Abstract
Background
HIV-infected patients who fail to normalize CD4 T cells despite suppressive antiretroviral therapy have impaired immune homeostasis: diminished naïve T cell numbers, elevated T cell turnover, senescence and inflammation.
Methods
Blood samples from immune failures (n=60), immune successes (n=20) and healthy controls (n=20) were examined for plasma IL-7 levels, for cellular expression of the IL-7Rα chain (CD127), for the exhaustion and senescence markers PD-1 and CD57, and for the survival factor Bcl2. As both inflammatory and homeostatic cytokines can induce T cell cycling, we also examined the effects of these mediators on exhaustion and senescence markers.
Results
Plasma levels of IL-7 were elevated and both CD4 and CD8 T cell CD127 expression was decreased in immune failure. Plasma levels of IL-7 correlated directly with naïve CD4 T cell counts in immune success and inversely with T cell cycling (Ki67) in healthy controls and immune success, but not in immune failure. CD4 T cell density of PD-1 was increased and Bcl2+ CD4 T cells were decreased in immune failure but not in immune success, while the proportion of T cells expressing CD57 was increased in immune failure. PD-1 and CD57 were induced on CD4 but not CD8 T cells by stimulation in vitro with inflammatory (IL-1β) or homeostatic (IL-7) cytokines.
Conclusions
Perturbation of the IL-7/IL-7 receptor axis, increased T cell turnover, and increased senescence may reflect dysregulated responses to both homeostatic and inflammatory cytokines in immune failure patients.
Introduction
Despite an increased survival , as many as 25% of HIV-infected patients with therapy-controlled viremia fail to recover CD4 T cell counts to “normal” levels [1-4]. Patients with low circulating CD4 T cell counts are at increased risk for cardiovascular disease, cancer, and liver disease [5, 6]. In earlier work, we and others found that these immune failure patients had decreased numbers of both CD4 and CD8 naïve T cells, increased activation of CD4 and CD8 T cells as measured by HLADR and CD38 expression, increased cycling of CD4 memory T cells as measured by Ki67 expression, and elevated plasma indices of inflammation and coagulation[7-10]. Immune failure patients may also have perturbations of immune homeostasis as T cell expression of the IL-7 receptor alpha chain (CD127) is diminished [11-13], and there is increased T cell expression of the immune senescence and exhaustion markers, CD57 and PD-1 [14-17]. Recovery of CD4 T cells may be impaired at many stages; naïve T cells may be less responsive to homeostatic signals, mature T cells may display increased turnover or increased sensitivity to death signals and more matured T cells may show signs of exhaustion and senescence. This study was designed to simultaneously explore aspects of CD4 T cell recovery, relating immune senescence, immune homeostasis, and inflammation in a well characterized group of treated patients. We found that markers of immune exhaustion (PD-1) and senescence (CD57) were elevated on T cells from immune failure patients and could be up-regulated when PBMCs from healthy subjects were stimulated with the homeostatic cytokine IL-7 or with the inflammatory cytokine IL-1β that we had shown earlier can drive CD4 T cell turnover [18]. Despite elevated plasma levels of IL-7 in immune failure, there was no association between IL-7 levels and T cell cycling or CD4 T cell numbers in immune failure. In contrast, plasma IL-7 levels correlated directly with naïve CD4 T cell numbers in immune success and inversely with CD4 T cell cycling in immune success and in healthy controls but not in immune failure. These findings support a model where homeostatic failure allows inflammatory mediators to drive cellular turnover in immune failure and both inflammation and homeostatic proliferation contribute to cellular exhaustion/senescence.
Methods
Patients
These studies were approved by the institutional review board (IRB) at University Hospitals/Case Medical Center and the Cleveland Clinic Foundation and all patients provided written informed consent in accordance with the Declaration of Helsinki. The Cleveland Immune Failure (CLIF) study examined immunologic indices in healthy controls and two groups of patients who had been receiving antiretroviral therapy for at least 2 years with plasma HIV RNA levels below detection using routine clinical assays; typically less than 50 copies/mL. Immune failure patients had CD4 T cells <350/uL and immune success patients had CD4 T cells >500/uL [7].
Cytokines
Plasma levels of IL-7 were measured by high sensitivity IL-7 ELISA (Quantikine HS, R&D Systems, Minneapolis, MN).
Cell preparation
Peripheral blood mononuclear cells (PBMCs) were prepared from whole blood by ficoll-hypaque density sedimentation and were cultured in RPMI medium supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin, and 1% L-glutamine at 37°C and 5% CO2, for functional assays or cryopreserved in 10% DMSO and 90% fetal bovine serum until thawing for phenotypic analysis. Induction of PD-1 and CD57 after stimulation with IL-6, IL-1β, (R&D Systems, Minneapolis, MN), or IL-7 (Cytheris, Issy les Moulineaux, France) was examined on freshly isolated PBMCs from healthy uninfected controls.
CFSE dye dilution
Cell division was assessed by labeling PBMCs with 5(6)-carboxyfluorescein diacetate, succinimidyl ester (CFSE) (Molecular Probes Invitrogen, Grand Island, NY) for 10 minutes at 37° C. Staining was quenched by the addition of FBS for 5 minutes on ice. Cells were then washed and cultured as described.
Flow cytometry
Viable cells were gated using live/dead- yellow viability dye (Invitrogen, Grand Island, NY). Lymphocytes were identified by forward and side scatter and T cell phenotype was assessed using the following fluorochrome-conjugated monoclonal antibodies: anti-CD3 peridinin chlorophyll protein (Percp), anti-CD8 allophycocyanin-cy7 (APC-Cy7), anti-CD127 phycoerythrin (PE), anti-CD45RA phycoerythrin cy7 (PE-Cy7), anti-CD27 AlexaFluor 700, (anti-CD279) anti-PD-1 allophycocyanin (APC), anti-CD57 fluorescein isothiocyanate (FITC), (all from BD Biosciences, San Jose, CA); and anti-CD4 Pacific Blue (Biolegend, San Diego, CA). Cells were incubated with monoclonal antibodies for 20 minutes in the dark at room temperature, washed, fixed in PBS containing 0.5% formaldehyde before analysis. For detection of the intracellular protein Bcl2, cells were surface stained, fixed, and permeabilized with a saponin-based buffer (BD Biosciences, San Jose, CA) followed by incubation with anti-Bcl2-FITC (BD Biosciences) for 40 minutes on ice. Samples were acquired on an LSRII flow cytometer (Becton Dickinson, San Jose, CA) and 30,000-50,000 live-gated events were collected. Data were analyzed using FACSDIVA, (Version 6.2 BD Biosciences) or Flow-Jo software (TreeStar Ashland, OR).
