Abstract
Exercise is an inexpensive intervention that may be used to reduce obesity and its consequences. In addition, many individuals who regularly exercise utilize dietary supplements to enhance their exercise routine and to accelerate fat loss or increase lean mass. Branched-chain amino acids (BCAAs) are a popular supplement and have been shown to produce a number of beneficial effects in rodent models and humans. Therefore, we hypothesized that BCAA supplementation would protect against high fat diet (HFD)-induced glucose intolerance and obesity in mice with and without access to exercise. We subjected 80 female C57BL/6 mice to a paradigm of HFD feeding, exercise in the form of voluntary wheel running, and BCAA supplementation in the drinking water for 16 weeks (n = 10 per group). Body weight was monitored weekly, while food and water consumption were recorded twice weekly. During the 5th, 10th, and 15th weeks of treatment, glucose tolerance and body composition were analyzed. Exercise significantly improved glucose tolerance in both control-fed and HFD-fed mice. BCAA supplementation, however, did not significantly alter glucose tolerance in any treatment group. While BCAA supplements did not improve lean to fat mass ratio in sedentary mice, it significantly augmented the effects of exercise on this parameter.
Keywords: BCAA, high fat diet, exercise, obesity, rodent, supplement
INTRODUCTION
Obesity is a growing epidemic in the United States (Flegal et al., 2012), due primarily to sedentary lifestyle and poor nutrition, such as diets high in fat content. It is well accepted that exercise is one way to promote a healthy calorie balance and body weight (Schmitz et al., 2000). Even in the absence of weight loss, exercise can lower blood pressure and improve insulin sensitivity (Carroll et al., 2002). The American Diabetes Association and the American College of Sports Medicine emphasize the utility of exercise in both prevention and management of type 2 diabetes (Colberg et al., 2010). Endurance exercise can reduce several of the parameters defining the metabolic syndrome (Pattyn et al., 2013), including improvements in insulin sensitivity (Bhat et al., 2012). Exercise is therefore an extremely important modality for the improvement and maintenance of glycemic control.
Voluntary wheel running is a frequently used model for exercise in rodents. Male mice provided with a running wheel have improved glucose tolerance and body composition (Bradley et al., 2008; Yan et al., 2012). Physical activity can protect male mice from diet-induced obesity (Huang et al., 2010; Yan et al., 2012). While the physical and metabolic effects of voluntary exercise have been studied extensively in male mice, evidence in female mice is mostly lacking.
Individuals who exercise often consume dietary supplements. Physical activity was associated with an estimated odds ratio of 2.45 for dietary supplementation (especially herbal stimulants) in a young adult population (Gardiner et al., 2007). A report in 2009 indicates that $14.8 billion was spent on non-vitamin, non-mineral supplements (Nahin et al., 2009). As the use of supplements is becoming ever more widespread, further research is warranted regarding their safety and efficacy. Studies report rates of protein or amino acid supplementation among exercising individuals from 42% to 77% (Morrison et al., 2004; Radimer et al., 2000). Branched-chain amino acids (BCAAs: leucine, valine, and isoleucine) are widely available for purchase and are a popular choice. Goston and Correia found that 6% of people exercising in gyms were taking BCAAs, a similar number to a report by de Silva et al in professional athletes (de Silva et al., 2010; Goston et al., 2010).
The BCAAs are touted for preventing muscle breakdown (Howatson et al., 2012). Indeed, BCAAs have been shown to activate enzymes involved in protein synthesis (Blomstrand et al., 2006), while interfering with BCAA metabolism induces exercise failure (She et al., 2010). Well-trained individuals subjected to calorie restriction and BCAA supplementation lost additional abdominal fat compared to those restricting calories alone (Mourier et al., 1997). BCAAs may play a role in the weight-management benefits of high protein diets (Qin et al., 2011). Additionally, male mice receiving BCAAs are protected from virally-initiated impaired glucose tolerance (Utsugi et al., 2000), which is not surprising as the BCAAs are implicated in glucose metabolism (Doi et al., 2007). D’Antona et al. reported that BCAA supplementation increases mean lifespan in male mice despite no significant changes in food intake or body weight (D'Antona et al., 2010).
The goal of this study was to investigate the effects of voluntary exercise and BCAA supplementation in combination with HFD consumption in female C57BL/6 mice. Eighty mice were subjected to a regimen of HFD feeding, exercise, and BCAA supplementation for 16 weeks (n = 10 per group). Drinking water was supplemented with BCAAs at a concentration of 2%, similar to the amount used by others (Arakawa et al., 2011; Guo et al., 2010; Macotela et al., 2011; Zhang et al., 2007). We hypothesized that voluntary exercise and BCAA supplementation, both in combination and separately, would improve body composition and glucose tolerance in HFD fed mice. Instead, we found that voluntary exercise significantly protected against HFD-induced obesity and glucose intolerance, and while BCAA supplementation did not significantly alter glucose tolerance, it did significantly improve lean to fat mass ratio in combination with voluntary exercise. We previously reported the body weight at the end of the study and glucose tolerance after 10 weeks in these same mice, but the focus was on the effects and correlation with Achilles tendon properties (Boivin et al., 2013).
RESEARCH DESIGN AND METHODS
Animals
This study was completed according to a protocol approved by the University of Kentucky Institutional Animal Care and Use Committee. Female C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME) and arrived at 9 weeks of age. Mice were individually housed in standard shoebox cages. The animals were housed with a 14:10 h light:dark cycle and ambient temperatures between 20–23°C. After one week acclimation, animals were maintained on their respective treatment regimen for 16 weeks. Animals were split into eight groups and rearranged such that mean body weight was approximately equal between groups as verified by one-way ANOVA (p = 0.999). Eight groups were then randomly assigned: 1) control diet (CD), sedentary (SED), water; 2) CD, SED, BCAA; 3) HFD, SED, water; 4) HFD, SED, BCAA; 5) CD, exercise (EX), water; 6) CD, EX, BCAA; 7) HFD, EX, water; 8) HFD, EX, BCAA. Animals were euthanized following a 3 h fast during week 16.
