Abstract
Purpose
To describe longitudinal changes in lens thickness in myopic children in the Correction of Myopia Evaluation Trial (COMET) and to investigate the association between these changes and myopia progression.
Methods
Four-hundred sixty-nine 6 to <12-year-old children with −1.25 to −4.50 D of myopia were enrolled in COMET, a clinical trial comparing single vision lenses (SVLs) versus progressive addition lenses (PALs) for slowing myopia. Children remained in their original lenses for 5 years and then could wear contact lenses, SVLs or PALs. Myopia by cycloplegic autorefraction (Nidek ARK 700A) and ocular components, including lens thickness, by A-scan ultrasound (Sonomed A2500) were measured annually over 11 years. Analyses of lens thickness were based on right eye data from 426 children with refractions fit with Gompertz functions. Longitudinal lens thickness measurements for each participant were fit with a third-degree polynomial function, and average polynomial functions were calculated for three groups of children previously identified based on Gompertz functions: 6–7 years at baseline (n = 40), ≥8 years with progressing myopia (n = 329), and ≥8 years with non-progressing myopia (n = 56). ANOVAs were used for comparing the lens curve-based parameters among the three groups. Associations between lens and Gompertz parameters were assessed using Pearson correlations.
Results
Overall, between 6 and 18 years the lenses thinned and then thickened, with the minimum value of 3.37 ± 0.15mm reached at 11.56 ± 2.04 years. The minimum lens thickness did not differ among the three myopia groups (p = 0.09), nor was it correlated with the amount of myopia at lens minimum or amount of final myopia (r's = −0.01 and −0.03, respectively, p's>0.05).
Conclusion
As a similar pattern of change in lens thickness with age was found in all children, whether their myopia progressed or not, these results suggest that the association of lens thinning and thickening with the course of myopia is coincidental rather than causal.
Keywords: Children's vision, lens, myopia
INTRODUCTION
It is well-documented that the lens in the adult eye gets thicker over time, adding approximately 20 microns per year from ages 18–75 years.1–5 Changes in lens thickness with age have also been reported in children, with the lens thinning up to the age of approximately 10–12 years and then thickening after that age.6–8 Early work was done by Larsen, who used ultrasonography to measure lens thickness in 80 newborns and 846 children aged 6 months to 18 years.6 These cross-sectional data showed that much of the decrease in lens thickness occurred in the first year and a half, with smaller additional decreases until 11–13 years.
It has been suggested that the pattern of lens thinning in children is related to myopia onset and progression. In a series of papers, Mutti, Zadnik and colleagues showed that lens thinning and thickening differed by the type of refractive error.9–13 The lens in hyperopes and emmetropes thinned until ~10 years of age and then thickened after that. In contrast, in myopic children the lens thinned between 6 and 10 years of age and stayed thin until at least 15 years of age (the oldest age tested), with myopes having the thinnest lenses of all refractive groups.
Two recent studies showed a pattern of thinning followed by thickening of the lens in children in all refractive groups.7,8 In 6- to 10-year-old Singaporean children with at least three study visits over 5 years, the lens showed a U-shaped growth curve, with a minimum thickness at approximately 9 years of age in hyperopes and emmetropes, and at 10 years in myopes.7 Children with myopia at all visits had the thinnest lenses. Analysis of cross-sectional data from Taiwanese children showed that the lens became thinner from 7 to 11 years of age and then subsequently thickened in all refractive groups, with myopic eyes having the thinnest lenses.8
Different theories have been proposed to explain lens thinning and its possible association with refractive errors in children. The mechanical tension hypothesis proposed by Mutti and colleagues suggests that the lens thinning found during childhood is produced by mechanical tension, conducted to the edges of the lens from the ciliary muscle via the suspensory zonules.9,10,14 In this theory, the failure of coordination of the ocular components to maintain emmetropia occurs because lens thinning reaches a physical limit and then is no longer able to sustain coordination with the increases in axial length. An alternative hypothesis for myopia development suggests that during near work, children with large lags of accommodation experience hyperopic defocus that, similar to animal models, can stimulate the emmetropization mechanism to increase axial elongation.15,16
A recent randomized trial, the Study of Theories about Myopia Progression (STAMP), tested these two hypotheses.14 Children who were already myopic (−0.75 to −4.50 D) and had large lags of accommodation were randomized to either single vision lenses (SVLs) or progressive addition lenses (PALs) for 1 year, and then required to wear SVLs in the second year of the study.14 The conclusion of the paper was that the lack of a rebound effect in the PAL group after switching to SVLs was “consistent with the accommodative lag theory of hyperopic defocus causing myopia progression” and not consistent with the mechanical tension theory involving the lens and ciliary body.
