Abstract
The paraventricular nucleus of the thalamus (PVT) is not traditionally considered part of the brain addiction neurocircuitry but has received growing attention with regard to a role in the modulation of drug-seeking behavior. This study sought to establish the pattern of neural activation induced by a response-reinstating discriminative stimulus (SD) conditioned to either cocaine (COC) or a conventional reinforcer using a palatable food substance, sweetened condensed milk (SCM). Male Wistar rats were trained to associate one SD (S+; COC or SCM availability) and a distinctly different SD (S−; non-reward; i.e., the availability of saline or the absence of SCM). Following extinction of COC- and SCM-reinforced responding, rats were presented with the respective S+ or S− alone and tested for the reinstatement of reward seeking. The COC S+ and SCM S+ elicited identical reinstatement, whereas the non-reward S− was behaviorally ineffective. PVT sections were obtained following completion of the reinstatement tests and labeled for Fos. The number of Fos+ neurons was compared among rats that were presented with the COC S+, SCM S+, or S−. Rats that were presented with the COC S+ exhibited a significant increase in Fos expression compared with rats that were presented with the S−. Moreover, Fos expression was significantly correlated with the number of reinstatement responses that were induced by the COC S+. In contrast, the SCM S+ and S− produced identical increases in Fos expression, without behaviorally relevant correlations. The findings implicate the PVT as an important site that is selectively recruited during COC-seeking behavior.
Keywords: cocaine, discriminative stimulus, reinstatement
INTRODUCTION
Drug addiction is a chronic relapsing disorder that is characterized by compulsive drug seeking and use (Leshner, 1997; McLellan et al., 2000; O’Brien et al., 1998; O’Brien and McLellan, 1996). Major advances have been made in elucidating the neurocircuitry that mediates drug craving and seeking. Compelling evidence suggests that these behaviors are controlled by interconnected cortical and limbic brain regions, including the medial prefrontal cortex, basolateral amygdala, hippocampus, nucleus accumbens (NAC), ventral tegmental area, and dorsal striatum (Belin and Everitt, 2008; Cardinal et al., 2002; Everitt et al., 2001; Goldstein and Volkow, 2002; Ito et al., 2002; Kalivas and Volkow, 2005; See, 2002; Weiss, 2005).
Recently, the paraventricular nucleus of the thalamus (PVT) has received attention as a brain site that participates in the regulation of drug-seeking behavior. Anatomically, the PVT is the target of numerous hypothalamic neuropeptidergic projections, with a major role in energy homeostasis (Freedman and Cassell, 1994; Otake, 2005). The PVT plays a role not only in energy homeostasis but also in arousal, endocrine regulation, and reward (Bhatnagar and Dallman, 1998; Kelley et al., 2005; Parsons et al., 2006; Van der Werf et al., 2002). The PVT receives a major orexin/hypocretin (Orx/Hcrt) projection from the lateral hypothalamus/perifornical hypothalamus (LH/PFA; Kirouac et al., 2005; Parsons et al., 2006). The PVT has been proposed to be a key relay station that gates Orx/Hcrt-coded reward-related communication between the LH/PFA and both the ventral and dorsal striatum (Kelley et al., 2005). The PVT also receives major glutamatergic projections from prelimbic and infralimbic cortical areas that may transmit information about emotional states (Heidbreder and Groenewegen, 2003; Kesner and Churchwell, 2011; Li and Kirouac, 2012).
Although the PVT has not traditionally been thought of as part of the addiction neurocircuitry, its connectivity, particularly with the hypothalamic Orx/Hcrt system and medial prefrontal cortex (mPFC), suggests a role in the motivational effects of drugs of abuse. Several recent findings support this hypothesis. The PVT is activated by the presentation of cocaine (COC)-paired cues (Brown et al., 1992), and PVT inactivation by tetrodotoxin prevents COC priming-induced reinstatement (James et al., 2010). The PVT is also the target of hypothalamic Orx/Hcrt projections that are activated by alcohol-related contextual cues. More specifically, rats that were exposed to contextual stimuli that were associated with alcohol availability exhibited a greater number of Fos+ hypothalamic Orx/Hcrt neurons than rats that were exposed to stimuli that were previously paired with non-reward. In the same rats, alcohol-related stimuli increased the number of Fos+ neurons in the PVT, neurons that have been shown to be closely associated with Orx/Hcrt afferents (Dayas et al., 2008). A role for the PVT in alcohol-seeking has also received direct support from findings that the context-induced reinstatement of alcoholic beer seeking is associated with activation of the PVT-ventral striatum pathway, which, if prevented by excitotoxic lesions of the PVT (Hamlin et al., 2009) or local injection of a κ opioid receptor agonist (Marchant et al., 2010), eliminates the context-induced reinstatement of alcohol seeking. Overall, these findings suggest that the PVT participates in mediating associations between drugs of abuse and drug-related environmental stimuli and their behavioral consequences.