Diet and Water Treatment
The mice were fed a 10 kcal% fat control diet (CD) and 60 kcal% HFD (D12450B and D12492; Research Diets, Inc., New Brunswick, NJ). A pre-weighed amount of food was given to each animal in such a quantity that they could eat ad libitum. Food remaining was weighed, discarded, and replaced twice weekly. BCAA supplement (NutraBio BCAA 5000, Nutrabio, Inc., Middlesex, NJ) was added to the drinking water at a concentration of 20 g/L, yielding a 2% solution. The BCAA consisted of a 2:1:1 ratio of leucine:valine:isoleucine. A sample of the supplement was sent to a third party lab (ALS Global, Salt Lake City, UT) for testing, which confirmed an approximate 2:1:1 ratio as claimed by the product label. The calorie intake from BCAA water consumption was included in the calorie consumption data (1 mL of the BCAA solution accounted for 0.08 kcal). Animals were given a known amount of control water or BCAA supplemented water. Water remaining was measured via graduated cylinder, discarded, and replaced with fresh solution twice weekly. The mice had 24 hour access to BCAA water except for brief fasting periods for testing and prior to euthanasia when they were given normal water.
Exercise
Exercise animals had free voluntary access to running wheels mounted within the home cages (Phenome Technologies, Inc., Lincolnshire, IL). Sedentary animals were housed in similar shoebox cages lacking running wheels. Wheel rotations were recorded mechanically and sent to a computer via ClockLab software (Actimetrics, Wilmette, IL). The mice had 24 hour access to running wheels except for brief periods of fasting prior to glucose tolerance testing and euthanasia as well as during glucose tolerance testing and body composition measurements.
Glucose Tolerance Testing
During the 5th, 10th, and 15th weeks of treatment, all animals were fasted in a clean cage lacking running wheels and provided water only. After 3 hours (5 and 10 weeks) or 6 hours (15 weeks), the tail was nicked and fasting blood glucose was recorded (Bayer Breeze 2 glucose meter, Bayer HealthCare, LLC, Tarrytown, NY) prior to intraperitoneal (IP) injection of D-glucose (Sigma-Aldrich Co., LLC, St. Louis, MO) at a concentration of 2 g/kg body weight. Blood glucose reading was repeated 15, 30, 60, and 120 minutes post-injection.
Body Composition
Body composition analysis was completed using EchoMRI-100 (Echo Systems, Houston, TX). After running an appropriate control sample, conscious animals were placed inside of a specifically designed tube and then inserted into the EchoMRI for analysis.
Statistics
Linear mixed modeling was used to analyze data in Figure 1, Figure 2, and Figure 3, as well as data on running speed and duration (not shown), with the basic structure of a two-way or three-way ANOVA plus random effects to capture correlations among repeated measurements, assuming within-group means were quadratic functions of time. Figure 4 employed three-way ANOVA with observations weighted by reciprocals of within-group variances. Subsequent Bonferroni post-hoc testing, when applicable, was utilized to assess between-group differences. Groups not carrying the same letter are significantly different from one another (a bar labeled only with a is significantly different from a bar labeled only with b, though neither is significantly different from a bar labeled with both a and b). Significance was considered as p < 0.05. Analyses were completed using SAS version 9.3.
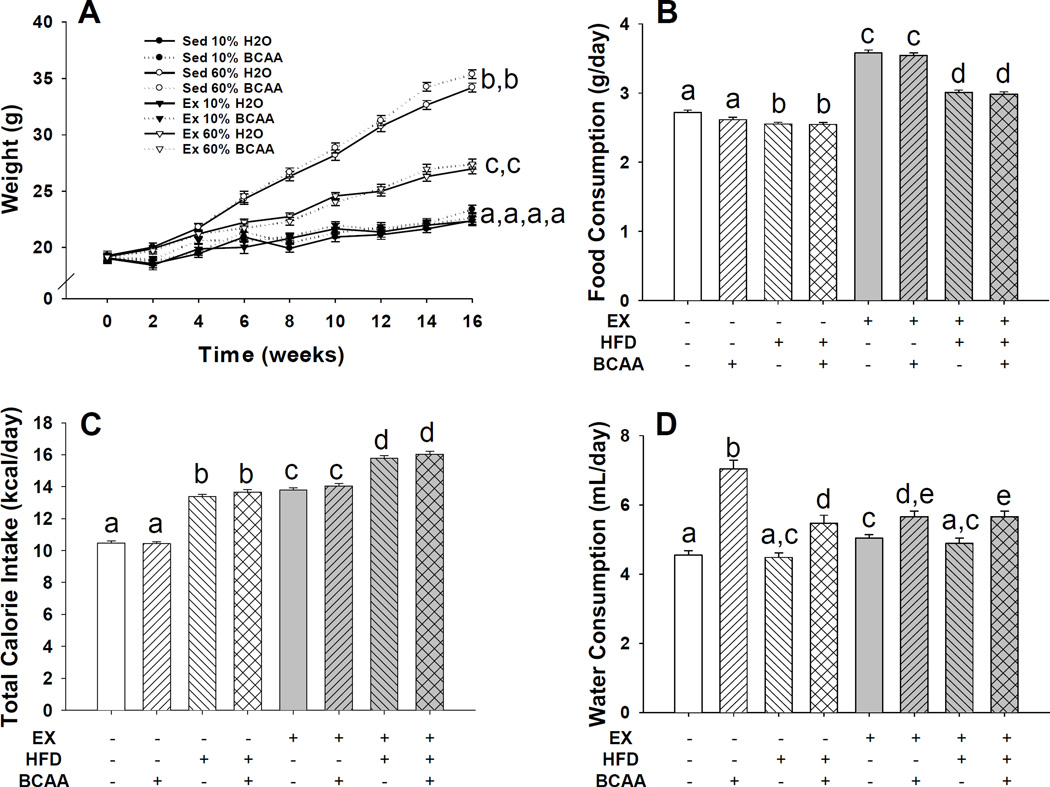
Figure 1. Female C57BL/6 body weights, food, calorie, and water consumption over 16 week time-course.
