Abstract
Background
If undetected, infrarenal Abdominal Aortic Aneurysm (AAA) growth can lead to rupture, a high-mortality complication. Some AAA patients exhibit inhomogeneous luminal contrast attenuation at first-pass CT angiography (CTA). This study assesses the association between this observation and aneurysm growth.
Methods
Sixty-seven consecutive pre-repair AAA CTAs were included in this retrospective study. The “Gravitational Gradient” (GG), defined as the ratio of the mean attenuation in a region-of-interest placed posteriorly to that in a region-of-interest placed anteriorly within the lumen of the aortic aneurysm on a single axial slice, and the maximum aneurysm diameter were measured from each CT data set. “AAA Contrast Inhomogeneity” was defined as the absolute value of the difference between the GG and 1.0. Univariate and multivariate logistic regression was used to assess the association of aneurysm growth >0.4 and >1.0 cm/year to AAA Contrast Inhomogeneity, aneurysm diameter, patient characteristics and cardiovascular co-morbidities.
Results
AAA Contrast Inhomogeneity was not correlated to aneurysm diameter (p=0.325). In multivariable analysis that included initial aneurysm diameter and AAA Contrast Inhomogeneity, both factors were significantly associated with rapid aneurysm growth (initial diameter: p=0.0029 and 0.011, and, AAA Contrast Inhomogeneity: p=0.045 and 0.048 for growth >0.4 cm/year and >1 cm/year respectively).
Conclusions
AAA Contrast Inhomogeneity is a common observation in first-pass CTA. It is associated with rapid aneurysm growth, independent of and incremental to aneurysm diameter.
Keywords: CT Angiography, Abdominal Aortic Aneurysm, Risk Stratification, Contrast Inhomogeneity, Aneurysm Growth
Introduction
The strongest predictor of abdominal aortic aneurysm (AAA) rupture is aneurysm diameter. Female sex, smoking, and high blood pressure also increase risk, while diabetes appears to reduce the risk of growth.1, 2 Mechanistic factors such as aortic wall stress also contribute to rupture.3, 4 Mortality is reduced with surveillance followed by elective intervention when the aneurysm diameter reaches 5–5.4 cm.5, 6 Improved patient-specific risk stratification is needed5, 7 as the rupture risk per year for aneurysms <5 cm in diameter was estimated to be up to 5% 8, while in one series, 6.8% of ruptured aneurysms were <5 cm in diameter. 9
Computed Tomography Angiography (CTA) is the appropriate imaging study to confirm a clinically suspected AAA and to plan intervention.10–12 Contrast enhancement is currently used for morphology assessment, for example to separate thrombus from lumen and to identify the presence of accessory renal arteries that would influence management decisions. To our knowledge, there is no peer-reviewed literature that describes the complex CTA enhancement patterns in the aneurysmal sac of AAA patients.
The “Gravitational Gradient”13 measures the ratio of mean attenuation between regions of interest (ROIs) placed posteriorly and anteriorly within the vessel lumen in large arteries imaged with first pass CTA and, if elevated, suggests slow blood flow. In aneuryms such as AAA, inhomogeneous contrast in the aneurysmal lumen likely represents turbulent flow that could be associated with higher-risk lesions. However, radiologists do not routinely report this observation as a clinical finding because it has not been studied systematically. We have anecdotally observed that some AAA patients with inhomogeneous enhancement have higher contrast in the independent portion of the aneurysm sac. Such a “reverse” Gravitational Gradient likely reflects complex blood flow patterns.
The purpose of this study is to investigate the observation of CTA contrast inhomogeneity in patients with infrarenal AAA. We describe the distribution of inhomogeneity patterns and the relationship of inhomogeneity to aneurysm growth.
Methods
Patient Selection
The institutional human research committee approved this retrospective study and waived the requirement for informed consent. All 67 CTAs of unrepaired infrarenal AAA performed at a single teaching hospital between June 2010 and December 2011 were included.
CTA Protocols
CT data were acquired helically using the central 64 detector rows of a first generation 320 (Aquilion ONE, Toshiba Medical Systems Corporation) or 2×32 (Sensation, Siemens Medical Solutions) detector row scanner. Standard institutional imaging and contrast injection protocols were used for all scans. Either 75 or 100 mL (depending on estimated glomerular filtration rate) iopamidol (370 mgI/ml, Isovue-370, Bracco Diagnostics, Princeton, NJ) was injected at 4 mL/s followed by 40 mL of saline. CT acquisition was timed for the arterial phase by automated bolus tracking in the abdominal aorta using a 180–200 Hounsfield unit (HU) threshold. Other parameters were 120 kVp tube potential, z-axis automatic tube current modulation, and 0.75–1 pitch. Images were reconstructed at 1–3 mm thickness using soft tissue kernels (B30f/FC08).