The goal of the present study was to advance our understanding of the role of the PVT in COC-seeking behavior, with the parallel objective of establishing the role of the PVT in the motivating effects of a highly potent food reinforcer relative to its role in COC seeking. This objective was considered relevant because the hypothalamic-PVT-striatal axis has been implicated in feeding behavior and the creation of energy reserves (e.g., Kelley et al., 2005). Quantitative Fos immunolabeling was used to determine neuronal activation of the PVT that is produced by the presentation of COC-related stimuli compared with stimuli that are conditioned to sweetened condensed milk (SCM) using an animal model of relapse.
MATERIALS AND METHODS
Rats
Forty-six male Wistar rats (Charles River, Wilmington, MA, USA), weighing 200–225 g upon arrival, were housed two per cage in a temperature- and humidity-controlled vivarium on a reverse 12 h/12 h light/dark cycle with ad libitum access to food and water. All of the procedures were conducted in strict adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute.
Self-administration, conditioning, and extinction
Behavioral training and testing were conducted as previously described (Martin-Fardon et al., 2009; Martin-Fardon et al., 2007). Rats that were designated for COC self-administration were surgically prepared with indwelling silastic catheters that were inserted in the right jugular vein. Rats that were designated for testing with SCM were not subjected to surgical procedures. Following 7 days of post-surgery recovery, the rats began self-administration training. Each session was initiated by the extension of two retractable levers into the operant conditioning chamber. The self-administration of COC (0.25 mg/0.1 ml infusion; delivered over 4 s) or SCM (2:1 [v/v] in water; 0.1 ml delivered into a 0.2 ml receptacle) began on a fixed-ratio 1 (FR1) schedule of reinforcement in daily 120-min (COC) or 40-min (SCM) sessions, 5 days per week. Responses at the right, active lever were reinforced, followed by a 20-s timeout (20 s TO) period that was signaled by illumination of a cue light above the active lever. During this time, the lever remained inactive to prevent accidental overdosing. To maintain identical training and experimental conditions, the signaled TO period was also implemented during SCM self-administration. Responses at the left, inactive lever remained without programmed consequences. Following 2 weeks of COC or SCM self-administration training, a contingency was introduced whereby responses at the active lever were differentially reinforced in the presence of discriminative stimuli (SD) that signaled reward availability (S+) vs. non-availability (S−). A constant 70 dB white noise served as the S+ for COC and SCM, whereas illumination of a 2.8 W house light located at the top of the chamber’s front panel served as the S− that signaled non-availability of the reinforcer (i.e., saline solution instead of COC and no consequence instead of SCM). Each session was initiated by presentation of the respective SD and extension of the levers. The SD remained present until termination of the session by retraction of the levers. In the presence of the S+, responses at the right, active lever were reinforced by COC or SCM on an FR1 schedule, followed by the 20 s TO period that was signaled by illumination of a cue light above the lever. In the presence of the S−, responses at the right, active lever were followed by an intermittent tone, during which the lever remained inactive for 20 s. Three daily sessions (each lasting 1 h for the COC group and 20 min for the SCM group), separated by 30 min intervals, were conducted, with two S+ (reward) sessions and one S− (non-reward) session sequenced in random order. The SCM sessions were restricted to 20 min to avoid satiety by the excessive ingestion of SCM and ensure that the number of responses were comparable during the first and second S+ sessions (Martin-Fardon et al., 2009; Martin-Fardon et al., 2007). After 8 training days (i.e., a total of 16 S+ and eight S− sessions), both the COC and SCM groups were placed on extinction (EXT) conditions in daily 30-min sessions. Each extinction session was initiated by extension of the active and inactive levers in the absence of either SD. During extinction sessions, responses at the lever remained non-reinforced. The extinction criterion was less than five responses per session for 3 consecutive days.
