | 7,385 (82.9%) | 0.187 | 1.206 | 0.978–1.487 | 0.080 | - | - | - | n.s. |
| Marijuana | 2,821 (31.7%) | 0.627 | 1.872 | 1.609–2.179 | <0.001 | 0.182 | 1.200 | 1.012–1.422 | 0.036 |
| Cocaine | 868 (9.7%) | 1.116 | 3.053 | 2.527–3.689 | <0.001 | 0.378 | 1.459 | 1.167–1.824 | 0.001 |
| Heroin | 44 (0.5%) | 1.332 | 3.789 | 1.902–7.547 | <0.001 | - | - | - | n.s. |
| Crack | 47 (0.5%) | 1.271 | 3.565 | 1.799–7.065 | <0.001 | - | - | - | n.s. |
| Ketamine | 161 (1.8%) | 1.522 | 4.582 | 3.227–6.505 | <0.001 | - | - | - | n.s. |
| Viagra/Cialis/Levitra | 630 (7.1%) | 0.851 | 2.343 | 1.870–2.935 | <0.001 | - | - | - | n.s. |
| Painkillers | 299 (3.4%) | 1.114 | 3.046 | 2.278–4.073 | <0.001 | - | - | - | n.s. |
Abbreviations: CI, confidence interval; GHB, gamma hydroxybutyrate; n.s., not significant; OR, odds ratio; SDET, San Diego Early Test.
Discussion
This is one of the largest studies to date to evaluate how recent methamphetamine use may impact sexual risk behavior among MSM. Perhaps unsurprisingly, this study found that sexual risk was significantly higher in MSM testers reporting recent methamphetamine use compared to those reporting that they never used methamphetamine (p<0.001). More interestingly, this study found that repeat testers who started using methamphetamine between testing had a significant increase in sexual risk, while there was no difference in sexual risk scores (i.e. SDET) between the first and most recent testing encounter for repeat testers who continued to use or never used methamphetamine. However, in those individuals who reported stopping the use of methamphetamine, their sexual risks partly declined. In the multivariable binary logistic regression model, this study also found that recent methamphetamine use had the strongest independent association with the highest SDET scores.
Importantly, 54% of MSM with recent-meth had SDET scores ≥5, a cutoff previously recommended to identify persons who require more intensive prevention interventions such as pre-exposure prophylaxis (PrEP)24. PrEP has been shown to be effective among MSM, reducing the risk of getting HIV infected by up to 92% in those with detectable plasma levels26. The finding that more than half of MSM with recent-meth had SDET scores ≥5 is in line with previous studies that have indicated that methamphetamine may also be associated with incident HIV infection5, 13, 27–29.While increased risk behavior may be the major contributing factor for high incidence of HIV among those using methamphetamine, another factor may be dry mucosa, a “side-effect” of methamphetamine use, which may lead to more chafing and abrasions, which, in turn, could provide an entry for HIV during sexual activity5.
Strikingly, we found that among repeat testers who started using methamphetamine between two tests, there was a significant increase of sexual risk behavior. In those who stopped using meth, number of sexual partners decreased by a third from median 15 male partners in the previous year to median 10. However, other risk behaviors such as CRAI and also reported STI rates did not decrease after stopping methamphetamine, which may indicate that some of those MSM may remain within high risk sexual networks and venues of methamphetamine using MSM. These finding indicates that meth use may be associated with a relatively rapid increase in risk behavior that may not be fully reversible. In a previous study meth use was associated with high-risk sexual behavior, and high risk behaviors were observed in both, those with occasional and those with frequent use19. The effect of meth on risk behavior may therefore differ from the effect of some other drugs, where dose-response or stepwise increases of risk behavior have been reported9, 30. If true, these finding may have important public health implications, as very early prevention interventions before MSM start using meth, may be needed.
Our study has several limitations including its single-center design, the 12-month recall to determine meth use and sexual behavior, and also the relatively small proportion of the cohort that was included in the analyses of those who started methamphetamine. Also frequency or patterns (e.g., binge use) of meth use were not assessed. Furthermore, self-reported measures for substance use and sexual risk behaviors may be subject to social desirability and recall biases. As this was a nonrandomized observational study, we also cannot rule out the possibility of unmeasured confounders may have been associated with both substance use and sexual risk behavior. Finally, this study focuses on methamphetamine use and sexual risk behavior only, while recent longitudinal evidence from methamphetamine treatment interventions suggests that methamphetamine use does not directly increase sexual risk behavior, but rather increases depression, which subsequently increases sexual risk taking31.
Given the ethical impossibility of conducting a randomized, controlled trial of the effects of methamphetamine use on sexual risk behavior, the results presented here provide the strongest evidence yet that initiation of methamphetamine use increases sexual risk behavior among HIV-uninfected MSM. Until more effective prevention or treatment interventions are available for methamphetamine users, HIV uninfected MSM who use methamphetamine may represent ideal candidates for alternative effective prevention interventions (i.e., PrEP).