Statistics
Continuous variables were compared between groups using the Mann-Whitney U test. Correlations between groups were assessed using a correlation matrix of the Spearman's rank correlation method (GraphPad Prism software, Version 5.04). P values of less than 0.05 were considered significant.
Results
This study was designed to ask if dysregulated immune homeostasis, and increased exhaustion and senescence might be related to an elevated inflammatory environment in well-characterized groups of patients with therapy-controlled viremia.
Immune failure is associated with perturbations in the IL-7/IL-7 receptor axis
As we had shown earlier that immune failure is associated with profound decreases in numbers of circulating naïve CD4 and CD8 T cells [7], it was important to monitor IL-7 and its receptor, key mediators of naïve T cell homeostasis [19-22].
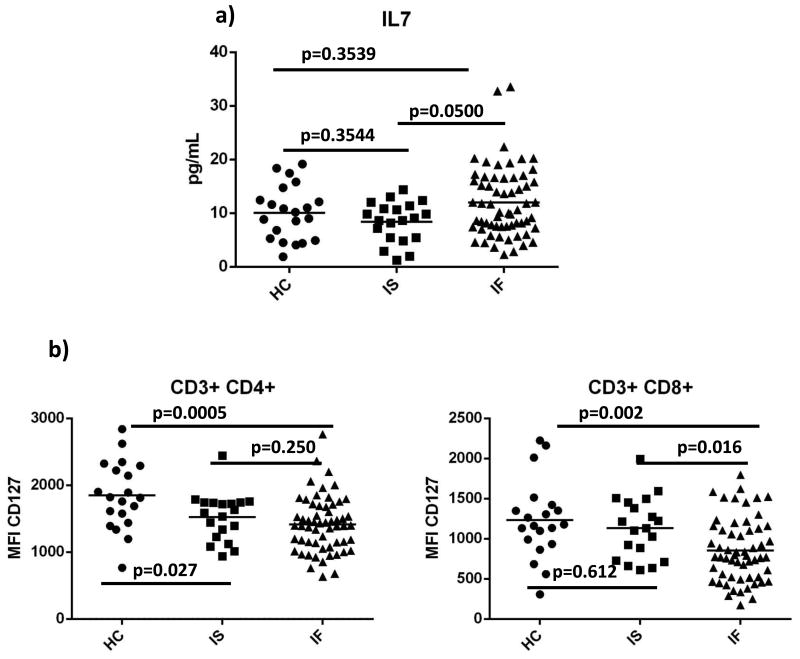
We found that plasma levels of IL-7 were higher in immune failure than in immune success (Fig. 1a; p=0.05). Earlier studies found decreased T cell expression of CD127 associated with low CD4 T cell numbers [11, 13, 23-25]. We found a significant reduction in CD127 density on CD4 T cells from both immune success and immune failure patients when compared to CD127 density among healthy controls (p=0.03; p=0.0005 respectively), but there was no difference between CD127 density in immune failure and immune success (p=0.25) (Fig. 1b, c). CD127 density on CD8 T cells was decreased significantly in immune failure when compared to CD127 density in healthy controls (p=0.002) and in immune success (p= 0.02) (Fig. 1d, e). We also found that in immune failure, CD8 CD127 density was inversely correlated with plasma levels of IL-6 (r= -0.270, p= 0.042), LPS (r= -0.277, p=0.037), and IP-10 (r= -0.276, p= 0.037) (not shown). We had previously reported that IL-6 or IL-1β exposure decreases T cell CD127 expression in vitro[18] but type1 interferon does not [26].
Figure 1. Plasma levels of IL-7 are elevated in immune failure and expression of the IL-7Rα chain (CD127) is decreased.
Plasma levels of IL-7 were measured by ELISA (a). Surface expression of CD127 on gated CD4 and CD8 T cells was measured by flow cytometry on cryopreserved PBMC (b,d; representative samples; c,e; summary data). HC= healthy controls (n=20); IS= immune success (n=20); IF= immune failure (n=60) Bars represent medians; groups compared by Mann-Whitney U test.
Discordant relationships among plasma IL-7, T cell numbers and cycling in immune failure
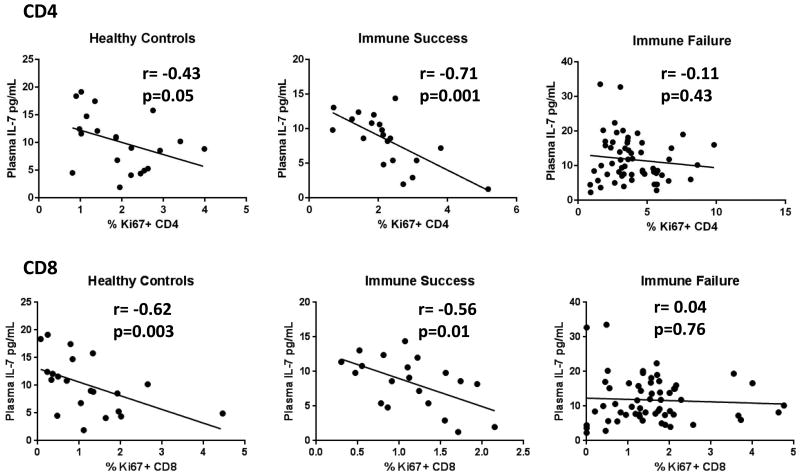
Iinverse relationships between plasma IL-7 and CD4 T cell numbers [24, 27, 28] have been reported largely among patients with incomplete virologic control. Here, we find that in persons with ART-mediated control of HIV replication, plasma levels of IL-7 are higher in immune failure than in immune success; nonetheless, CD4 T cell counts and plasma IL-7 levels were not correlated in immune failure (r= 0.19, p=0.15); or when immune failures and immune successes were combined (r= 0.03, p=0.77). Plasma IL-7 rises dramatically only as CD4 T cell numbers fall below 100 cells/uL and, here CD4 T cell numbers in immune failure averaged 252 cells/uL and were not as low as in the earlier studies where this relationship was identified [24, 27, 28]. Interestingly, although plasma IL-7 levels correlated inversely with cycling (Ki67+) CD4 and CD8 T cells in immune success (r= -0.71, p=0.001; r= -0.56, p=0.01 respectively) and in healthy controls (r= -0.43, p=0.05; r= -0.62, p=0.003 respectively), they did not correlate in the immune failure patients (Fig. 2) uncovering a novel and interesting relationship between IL-7 and T cell cycling in health and successfully treated HIV infection that is perturbed in the setting of immune failure.