Another theory to explain lens thinning in childhood is based on nuclear compaction.17–20 Early work by Brown and colleagues showed that the nucleus of the lens in children with lamellar cataracts thinned during infancy, and further work by the same group led to the suggestion that nuclear compaction and cortical growth were balanced such that lens thickness did not change in childhood.17,18 More recently, based on an analysis of data from predominately myopic children in Singapore, Iribarren et al. suggested that a greater rate of nuclear compaction compared with slower cortical growth produced the lens thinning seen between birth and 10 years.20 They also found “limited evidence for active regulation of the changes in the lens in relation to axial elongation and refractive development”.20
A further test of a possible active role for the lens in myopia after onset is whether there are systematic changes in lens thickness that are related to myopia progression and stabilization. None of the above studies followed individual myopic children for a sufficiently long time, through the period of rapid progression and eventual stabilization of myopia, to determine what happens to the lens during these phases. The present study was undertaken to investigate changes in lens thickness in children who participated in the Correction of Myopia Evaluation Trial (COMET) and the association of these changes with myopia progression and stabilization.
MATERIALS AND METHODS
Overview
Details of the study design and demographic characteristics of the study population have been presented previously21–23 and are briefly summarized here. Four clinical centers located at schools/colleges of optometry in Birmingham, Alabama; Boston, Massachusetts; Houston, Texas; and Philadelphia, Pennsylvania enrolled 469 children between September 1997 and September 1998, and followed them up to 14 years. Children enrolled in COMET met specific inclusion criteria: age 6 to <12 years old, spherical equivalent between −1.25 and −4.50 D in each eye, astigmatism ≤1.50 D in either eye, anisometropia ≤1.00 D SER, birth weight ≥1250 g, and visual acuity with distance correction of 0.20 LogMAR (20/32) or better. Children agreed to wear their randomly assigned COMET glasses (PALs: Varilux comfort with + 2.0 D add or SVLs) during all waking hours.
The children remained in their original lens assignments for 5 years at which point the clinical trial phase ended and COMET became an observational study of factors associated with myopia progression and stabilization. During this period, the children, in consultation with their study optometrist, were allowed to choose to wear contact lenses, SVLs or PALs. Switching to contact lenses had little impact on progression.24
The COMET study and protocols conformed to the tenets of the Declaration of Helsinki. The institutional review boards of each participating center approved the research protocols. Informed consent (parents) and assent (children) were obtained after verbal and written explanation of the nature and possible consequences of the study. When children turned 18 years old, they were re-consented as adults.
Procedures
Cycloplegic autorefraction (Nidek ARK 700A) was used to measure progression of myopia, the primary outcome measure in COMET. However, non-cycloplegic autorefraction measurements were used in the Gompertz curve fits described below because they were taken every 6 months in contrast to the cycloplegic autorefraction which was done annually. Comparison of non-cycloplegic and cycloplegic measures showed mean (SD) differences of 0.19 (0.22) D at baseline21 and 0.23 (0.27) D on average throughout the 11-year follow-up period reported here,25 with non-cycloplegic measurements more myopic than cycloplegic ones. Following cycloplegic autorefraction, ocular components (anterior chamber depth, lens thickness, vitreous chamber depth, and overall axial length) were measured by Sonomed A-2500 ultrasonography. Lens thickness is presented as the average of three to five reliable measurements.
Myopia Curve Fitting (Gompertz Function)
To evaluate the course of myopia for each participant, the spherical equivalent refractions at each visit were fit with a double-exponential Gompertz function that was used to estimate the age and amount of myopia at stabilization and the age at which the slowing of myopia progression was at its maximum (the second inflection point of the Gompertz function).25 Right eye data were fit to individual Gompertz functions in participants with at least 6 years of follow-up and at least seven refraction measurements over 11 years. Four-hundred twenty-six of the 469 participants had valid curve fits. As reported previously,25 the participants were classified into three categories: (1) children who were 6–7 years of age at baseline, (2) children ≥8 years of age at baseline with progressing myopia and (3) children ≥8 years old at baseline with myopia that did not progress.