Acknowledgments
Funding
This work was supported by funds from the following: the Max Kade Foundation, New York (Max Kade Postdoctoral Research grant), International Research Fellowship in Neuro AIDS (R25-MH081482), the Bettencourt-Schueller Foundation, the California HIV/AIDS Research Program (CHRP) Grant F13SD321, the Department of Veterans Affairs and grants from the National Institutes of Health: AI43638, AI074621, AI106039. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Dr. Hoenigl served on the speakers’ bureau of Merck. Dr. Little received research grant from Gilead. Dr. Smith has served as consultant for Testing Talent Services.
Footnotes
Conflicts of interest
All other authors no conflicts.
References
- 1.Clark T, Marquez C, Hare CB, et al. Methamphetamine use, transmission risk behavior and internet use among HIV-infected patients in medical care, San Francisco, 2008. AIDS Behav. 2012;16:396–403. doi: 10.1007/s10461-010-9869-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freeman P, Walker BC, Harris DR, et al. Methamphetamine use and risk for HIV among young men who have sex with men in 8 US cities. Arch Pediatr Adolesc Med. 2011;165:736–740. doi: 10.1001/archpediatrics.2011.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reback CJ, Fletcher JB, Shoptaw S, et al. Methamphetamine and other substance use trends among street-recruited men who have sex with men, from 2008 to 2011. Drug Alcohol Depend. 2013;133:262–265. doi: 10.1016/j.drugalcdep.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koblin BA, Murrill C, Camacho M, et al. Amphetamine use and sexual risk among men who have sex with men: results from the National HIV Behavioral Surveillance study--New York City. Subst Use Misuse. 2007;42:1613–1628. doi: 10.1080/10826080701212519. [DOI] [PubMed] [Google Scholar]
- 5.Buchbinder SP, Vittinghoff E, Heagerty PJ, et al. Sexual risk, nitrite inhalant use, and lack of circumcision associated with HIV seroconversion in men who have sex with men in the United States. J Acquir Immune Defic Syndr. 2005;39:82–89. doi: 10.1097/01.qai.0000134740.41585.f4. [DOI] [PubMed] [Google Scholar]
- 6.Drumright LN, Gorbach PM, Little SJ, et al. Associations between substance use, erectile dysfunction medication and recent HIV infection among men who have sex with men. AIDS Behav. 2009;13:328–336. doi: 10.1007/s10461-007-9330-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drumright LN, Little SJ, Strathdee SA, et al. Unprotected anal intercourse and substance use among men who have sex with men with recent HIV infection. J Acquir Immune Defic Syndr. 2006;43:344–350. doi: 10.1097/01.qai.0000230530.02212.86. [DOI] [PubMed] [Google Scholar]
- 8.Drumright LN, Patterson TL, Strathdee SA. Club drugs as causal risk factors for HIV acquisition among men who have sex with men: a review. Subst Use Misuse. 2006;41:1551–1601. doi: 10.1080/10826080600847894. [DOI] [PubMed] [Google Scholar]
- 9.Santos GM, Coffin PO, Das M, et al. Dose-response associations between number and frequency of substance use and high-risk sexual behaviors among HIV-negative substance-using men who have sex with men (SUMSM) in San Francisco. J Acquir Immune Defic Syndr. 2013;63:540–544. doi: 10.1097/QAI.0b013e318293f10b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghanem A, Little SJ, Drumright L, et al. High-risk behaviors associated with injection drug use among recently HIV-infected men who have sex with men in San Diego, CA. AIDS Behav. 2011;15:1561–1569. doi: 10.1007/s10461-011-9970-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Semple SJ, Zians J, Grant I, et al. Methamphetamine use, impulsivity, and sexual risk behavior among HIV-positive men who have sex with men. J Addict Dis. 2006;25:105–114. doi: 10.1300/J069v25n04_10. [DOI] [PubMed] [Google Scholar]
- 12.Plankey MW, Ostrow DG, Stall R, et al. The relationship between methamphetamine and popper use and risk of HIV seroconversion in the multicenter AIDS cohort study. J Acquir Immune Defic Syndr. 2007;45:85–92. doi: 10.1097/QAI.0b013e3180417c99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ostrow DG, Plankey MW, Cox C, et al. Specific sex drug combinations contribute to the majority of recent HIV seroconversions among MSM in the MACS. J Acquir Immune Defic Syndr. 2009;51:349–355. doi: 10.1097/QAI.0b013e3181a24b20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorbach PM, Drumright LN, Javanbakht M, et al. Antiretroviral drug resistance and risk behavior among recently HIV-infected men who have sex with men. J Acquir Immune Defic Syndr. 2008;47:639–643. doi: 10.1097/QAI.0b013e3181684c3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colfax GN, Vittinghoff E, Grant R, et al. Frequent methamphetamine use is associated with primary non-nucleoside reverse transcriptase inhibitor resistance. AIDS. 2007;21:239–241. doi: 10.1097/QAD.0b013e3280114a29. [DOI] [PubMed] [Google Scholar]