Figure 2. Plasma IL-7 correlates inversely with cycling T cells in immune success and healthy controls but not in immune failure.
Correlations between plasma IL-7 levels and % Ki67+ CD4 and CD8 T cells were assessed by Spearman's rank test. Linear regression curves are displayed in graphs. P values of 0.05 or less were considered significant.
Immune exhaustion and senescence markers, PD-1 and CD57, are elevated on most T cell subpopulations in immune failure
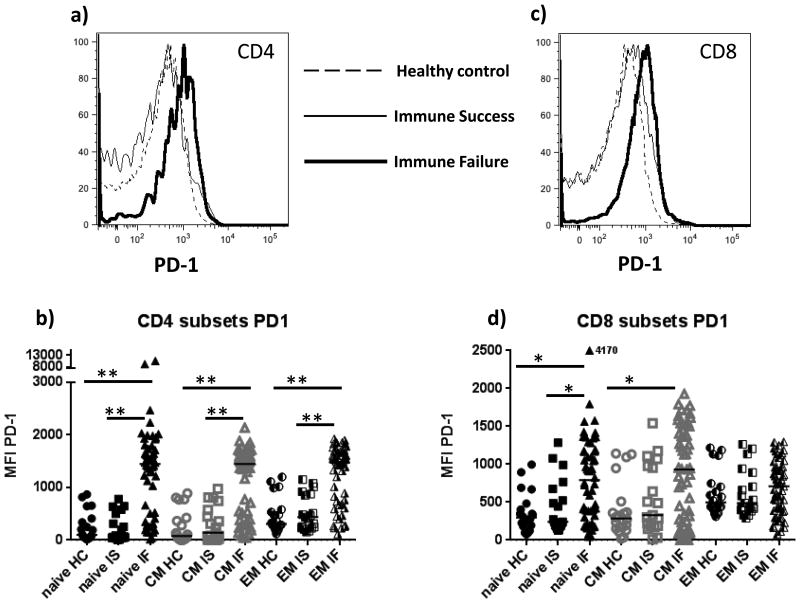
As immune failure is associated with naïve T cell restoration failure and sustained turnover and relative expansion of more mature T cells, we examined T cell expression of PD-1 and CD57. Programmed death 1 (PD-1) is an inhibitory receptor expressed after T cell activation [29] and in HIV infection increased T cell expression of PD-1 is associated with functional exhaustion and disease progression [15, 16, 29-31]. Immune failure patients had greater PD-1 density on all CD4 T cell subsets than did healthy controls and immune successes indicating that irrespective of maturation phenotype, activation and exhaustion was characteristic of all helper subpopulations in immune failure (Fig. 3a, b). PD-1 density was greater on naïve CD8 T cells in immune failure than among immune successes (p= 0.013) and among healthy controls and was greater on central memory CD8 T cells in immune failure than among healthy controls (p= 0.024) and tended to also be greater than among immune successes but not significantly (p= 0.129, Fig. 3c,d), while among more matured effector memory CD8 T cells, PD-1 densities were comparable in all patient groups (Fig 3d).
Figure 3. Immune senescence markers PD-1 and CD57 are elevated on T cells in immune failure.
Surface expression of PD-1 is expressed as mean fluorescent intensity (MFI) (a,c, representative samples; b,d, summary data). Surface expression of CD57 is shown as proportion of stained cells (e,g, representative samples; f,h, summary data). Maturation subsets defined using CD45RA and CCR7 staining (naïve= CD45RA+CCR7+; central memory (CM) CD45RA-CCR7+; effector memory (EM) CD45RA-CCR7-) HC= healthy controls (n=20); IS= immune success (n=20); IF= immune failure (n=60) Bars represent medians; groups are compared by Mann-Whitney U test. **p= <0.001; *p= <0.05
CD57 is a cell surface marker of cells that have undergone multiple rounds of cell division, have shortened telomeres, and are unable to proliferate [32]. In HIV infection, T cell expression of CD57 is elevated [13, 14, 16, 32, 33]. Here, we found that populations of CD57 negative and positive cells were readily distinguished (Fig 3 e, g) and so report these results as proportions of expressing and non-expressing cells. The proportion of CD57 positive CD4 and CD8 T cells was elevated in all maturation subsets in immune failure patients compared to proportions in healthy controls (Fig. 3e-h) and when compared to immune successes, was increased in naïve and central memory CD4 and CD8 T cells, but not among effector memory cells. (Fig. 3 e-h). In immune failures the proportion of CD4 T cells expressing CD57 was correlated with plasma levels of IL-6 (r= 0.341, p= 0.009) and CD4 and CD8 T cell activation (r= 0.397, p=0.002; r= 0.267, p=0.045, respectively) and inversely with absolute naïve CD4 and CD8 counts (r= -0.454, p= <0.001; r= -0.432, p= 0.001, respectively) (not shown).