CTA Measurements
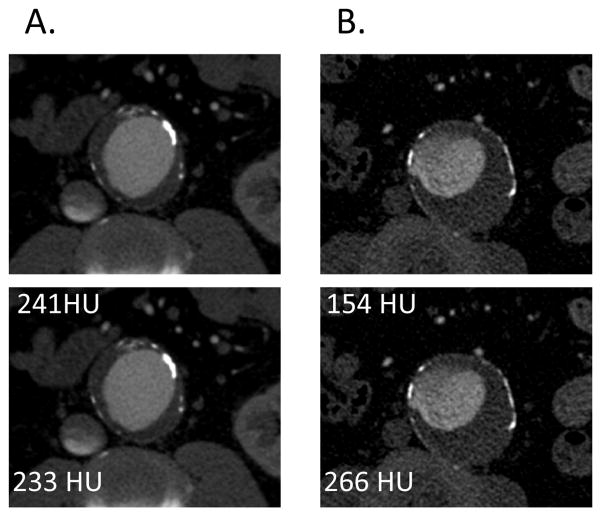
Two readers independently performed all measurements. Two ROIs, each 60 mm2 in size, were placed in the axial image of the AAA where the sac had the largest short axis dimension. The posterior ROI was placed in the posterior one-third of the lumen, and the anterior ROI was placed in the anterior one-third of the lumen (figure 1) with care to exclude intraluminal thrombus and aorta wall.
Figure 1.
(A) Axial CTA images of a patient with aa AAA of 5.4 cm diameter and homogeneous luminal contrast attenuation (AAA Contrast Inhomogeneity = 0.03). (B) CTA of a patient with a similar-sized AAA (5.6 cm diameter) with inhomogeneous contrast attenuation and correspondingly higher AAA Contrast Inhomogeneity (0.73).
The “AAA Contrast Inhomogeneity” was defined as the absolute value (denoted “ABS” in the equations below) of the difference between the gravitational gradient13 and one. This follows the simple mathematical equation:
or, expanding out the definition of the gravitational gradient,
For each aneurysm included in the study, each reader measured and recorded the maximum aneurysm diameter and AAA Contrast Inhomogeneity.
Patient Characteristics
The following patient parameters were obtained from the electronic medical record: age, sex, body mass index, history of coronary artery disease (CAD), hypertension, diabetes mellitus, lower extremity peripheral artery disease (PAD), and congestive heart failure.
Aneurysm Growth
An average rate of aneurysm growth (cm/year) for each patient was determined by linear regression of all diameter measurements reported in CTA and/or ultrasound (US) studies in a 3-year period prior to repair or, for patients who did not undergo repair, the last imaging study available. The first diameter measurement in this period was demarcated as the initial AAA diameter. Patients who did not have at least two measurements separated by >1 month were excluded from analysis.
Statistical Analysis
Analyses were performed in STATA (version 11.2, Stata Corp). Agreement of AAA Contrast Inhomogeneity and maximum diameter amongst readers was assessed using Pearson correlation. Subsequent statistical analyses were performed using the average of readers’ CTA measurements. Correlation was assessed using the Pearson correlation coefficient (r).
Univariate logistic regression was used to assess the association of all measured variables with aneurysm growth >0.4 and >1.0 cm/year.4, 14 All variables significantly associated with the outcome were then included in a multivariate logistic regression model. For patients with more than one CTA during the study period, AAA Contrast Inhomogeneity measured on the earliest CTA was used in this analysis. A value p<0.05 was considered significant.
Results
Agreement between CTA measurements among readers was excellent (Pearson r=0.85 for AAA Contrast Inhomogeneity and 0.84 for maximum diameter, both p<0.001). AAA Contrast Inhomogeneity was not correlated to either maximum aneurysm diameter at the CTA that it was measured on (Pearson r=0.12, p=0.325), or to initial AAA diameter (Pearson r=0.16, p=0.206).