Conditioned reinstatement
Reinstatement tests were conducted under extinction conditions but with reintroduction of the SD, such that the COC and SCM groups were further divided into S+ and S− test groups (four groups total, two for COC and two for SCM). The reinstatement tests lasted 30 min, after which the animals were returned to the vivarium for an additional hour (corresponding to 90 min after the beginning of the behavioral test). Four rats were lost in the COC group (two because of health complications and two that failed to acquire COC self-administration), reducing the number of animals to n = 18 for COC (9 for S+ and 9 for S−), n = 16 for SCM (8 for S+ and 8 for S−), and n = 8 for naive.
Immunocytochemistry
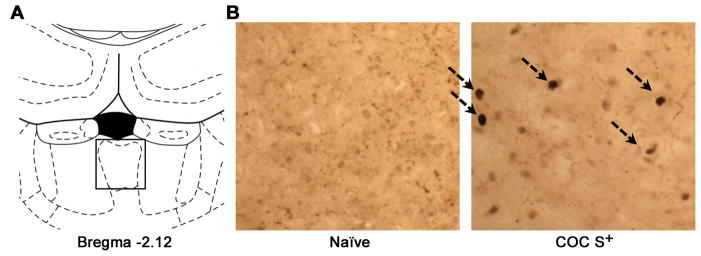
One hour after the reinstatement session, the rats were deeply anesthetized and transcardially perfused with cold 4% paraformaldehyde in 0.1 mM sodium tetraborate, pH 9.5. Brains were removed, postfixed in 4% paraformaldehyde overnight, and stored in 30% (w/v) sucrose, 0.1% (w/v) sodium azide, and KPBS solution. The brains were coronally sectioned in a one-in-six series of adjacent 40 μm sections on a cryostat at −20ºC and collected in strict anatomical order. The sections were then processed for Fos immunodetection. Briefly, one series of coronal sections were blocked for 1 h using 3% bovine serum albumin (BSA)/1% Triton-X/phosphate-buffered saline (PBS), followed by incubation for 48 h at 4ºC with anti-Fos (1:20000, sc-52, Santa Cruz Biotechnology, Santa Cruz, CA, USA). The tissue sections were then incubated with biotinylated secondary donkey anti-rabbit antibody for 2 h (1:300, 711-065-152, Jackson Immuno-Research, West Grove, PA, USA), followed by ABC-Elite (Vector Laboratories). Nuclear Fos immunostaining was visualized using DAB-Ni. Fos+ PVT neurons (typical range: −1.8 and −3.3 from bregma; (Paxinos, 1997); Fig. 1) were counted manually. For immunocytochemistry, an age-matched naive group of rats was prepared (never exposed to the behavioral chambers), and the brain tissues were processed as above for the other animals.
Fig. 1.
(A) Schematic illustration of the rostrocaudal level at which Fos+ cells were counted in the paraventricular nucleus of the thalamus (PVT) within the defined square. (B) Typical photomicrographs that illustrate Fos activation in the PVT induced by the COC S+ (right) vs. naive (left).
Statistical analysis
Conditioned reinstatement data and the percentage of naive Fos+ cells were analyzed using two-way analysis of variance (ANOVA), with group (COC and SCM) and reinstatement test (behavior following S+ or S− presentation) as independent factors. Raw Fos data were analyzed using between-subjects one-way ANOVA. Significant main effects or interactions in the ANOVA were followed by Bonferroni post hoc tests. Differences in responding at the active lever between the respective reward and non-reward conditions during the last day of the training/conditioning phase were analyzed using paired t-tests. Pearson’s r correlation coefficients were determined to establish the linear dependence between the number of operant responses during the reinstatement tests and the number of Fos+ cells.
RESULTS
Self-administration, conditioning, and extinction
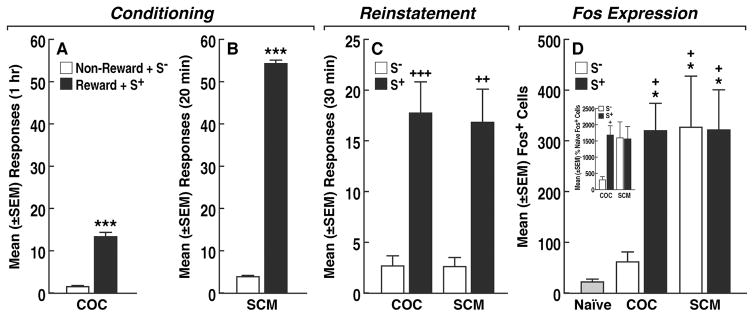
Rats in both the COC (n = 18) and SCM (n = 16) groups acquired robust and stable self-administration, with only negligible responding during non-reward sessions (paired t-test: COC, t17 = 9.8, p < 0.0001; SCM, t15 = 78.8, p < 0.0001; Fig. 2A, B). The number of days to reach the extinction criterion (≤ 5 responses) was similar for both COC and SCM animals (12 ± 3 days and 13 ± 5 days, respectively).