Both homeostatic and inflammatory cytokines drive expression of exhaustion/senescence markers on CD4 T cells in vitro
While elevated T cell expression of PD-1 and CD57 has been demonstrated in HIV infection [14-16, 30, 31, 34] and here we report elevated expression of these markers in distinct T cell maturation subsets in immune failure patients. The drivers of exhaustion and senescence in this setting are not well-defined. As elevated levels of IL-7 [27, 28] and IL-6 levels are seen in immune failure [7] predictmorbid outcomes [35-37] and as increased and as increased expression of IL-1β in lymphoid tissues may drive memory CD4 T cell cycling[18], we examined the effects of these cytokines on exhaustion and senescence marker expression.
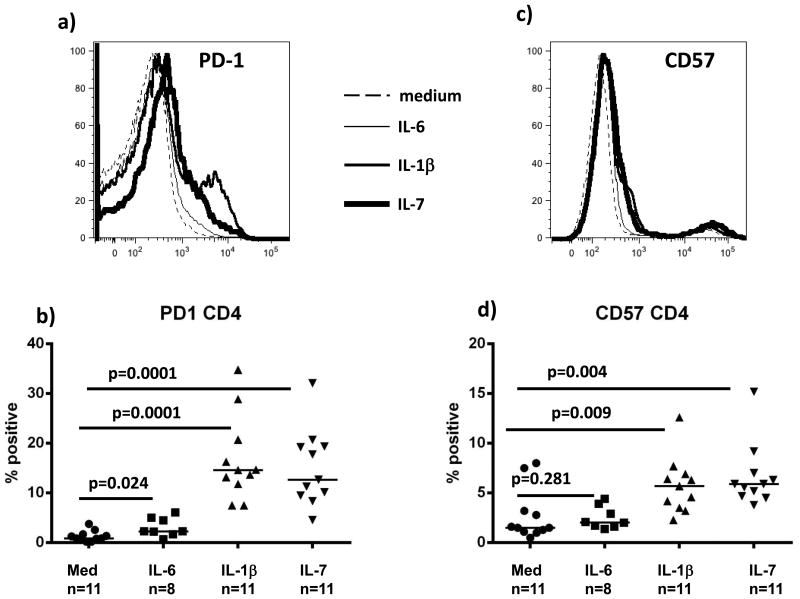
PBMCs from healthy uninfected controls were labeled with CFSE dye, stimulated with IL-6 (10ng/mL), IL-1β (10ng/mL), or IL-7 (5ng/mL) for 7 days, and then dilution of dye and proportions of PD-1 and CD57 positive cells were measured. Both IL-1β and IL-7 increased proportions of PD-1 (Fig. 4a, b) and CD57 (Fig. 4c, d) positive CD4 T cells and these increases were greater in cells that had proliferated than among cells that had not divided (Fig 4e, f). A more modest increase in the proportion of PD-1 expressing cells was seen after IL-6 exposure, and there was no effect of IL-6 on CD57. In contrast, CD8 T cells were unaffected after stimulation with IL-1β, IL-6 or IL-7 (not shown). Both CD4 and CD8 T cells divide after exposure to IL-7 while IL-6 and IL-1β induce division of CD4 but not CD8 T cells [18].
Figure 4. Immune exhaustion/senescence markers PD-1 and CD57 are inducible by IL-1β and IL-7 in vitro.
PBMCs from healthy uninfected subjects were labeled with CFSE, stimulated in vitro for 7 days with IL-6 (10ng/mL), IL-1β (10ng/mL), or IL-7 (5ng/mL), then examined for proliferation by dye dilution and for surface protein expression by flow cytometry on gated CD4 T cells (a,c representative samples; b,d summary data; e,f surface antigen expression among non-proliferating (CSFE high) and proliferating (CFSE low cells) cells) Bars represent medians; groups are compared by Mann-Whitney U test.
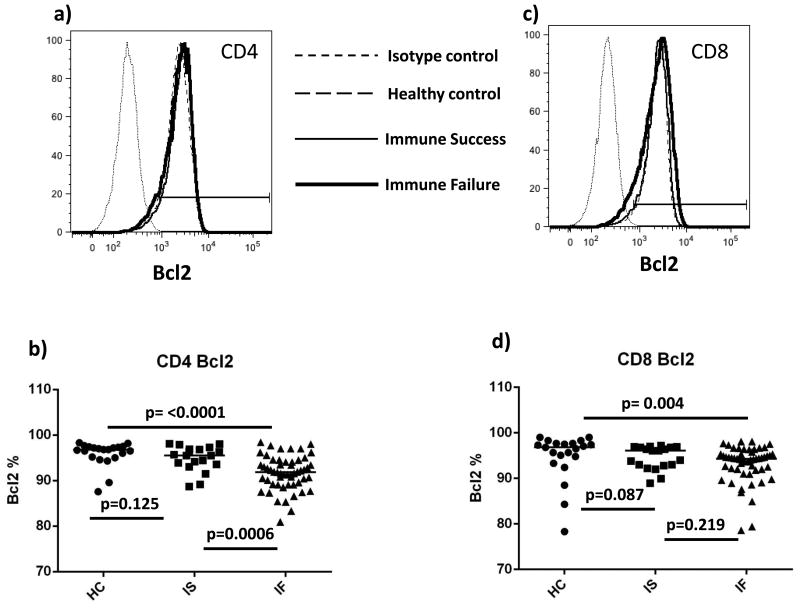
T cell Bcl2 levels are diminished in immune failure
Reduced levels of the pro-survival factor Bcl2 have been found in CD4 T cells from viremic HIV-infected patients [11] and immune failure patients [38], and CD4 T cells from these patients have a reduced ability to increase Bcl2 after IL-7 stimulation [11, 24]. Most CD4 T cells expressed detectable levels of Bcl2, ranging from 88%-98% in healthy controls, 89%-98% in immune success patients, and 81%-98% in immune failure patients; however the median proportion of Bcl2+ CD4 T cells was lower in immune failure patients (92%) than among healthy controls (97%, p = <0.0001) or among immune success patients (96%, p= 0.0006) (Fig. 5).
Figure 5. Fewer CD4 T cells express detectable Bcl2 in immune failure.