Female mice were divided into groups ensuring no significant initial body weight differences. Biweekly animal body weight is presented (A). As time progressed, HFD fed animals gained significantly more weight, independent of water treatment. Exercise attenuated this weight gain. Exercise had no effect on the body weight of animals fed the CD while BCAA supplementation did not significantly affect body weight in any of the comparisons. Food remaining was weighed and replaced twice weekly and calculated as grams per day (B). Calorie consumption was calculated by multiplying grams of food consumed by calories per gram, plus calories from water intake (C). Water remaining was measured by graduated cylinder and replaced twice weekly (D). Sedentary control animals consuming HFD decreased the quantity of food consumed, but ingested more calories. Exercise animals consuming the HFD similarly decreased their quantity of intake, but received the most calories of any group. The BCAA water groups consistently drank more than their respective water-consuming group, regardless of diet or exercise treatment. Different letters indicate p < 0.05 between groups (i.e. bars labeled only with a are significantly different from bars labeled only with b, while neither is significantly different from a bar labeled with both an a and b, et cetera). Error bars indicate S.E.M. n = 10 for all groups.
Figure 2. Average daily running distance.
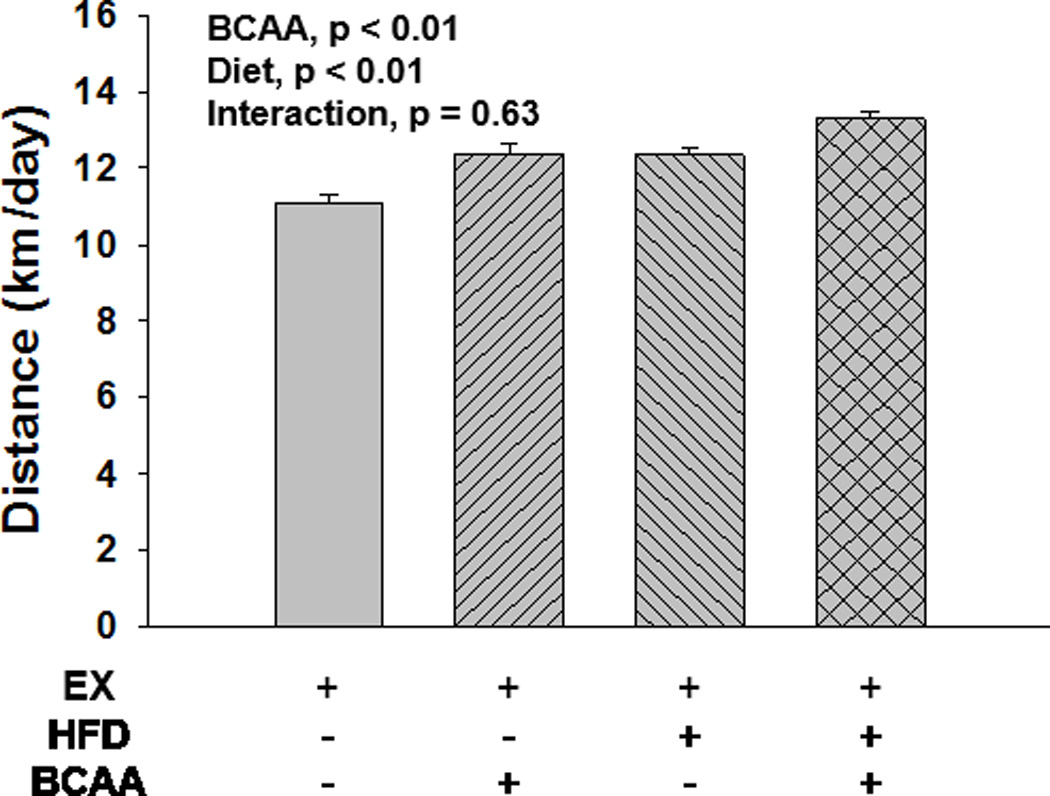
Record of distance ran was kept digitally using Actimetrics ClockLab software. Data analysis was by linear mixed modeling; but for visualization purposes, running distance was averaged per day per mouse, and the average was subsequently taken across all days for each of the four groups. HFD-fed animals ran further than CD animals. BCAA supplementation resulted in an increased distance ran over the course of the 16 week study, independent of diet treatment. Error bars indicate S.E.M. n = 10 per group except for HFD + BCAA n = 8.
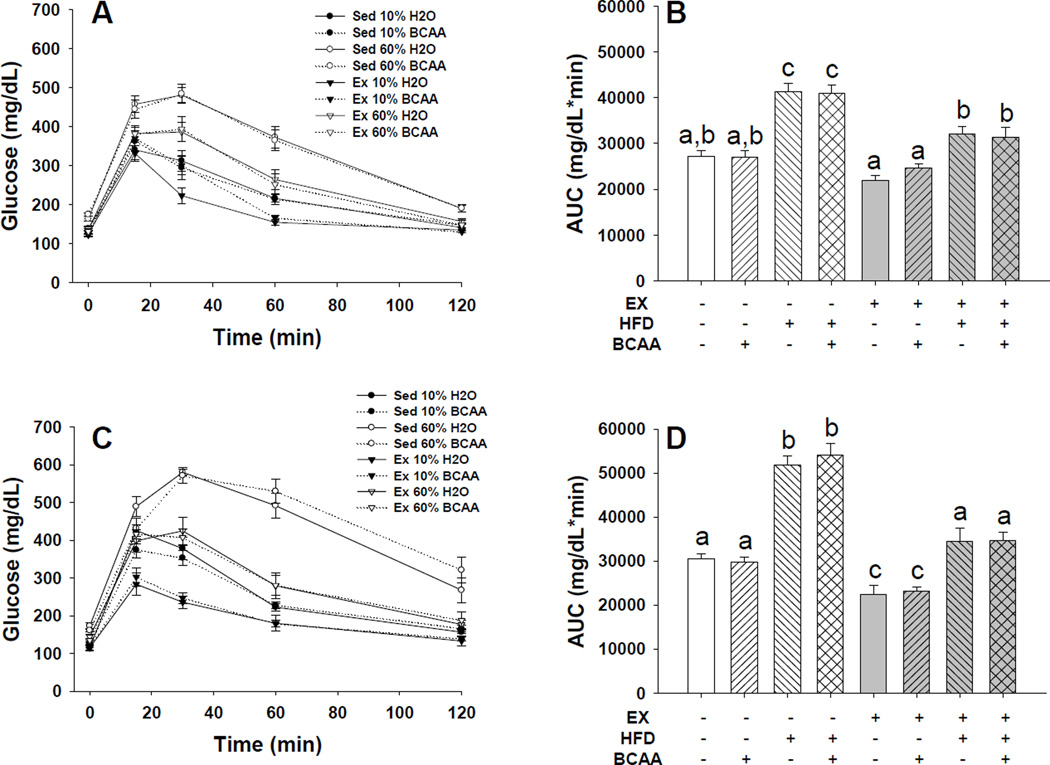
Figure 3. Glucose tolerance during the 5th and 15th weeks of treatment.