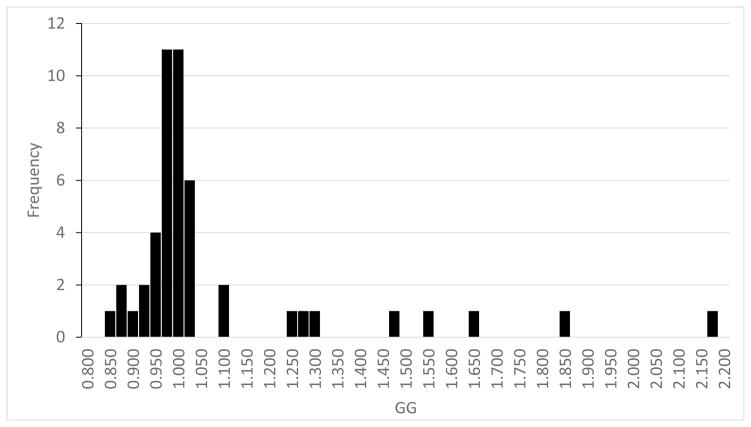
Of the 67 CTAs included in the study, 19 were excluded from regression analyses due to insufficient follow-up time. For the remaining 48 patients, patient characteristics are provided in table 1. Thirteen of the 48 patients (27%) had AAA Contrast Inhomogeneity values >10% (figure 2; four with Gravitational Gradient <0.9 and nine with Gravitational Gradient >1.1). For regression analyses, AAA Contrast Inhomogeneity was considered as a continuous, real-valued predictive variable.
Table 1.
Patient characteristics of the study cohort.
| N=48 | |
|---|---|
|
| |
| Age (yrs) | 72.52±10.44 |
|
| |
| Female | 14 (29.2) |
|
| |
| Race | |
| Caucasian | 43 (89.5) |
| African-American | 2 (4.2) |
| Hispanic | 3 (6.3) |
| Other | 0 |
|
| |
| Hypertension | 42 (87.5) |
|
| |
| CAD | 16 (33.3) |
|
| |
| Aortic valve disease | 4 (8.3) |
|
| |
| Lower extremity PAD | 9 (18.8) |
|
| |
| Congestive Heart Failure | 2 (4.2) |
|
| |
| Body weight* (kg) | 80.18±18.45 |
|
| |
| BMI$ (kg/m2) | 27.82±5.13 |
|
| |
| Diabetes Mellitus | 9 (18.8) |
CAD: Coronary artery disease, PAD: Lower extremity peripheral artery disease, BMI: Body mass index,
Data missing in 2 patients
Data missing in 6 patients
Figure 2.
Distribution of the Gravitational Gradient in the study cohort.
The average time interval of AAA diameter measurements available for analysis was 791 days (interquartile range: 456–1008 days). Fifteen of the 48 patients (31%) had >0.4 cm/year growth rate and 8 (16%) had >1cm/year growth rate. Univariate analyses revealed that only initial AAA diameter and AAA Contrast Inhomogeneity were significantly associated with rapid aneurysm growth (table 2). In multivariable analyses, both parameters remained significantly associated with growth (table 2).
Table 2.
Univariate and multivariate analysis for the association of patient characteristics, cardiovascular co-morbidities, AAA Contrast Inhomogeneity, and aneurysm diameter with rapid aneurysm growth (n=48).
| Univariate Analyses | Multivariate Analyses | ||||
|---|---|---|---|---|---|
| p | Regression Coefficient [95%CI] | p | Regression Coefficient [95%CI] | ||
| >1cm/yr growth | Initial AAA diameter | 0.006 | 2.079 [0.592–3.566] | 0.011 | 1.848 [0.425–3.271] |
| Contrast Inhomogeneity | 0.010 | 5.060 [1.193–8.927] | 0.048 | 4.510 [0.039–8.980] | |
| Diabetes | 0.152 | - | |||
| Age | 0.579 | - | |||
| CAD | 0.784 | - | |||
| PAD | 0.623 | - | |||
| Hypertension | 0.258 | - | |||
| Female sex | 0.777 | - | |||
| BMI | 0.859 | - | |||
| CHF | 0.243 | - | |||
| >0.4 cm/yr growth | Initial AAA diameter | 0.005 | 1.488 [0.455–2.522] | 0.029 | 1.330 [0.135–2.525] |
| Contrast Inhomogeneity | 0.016 | 10.612 [0.993–19.231] | 0.045 | 8.869 [0.180–17.560] | |
| Diabetes | 0.349 | - | |||
| Age | 0.310 | - | |||
| CAD | 0.511 | - | |||
| PAD | 0.881 | - | |||
| Hypertension | 0.301 | - | |||
| Female sex | 0.669 | - | |||
| BMI | 0.654 | - | |||
| CHF | 0.569 | - | |||
AAA: Abdominal aortic aneurysm, CAD: Coronary artery disease, PAD: Lower extremity peripheral artery disease, BMI: Body mass index, CHF: Congestive heart failure
Discussion
Sheiman et al15 first studied contrast layering in the abdominal aorta of 22 patients with varied pathologies, including AAA, using a small bolus (10 ml) of contrast. Higher peak enhancement was observed in the dependent compared to the non-dependent ROI (48.3±19.0 versus 33.0±19.7 HU). These measurements correspond to AAA Contrast Inhomogeneity values of approximately 0.16, assuming 60 HU for unenhanced blood, similar to the average of 0.13±0.24 in our AAA cohort.