Fig. 2.
(A, B) Differences in responding between S+ and S− sessions at the end of the conditioning phase for COC and SCM, respectively. ***p < 0.001, vs. respective S−. (C) Following conditioning and after extinction, presentation of the COC S+ or SCM S+ (but not the non-reward S−) produced robust reinstatement. +++p < 0.001, ++p < 0.01, vs. respective S−. (D) Fos+ cells in the PVT following SD presentation. +p < 0.05, vs. COC S−; *p < 0.05, vs. naive. (Inset) Percentage of naive Fos+ cells. +p < 0.05, vs. COC S−.
Conditioned reinstatement
Following extinction, presentation of the COC S+ or SCM S+ (Fig. 2C) elicited statistically identical recovery of responding, whereas responding in the presence of the non-reward S− remained at extinction levels (two-way ANOVA: COC vs. SCM, F1,30 = 0.05, p = 0.80; S+ vs. S−, F1,30 = 37.7, p < 0.0001; group [COC vs. SCM] × reinstatement test (S+ vs. S−) interaction, F1,30 = 0.03, p = 0.87), which was confirmed by the Bonferroni post hoc test (p < 0.001, vs. COC S−; p < 0.01, vs. SCM S−; Fig. 2C).
Effect of cocaine and SCM cues on Fos immunoreactivity
In COC animals, only rats that were presented with the COC S+ exhibited a significant increase in Fos expression in the PVT compared with the non-reward S− and naive control group (one-way ANOVA: F4,37 = 6.5, p = 0.0005; Bonferroni: p < 0.05, vs. COC S−; p < 0.05, vs. naive; Fig. 2D). In contrast, both the SCM S+ and non-reward S− produced similar increases in Fos expression compared with naive animals (Bonferroni: p < 0.05; Fig. 2D). Transformation of the data into percent changes relative to the naive group confirmed that the COC S+ selectively increased Fos expression (p < 0.05, vs. COC S−; two-way ANOVA followed by Bonferroni: COC vs. SCM, F1,30 = 3.50, p = 0.07; S+ vs. S−, F1,30 = 3.77, p = 0.06; group [COC vs. SCM] × reinstatement test [S+ vs. S−] interaction, F1,30 = 4.15, p = 0.05; Fig. 2D inset).
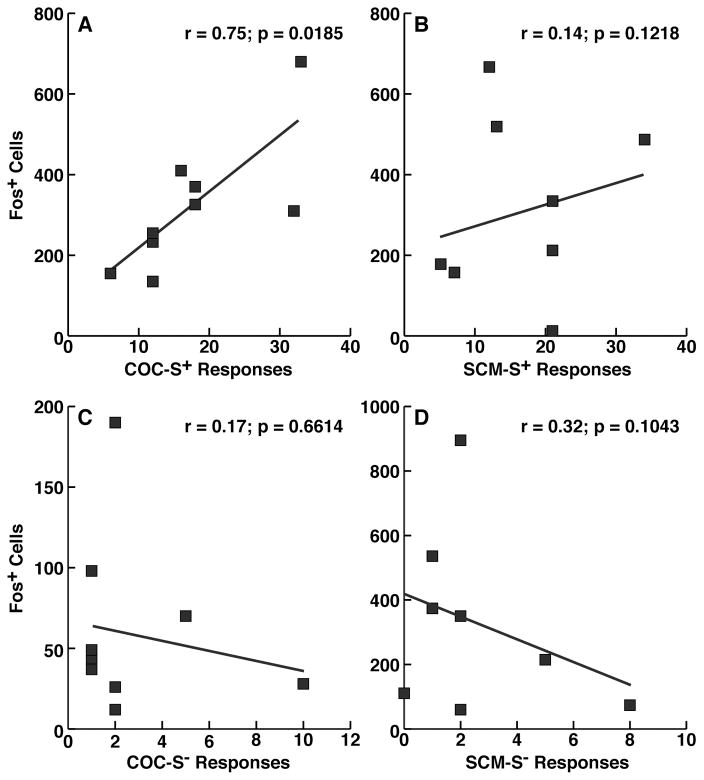
Correlational analysis revealed a significant relationship between the number of Fos+ cells and number of reinstatement responses in rats that were presented with the COC S+ (r = 0.75, p = 0.0185; Fig. 3A) but not in rats that were presented with the SCM S+ (Fig. 3B). No correlations were found between the number of Fos-expressing neurons and COC S− or SCM S− responses (Fig. 3C, D).