Animals were fasted for three hours at 5 weeks (A,B) and six hours at 15 weeks (C,D). Blood glucose was recorded at baseline, animals received an IP injection of 2 g/kg D-Glucose, and the blood glucose measurement was repeated 15, 30, 60, and 120 minutes post-injection (A,C). HFD consumption resulted in impaired glucose tolerance, independent of water treatment. Exercise attenuated these changes to the level of controls. Exercise, in combination with CD, improved glucose tolerance compared to controls at week 15, but not at week 5. BCAA water supplementation had no impact on glucose tolerance, regardless of diet or exercise treatment. Area under the curve was significantly increased for sedentary HFD fed animals and rescued by exercise (B,D). Letters indicate p < 0.05 between groups. Error bars indicate S.E.M. n = 10 for all groups.
Figure 4. Body composition analysis after 15th weeks of treatment.
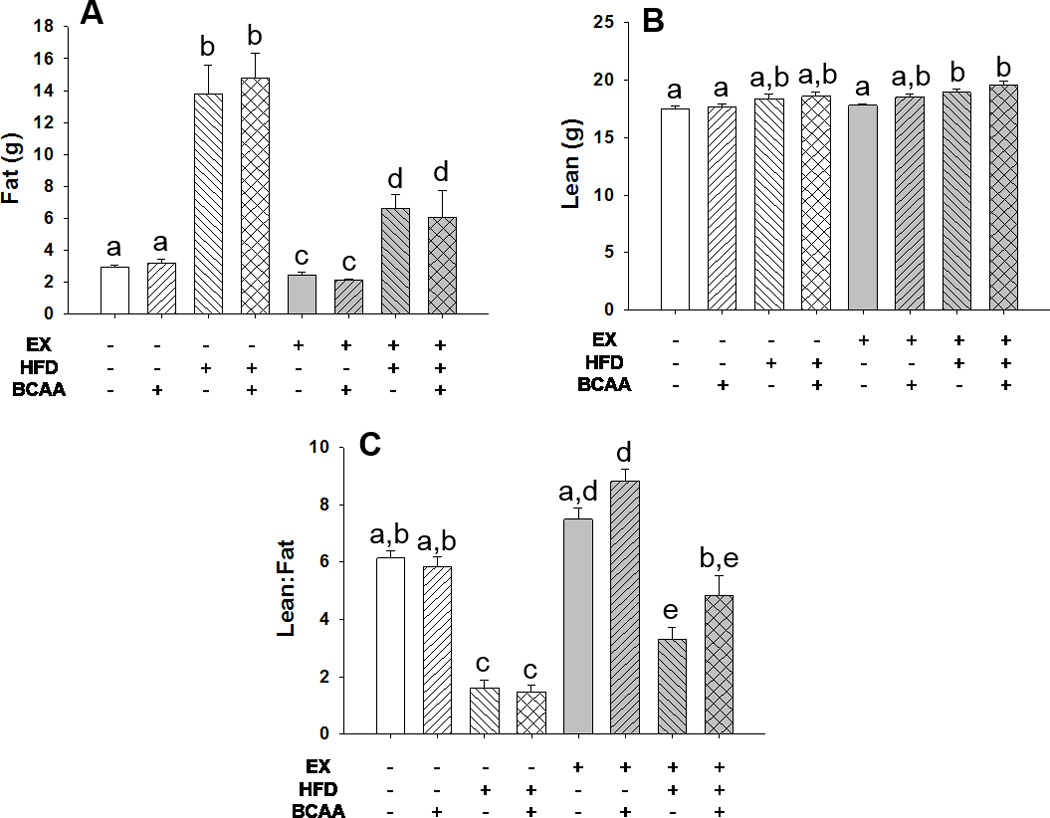
Animals were subjected to EchoMRI for body composition analysis. HFD resulted in an increase in fat mass (grams) compared to control animals (A). Exercise attenuated some of this increase. HF feeding plus exercise resulted in an increase in lean mass compared to CD animals (B). HFD significantly reduced lean-to-fat mass ratio (C). Exercise plus CD did not increase lean to fat mass ratio compared to SED CD animals, but EX CD BCAA did. Similarly, exercise plus BCAA supplementation resulted in recovery of lean to fat mass ratio to SED CD water levels, when EX HFD alone did not. Different letters indicate p < 0.05 between groups. Error bars indicate S.E.M. n = 10 for all groups.
RESULTS
General
Body weight was recorded weekly for the duration of the study (Figure 1A), though it is presented as biweekly for clarity in graphical representation. Exercise significantly attenuated body weight increases that were associated with HFD consumption (p = 0.0002 for the two-way interaction between diet and exercise), though not to the levels of the CD fed mice. BCAA supplementation had no significant impact on body weight (p = 0.57 for main effect of BCAA by three-way ANOVA). HFD fed mice ate significantly less food in grams (p < 0.0001) (Figure 1B) but took in significantly more calories compared with the CD fed animals (p < 0.0001) (Figure 1C). Exercised animals consumed more food in grams and calories than their respective sedentary CD and HFD fed mice (p < 0.0001 for both comparisons) (Figure 1B and C). BCAA supplementation did not significantly impact food or calorie consumption (p = 0.33 and p = 0.24, respectively). The BCAA supplementation did, however, significantly increase water consumption (p < 0.0001 for main effect of BCAA) (Figure 1D). All BCAA supplemented animals drank more than their respective control water group. Further, sedentary CD BCAA drank significantly more than any other group. Serum creatinine levels were measured, and we found no indication of kidney damage as a consequence of BCAA supplementation (data not shown). Both HFD and BCAA caused an increase in distance run (p < 0.0001 for main effect of diet, p = 0.0056 for main effect of BCAA) (Figure 2). There was no significant interaction of diet and BCAA supplement (p = 0.63). This increase in distance ran was due to a combination effect of both an increase in duration of running (p = 0.025 for main effect of diet, p = 0.010 for main effect of BCAA), as well as an increase in speed of running (p < 0.001 for main effect of diet, p < 0.001 for main effect of BCAA). There was no significant interaction of diet with BCAA supplementation regarding either time (p = 0.53) or speed (p = 0.56).