Cardiovascular imagers have for roughly a decade measured the contrast enhanced aneurysm sac from rapidly acquired first-pass CTA without studying or reporting the pattern of enhancement. Our newly introduced parameter, “AAA Contrast Inhomogeneity”, provides a straightforward quantification of a recognized observation in the aneurysm sac (Figure 1). While we used ROIs for quantification, with experience, a cardiovascular imager can probably readily separate those patients with homogeneous versus inhomogeneous contrast enhancement without post-processing.
We investigated this new finding to potentially improve risk stratification for AAA patients. Rupture risk can be significant even for small aneurysms,9, 16 while growth rates can vary among individuals and over time,5, 7, 17 which can be a limitation for surveillance strategies. Many anatomic, risk-factor related, and hemodynamic variables have been investigated to enhance risk assessment,1, 3, 4, 18 but clinically-applicable algorithms remain elusive.6 In our cohort, AAA Contrast Inhomogeneity was not correlated to AAA diameter and was independently predictive of a period of clinically significant AAA enlargement. The fundamental link between hemodynamics and cardiovascular disease onset and progression19 support a potential relationship between inhomogeneous contrast distribution and blood flow patterns. In general, regions of slow or turbulent flow are considered to activate inflammatory mechanisms and promote lipid deposition and thrombus formation.19 These can lead to eventual degradation of extracellular matrix and wall mechanical properties, potentially increasing AAA size and rupture risk.17, 20, 21 In a recent series of 7 ruptured AAAs, the location of rupture was found at or near flow recirculation zones, regardless of aneurysm size and configuration.18
Study Limitations
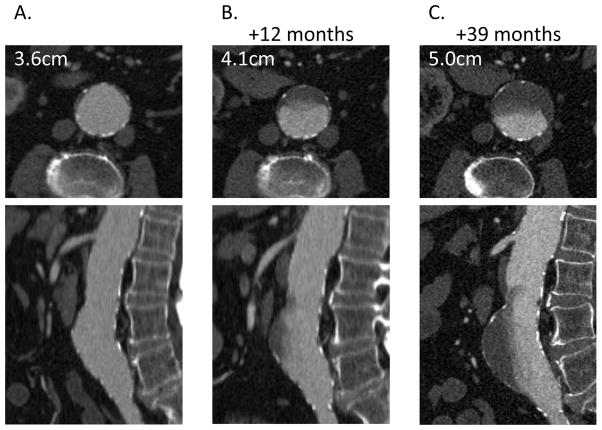
This is the first study of contrast enhancement patterns in AAA; we reported on 67 consecutive patients from a large, urban teaching hospital. Because the study is retrospective, the timing between studies and the follow-up was inconsistent, leading to the exclusion of 19 patients. It was also necessary to treat US- and CTA-reported diameters equivalently and to use a linear fit to define average growth rate. Although this is the most commonly used method,7 it can be inaccurate as AAAs often exhibit periods of sudden expansion and plateaus.7 Furthermore, although the average interval available for analysis after the CTA study was 201 days, in some patients it was the last exam. It is thus unclear whether AAA Contrast Inhomogeneity can predict growth or whether it may be better suited toward optimizing surveillance intervals.5, 7 This should be further assessed with an appropriate, prospective study design. In one patient with follow-up CTA, the region of reduced contrast enhancement causing inhomogeneity correlated with subsequent thrombus formation and aneurysm expansion (figure 3), suggesting that it may be a relatively early finding. We also note that ROI locations were standardized for consistency; namely, we used the mean HU measurements on the axial slice where the aneurysm was largest. This may not be the ideal location to measure contrast inhomogeneity, and may require optimization with respect to aneurysm and thrombus geometry and asymmetry. Finally, other variables that affect hemodynamics, such as cardiac output, were not available in this retrospective cohort and were thus not included in the analysis.