Fig. 3.
Correlation between S+- and S−-induced behavior and Fos expression in the PVT. The figure shows correlation plots between the number of reinstatement responses and number of Fos+ cells, confirming behaviorally correlated recruitment of the PVT during S+-induced reinstatement of cocaine seeking (A) but not SCM seeking (B). No correlations were found between Fos-expressing neurons and COC S− or SCM S− responses (C, D).
DISCUSSION
The results confirmed that response-reinstating exposure to a COC-predictive stimulus (S+) is associated with significant neural activation of the PVT and extend similar previous findings (Brown et al., 1992) by establishing that this effect is positively correlated with the number of COC-seeking responses induced by the COC cue. Presentation of the stimulus that was predictive of potent food reward (SCM S+) elicited reinstatement that was statistically identical to reinstatement produced by the COC S+ but induced only nonspecific Fos expression that was not correlated with behavior and similar to the effects of the stimulus that was predictive of non-reward (SCM S−). These findings implicate the PVT as an important site that regulates the control of COC seeking induced by environmental stimuli but not behavior controlled by stimuli conditioned to a highly palatable food reward.
One may argue that the differences observed between the COC and SCM groups on PVT activation may be attributable to procedural differences. Oral availability of SCM provided olfactory cues during self-administration, possibly adding a salient stimulus dimension to the S+ context not present during intravenous self-administration of COC. The absence of this stimulus dimension during reinstatement testing may have resulted in a weaker reinstatement compared to COC seeking, however, the data and previous findings do not support this interpretation. The magnitude of the responses induced by the COC S+ and SCM S+ during the reinstatement tests was statistically identical, suggesting that reliable and comparable conditioning occurred for SCM and COC as also documented in several earlier studies (Baptista et al., 2004; Martin-Fardon et al., 2009; Martin-Fardon et al., 2007).
Activation (i.e., increased Fos expression) of the PVT showed a strong positive correlation with the degree of COC seeking and therefore is indicative of a role of neuronal activation in COC-seeking behavior. This interpretation is consistent with previous findings that showed a positive correlation between morphine preference scores and Fos expression in the anterior cingulate, basolateral amygdala, and ventral lateral bed nucleus of the stria terminalis (Harris and Aston-Jones, 2003) as well as a correlation between the activation of hypothalamic Orx/Hcrt neurons and reinstatement of morphine-induced conditioned place preference (Harris et al., 2005).
The response-reinstating actions of the SCM S+ were associated with significant activation of the PVT, similar to the effects of the COC S+. The PVT has been implicated in the regulation of feeding (Kelley et al., 2005) and is a critical site for the mediation of reward-based feeding (Choi et al., 2012). Moreover, the expectation of a highly palatable natural reward (e.g., chocolate) has been shown to produce neuronal activation in the PVT (Choi et al., 2010). This effect is consistent with the present findings of PVT activation by the SCM-predictive stimulus (S+). However, activation of the PVT by the SCM S+ was not correlated with the reinstatement of SCM seeking, and the SCM S− that did not elicit reinstatement produced the same degree of PVT activation as the SCM S+. These observations argue against a role for the PVT in behavior controlled by stimuli that are conditioned to highly palatable food reward. However, because the PVT is activated by the expectation to receive highly palatable food, one possibility for the comparable activation of the PVT by both the SCM S+ and SCM S− is that the operant chamber itself, as part of the stimulus environment associated with the availability of highly palatable food, is a sufficiently strong stimulus to elicit neuronal activation. Another possible explanation for the equivalent activation of the PVT by the SCM reward-predictive and non-predictive stimuli is that these effects reflect nonspecific arousal rather than direct responses to the stimuli. However, if this were the case, then one would expect that the COC S− would also induce neural activation related to nonspecific arousal, which did not occur.