Glucose tolerance
Glucose tolerance following an IP glucose challenge was measured in fasting mice, and the time course and area under the curves (AUC) are shown at the 5 and 15 week time points to illustrate progression over time (Figure 3). Glucose disposal was significantly impaired by HFD feeding during the 5th (Figure 3A and B), 10th (not shown), and 15th weeks of dietary treatment (Figure 3C and D). The impairment was worse at week 15 than at week 5. Exercise significantly improved glucose disposal under both dietary conditions (p < 0.0001 for main effect of exercise at both 5 and 15 weeks). The glucose tolerance impairment induced by the HFD was attenuated such that they were not significantly different than CD fed sedentary mice. BCAA supplementation did not significantly affect glucose tolerance (p = 0.93, p = 0.70 for main effect of BCAA at 5 and 15 weeks, respectively). Data at the 10 week time point followed a similar trend to the 5 and 15 week data (Boivin et al., 2013).
Body composition
Body composition data was collected two days after glucose tolerance testing during weeks 5 (not shown), 10 (not shown) and 15 (Figure 4). HFD significantly increased fat mass (p < 0.0001 for main effect of HFD by three-way ANOVA) (Figure 4A). Exercise significantly decreased fat mass in both the CD and HFD fed mice (p < 0.0001 for main effect of exercise) (Figure 4A). HFD resulted in a significant increase in lean mass when compared with their respective control (p < 0.0001 for main effect of HFD by three-way ANOVA) (Figure 4B). When looked at in terms of lean to fat mass ratio (Figure 4C), the differences in body composition are more notable. HFD dramatically decreased lean to fat mass ratio (p < 0.0001 for main effect of HFD by three-way ANOVA). Exercise in combination with HFD served to significantly increase lean to fat mass ratio when compared with sedentary HFD animals, and exercise HFD BCAA further recovered lean to fat mass ratio to equivalent levels of the sedentary CD animals. Similarly, exercise CD water did not significantly increase lean to fat mass ratio beyond sedentary CD animals, but exercise CD BCAA did induce a significant increase therein. Similar patterns were also true in the data from week 5 and week 10, though the degree of separation increased over time.
DISCUSSION
The benefits of BCAA supplementation are somewhat controversial. Some studies in human subjects suggest that BCAA supplementation in combination with exercise can decrease muscle fatigue, decrease muscle soreness, and improve endurance (Leahy et al., 2013; Matsumoto et al., 2009; Shimomura et al., 2010). Interestingly, both the Shimomura and the Leahy reports found the reported improvements in muscle soreness in female subjects. Other reports indicate no significant effect of BCAA supplementation on similar outcomes (Areces et al., 2014; Knechtle et al., 2012; Spillane et al., 2012). Regardless, BCAA supplementation is relatively common amongst exercising individuals and their potential use for beneficial effects warrants further study.
The benefits of voluntary wheel running on glucose tolerance and body composition have been explored in several studies using male mice (Bradley et al., 2008; Yan et al., 2012). Exercise training improved cardiovascular outcomes and glucose tolerance in male C57BL/6 mice fed a HFD (Hafstad et al., 2013). Voluntary wheel running in female A/J mice decreased fat mass and liver triglycerides (Takeshita et al., 2012). Therefore, it is not surprising that we found improvements in several outcomes due to free choice exercise in the current study. The degree of the improvements, however, is notable. Exercise improved glucose tolerance in HFD-fed animals to approximately the level of sedentary control diet animals, which is a profound improvement, especially considering the difference in body weight.
Of the three BCAAs, leucine especially has been proposed to impart important effects (Layman et al., 2006). At least two studies have used 1.5% leucine in the drinking water and the same HFD as the current study and observed improved glucose tolerance as a consequence of supplementation (Macotela et al., 2011; Zhang et al., 2007). Guo and colleagues also showed decreased hemoglobin A1C levels in two strains of male mice as a consequence of 1.5% leucine supplementation in the drinking water for 8 months (Guo et al., 2010). Yet, we found no significant impact on glucose tolerance using female mice supplemented with 2% BCAA (approximately 1% leucine) in the drinking water. Perhaps this could be due to differences in BCAA supplement/dose or sex differences between the studies. Importantly, the 16 week duration for the BCAA supplementation did not negatively impact glucose tolerance or weight gain. Different doses of BCAAs or leucine alone could potentially be explored in the future.
Freudenberg and colleagues found that a 6% leucine supplement in the diet for 20 weeks decreased food intake and partially ameliorated HFD (20% w/w) induced weight gain in male C57BL/6 mice (Freudenberg et al., 2012). Using the same HFD as the current study and a comparable 1.5% leucine supplement in the water for a 14 week duration, another study suggested this same protection from weight gain with leucine supplemented water in male C57BL/6 mice (Zhang et al., 2007). However, our study resulted in no significant decrease in food intake or in body weight as a consequence of BCAA supplementation.
Other studies have suggested a lack of effect of amino acid supplementation. Nairizi and coworkers found no difference in body weight or food consumption as a consequence of 150 mmol/L (1.97%) leucine supplement in the water for 14 weeks in male C57BL/6 mice, and again, the same HFD used in the current study (Nairizi et al., 2009). Similarly, male C57BL/6 mice consuming a diet supplemented with 4.5% leucine did not have different weight or glucose handling response (Noatsch et al., 2011). A similar study also found no change in male mouse food intake or body weight with BCAA supplementation in the water at 1.5 mg/g body weight (D'Antona et al., 2010). These findings are in agreement with our lack of significant differences in body weight and food consumption due to BCAA supplementation.