Figure 3.
Serial CTA images in one patient demonstrate luminal contrast attenuation that is (A) homogeneous at initial CTA but (B) becomes visibly inhomogeneous at 12 months (AAA Contrast Inhomogeneity=0.6) and (C) further increases at 39 months (AAA Contrast Inhomogeneity=0.86). Anterior thrombus formation and aneurysm growth at the region corresponding to the observed inhomogeneity in attenuation is noted over time.
Conclusions
AAA Contrast Inhomogeneity is a simple quantification of a common observation. It can be measured with two ROIs placed anteriorly and posteriorly in the AAA lumen at first pass CTA. It appears associated with AAA progression independent of AAA diameter and should be further tested toward its potential applications for risk assessment in patients with abdominal aortic aneurysms.
Acknowledgments
Funding Sources: NIH NIBIB Grant #EB015868; RSNA Grant #RSD1403
Footnotes
Conflicts of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sweeting MJ, Thompson SG, Brown LC, Powell JT Rescan collaborators. Meta-analysis of individual patient data to examine factors affecting growth and rupture of small abdominal aortic aneurysms. Br J Surg. 2012;99:655–665. doi: 10.1002/bjs.8707. [DOI] [PubMed] [Google Scholar]
- 2.Brown LC, Powell JT. Risk factors for aneurysm rupture in patients kept under ultrasound surveillance. Uk small aneurysm trial participants. Ann Surg. 1999;230:289–296. doi: 10.1097/00000658-199909000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fillinger MF, Raghavan ML, Marra SP, Cronenwett JL, Kennedy FE. In vivo analysis of mechanical wall stress and abdominal aortic aneurysm rupture risk. J Vasc Surg. 2002;36:589–597. doi: 10.1067/mva.2002.125478. [DOI] [PubMed] [Google Scholar]
- 4.Li ZY, Sadat U, U-King-Im J, Tang TY, Bowden DJ, Hayes PD, Gillard JH. Association between aneurysm shoulder stress and abdominal aortic aneurysm expansion: A longitudinal follow-up study. Circulation. 2010;122:1815–1822. doi: 10.1161/CIRCULATIONAHA.110.939819. [DOI] [PubMed] [Google Scholar]
- 5.Bown MJ, Sweeting MJ, Brown LC, Powell JT, Thompson SG. Surveillance intervals for small abdominal aortic aneurysms: A meta-analysis. JAMA. 2013;309:806–813. doi: 10.1001/jama.2013.950. [DOI] [PubMed] [Google Scholar]
- 6.Chaikof EL, Brewster DC, Dalman RL, Makaroun MS, Illig KA, Sicard GA, Timaran CH, Upchurch GR, Jr, Veith FJ Society for Vascular S. The care of patients with an abdominal aortic aneurysm: The society for vascular surgery practice guidelines. J Vasc Surg. 2009;50:S2–49. doi: 10.1016/j.jvs.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Brady AR, Thompson SG, Fowkes FG, Greenhalgh RM, Powell JT Participants UKSAT. Abdominal aortic aneurysm expansion: Risk factors and time intervals for surveillance. Circulation. 2004;110:16–21. doi: 10.1161/01.CIR.0000133279.07468.9F. [DOI] [PubMed] [Google Scholar]
- 8.Brewster DC, Cronenwett JL, Hallett JW, Jr, Johnston KW, Krupski WC, Matsumura JS. Guidelines for the treatment of abdominal aortic aneurysms. Report of a subcommittee of the joint council of the american association for vascular surgery and society for vascular surgery. Journal of vascular surgery. 2003;37:1106–1117. doi: 10.1067/mva.2003.363. [DOI] [PubMed] [Google Scholar]
- 9.Nicholls SC, Gardner JB, Meissner MH, Johansen HK. Rupture in small abdominal aortic aneurysms. J Vasc Surg. 1998;28:884–888. doi: 10.1016/s0741-5214(98)70065-5. [DOI] [PubMed] [Google Scholar]