Another hypothesis to explain the similar Fos expression induced by the SCM S+ and SCM S− could be that the SCM S+ and SCM S− activated a similar number of neurons, but from distinct neuronal populations of which only one mediates active SCM seeking. This hypothesis could be confirmed in the future by selectively inactivating neurons that are activated by the SCM S− using the Daun02 method (Koya et al., 2009) and assessing whether reinstatement induced by the SCM S+ is conserved in subsequent tests. Another hypothesis to explain the strong neural activation by the SCM S− is that increased Fos expression reflects active response inhibition mediated by a neural population distinct from that activated by the S+. However, this hypothesis is difficult to reconcile with the lack of neural activation produced by the COC S−. Thus, the reason for the equivalent activation of the PVT by the SCM S+ and SCM S− remains presently unclear and sharply contrasts the selective and behaviorally correlated PVT activation by the COC S+.
A possible explanation for why a significant correlation between Fos expression and conditioned reinstatement occurred only in the COC group and not in the SCM group is that during self-administration, COC altered PVT neurotransmission. Supporting this hypothesis, as yet unpublished data from this laboratory show that intra-PVT Orx-A/Hcrt-1 injections exert a priming-like effect (i.e., reinstatement of both COC and SCM seeking), but with a vastly different dose-response profile, characterized by substantially stronger reinstatement of COC vs. SCM seeking at moderate doses (Matzeu, 2013). Although the data do not permit definitive conclusions about the dissociation between the sole effects of the COC S+ on Fos expression vs. the effects of COC-seeking behavior itself on inducing Fos expression in the PVT, the present findings support the existing data on the importance of the PVT in drug-seeking behavior.
The behaviorally correlated increase in Fos immunoreactivity induced by the COC S+ supports the hypothesis that the PVT plays a specific role in COC-seeking behavior. This finding also suggests that the PVT is an integral part of the brain addiction neurocircuitry. In fact, the PVT connects with brain structures that mediate craving and COC seeking, such as the mPFC, NAC, and amygdala (Chen and Su, 1990; Moga et al., 1995; Vertes and Hoover, 2008). The PVT has been shown to receive very dense inputs from the mPFC (Li and Kirouac, 2012), suggesting that these connections may modulate the expression of COC-seeking behavior (Di Ciano et al., 2007; Di Pietro et al., 2006). The PVT also sends glutamatergic projections to the NAC. Stimulation of this projection increases glutamate levels in the NAC (Jones et al., 1989; Parsons et al., 2007), an effect thought to be linked to COC-seeking behavior (Cornish and Kalivas, 2000). It has also been shown that the context-induced reinstatement of alcoholic beer seeking is associated with PVT-NAC pathway recruitment (Hamlin et al., 2009). Thus, growing evidence supports a role for PVT projections to the NAC in the regulation of drug-seeking behavior.
In conclusion, the results show that the PVT is selectively recruited during COC-seeking behavior, reflected by a positive correlation between COC-seeking behavior and PVT activation. However, a “selective” role of the PVT in COC seeking will require verification by direct experimental manipulations of the PVT, such as transient inactivation or selective inactivation of neuronal ensembles that are activated by COC and SCM cues using the Daun02 inactivation method to confirm that non-functionality of the PVT prevents COC- but not SCM-seeking behavior.
Acknowledgments
This is publication number 25038 from The Scripps Research Institute. Research support: NIH/NIDA DA033344 (R.M.F.), DA08467, and DA07348 (F.W.). We thank E. Strong and B. Leos for technical assistance and M. Arends for assistance with preparation of the manuscript.
Footnotes
Author contributions
A.M., F.W., and R.M.F. participated in the study concept and design. A.M., G.C., T.M.K., and R.M.F. performed the experiments and statistical analyses, interpreted the findings, and drafted the manuscript. All of the authors critically reviewed the content of the manuscript and approved the final version for publication.