Lens Thickness Curve Fitting (Polynomial Function)
The observed longitudinal lens thickness values from the COMET cohort demonstrated a general pattern of lens thinning in the early school years until reaching a nadir at a specific age, after which the lens began to thicken. Different modeling approaches were evaluated to determine which one provided the best fit for this pattern of changes in the lens with age. Although the Gompertz function, which has a pattern of rapid acceleration followed by slowing and stabilization, was used to model the course of myopia, this function could not be applied to the longitudinal lens data with its pattern of thinning and thickening. Therefore, the approaches that were evaluated included a linear model,10 2nd degree polynomial modeling and 3rd degree polynomial functions. Two fitting indices, Akaike's Information Criterion and Bayesian Information Criterion, were used to compare the quality of the model fits for the three different modeling approaches. The 3rd degree polynomial function was selected as the modeling approach for these analyses because it showed the best fitting indices in 99% (422/426) of the participants' curves. For each subject, the 3rd degree polynomial function was specified as follows:
where age is the actual age (in decimal years) for each subject, LT represents the lens thickness value in mm at each age, and a, b, c, d are polynomial coefficients to be estimated. Polynomial coefficients were estimated using the “MIXED” procedure in SAS version 9.2 (SAS institute, Cary, NC) and within-subject correlations were adjusted using the autoregressive model. Two curve-based parameters, (1) minimum lens thickness value (a local minimum) and (2) age at minimum lens thickness value, were calculated based on estimated individual polynomial coefficients using the first derivative of the 3rd degree polynomial function: b+2c * age + 3d * age2. After the age at minimum lens thickness was identified, the corresponding level of myopia at minimum lens thickness was calculated using the fitted Gompertz function described above. For children ≥8 years old at baseline with myopia that did not progress, their baseline myopia was used for the amount of myopia at minimum lens thickness.
To ensure comparability of lens fits for participants of all ages, the lens curves were truncated at the age of 18 years, the maximum age with data for the youngest participants. Of the 426 lens curves, only five were affected by the truncation, i.e. their ages at minimum lens thickness were older than 18 years. However, their ages and lens thickness values at lens minimum with truncation at 18 years did not differ significantly from their values using all available data (p>0.50), so their truncated values were used for data analysis.
Following the completion of the curve fitting for each participant's longitudinal lens values, lens curve-based parameters were summarized across all curves using means and standard deviations. Group curves then were estimated for each of the three Gompertz fit myopia groups (children 6–7 years of age at baseline, children ≥8 years of age at baseline with progressing myopia, and children ≥8 years old at baseline with myopia that did not progress) by averaging the polynomial coefficients from all participants' curves. An overall comparison of the three subgroup curves was performed using the MANOVA model to compare the four polynomial coefficients which control the shape of the curves. Summary statistics were also calculated for baseline characteristic categories (e.g. gender, ethnicity and treatment groups). In addition, ANOVA models were used for overall and pair-wise comparisons of the lens curve-based parameters between the three myopia subgroups and baseline characteristic categories. Associations between the lens curve-based parameters (e.g. age at minimum lens thickness and minimum lens thickness values) and the Gompertz curve-based parameters (e.g. age at peak deceleration, age at stabilization, and the amount of myopia at stabilization) were assessed using Pearson correlations. For all of the above analyses, p values less than 0.05 were considered statistically significant. P values based on the ANOVA pair-wise comparisons between Gompertz fit subgroups and baseline characteristic categories were adjusted by the Bonferroni correction.
Definition of Minimal or No Change in Lens Thickness
During the curve fitting process, a few participants were observed to have minimal or no change in lens thickness during follow-up. To establish a definition of no change in lens thickness, measurement variability was evaluated using cross-sectional and longitudinal analyses. First, within-subject variability was considered at baseline26 and at each of the 11 follow-up visits. At baseline, the average within-subject variability was 0.06 mm based on five repeated measures of lens thickness.26 At each of the 11 follow-up visits, within-subject variability of the five repeated lens measures was calculated for each participant. The variability at each visit ranged from 0.03 to 0.05 mm. Second, for each participant, longitudinal within-subject variability was calculated as the standard deviation of the longitudinal curve-based change in lens thickness from baseline age to 18 years old, resulting in an average longitudinal within-subject variability across all participants of 0.07 mm. Third, between-subject variability of the maximum changes in lens thickness over time was determined using the maximum curve-based change in lens thickness for each participant (maximum minus minimum lens thickness values) between the baseline age and 18 years and calculating the standard deviation of the maximum changes across all participants, also resulting in a standard deviation of the maximum changes of 0.07 mm. Based on these measurement variability results, to be conservative, a cutoff point of 0.06 mm or less was selected to classify participants as “no change” or “change” in lens thickness. For the 14% (61/426) of participants classified as “no change” in lens thickness, their baseline values were used for their minimum lens thickness, similar to what was done previously to define myopia for the group with no progression.25
RESULTS
Baseline characteristics of all participants (n = 426) with Gompertz curve fits and lens thickness data, whether there was a change in thickness or not, are presented in Table 1. The distribution of ethnicity in the three Gompertz curve fit groups is significantly different (p = 0.007), with more African-Americans in the group having no myopia progression after baseline, as reported previously.25 The gender distribution did not vary by group (p = 0.46).