Both Noatsch et al. and Nairizi et al. found no difference in body composition of male C57BL/6 mice as a consequence of increased leucine intake (Nairizi et al., 2009; Noatsch et al., 2011). On the contrary, Zhang et al reported a decrease in fat mass gained by C57BL/6 males in response to leucine supplementation (Zhang et al., 2007). While the exercise control diet water group did not have significantly increased lean to fat mass ratio over sedentary control diet water in female C57BL/6 mice in the current study, exercise control diet plus BCAAs did. Additionally, while exercise HFD water did not rescue lean to fat mass ratio back to sedentary control diet water levels, exercise HFD BCAA did return lean to fat mass ratio to nearly equivalent of control animals. These two findings are particularly interesting in that they acknowledge that BCAAs do not significantly increase lean to fat mass ratio within the exercise strata, but that they may increase lean to fat mass ratio above sedentary levels where water and exercise alone failed to do so.
Studies similar to the current study report no change in water consumption using BCAA or leucine supplemented water (Nairizi et al., 2009; Zhang et al., 2007). Similarly, a study specifically looking at the impact of various amino acids on water intake in rats reported no change in water consumption when the animals were provided BCAAs (Anderson et al., 1994). Of interest, however, exercising rats given the choice between plain water versus a BCAA enriched solution prefer the latter (Smriga et al., 2006). The impact that the BCAA supplement had on water consumption in the current study is therefore notable. It is possible that the mice enjoyed the taste of the BCAA solution and consumed more for this reason. The mice were not offered both regular water and BCAA separately so we cannot be certain about why the mice drank more of the BCAA supplemented water. We suspect that the increase in water consumption is not likely to be a consequence of kidney damage based on serum creatinine data (not shown). However, investigation of kidney histology and urinary measures would be required to confirm this interpretation. It is also feasible that the increase in amino acid intake resulted in an increased blood urea level, which could trigger an increase in glomerular filtration rate, ultimately resulting in increased water intake to maintain hydration status (Bankir et al., 1996). Further analyses would be required to substantiate any of these suggestions.
Finally, we did observe a significant increase in running distance as a consequence of HFD consumption, and a further significant increase when HFD was combined with the BCAA supplement. This increase in distance ran was a consequence of longer running duration (BCAA supplement and high fat diet both significantly increased run time, though there was no interaction of the two) combined with a faster running speed (BCAA supplement and high fat diet both significantly increased run speed, though again there was no interaction). It has been shown that HFD can cause this increase in running activity (Meek et al., 2010). Also, it has been suggested that BCAA supplementation can decrease the buildup of lactate in the muscle and therefore may decrease muscle fatigue (Koba et al., 2007). Consequently, it is plausible that the mice that received BCAA supplement in the current study ran farther because they did not fatigue as quickly. Additional investigation is warranted to determine whether or not this is true.
Future studies should include different doses of BCAAs, and either BCAA supplemented food or a choice-based water experiment. It would also be interesting to examine the impact that the BCAA supplement may be having on skeletal muscle, if any. As suggested by the body composition data, BCAA supplementation may provide the most benefit in combination with exercise. However, future studies should also focus on alternative forms of exercise, such as resistance training, in combination with BCAA supplementation. This study further supports the idea that exercise is extremely efficacious for ameliorating the weight gain and glucose intolerance induced by HFD, while BCAA supplementation had no impact on these parameters in female C57BL/6 mice.
NOVELTY STATEMENT.
Exercise had a dramatic impact on the weight gain, glucose tolerance, and body composition of female C57BL/6 mice fed a HFD. BCAA supplementation impacted water intake, and, excitingly, improved body composition when combined with exercise when exercise alone did not.
Acknowledgments
K.M.P., H.G.S, and K.J.P. designed research. K.M.P. performed research. K.M.P. and K.J.P. analyzed data. R.J.C. performed statistical analyses. K.M.P., R.J.C., H.G.S., and K.J.P. wrote the manuscript. K.J.P. is the guarantor and takes responsibility for the contents of the article. This study and core services for this study were supported by US NIH grants (National Center for Research Resources, 5P20 RR021954-05, and an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences under grant number 8P20 GM103527-05). Further support was from The National Institute of Diabetes and Digestive and Kidney Diseases, R01 DK090460, and National Institute of Environmental Health Sciences, P42 ES007380. K.M.P. was supported by an NIH training grant (DK07778).
ABBREVIATIONS
- CD
control diet
- HFD
high fat diet
- BCAA
branched-chain amino acid
- SED
sedentary
- EX
exercise
Footnotes
DECLARATION OF INTERESTS
The authors report no conflicts of interest.