(a,c, representative samples; b,d, summary data) HC= healthy controls (n=20); IS= immune success (n=20); IF= immune failure (n=60) Bars represent medians; groups are compared by Mann-Whitney U test.
Discussion
The responsiveness of T cells to IL-7 is essential for the maintenance of T cell homeostasis and for recovery from CD4 T cell deficiency [19, 21, 28, 39]. In HIV infection, plasma IL-7 levels are often elevated and correlate inversely with CD4 T cell counts [19, 24, 27]. Although we found elevated levels of plasma IL-7 in the immune failure patients, there was no correlation between plasma levels of IL-7 and CD4 T cell counts, perhaps because the CD4 T cell counts in our study were not as low as those seen in earlier studies[19, 24, 27]. Yet, in immune success but not in immune failure, IL-7 levels correlated with naïve CD4 T cell counts (r= 0.47, p= 0.04).
Reduced thymic output [40], lymph node fibrosis [41-43], and increased T cell turnover [8, 18] are all plausible contributors to immune restoration failure. Earlier studies also found decreased CD4 T cell expression of CD127 in immune failure [11, 23, 24] and linked decreased receptor expression and function to immune activation [11, 25]. We found decreased expression of the IL-7Rα chain (CD127) in T cells from both immune failure and immune success patients and that expression of CD127 on CD8 T cells was inversely correlated with plasma levels of inflammatory mediators, IL-6, LPS, and IP-10 in immune failure. In immune success, expression of CD127 on both CD8 and CD4 T cells was inversely correlated with plasma levels of IP-10. This suggests that inflammation may be driving decreased CD127 expression and inhibiting responsiveness to IL-7 and CD4 recovery in immune failure as suggested earlier by our in vitro studies where both IL-1β and IL-6 decreased CD127 expression and these cytokines as well as type 1 interferon could impair IL-7 responsiveness [18, 26]. Other studies have demonstrated that T cells from immune failure patients show reduced proliferation [11, 12] and fail to up-regulate Bcl2 [11, 12, 38] in response to IL-7. Here, we saw significantly lower proportions of Bcl2 positive CD4 T cells in immune failure, but in vitro responses to IL-7 were not examined. Colle et. al. found that CD4 T cell expression of CD127 predicted the IL-7-induced increase of Bcl2 and CD25 among healthy controls [44]. This association was lost in viremic and ART treated patients [44].
We found that in both healthy controls and in immune success, plasma IL-7 levels correlated inversely with T cell cycling and in immune success, plasma IL-7 directly correlated with naïve CD4 T cell counts as might be expected in the setting of a successful homeostatic response. These associations were lost in immune failure. It may seem counterintuitive that plasma levels of IL-7 are inversely correlated with T cell cycling in health and immune success. Yet, as IL-7-induced cycling is initiated in lymphoid tissues, Ki-67+ cells in blood have mostly completed division and in these two settings, have consumed IL-7 in order to do so. As IL-7 levels are regulated largely by receptor mediated uptake [21], this impact on plasma levels of IL-7 may be more demonstrable at the lower levels of IL-7 seen in healthy controls and in immune success patients than in patients with immune failure where low levels of CD127 keep IL-7 levels high. Also, the lack of correlation between plasma IL-7 levels and T cell cycling in immune failure patients suggests that other factors may be driving T cell cycling in immune failure and are masking any relationship among IL-7 levels, T cell cycling and CD127 expression. In the setting of immune failure, cycling of memory CD4 T cells has the characteristics of broad bystander activation [45] and may be driven at least in part by exposure to IL-1β [18] and other inflammatory mediators such as IL-2 and IL-15, common gamma receptor cytokines whose expression is also elevated in lymphoid tissues [46]. Moreover, our new data demonstrating an inverse correlation between plasma levels of IL-6, LPS, and IP-10 with CD127 expression on CD8 T cells in immune failure suggests that the IL-7 axis is impaired in this inflammatory environment. Indeed, our previous studies suggest that the inflammatory cytokines IL-6 and IL-1β can down-regulate the expression of CD127 on T cells and both these cytokines and type 1 interferons can block T cell responses to IL-7 [18, 26]. Expression of each of these cytokines is increased in treated HIV infection [4, 9, 18, 35, 37, 47]. Thus, failure to reconstitute CD4 T cells on therapy may be related at least in part to inflammation-driven impaired responses to IL-7.
Memory T cells in HIV infection often express markers of senescence/exhaustion [13-16, 30-33] that is seen in settings of sustained inflammation and T cell activation [14, 48]. We refer to the PD-1+ T cells as exhausted and the CD57+ T cells as senescent , yet there is overlap between these phenotypes that is associated with growth arrest [49-51]. One difference, however, is that while “exhausted” cells demonstrate decreased cytokine production [49], “senescent cells may divide poorly, yet continue to produce inflammatory cytokines [48, 51], and by filling “T cell space” may interfere with the homeostatic regulation of T cell numbers. Markers of immune senescence and exhaustion are particularly elevated on T cells from immune failure patients [13-15] and our measures of PD-1 and CD57 on circulating T cells are confirmatory. We showed a greater proportion of CD57+ “naïve” and “central memory” CD4 and CD8 T cells in immune failure. An earlier study also showed an increased proportion of CD57+ “central memory” and “naïve” CD4 T cells in HIV infection and that patients with CD4 T cells < 200/uL had the highest proportions of senescent cells [33]. We also found elevated expression of PD-1 on all maturation subsets of CD4 T cells and on “naïve” and “central memory” CD8 T cells in immune failure. Increased PD-1 expression was reported earlier in treated and untreated HIV infection but those patients were not segregated according to the success of immune restoration [29]. Here we show that immune failure is associated with dramatic increases in PD-1 expression on T lymphocyte subpopulations despite virologic control, while among immune successes PD-1 expression is normal. As both CD57 and PD-1 expression are associated with decreased proliferative capability, their elevated expression on “naïve” and “central memory” CD4 T cells in the immune failure patients may implicate an additional mechanism impairing CD4 T cell recovery in these patients that does not resolve with ART. It is likely that other factors are more important in driving senescence and exhaustion in CD8 T cells and it should be noted that CD8 T cell numbers do not durably increase during immune recovery on ART [52].