TABLE 1.
Characteristics of COMET participants with Gompertz curve fits (N= 426).
| Age at baseline |
||||
|---|---|---|---|---|
| ≥8 Years |
||||
| Overall (N= 426) | 6–7 Years progressing myopia post baseline (group A) (N=40a) | Progressing myopia post baseline (group B) (N= 329) | No myopia progression post baseline (group C) (N= 56) | |
| Ethnicityb | n (%) | n (%) | n (%) | n (%) |
| African American | 112 (26.3) | 11 (27.5) | 72 (21.9) | 28 (50.0) |
| Asian | 33 (7.8) | 2 (5.0) | 29 (8.8) | 2 (3.6) |
| Hispanic | 62 (14.6) | 6 (15.0) | 49 (14.9) | 7 (12.5) |
| Mixed race | 21 (4.9) | 2 (5.0) | 18 (5.5) | 1 (1.8) |
| White | 198 (46.5) | 19 (47.5) | 161 (48.9) | 18 (32.1) |
| Genderb | ||||
| Male | 198 (46.5) | 15 (37.5) | 157 (47.7) | 25 (44.6) |
| Female | 228 (53.5) | 25 (62.5) | 172 (52.3) | 31 (55.4) |
| Refractive errorc | Mean (SD) | |||
| Baseline | −2.39 (0.83) | −2.37 (0.78) | −2.40 (0.85) | −2.52 (0.72) |
| Final | −5.37 (2.01) | −7.35 (2.29) | −5.54 (1.74) | −3.02 (1.72) |
Excludes one participant without progression after baseline.
p Values based on chi-square tests comparing proportions for ethnicity and gender across the three myopia groups are 0.007 and 0.46, respectively.
p Values based on ANOVA for comparing baseline and final refractive error among three myopia groups are 0.58 and <0.0001, respectively.
Table 2 presents comparisons at baseline and at lens minimum. Overall, the lens reached a mean minimum value of 3.37 ± 0.15mm at 11.56 ± 2.04 years. As shown in the table, the mean baseline lens thickness in the 6–7 year olds (3.50 mm) was significantly greater than in either of the other two groups (≥8 years old with progressing myopia and ≥8 years old with myopia that did not progress), in which both had a mean baseline lens thickness of 3.40 mm (overall p = 0.0009). The age at which the minimum lens thickness occurred was significantly different across the three groups (p = 0.007), with the 6–7 year olds younger than the other two groups when the lens reached its minimum thickness. However, the values of the minimum lens thickness were similar across the three groups and not significantly different from each other (p = 0.09). The 6–7 year group, which was shown previously to have a faster rate of myopia progression (and an earlier age of myopia stabilization with more myopia) than the other groups,25 had significantly more myopia at the lens minimum (mean ± SD: −4.55D± 1.82) compared to the other two groups: −3.26D± 1.36 for the group ≥8 years at baseline with progressing myopia and −2.52D± 0.72 for the group ≥8 years at baseline with myopia that did not progress, p<0.0001. The largest difference in the amount of myopia at the minimum lens thickness was between the younger group and the older group with no progression (approximately 2.0 D, p<0.0001). The other pairwise comparisons were also statistically significant, as shown in Table 2. However, there were no significant differences in any of these values by ethnicity, gender or treatment group.
TABLE 2.
Baseline values and values at minimum lens thickness (age, lens thickness, myopia) by baseline characteristics (N= 426).