REFERENCES
- Anderson GH, Luo S, Trigazis L, Kubis G, Li ET. Effects of essential amino acids on food and water intake of rats. Can J Physiol Pharmacol. 1994;72(8):841–848. doi: 10.1139/y94-119. [DOI] [PubMed] [Google Scholar]
- Arakawa M, Masaki T, Nishimura J, Seike M, Yoshimatsu H. The effects of branched-chain amino acid granules on the accumulation of tissue triglycerides and uncoupling proteins in diet-induced obese mice. Endocr J. 2011;58(3):161–170. doi: 10.1507/endocrj.k10e-221. doi:JST.JSTAGE/endocrj/K10E-221. [DOI] [PubMed] [Google Scholar]
- Areces F, Salinero JJ, Abian-Vicen J, Gonzalez-Millan C, Gallo-Salazar C, Ruiz-Vicente D, Del Coso J. A 7-day oral supplementation with branched-chain amino acids was ineffective to prevent muscle damage during a marathon. Amino Acids. 2014;46(5):1169–1176. doi: 10.1007/s00726-014-1677-3. [DOI] [PubMed] [Google Scholar]
- Bankir L, Bouby N, Trinh-Trang-Tan MM, Ahloulay M, Promeneur D. Direct and indirect cost of urea excretion. Kidney Int. 1996;49(6):1598–1607. doi: 10.1038/ki.1996.232. [DOI] [PubMed] [Google Scholar]
- Bhat G, Baba CS, Pandey A, Kumari N, Choudhuri G. Life style modification improves insulin resistance and liver histology in patients with non-alcoholic fatty liver disease. World J Hepatol. 2012;4(7):209–217. doi: 10.4254/wjh.v4.i7.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomstrand E, Eliasson J, Karlsson HK, Kohnke R. Branched-chain amino acids activate key enzymes in protein synthesis after physical exercise. J Nutr. 2006;136(1 Suppl):269S–273S. doi: 10.1093/jn/136.1.269S. doi:136/1/269S. [DOI] [PubMed] [Google Scholar]
- Boivin GP, Platt KM, Corbett J, Reeves J, Hardy AL, Elenes EY, Pearson KJ. The effects of high-fat diet, branched-chainamino acids and exercise on female C57BL/6 mouse Achilles tendon biomechanical properties. Bone Joint Res. 2013;2(9):186–192. doi: 10.1302/2046-3758.29.2000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley RL, Jeon JY, Liu FF, Maratos-Flier E. Voluntary exercise improves insulin sensitivity and adipose tissue inflammation in diet-induced obese mice. Am J Physiol Endocrinol Metab. 2008;295(3):E586–E594. doi: 10.1152/ajpendo.00309.2007. doi:00309.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JF, Kyser CK. Exercise training in obesity lowers blood pressure independent of weight change. Med Sci Sports Exerc. 2002;34(4):596–601. doi: 10.1097/00005768-200204000-00006. [DOI] [PubMed] [Google Scholar]
- Colberg SR, Albright AL, Blissmer BJ, Braun B, Chasan-Taber L, Fernhall B, Sigal RJ. Exercise and type 2 diabetes: American College of Sports Medicine and the American Diabetes Association: joint position statement. Exercise and type 2 diabetes. Med Sci Sports Exerc. 2010;42(12):2282–2303. doi: 10.1249/MSS.0b013e3181eeb61c. [DOI] [PubMed] [Google Scholar]
- D'Antona G, Ragni M, Cardile A, Tedesco L, Dossena M, Bruttini F, Nisoli E. Branched-chain amino acid supplementation promotes survival and supports cardiac and skeletal muscle mitochondrial biogenesis in middle-aged mice. Cell Metab. 2010;12(4):362–372. doi: 10.1016/j.cmet.2010.08.016. doi:S1550-4131(10)00304-9. [DOI] [PubMed] [Google Scholar]
- de Silva A, Samarasinghe Y, Senanayake D, Lanerolle P. Dietary supplement intake in national-level Sri Lankan athletes. Int J Sport Nutr Exerc Metab. 2010;20(1):15–20. doi: 10.1123/ijsnem.20.1.15. [DOI] [PubMed] [Google Scholar]
- Doi M, Yamaoka I, Nakayama M, Sugahara K, Yoshizawa F. Hypoglycemic effect of isoleucine involves increased muscle glucose uptake and whole body glucose oxidation and decreased hepatic gluconeogenesis. Am J Physiol Endocrinol Metab. 2007;292(6):E1683–E1693. doi: 10.1152/ajpendo.00609.2006. doi:00609.2006. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307(5):491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- Freudenberg A, Petzke KJ, Klaus S. Comparison of high-protein diets and leucine supplementation in the prevention of metabolic syndrome and related disorders in mice. J Nutr Biochem. 2012;23(11):1524–1530. doi: 10.1016/j.jnutbio.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Gardiner P, Kemper KJ, Legedza A, Phillips RS. Factors associated with herb and dietary supplement use by young adults in the United States. BMC Complement Altern Med. 2007;7:39. doi: 10.1186/1472-6882-7-39. doi:1472-6882-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goston JL, Correia MI. Intake of nutritional supplements among people exercising in gyms and influencing factors. Nutrition. 2010;26(6):604–611. doi: 10.1016/j.nut.2009.06.021. [DOI] [PubMed] [Google Scholar]
- Guo K, Yu YH, Hou J, Zhang Y. Chronic leucine supplementation improves glycemic control in etiologically distinct mouse models of obesity and diabetes mellitus. Nutr Metab (Lond) 2010;7:57. doi: 10.1186/1743-7075-7-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafstad AD, Lund J, Hadler-Olsen E, Hoper AC, Larsen TS, Aasum E. High and moderate intensity training normalizes ventricular function and mechanoenergetics in diet-induced obese mice. Diabetes. 2013 doi: 10.2337/db12-1580. doi:db12-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howatson G, Hoad M, Goodall S, Tallent J, Bell PG, French DN. Exercise-induced muscle damage is reduced in resistance-trained males by branched chain amino acids: a randomized, double-blind, placebo controlled study. J Int Soc Sports Nutr. 2012;9(1):20. doi: 10.1186/1550-2783-9-20. doi:1550-2783-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, Li S, Shao M, Qi Q, Zhao F, You J, Liu Y. Calorie restriction and endurance exercise share potent anti-inflammatory function in adipose tissues in ameliorating diet-induced obesity and insulin resistance in mice. Nutr Metab (Lond) 2010;7:59. doi: 10.1186/1743-7075-7-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knechtle B, Mrazek C, Wirth A, Knechtle P, Rust CA, Senn O, Ballmer P. Branched-chain amino acid supplementation during a 100-km ultra-marathon--a randomized controlled trial. J Nutr Sci Vitaminol (Tokyo) 2012;58(1):36–44. [PubMed] [Google Scholar]