Earlier studies have shown that common gamma chain cytokines such as IL-7 could increase expression of senescence markers PD-1 and Tim-3 on T cells, but IL-1β could not [53, 54] . Here, we demonstrate the novel finding that the inflammatory cytokine IL-1β, as well as the homeostatic cytokine IL-7 could induce expression of the exhaustion and senescence markers PD-1 and CD57 on CD4 T cells from healthy uninfected subjects while IL-6 increased CD4 T cell expression of PD-1 only modestly. These effects were most dramatic on cells that had undergone cell division. Further study will be needed to compare the functional phenotype of CD4 T cells that have proliferated in response to these inflammatory and homeostatic stimuli. By simultaneously examining these indices, in the same study, we could demonstrate associations among inflammation, exhaustion/senescence, and the IL-7/IL-7 receptor axis in patients who had recovered CD4 T cells and those who had not.
Thus, immune failure in treated HIV infection is characterized by increased levels of circulating IL-7, decreased cell surface expression of the IL-7 receptor alpha chain (CD127) in a setting characterized by naïve T cell restoration failure and increased turnover and senescence of memory CD4 T cells. A heightened inflammatory environment in treated HIV infection [35, 37] may contribute to this scenario, with inflammatory mediators IL-1β and IL-6 blunting responses to IL-7 by decreasing expression of CD127 [18] and increasing both CD4 T cell turnover [18] and senescence, and with type 1 interferon providing additional blockade of IL-7 signaling [55].
It remains to be seen whether cellular division induced by IL-7 is more likely to result in cell death in the presence of these inflammatory mediators. Markers of immune exhaustion/senescence, PD-1 and CD57, are broadly elevated on both CD8 and CD4 maturation subsets in immune failure patients and the inflammatory cytokine IL-1β and the homeostatic cytokine IL-7, that can both drive memory cell turnover, also can increase expression of PD-1 and CD57 on CD4 T cells in vitro. Thus, inflammatory cytokines may further disrupt T cell homeostasis at multiple stages of CD4 T cell recovery by promoting T cell activation and immune senescence.
Acknowledgments
This work was supported by grants from the National Institutes of Health [AI 076174, AI 68636, AI 105937] the CWRU Center for AIDS Research – [AI 36219] and the Fasenmyer Foundation.
Research reported in this publication was also supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number R00HL108743. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
The authors have no commercial or other associations that might pose a conflict of interest.
Literature Cited
- 1.Benveniste O, Flahault A, Rollot F, et al. Mechanisms involved in the low-level regeneration of CD4+ cells in HIV-1-infected patients receiving highly active antiretroviral therapy who have prolonged undetectable plasma viral loads. J Infect Dis. 2005;191:1670–9. doi: 10.1086/429670. [DOI] [PubMed] [Google Scholar]
- 2.Gazzola L, Tincati C, Bellistri GM, Monforte A, Marchetti G. The absence of CD4+ T cell count recovery despite receipt of virologically suppressive highly active antiretroviral therapy: clinical risk, immunological gaps, and therapeutic options. Clin Infect Dis. 2009;48:328–37. doi: 10.1086/595851. [DOI] [PubMed] [Google Scholar]
- 3.Kelley CF, Kitchen CM, Hunt PW, et al. Incomplete peripheral CD4+ cell count restoration in HIV-infected patients receiving long-term antiretroviral treatment. Clin Infect Dis. 2009;48:787–94. doi: 10.1086/597093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lederman MM, Funderburg NT, Sekaly RP, Klatt NR, Hunt PW. Residual immune dysregulation syndrome in treated HIV infection. Advances in immunology. 2013;119:51–83. doi: 10.1016/B978-0-12-407707-2.00002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith C, Sabin CA, Lundgren JD, et al. Factors associated with specific causes of death amongst HIV-positive individuals in the D:A:D Study. AIDS. 2010;24:1537–48. doi: 10.1097/QAD.0b013e32833a0918. [DOI] [PubMed] [Google Scholar]
- 6.Engsig FN, Zangerle R, Katsarou O, et al. Long-term mortality in HIV-positive individuals virally suppressed for >3 years with incomplete CD4 recovery. Clin Infect Dis. 2014;58:1312–21. doi: 10.1093/cid/ciu038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lederman MM, Calabrese L, Funderburg NT, et al. Immunologic failure despite suppressive antiretroviral therapy is related to activation and turnover of memory CD4 cells. J Infect Dis. 2011;204:1217–26. doi: 10.1093/infdis/jir507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marchetti G, Gori A, Casabianca A, et al. Comparative analysis of T-cell turnover and homeostatic parameters in HIV-infected patients with discordant immune-virological responses to HAART. AIDS. 2006;20:1727–36. doi: 10.1097/01.aids.0000242819.72839.db. [DOI] [PubMed] [Google Scholar]
- 9.Fernandez S, Tanaskovic S, Helbig K, et al. CD4+ T-cell deficiency in HIV patients responding to antiretroviral therapy is associated with increased expression of interferon-stimulated genes in CD4+ T cells. J Infect Dis. 2011;204:1927–35. doi: 10.1093/infdis/jir659. [DOI] [PubMed] [Google Scholar]
- 10.French MA, King MS, Tschampa JM, da Silva BA, Landay AL. Serum immune activation markers are persistently increased in patients with HIV infection after 6 years of antiretroviral therapy despite suppression of viral replication and reconstitution of CD4+ T cells. J Infect Dis. 2009;200:1212–5. doi: 10.1086/605890. [DOI] [PubMed] [Google Scholar]