| Baseline values |
Values at minimum lens thickness |
||||
|---|---|---|---|---|---|
| Lens thickness (mm) | Age (years)a | Lens thicknessa (mm) | Myopia (D) | ||
| N | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| Overall | 426 | 3.41 (0.16) | 11.56 (2.04) | 3.37 (0.15) | −3.28 (1.42) |
| Baseline age and progression status group | |||||
| 6–7 Years progressing myopia (group A) | 40b | 3.50 (0.17) | 10.69 (2.64) | 3.42 (0.17) | −4.55 (1.82) |
| ≥8 Years progressing myopia (group B) | 329 | 3.40 (0.16) | 11.58 (1.92) | 3.36 (0.15) | −3.26 (1.36) |
| ≥8 Years no myopia progression (group C) | 56 | 3.40 (0.18) | 11.97 (1.90) | 3.37 (0.17) | −2.52 (0.72) |
| p Valuec (overall) | 0.0009 | 0.007 | 0.09 | <0.0001 | |
| Pair-wise comparisonsd | |||||
| A versus B: mean difference (SE) | 0.10 (0.03) | −0.89 (0.33) | 0.06 (0.03) | −1.29 (0.23) | |
| p Value | 0.0002 d | 0.008 | 0.03 | <0.0001d | |
| A versus C: mean difference (SE) | 0.10 (0.03) | −1.28 (0.41) | 0.05 (0.03) | −2.03 (0.28) | |
| p Value | 0.004 | 0.002 d | 0.14 | <0.0001d | |
| B versus C: mean difference (SE) | 0.00 (0.02) | −0.39 (0.29) | −0.01 (0.02) | −0.74 (0.19) | |
| p Value | 0.90 | 0.18 | 0.70 | 0.0002 d | |
| Ethnicity | |||||
| African American | 112 | 3.40 (0.17) | 11.67 (2.09) | 3.37 (0.17) | −3.32 (1.26) |
| Asian | 33 | 3.40 (0.14) | 11.68 (2.27) | 3.35 (0.11) | −3.76 (1.68) |
| Hispanic | 62 | 3.44 (0.17) | 11.56 (1.69) | 3.39 (0.15) | −3.15 (1.49) |
| Mixed | 21 | 3.38 (0.13) | 11.65 (1.67) | 3.34 (0.13) | −3.51 (1.71) |
| White | 198 | 3.41 (0.16) | 11.48 (2.11) | 3.36 (0.16) | −3.20 (1.41) |
| p Valuec (overall) | 0.55 | 0.94 | 0.58 | 0.23 | |
| Gender | |||||
| Male | 198 | 3.40 (0.17) | 11.57 (2.04) | 3.35 (0.16) | −3.34 (1.41) |
| Female | 228 | 3.42 (0.16) | 11.55 (2.04) | 3.38 (0.15) | −3.22 (1.45) |
| p Valuee | 0.20 | 0.91 | 0.12 | 0.38 | |
| Treatment Group | |||||
| PVL | 211 | 3.42 (0.15) | 11.48 (1.95) | 3.38 (0.14) | −3.26 (1.39) |
| SVL | 215 | 3.40 (0.18) | 11.64 (2.12) | 3.36 (0.16) | −3.31 (1.46) |
| p Valuee | 0.23 | 0.42 | 0.17 | 0.69 | |
For the 61 participants without lens thickness changes, curve-based baseline values were used for minimum lens thickness values.
Excludes one participant without progression after baseline.
Main effects were tested based on ANOVA models comparing the above categories (e.g. three myopia groups, and ethnicity groups). Bolded p values are significant (p<0.05).
Pairwise comparisons were tested based on post-hoc t-tests from ANOVA models. Bolded p values remained significant after the Bonferroni adjustment for multiple comparisons (the significance level is p<0.05/15 = 0.003).
Based on two sample t-tests.
Additional analyses showed that the minimum lens thickness in each child was not correlated with the amount of myopia at the lens minimum or the amount of final myopia (r =−0.01 and −0.03, respectively, both p values >0.05).
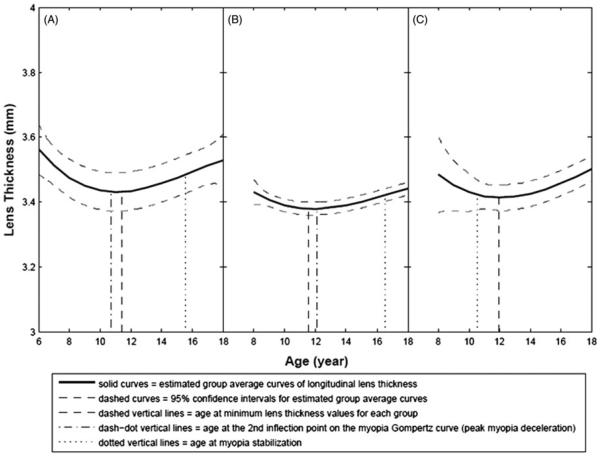
Lens thickness by age for the three myopia groups is shown graphically in Figure 1. In all three groups, the lenses thinned and then thickened, with the minimum overall value observed at a mean ± SD age of 11.56 ± 2.04 years. For the first two curves shown in Figure 1(A and B), the mean age at lens minimum is within a year of the age of the second inflection point of the Gompertz function fit to the myopia progression data. As described previously,27 the second inflection is the age at which myopia progression slows most strongly, suggesting the presence of biological influences that foreshadow myopia cessation. As the third curve shown in Figure 1(C) presents lens thickness in eyes with myopia that did not progress, it has an earlier age of myopia stabilization compared to the other two, and does not show an age for a second inflection point, which is related to progression. For myopia progression curves with a second inflection point (i.e. those shown in Figure 1A and B), there was a low but significant correlation (r = 0.18, p = 0.0004) between the age at minimum lens thickness and age at the second inflection point.