References
- Baptista MA, Martin-Fardon R, Weiss F. Preferential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on conditioned reinstatement versus primary reinforcement: comparison between cocaine and a potent conventional reinforcer. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:4723–4727. doi: 10.1523/JNEUROSCI.0176-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57:432–441. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Dallman M. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience. 1998;84:1025–1039. doi: 10.1016/s0306-4522(97)00577-0. [DOI] [PubMed] [Google Scholar]
- Brown EE, Robertson GS, Fibiger HC. Evidence for conditional neuronal activation following exposure to a cocaine-paired environment: role of forebrain limbic structures. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1992;12:4112–4121. doi: 10.1523/JNEUROSCI.12-10-04112.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Chen S, Su HS. Afferent connections of the thalamic paraventricular and parataenial nuclei in the rat--a retrograde tracing study with iontophoretic application of Fluoro-Gold. Brain research. 1990;522:1–6. doi: 10.1016/0006-8993(90)91570-7. [DOI] [PubMed] [Google Scholar]
- Choi DL, Davis JF, Fitzgerald ME, Benoit SC. The role of orexin-A in food motivation, reward-based feeding behavior and food-induced neuronal activation in rats. Neuroscience. 2010;167:11–20. doi: 10.1016/j.neuroscience.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Choi DL, Davis JF, Magrisso IJ, Fitzgerald ME, Lipton JW, Benoit SC. Orexin signaling in the paraventricular thalamic nucleus modulates mesolimbic dopamine and hedonic feeding in the rat. Neuroscience. 2012;210:243–248. doi: 10.1016/j.neuroscience.2012.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:RC89. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayas CV, McGranahan TM, Martin-Fardon R, Weiss F. Stimuli linked to ethanol availability activate hypothalamic CART and orexin neurons in a reinstatement model of relapse. Biol Psychiatry. 2008;63:152–157. doi: 10.1016/j.biopsych.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Benham-Hermetz J, Fogg AP, Osborne GE. Role of the prelimbic cortex in the acquisition, re-acquisition or persistence of responding for a drug-paired conditioned reinforcer. Neuroscience. 2007;150:291–298. doi: 10.1016/j.neuroscience.2007.09.016. [DOI] [PubMed] [Google Scholar]
- Di Pietro NC, Black YD, Kantak KM. Context-dependent prefrontal cortex regulation of cocaine self-administration and reinstatement behaviors in rats. The European journal of neuroscience. 2006;24:3285–3298. doi: 10.1111/j.1460-9568.2006.05193.x. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Res Brain Res Rev. 2001;36:129–138. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- Freedman LJ, Cassell MD. Relationship of thalamic basal forebrain projection neurons to the peptidergic innervation of the midline thalamus. J Comp Neurol. 1994;348:321–342. doi: 10.1002/cne.903480302. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin AS, Clemens KJ, Choi EA, McNally GP. Paraventricular thalamus mediates context-induced reinstatement (renewal) of extinguished reward seeking. Eur J Neurosci. 2009;29:802–812. doi: 10.1111/j.1460-9568.2009.06623.x. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Enhanced morphine preference following prolonged abstinence: association with increased Fos expression in the extended amygdala. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2003;28:292–299. doi: 10.1038/sj.npp.1300037. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Groenewegen HJ. The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neuroscience and biobehavioral reviews. 2003;27:555–579. doi: 10.1016/j.neubiorev.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Robbins TW, Everitt BJ. Dopamine release in the dorsal striatum during cocaine-seeking behavior under the control of a drug-associated cue. J Neurosci. 2002;22:6247–6253. doi: 10.1523/JNEUROSCI.22-14-06247.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, Charnley JL, Jones E, Levi EM, Yeoh JW, Flynn JR, Smith DW, Dayas CV. Cocaine- and amphetamine-regulated transcript (CART) signaling within the paraventricular thalamus modulates cocaine-seeking behaviour. PLoS One. 2010;5:e12980. doi: 10.1371/journal.pone.0012980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MW, Kilpatrick IC, Phillipson OT. Regulation of dopamine function in the nucleus accumbens of the rat by the thalamic paraventricular nucleus and adjacent midline nuclei. Experimental brain research. 1989;76:572–580. doi: 10.1007/BF00248914. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE. A proposed hypothalamic-thalamic-striatal axis for the integration of energy balance, arousal, and food reward. J Comp Neurol. 