- 11.Colle JH, Moreau JL, Fontanet A, et al. Regulatory dysfunction of the interleukin-7 receptor in CD4 and CD8 lymphocytes from HIV-infected patients--effects of antiretroviral therapy. J Acquir Immune Defic Syndr. 2006;42:277–85. doi: 10.1097/01.qai.0000214823.11034.4e. [DOI] [PubMed] [Google Scholar]
- 12.Bellistri GM, Casabianca A, Merlini E, et al. Increased bone marrow interleukin-7 (IL-7)/IL-7R levels but reduced IL-7 responsiveness in HIV-positive patients lacking CD4+ gain on antiviral therapy. PLoS One. 2010;5:e15663. doi: 10.1371/journal.pone.0015663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanaskovic S, Fernandez S, Price P, French MA. Interleukin-7 signalling defects in naive CD4+ T cells of HIV patients with CD4+ T-cell deficiency on antiretroviral therapy are associated with T-cell activation and senescence. AIDS. 2014;28:821–30. doi: 10.1097/QAD.0000000000000213. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez S, Price P, McKinnon EJ, Nolan RC, French MA. Low CD4+ T-cell counts in HIV patients receiving effective antiretroviral therapy are associated with CD4+ T-cell activation and senescence but not with lower effector memory T-cell function. Clin Immunol. 2006;120:163–70. doi: 10.1016/j.clim.2006.04.570. [DOI] [PubMed] [Google Scholar]
- 15.Grabmeier-Pfistershammer K, Steinberger P, Rieger A, Leitner J, Kohrgruber N. Identification of PD-1 as a unique marker for failing immune reconstitution in HIV-1-infected patients on treatment. J Acquir Immune Defic Syndr. 2011;56:118–24. doi: 10.1097/QAI.0b013e3181fbab9f. [DOI] [PubMed] [Google Scholar]
- 16.Petrovas C, Chaon B, Ambrozak DR, et al. Differential association of programmed death-1 and CD57 with ex vivo survival of CD8+ T cells in HIV infection. J Immunol. 2009;183:1120–32. doi: 10.4049/jimmunol.0900182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernandez S, French MA, Price P. Immunosenescent CD57+CD4+ T-cells accumulate and contribute to interferon-gamma responses in HIV patients responding stably to ART. Disease markers. 2011;31:337–42. doi: 10.3233/DMA-2011-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shive CL, Mudd JC, Funderburg NT, et al. Inflammatory Cytokines Drive CD4+ T-Cell Cycling and Impaired Responsiveness to Interleukin 7: Implications for Immune Failure in HIV Disease. J Infect Dis . 2014 doi: 10.1093/infdis/jiu125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gougeon ML, Chiodi F. Impact of gamma-chain cytokines on T cell homeostasis in HIV-1 infection: therapeutic implications. J Intern Med. 2010;267:502–14. doi: 10.1111/j.1365-2796.2010.02221.x. [DOI] [PubMed] [Google Scholar]
- 20.Fry TJ, Mackall CL. Interleukin-7: master regulator of peripheral T-cell homeostasis? Trends Immunol. 2001;22:564–71. doi: 10.1016/s1471-4906(01)02028-2. [DOI] [PubMed] [Google Scholar]
- 21.Fry TJ, Mackall CL. The many faces of IL-7: from lymphopoiesis to peripheral T cell maintenance. J Immunol. 2005;174:6571–6. doi: 10.4049/jimmunol.174.11.6571. [DOI] [PubMed] [Google Scholar]
- 22.Takada K, Jameson SC. Naive T cell homeostasis: from awareness of space to a sense of place. Nat Rev Immunol. 2009;9:823–32. doi: 10.1038/nri2657. [DOI] [PubMed] [Google Scholar]
- 23.Marziali M, De Santis W, Carello R, et al. T-cell homeostasis alteration in HIV-1 infected subjects with low CD4 T-cell count despite undetectable virus load during HAART. AIDS. 2006;20:2033–41. doi: 10.1097/01.aids.0000247588.69438.fd. [DOI] [PubMed] [Google Scholar]
- 24.Rethi B, Fluur C, Atlas A, et al. Loss of IL-7Ralpha is associated with CD4 T-cell depletion, high interleukin-7 levels and CD28 down-regulation in HIV infected patients. AIDS. 2005;19:2077–86. doi: 10.1097/01.aids.0000189848.75699.0f. [DOI] [PubMed] [Google Scholar]
- 25.Kiazyk SA, Fowke KR. Loss of CD127 expression links immune activation and CD4(+) T cell loss in HIV infection. Trends Microbiol. 2008;16:567–73. doi: 10.1016/j.tim.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen TP, Bazdar DA, Mudd JC, et al. Interferon-alpha inhibits CD4 T cell responses to interleukin-7 and interleukin-2 and selectively interferes with Akt signaling. J Leukoc Biol. 2015;97:1139–46. doi: 10.1189/jlb.4A0714-345RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Napolitano LA, Grant RM, Deeks SG, et al. Increased production of IL-7 accompanies HIV-1-mediated T-cell depletion: implications for T-cell homeostasis. Nat Med. 2001;7:73–9. doi: 10.1038/83381. [DOI] [PubMed] [Google Scholar]
- 28.Fry TJ, Connick E, Falloon J, et al. A potential role for interleukin-7 in T-cell homeostasis. Blood. 2001;97:2983–90. doi: 10.1182/blood.v97.10.2983. [DOI] [PubMed] [Google Scholar]
- 29.Breton G, Chomont N, Takata H, et al. Programmed death-1 is a marker for abnormal distribution of naive/memory T cell subsets in HIV-1 infection. J Immunol. 2013;191:2194–204. doi: 10.4049/jimmunol.1200646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El-Far M, Halwani R, Said E, et al. T-cell exhaustion in HIV infection. Curr HIV/AIDS Rep. 2008;5:13–9. doi: 10.1007/s11904-008-0003-7. [DOI] [PubMed] [Google Scholar]
- 31.Day CL, Kaufmann DE, Kiepiela P, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–4. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 32.Brenchley JM, Karandikar NJ, Betts MR, et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003;101:2711–20. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- 33.Mojumdar K, Vajpayee M, Chauhan NK, Singh A, Singh R, Kurapati S. Loss of CD127 & increased immunosenescence of T cell subsets in HIV infected individuals. The Indian journal of medical research. 2011;134:972–81. doi: 10.4103/0971-5916.92645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palmer BE, Blyveis N, Fontenot AP, Wilson CC. Functional and phenotypic characterization of CD57+CD4+ T cells and their association with HIV-1-induced T cell dysfunction. J Immunol. 2005;175:8415–23. doi: 10.4049/jimmunol.175.12.8415. [DOI] [PubMed] [Google Scholar]