- Koba T, Hamada K, Sakurai M, Matsumoto K, Hayase H, Imaizumi K, Mitsuzono R. Branched-chain amino acids supplementation attenuates the accumulation of blood lactate dehydrogenase during distance running. J Sports Med Phys Fitness. 2007;47(3):316–322. [PubMed] [Google Scholar]
- Layman DK, Walker DA. Potential importance of leucine in treatment of obesity and the metabolic syndrome. J Nutr. 2006;136(1 Suppl):319S–323S. doi: 10.1093/jn/136.1.319S. doi:136/1/319S [pii] [DOI] [PubMed] [Google Scholar]
- Leahy DT, Pintauro SJ. Branched-chain amino Acid plus glucose supplement reduces exercise-induced delayed onset muscle soreness in college-age females. ISRN Nutr. 2013;2013:921972. doi: 10.5402/2013/921972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macotela Y, Emanuelli B, Bang AM, Espinoza DO, Boucher J, Beebe K, Kahn CR. Dietary leucine--an environmental modifier of insulin resistance acting on multiple levels of metabolism. PLoS One. 2011;6(6):e21187. doi: 10.1371/journal.pone.0021187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Koba T, Hamada K, Sakurai M, Higuchi T, Miyata H. Branched-chain amino acid supplementation attenuates muscle soreness, muscle damage and inflammation during an intensive training program. J Sports Med Phys Fitness. 2009;49(4):424–431. [PubMed] [Google Scholar]
- Meek TH, Eisenmann JC, Garland T., Jr Western diet increases wheel running in mice selectively bred for high voluntary wheel running. Int J Obes (Lond) 2010;34(6):960–969. doi: 10.1038/ijo.2010.25. [DOI] [PubMed] [Google Scholar]
- Morrison LJ, Gizis F, Shorter B. Prevalent use of dietary supplements among people who exercise at a commercial gym. Int J Sport Nutr Exerc Metab. 2004;14(4):481–492. doi: 10.1123/ijsnem.14.4.481. [DOI] [PubMed] [Google Scholar]
- Mourier A, Bigard AX, de Kerviler E, Roger B, Legrand H, Guezennec CY. Combined effects of caloric restriction and branched-chain amino acid supplementation on body composition and exercise performance in elite wrestlers. Int J Sports Med. 1997;18(1):47–55. doi: 10.1055/s-2007-972594. [DOI] [PubMed] [Google Scholar]
- Nahin RL, Barnes PM, Stussman BJ, Bloom B. Costs of complementary and alternative medicine (CAM) and frequency of visits to CAM practitioners: United States, 2007. Natl Health Stat Report. 2009;(18):1–14. [PubMed] [Google Scholar]
- Nairizi A, She P, Vary TC, Lynch CJ. Leucine supplementation of drinking water does not alter susceptibility to diet-induced obesity in mice. J Nutr. 2009;139(4):715–719. doi: 10.3945/jn.108.100081. doi:jn.108.100081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noatsch A, Petzke KJ, Millrose MK, Klaus S. Body weight and energy homeostasis was not affected in C57BL/6 mice fed high whey protein or leucine-supplemented low-fat diets. Eur J Nutr. 2011;50(6):479–488. doi: 10.1007/s00394-010-0155-2. [DOI] [PubMed] [Google Scholar]
- Pattyn N, Cornelissen VA, Eshghi SR, Vanhees L. The effect of exercise on the cardiovascular risk factors constituting the metabolic syndrome: a meta-analysis of controlled trials. Sports Med. 2013;43(2):121–133. doi: 10.1007/s40279-012-0003-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin LQ, Xun P, Bujnowski D, Daviglus ML, Van Horn L, Stamler J, He K. Higher branched-chain amino acid intake is associated with a lower prevalence of being overweight or obese in middle-aged East Asian and Western adults. J Nutr. 2011;141(2):249–254. doi: 10.3945/jn.110.128520. doi:jn.110.128520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radimer KL, Subar AF, Thompson FE. Nonvitamin, nonmineral dietary supplements: issues and findings from NHANES III. J Am Diet Assoc. 2000;100(4):447–454. doi: 10.1016/S0002-8223(00)00137-1. [DOI] [PubMed] [Google Scholar]
- Schmitz KH, Jacobs DR, Jr, Leon AS, Schreiner PJ, Sternfeld B. Physical activity and body weight: associations over ten years in the CARDIA study. Coronary Artery Risk Development in Young Adults. Int J Obes Relat Metab Disord. 2000;24(11):1475–1487. doi: 10.1038/sj.ijo.0801415. [DOI] [PubMed] [Google Scholar]
- She P, Zhou Y, Zhang Z, Griffin K, Gowda K, Lynch CJ. Disruption of BCAA metabolism in mice impairs exercise metabolism and endurance. J Appl Physiol. 2010;108(4):941–949. doi: 10.1152/japplphysiol.01248.2009. doi:01248.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura Y, Inaguma A, Watanabe S, Yamamoto Y, Muramatsu Y, Bajotto G, Mawatari K. Branched-chain amino acid supplementation before squat exercise and delayed-onset muscle soreness. Int J Sport Nutr Exerc Metab. 2010;20(3):236–244. doi: 10.1123/ijsnem.20.3.236. [DOI] [PubMed] [Google Scholar]
- Smriga M, Kameishi M, Torii K. Exercise-dependent preference for a mixture of branched-chain amino acids and homeostatic control of brain serotonin in exercising rats. J Nutr. 2006;136(2):548S–552S. doi: 10.1093/jn/136.2.548S. doi:136/2/548S. [DOI] [PubMed] [Google Scholar]
- Spillane M, Emerson C, Willoughby DS. The effects of 8 weeks of heavy resistance training and branched-chain amino acid supplementation on body composition and muscle performance. Nutr Health. 2012;21(4):263–273. doi: 10.1177/0260106013510999. [DOI] [PubMed] [Google Scholar]
- Takeshita H, Horiuchi M, Izumo K, Kawaguchi H, Arimura E, Aoyama K, Takeuchi T. Long-term voluntary exercise, representing habitual exercise, lowers visceral fat and alters plasma amino acid levels in mice. Environ Health Prev Med. 2012;17(4):275–284. doi: 10.1007/s12199-011-0249-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsugi T, Yoshida A, Kanda T, Kobayashi I, Kurabayashi M, Tomono S, Nagai R. Oral administration of branched chain amino acids improves virus-induced glucose intolerance in mice. Eur J Pharmacol. 2000;398(3):409–414. doi: 10.1016/s0014-2999(00)00221-1. doi:S0014299900002211. [DOI] [PubMed] [Google Scholar]
- Yan L, DeMars LC, Johnson LK. Long-term voluntary running improves diet-induced adiposity in young adult mice. Nutr Res. 2012;32(6):458–465. doi: 10.1016/j.nutres.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Guo K, LeBlanc RE, Loh D, Schwartz GJ, Yu YH. Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanisms. Diabetes. 2007;56(6):1647–1654. doi: 10.2337/db07-0123. doi:db07-0123. [DOI] [PubMed] [Google Scholar]