FIGURE 1.
Estimated average curves for longitudinal lens thickness for three myopia groups. (A) 6–7 Years old at baseline, (B) 8 to <12 years old at baseline with myopia progression and (C) 8 to <12 years old at baseline with no myopia progression. Note that age at the 2nd inflection point is not presented for the 8+ year olds with no myopia progression because without progression there is no 2nd inflection point.
Overall, the annual thickening rate for the lens is 0.012 mm/year from 11.5 to 18 years of age. For the three myopia groups, the rates are 0.015 mm/year, 0.012 mm/year and 0.011 mm/year, respectively, with no significant difference between the groups.
Eighty-six percent of the group had a change in lens thickness over the observation period, while 14% did not, using 0.06 mm or less to classify participants as “no change”. When analyses were done based only on the subgroup with a change in lens thickness, results were the same as those based on all available data presented above. There was a significant difference in the amount of myopia progression from baseline to stabilization between these two groups: the mean (±SD) myopia progression in participants with no change in lens thickness was −2.00 ± 1.73 D, which was significantly less than the mean myopia progression of −2.54 ± 1.81 D in the group with a change in lens thickness (p = 0.03).
DISCUSSION
The main result from the analysis of changes in lens thickness over the course of 11 years of myopia progression and stabilization in the COMET cohort was that the pattern of lens thinning followed by thickening was the same whether myopia progressed or not. For the overall cohort, the lens reached a minimum thickness at an average age of 11.56 years and then subsequently thickened. Additional analyses showed that the minimum lens thickness in each child was not correlated with the amount of myopia at the lens minimum or the amount of final myopia. These results suggest that the change in lens thickness is not related to the course of myopia, but instead varies with age.
The pattern of lens thinning followed by thickening found in the eyes of the COMET cohort with a range of myopia from low to high (final spherical equivalent from −1.8 to −13.6 D) agrees with data from two studies of Chinese children having a range of refractive errors from hyperopia to myopia. The data from Wong et al. showed a two phase process in the growth of the lens in all refractive groups, with the lens thinning in the early school years, reaching a minimum thickness at 10 years in myopes, and getting thicker after that age.7 However, that study only followed children until the age of 12 years. Using cross-sectional data, Shih et al. also showed that the lens thinned (until about the age of 11 years in myopes) and then grew thicker, but the lack of longitudinal data was a limitation.8 A common finding is that the eyes of myopic children first showed a pattern of lens thinning, with the lens reaching its thinnest value between 10 and 11.5 years of age, and then grew thicker up to the age of 18 years, after which it is known that the lens continues to thicken from 18 to 75 years.1–5
A different pattern in myopic eyes was reported in a series of papers by Mutti, Zadnik and colleagues: the lens in myopic children thinned between 6 and 10 years of age, with little change in the subsequent 5 years,9–13 lending support for the mechanical tension theory of myopia. However, the current data and those from the Study of Theories about Myopia Progression do not support this theory.14 Possible reasons for the different results in these studies include the use of different methods for measuring and analyzing lens thickness, as well as shorter lengths of follow-up in all studies prior to COMET, resulting in datasets with limited data from each participant.
Other evidence from the COMET cohort for the lack of an association between myopia progression and change in lens thickness is found in the fact that eyes with lenses that maintained the same thickness over the course of the study (14% of the group) had myopia that progressed an average of 2.00 D from baseline to stabilization. These data agree with those from a study of 7–9-year-old myopic children in Singapore followed for 3 years in which myopia progression was not associated with the rate of change in lens thickness.28 An additional piece of evidence comes from data collected by the COMET study group from a sample of 204 young adult non-myopes matched by age, gender and ethnicity to the COMET myopic cohort in year 12 of the study (unpublished data). The mean lens thickness (3.57 ± 0.20 mm) in these non-myopes at a mean age of 21.4 years did not differ significantly from the lens thickness of 3.54 ± 0.19 mm in the COMET group at the same mean age (p = 0.17), even though one group included only non-myopes and the other only myopes with data at that age (n = 361). All these results, taken together, suggest that age is a more relevant factor than refractive error in changes in lens thickness.
The data from human eyes showing that changes in lens thickness are not related to myopia progression are similar to what has been reported in animal models of eye growth. A review paper on the role of the lens in refractive development concluded that “its participation is non-existent or minimal at best”.29 This agrees with the finding of no association between refractive error and lens thickness in 210 rhesus monkeys,30 although it should be noted that most of the monkeys were younger than the children tested in the current study even when age was adjusted for the difference between monkey and human years. Another caveat is that most of the experiments using animals lasted for relatively short periods of time and changes in the lens may take longer to appear.31 Therefore, while the current results and most of the animal data suggest a developmental process for changes in the lens, a possible environmental influence cannot be ruled out.