- 16.Moore DJ, Montoya JL, Blackstone K, et al. Preliminary Evidence for Feasibility Use, and Acceptability of Individualized Texting for Adherence Building for Antiretroviral Adherence and Substance Use Assessment among HIV-Infected Methamphetamine Users. AIDS Res Treat. 2013;2013:585143. doi: 10.1155/2013/585143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rajasingham R, Mimiaga MJ, White JM, et al. A systematic review of behavioral and treatment outcome studies among HIV-infected men who have sex with men who abuse crystal methamphetamine. AIDS Patient Care STDS. 2012;26:36–52. doi: 10.1089/apc.2011.0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiasson MA, Hirshfield S, Remien RH, et al. A comparison of on-line and off-line sexual risk in men who have sex with men: an event-based on-line survey. J Acquir Immune Defic Syndr. 2007;44:235–243. doi: 10.1097/QAI.0b013e31802e298c. [DOI] [PubMed] [Google Scholar]
- 19.Colfax G, Coates TJ, Husnik MJ, et al. Longitudinal patterns of methamphetamine, popper (amyl nitrite), and cocaine use and high-risk sexual behavior among a cohort of san francisco men who have sex with men. J Urban Health. 2005;82:i62–i70. doi: 10.1093/jurban/jti025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thu Vu NT, Maher L, Zablotska I. Amphetamine-type stimulants and HIV infection among men who have sex with men: implications on HIV research and prevention from a systematic review and meta-analysis. J Int AIDS Soc. 2015;18:19273. doi: 10.7448/IAS.18.1.19273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prestage G, Grierson J, Bradley J, et al. The role of drugs during group sex among gay men in Australia. Sex Health. 2009;6:310–317. doi: 10.1071/SH09014. [DOI] [PubMed] [Google Scholar]
- 22.Morris SR, Little SJ, Cunningham T, et al. Evaluation of an HIV nucleic acid testing program with automated Internet and voicemail systems to deliver results. Ann Intern Med. 2010;152:778–785. doi: 10.1059/0003-4819-152-12-201006150-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoenigl M, Green N, Mehta SR, et al. Risk Factors for Acute and Early HIV Infection Among Men Who Have Sex With Men (MSM) in San Diego, 2008 to 2014: A Cohort Study. Medicine (Baltimore) 2015;94:e1242. doi: 10.1097/MD.0000000000001242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoenigl M, Weibel N, Mehta SR, et al. Development and Validation of the San Diego Early Test (SDET) Score to Predict Acute and Early HIV Infection Risk in Men who have Sex with Men. Clin Infect Dis. 2015 doi: 10.1093/cid/civ335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoenigl M, Anderson CM, Green N, et al. Repeat HIV-testing is associated with an increase in behavioral risk among men who have sex with men: a cohort study. BMC Med. 2015;13 doi: 10.1186/s12916-015-0458-5. 218,015-0458-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hightow-Weidman LB, Golin CE, Green K, et al. Identifying people with acute HIV infection: demographic features, risk factors, and use of health care among individuals with AHI in North Carolina. AIDS Behav. 2009;13:1075–1083. doi: 10.1007/s10461-008-9519-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith DK, Pals SL, Herbst JH, et al. Development of a clinical screening index predictive of incident HIV infection among men who have sex with men in the United States. J Acquir Immune Defic Syndr. 2012;60:421–427. doi: 10.1097/QAI.0b013e318256b2f6. [DOI] [PubMed] [Google Scholar]
- 29.Thiede H, Jenkins RA, Carey JW, et al. Determinants of recent HIV infection among Seattle-area men who have sex with men. Am J Public Health. 2009;99(Suppl 1):S157–S164. doi: 10.2105/AJPH.2006.098582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cavazos-Rehg PA, Krauss MJ, Spitznagel EL, et al. Substance use and the risk for sexual intercourse with and without a history of teenage pregnancy among adolescent females. J Stud Alcohol Drugs. 2011;72:194–198. doi: 10.15288/jsad.2011.72.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fletcher JB, Reback CJ. Depression mediates and moderates effects of methamphetamine use on sexual risk taking among treatment-seeking gay and bisexual men. Health Psychol. 2015;34:865–869. doi: 10.1037/hea0000207. [DOI] [PMC free article] [PubMed] [Google Scholar]