2005;493:72–85. doi: 10.1002/cne.20769. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Churchwell JC. An analysis of rat prefrontal cortex in mediating executive function. Neurobiology of learning and memory. 2011;96:417–431. doi: 10.1016/j.nlm.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Kirouac GJ, Parsons MP, Li S. Orexin (hypocretin) innervation of the paraventricular nucleus of the thalamus. Brain Res. 2005;1059:179–188. doi: 10.1016/j.brainres.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Koya E, Golden SA, Harvey BK, Guez-Barber DH, Berkow A, Simmons DE, Bossert JM, Nair SG, Uejima JL, Marin MT, Mitchell TB, Farquhar D, Ghosh SC, Mattson BJ, Hope BT. Targeted disruption of cocaine-activated nucleus accumbens neurons prevents context-specific sensitization. Nature neuroscience. 2009;12:1069–1073. doi: 10.1038/nn.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshner AI. Addiction is a brain disease, and it matters. Science. 1997;278:45–47. doi: 10.1126/science.278.5335.45. [DOI] [PubMed] [Google Scholar]
- Li S, Kirouac GJ. Sources of inputs to the anterior and posterior aspects of the paraventricular nucleus of the thalamus. Brain structure & function. 2012;217:257–273. doi: 10.1007/s00429-011-0360-7. [DOI] [PubMed] [Google Scholar]
- Marchant NJ, Furlong TM, McNally GP. Medial dorsal hypothalamus mediates the inhibition of reward seeking after extinction. J Neurosci. 2010;30:14102–14115. doi: 10.1523/JNEUROSCI.4079-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fardon R, Baptista MA, Dayas CV, Weiss F. Dissociation of the effects of MTEP [3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]piperidine] on conditioned reinstatement and reinforcement: comparison between cocaine and a conventional reinforcer. The Journal of pharmacology and experimental therapeutics. 2009;329:1084–1090. doi: 10.1124/jpet.109.151357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fardon R, Maurice T, Aujla H, Bowen WD, Weiss F. Differential effects of sigma1 receptor blockade on self-administration and conditioned reinstatement motivated by cocaine vs natural reward. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2007;32:1967–1973. doi: 10.1038/sj.npp.1301323. [DOI] [PubMed] [Google Scholar]
- Matzeu A, Kerr T, Weiss F, Martin-Fardon R. Orexin/hypocretin in the paraventricular nucleus of the thalamus mediates cocaine-seeking behavior in rats. Program No. 350.19. 2013 Neuroscience Meeting Planner; San Diego, CA: Society for Neuroscience 2013; 2013. Online. [Google Scholar]
- McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA : the journal of the American Medical Association. 2000;284:1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- Moga MM, Weis RP, Moore RY. Efferent projections of the paraventricular thalamic nucleus in the rat. The Journal of comparative neurology. 1995;359:221–238. doi: 10.1002/cne.903590204. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion? Journal of psychopharmacology. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, McLellan AT. Myths about the treatment of addiction. Lancet. 1996;347:237–240. doi: 10.1016/s0140-6736(96)90409-2. [DOI] [PubMed] [Google Scholar]
- Otake K. Cholecystokinin and substance P immunoreactive projections to the paraventricular thalamic nucleus in the rat. Neurosci Res. 2005;51:383–394. doi: 10.1016/j.neures.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Parsons MP, Li S, Kirouac GJ. The paraventricular nucleus of the thalamus as an interface between the orexin and CART peptides and the shell of the nucleus accumbens. Synapse. 2006;59:480–490. doi: 10.1002/syn.20264. [DOI] [PubMed] [Google Scholar]
- Parsons MP, Li S, Kirouac GJ. Functional and anatomical connection between the paraventricular nucleus of the thalamus and dopamine fibers of the nucleus accumbens. The Journal of comparative neurology. 2007;500:1050–1063. doi: 10.1002/cne.21224. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 1997. [DOI] [PubMed] [Google Scholar]
- See RE. Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacol Biochem Behav. 2002;71:517–529. doi: 10.1016/s0091-3057(01)00682-7. [DOI] [PubMed] [Google Scholar]
- Van der Werf YD, Witter MP, Groenewegen HJ. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Brain Res Rev. 2002;39:107–140. doi: 10.1016/s0165-0173(02)00181-9. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB. Projections of the paraventricular and paratenial nuclei of the dorsal midline thalamus in the rat. The Journal of comparative neurology. 2008;508:212–237. doi: 10.1002/cne.21679. [DOI] [PubMed] [Google Scholar]
- Weiss F. Neurobiology of craving, conditioned reward and relapse. Curr Opin Pharmacol. 2005;5:9–19. doi: 10.1016/j.coph.2004.11.001. [DOI] [PubMed] [Google Scholar]