CONCLUSIONS
In conclusion, the pattern of change in lens thickness appears to vary with age and does not appear to be related to the course of myopia. As a similar lens pattern was found in children with myopia that progressed and in those with myopia that did not progress over 11 years of follow-up, the lens thinning/thickening that occurs during myopia progression may be coincidental rather than causal.
Acknowledgments
This work was supported by NEI/NIH grants EY11740, 11805, 11756, 11754, 11752, and 11755.
Footnotes
DECLARATION OF INTEREST The authors report no commercial relationships or conflicts of interest.
REFERENCES
- 1.Augusteyn RC. On the growth and internal structure of the human lens. Exp Eye Res. 2010;90:643–654. doi: 10.1016/j.exer.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dubbelman M, van der Heijde GL, Weeber HA. The thickness of the aging human lens obtained from corrected Scheimpflug images. Optom Vis Sci. 2001;78:411–416. doi: 10.1097/00006324-200106000-00013. [DOI] [PubMed] [Google Scholar]
- 3.Koretz JE, Strenk SA, Strenk LM, Semmlow JL. Scheimpflug and high-resolution magnetic resonance imaging of the anterior segment: a comparative study. J Opt Soc Am A. 2004;21:346–354. doi: 10.1364/josaa.21.000346. [DOI] [PubMed] [Google Scholar]
- 4.Doyle L, Little JA, Saunders KJ. Repeatability of OCT lens thickness measures with age and accommodation. Optom Vis Sci. 2013;90:1396–1405. doi: 10.1097/OPX.0000000000000080. [DOI] [PubMed] [Google Scholar]
- 5.Richdale K, Sinnott LT, Bullimore MA, Wassenaar PA, Schmalbrock P, Kao CY, et al. Quantification of age-related and per diopter accommodative changes of the lens and ciliary muscle in the emmetropic human eye. Invest Ophthalmol Vis Sci. 2013;54:1095–1105. doi: 10.1167/iovs.12-10619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larsen JS. The sagittal growth of the eye. II: Ultrasonic measurement of the axial diameter of the lens and the anterior segment from birth to puberty. Acta Ophthalmol. 1971;49:427–440. doi: 10.1111/j.1755-3768.1971.tb00968.x. [DOI] [PubMed] [Google Scholar]
- 7.Wong HB, Machin D, Tan SB, Wong TY, Saw SM. Ocular component growth curves among Singaporean children with different refractive error status. Invest Ophthalmol Vis Sci. 2010;51:1341–1347. doi: 10.1167/iovs.09-3431. [DOI] [PubMed] [Google Scholar]
- 8.Shih YF, Chiang TH, Lin LL. Lens thickness changes among schoolchildren in Taiwan. Invest Ophthalmol Vis Sci. 2009;50:2637–2644. doi: 10.1167/iovs.08-3090. [DOI] [PubMed] [Google Scholar]
- 9.Mutti DO, Zadnik K, Fusaro RE, Friedman NE, Sholtz RI, Adams AJ. Optical and structural development of the crystalline lens in childhood. Invest Ophthalmol Vis Sci. 1998;39:120–133. [PubMed] [Google Scholar]
- 10.Mutti DO, Mitchell GL, Sinnot LT, Jones-Jordan LA, Moeschberger ML, Cotter SA, et al. Corneal and crystalline lens dimensions before and after myopia onset. Optom Vis Sci. 2012;89:251–262. doi: 10.1097/OPX.0b013e3182418213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones LA, Mitchell GL, Mutti DO, Hayes JR, Moeschberger ML, Zadnik K. Comparison of ocular component growth curves among refractive error groups in children. Invest Ophthalmol Vis Sci. 2005;46:2317–2327. doi: 10.1167/iovs.04-0945. [DOI] [PubMed] [Google Scholar]
- 12.Zadnik K, Mutti DO, Fusaro RE, Adams AJ. Longitudinal evidence of crystalline lens thinning in children. Invest Ophthalmol Vis Sci. 1995;36:1581–1587. [PubMed] [Google Scholar]
- 13.Zadnik K, Manny RE, Yu JA, Mitchell GL, Cotter SA, Quiralte JC, et al. Ocular component data in school children as a function of age and gender. Optom Vis Sci. 2002;80:226–236. doi: 10.1097/00006324-200303000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Berntsen D, Sinnott LT, Mutti DO, Zadnik K. A randomized trial using progressive addition lenses to evaluate theories of myopia progression in children with a high lag of accommodation. Invest Ophthalmol Vis Sci. 2012;53:640–649. doi: 10.1167/iovs.11-7769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goss DA. Clinical accommodation and heterophoria findings preceding juvenile onset of myopia. Optom Vis Sci. 1991;68:110–116. doi: 10.1097/00006324-199102000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Gwiazda J, Thorn F, Bauer J, Held R. Myopic children show insufficient accommodative response to blur. Invest Ophthalmol Vis Sci. 1993;34:690–694. [PubMed] [Google Scholar]