- 10.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM, Jr, White CJ, White J, White RA, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. Acc/aha guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic) J Vasc Interv Radiol. 2006;17:1383–1397. doi: 10.1097/01.RVI.0000240426.53079.46. [DOI] [PubMed] [Google Scholar]
- 11.Desjardins B, Dill KE, Flamm SD, Francois CJ, Gerhard-Herman MD, Kalva SP, Mansour MA, Mohler ER, 3rd, Oliva IB, Schenker MP, Weiss C, Rybicki FJ. Acr appropriateness criteria® pulsatile abdominal mass, suspected abdominal aortic aneurysm. The international journal of cardiovascular imaging. 2013;29:177–183. doi: 10.1007/s10554-012-0044-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Francois CJ, Kramer H, Rybicki FJ, Ray CE, Bandyk DF, Burke CT, Dill KE, Gerhard-Herman MD, Hanley M, Hohenwalter EJ, Mohler ER, Rochon PJ, Schenker MP. Acr appropriateness criteria®: Abdominal aortic aneurysm: Interventional planning and follow-up. 2012. [Google Scholar]
- 13.Hanley M, Mitsouras D, Steigner ML, Demehri S, Signorelli J, Kumamaru KK, Rybicki FJ. Computed tomography angiography gravitational gradient as an imaging sign of slow flow. Journal of computer assisted tomography. 2013;37:297–300. doi: 10.1097/RCT.0b013e31827bc476. [DOI] [PubMed] [Google Scholar]
- 14.Lederle FA, Wilson SE, Johnson GR, Reinke DB, Littooy FN, Acher CW, Ballard DJ, Messina LM, Gordon IL, Chute EP, Krupski WC, Busuttil SJ, Barone GW, Sparks S, Graham LM, Rapp JH, Makaroun MS, Moneta GL, Cambria RA, Makhoul RG, Eton D, Ansel HJ, Freischlag JA, Bandyk D. Immediate repair compared with surveillance of small abdominal aortic aneurysms. The New England journal of medicine. 2002;346:1437–1444. doi: 10.1056/NEJMoa012573. [DOI] [PubMed] [Google Scholar]
- 15.Sheiman RG, Prassopoulos P, Raptopoulos V. Ct detection of layering of i.V. Contrast material in the abdominal aorta. AJR. American journal of roentgenology. 1998;171:1291–1295. doi: 10.2214/ajr.171.5.9798864. [DOI] [PubMed] [Google Scholar]
- 16.Zarins CK, Crabtree T, Arko FR, Heikkinen MA, Bloch DA, Ouriel K, White RA. Endovascular repair or surveillance of patients with small aaa. Eur J Vasc Endovasc Surg. 2005;29:496–503. doi: 10.1016/j.ejvs.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Blanchard JF. Epidemiology of abdominal aortic aneurysms. Epidemiologic reviews. 1999;21:207–221. doi: 10.1093/oxfordjournals.epirev.a017997. [DOI] [PubMed] [Google Scholar]
- 18.Sho E, Sho M, Nanjo H, Kawamura K, Masuda H, Dalman RL. Hemodynamic regulation of cd34+ cell localization and differentiation in experimental aneurysms. Arteriosclerosis, thrombosis, and vascular biology. 2004;24:1916–1921. doi: 10.1161/01.ATV.0000142805.20398.74. [DOI] [PubMed] [Google Scholar]
- 19.Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nature clinical practice. Cardiovascular medicine. 2009;6:16–26. doi: 10.1038/ncpcardio1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buijs RV, Willems TP, Tio RA, Boersma HH, Tielliu IF, Slart RH, Zeebregts CJ. Current state of experimental imaging modalities for risk assessment of abdominal aortic aneurysm. J Vasc Surg. 2013;57:851–859. doi: 10.1016/j.jvs.2012.10.097. [DOI] [PubMed] [Google Scholar]
- 21.Lukasiewicz A, Reszec J, Kowalewski R, Chyczewski L, Lebkowska U. Assessment of inflammatory infiltration and angiogenesis in the thrombus and the wall of abdominal aortic aneurysms on the basis of histological parameters and computed tomography angiography study. Folia Histochemica et Cytobiologica. 2012;50:547–553. doi: 10.5603/20323. [DOI] [PubMed] [Google Scholar]