- 17.Brown N, Sparrow JM, Bron AJ. Central compaction in the process of lens growth as indicated by lamellar cataract. Br J Ophthalmol. 1988;72:538–544. doi: 10.1136/bjo.72.7.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown N, Bron AJ. Lens disorders: a clinical manual of cataract diagnosis. Butterworth-Heineman; Oxford, UK: 1996. Chapter 3. Lens growth; pp. 17–24. [Google Scholar]
- 19.Cook CA, Koretz JF, Pfahnl A, Hyun J, Kaufman P. Aging of the human crystalline lens and anterior segment. Vision Res. 1994;34:2945–2954. doi: 10.1016/0042-6989(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 20.Iribarren R, Morgan I, Chan Y, Lin X, Saw SM. Changes in lens power in Singapore Chinese children during refractive development. Invest Ophthalmol Vis Sci. 2012;53:5124–5130. doi: 10.1167/iovs.12-9637. [DOI] [PubMed] [Google Scholar]
- 21.Gwiazda J, Marsh-Tootle WL, Hyman L, Hussein M, Norton TT. Baseline refractive and ocular component measures of children enrolled in the correction of myopia evaluation trial (COMET) Invest Ophthalmol Vis Sci. 2002;43:314–321. [PubMed] [Google Scholar]
- 22.Gwiazda J, Hyman L, Hussein M, Everett D, Norton TT, Kurtz D, et al. A randomized clinical trial of progressive addition lenses versus single vision lenses on the progression of myopia in children. Invest Ophthalmol Vis Sci. 2003;44:1492–1500. doi: 10.1167/iovs.02-0816. [DOI] [PubMed] [Google Scholar]
- 23.Hyman L, Gwiazda J, Marsh-Tootle WL, Norton TT, Hussein M. Control The Correction of Myopia Evaluation Trial (COMET): design and general baseline characteristics. Clin Trials. 2001;22:573–592. doi: 10.1016/s0197-2456(01)00156-8. [DOI] [PubMed] [Google Scholar]
- 24.Marsh-Tootle WL, Dong LM, Hyman L, Gwiazda J, Weise KK, Dias L, et al. Myopia progression in children wearing spectacles vs. switching to contact lenses. Optom Vis Sci. 2009;86:741–747. doi: 10.1097/OPX.0b013e3181a6a250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.COMET Group Myopia stabilization and associated factors among participants in the Correction of Myopia Evaluation Trial (COMET) Invest Ophthalmol Vis Sci. 2013;54:7871–7884. doi: 10.1167/iovs.13-12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurtz D, Manny R, Hussein M, COMET Study Group Variability of the ocular component measurements in children using A-scan ultrasonography. Optom Vis Sci. 2004;81:35–43. doi: 10.1097/00006324-200401000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Thorn F, Gwiazda J, Held R. Myopia progression is specified by a double exponential growth function. Optom Vis Sci. 2005;82:286–297. doi: 10.1097/01.opx.0000159370.66540.34. [DOI] [PubMed] [Google Scholar]
- 28.Saw SM, Chua WH, Gazzard G, Koh D, Tan D, Stone R. Eye growth changes in myopic children in Singapore. Br J Ophthalmol. 2005;89:1489–1484. doi: 10.1136/bjo.2005.071118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sivak JG. The role of the lens in refractive development of the eye: animal models of ametropia. Exp Eye Res. 2008;87:3–8. doi: 10.1016/j.exer.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Qiao-Grider Y, Hung LF, Kee CS, Ramamirtham R, Smith EL., III Nature of the refractive errors in rhesus monkeys (Macaca mulatta) with experimentally induced ametropias. Vision Res. 2010;50:1867–1881. doi: 10.1016/j.visres.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen Y, Belkin M, Yehezkel O, Solomon A, Polat U. Dependency between light intensity and refractive development under light-dark cycles. Exp Eye Res. 2011;92:40–46. doi: 10.1016/j.exer.2010.10.012. [DOI] [PubMed] [Google Scholar]