- 35.Hunt PW, Sinclair E, Rodriguez B, et al. Gut Epithelial Barrier Dysfunction and Innate Immune Activation Predict Mortality in Treated HIV Infection J Infect Dis . 2014 doi: 10.1093/infdis/jiu238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tenorio AR, Zheng Y, Bosch RJ, et al. Soluble Markers of Inflammation and Coagulation but Not T-Cell Activation Predict Non-AIDS-Defining Morbid Events During Suppressive Antiretroviral Treatment. J Infect Dis . 2014 doi: 10.1093/infdis/jiu254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.David D, Keller H, Nait-Ighil L, et al. Involvement of Bcl-2 and IL-2R in HIV-positive patients whose CD4 cell counts fail to increase rapidly with highly active antiretroviral therapy. AIDS. 2002;16:1093–101. doi: 10.1097/00002030-200205240-00002. [DOI] [PubMed] [Google Scholar]
- 39.Goldrath AW. Maintaining the status quo: T-cell homeostasis. Microbes and infection / Institut Pasteur. 2002;4:539–45. doi: 10.1016/s1286-4579(02)01570-8. [DOI] [PubMed] [Google Scholar]
- 40.Li T, Wu N, Dai Y, et al. Reduced thymic output is a major mechanism of immune reconstitution failure in HIV-infected patients after long-term antiretroviral therapy. Clin Infect Dis. 2011;53:944–51. doi: 10.1093/cid/cir552. [DOI] [PubMed] [Google Scholar]
- 41.Lederman MM, Margolis L. The lymph node in HIV pathogenesis. Semin Immunol. 2008;20:187–95. doi: 10.1016/j.smim.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schacker TW, Nguyen PL, Beilman GJ, et al. Collagen deposition in HIV-1 infected lymphatic tissues and T cell homeostasis. J Clin Invest. 2002;110:1133–9. doi: 10.1172/JCI16413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zeng M, Smith AJ, Wietgrefe SW, et al. Cumulative mechanisms of lymphoid tissue fibrosis and T cell depletion in HIV-1 and SIV infections. J Clin Invest. 2011;121:998–1008. doi: 10.1172/JCI45157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Colle JH, Moreau JL, Fontanet A, Lambotte O, Delfraissy JF, Theze J. The correlation between levels of IL-7Ralpha expression and responsiveness to IL-7 is lost in CD4 lymphocytes from HIV-infected patients. AIDS. 2007;21:101–3. doi: 10.1097/QAD.0b013e3280115b6a. [DOI] [PubMed] [Google Scholar]
- 45.Jiang W, Younes SA, Funderburg NT, et al. Cycling memory CD4+ T cells in HIV disease have a diverse TCR repertoire and a phenotype consistent with bystander activation. J Virol . 2014 doi: 10.1128/JVI.00017-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Biancotto A, Grivel JC, Iglehart SJ, et al. Abnormal activation and cytokine spectra in lymph nodes of people chronically infected with HIV-1. Blood. 2007;109:4272–9. doi: 10.1182/blood-2006-11-055764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hardy GA, Sieg S, Rodriguez B, et al. Interferon-alpha is the primary plasma type-I IFN in HIV-1 infection and correlates with immune activation and disease markers. PLoS One. 2013;8:e56527. doi: 10.1371/journal.pone.0056527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adams PD. Healing and hurting: molecular mechanisms, functions, and pathologies of cellular senescence. Mol Cell. 2009;36:2–14. doi: 10.1016/j.molcel.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 49.Akbar AN, Henson SM. Are senescence and exhaustion intertwined or unrelated processes that compromise immunity? Nat Rev Immunol. 2011;11:289–95. doi: 10.1038/nri2959. [DOI] [PubMed] [Google Scholar]
- 50.Mudd JC, Lederman MM. CD8 T cell persistence in treated HIV infection. Curr Opin HIV AIDS. 2014;9:500–5. doi: 10.1097/COH.0000000000000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weyand CM, Yang Z, Goronzy JJ. T-cell aging in rheumatoid arthritis. Current opinion in rheumatology. 2014;26:93–100. doi: 10.1097/BOR.0000000000000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Serrano-Villar TS, Lee Sulggi A, Hunt Peter W, Sinclair Elizabeth, Shacklett Barbara L, Ferre April L, Hayes Timothy L, Somsouk Ma, Hsue Priscilla Y, Van Natta Mark L, Meinert Curtis L, Lederman Michael M, Hatano Hiroyu, Jain Vivek, Huang Yong, Hecht Frederick M, Martin Jeffrey N, McCune Joseph M, Moreno Santiago, Deeks Steven G. HIV-Infected Individuals with Low CD4/CD8 Ratio despite Effective Antiretroviral Therapy Exhibit Altered T Cell Subsets, Heightened CD8+ T Cell Activation, and Increased Risk of Non-AIDS Morbidity and Mortality. PLOS Pathogens . 2014;10 doi: 10.1371/journal.ppat.1004078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kinter AL, Godbout EJ, McNally JP, et al. The common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 induce the expression of programmed death-1 and its ligands. J Immunol. 2008;181:6738–46. doi: 10.4049/jimmunol.181.10.6738. [DOI] [PubMed] [Google Scholar]
- 54.Mujib S, Jones RB, Lo C, et al. Antigen-independent induction of Tim-3 expression on human T cells by the common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 is associated with proliferation and is dependent on the phosphoinositide 3-kinase pathway. J Immunol. 2012;188:3745–56. doi: 10.4049/jimmunol.1102609. [DOI] [PubMed] [Google Scholar]
- 55.Nguyen T. Interferon-a inhibits CD4 T cell responses to interleukin-7 and interleukin-2 and selectively interferes with Akt signaling. Journal of Leukocyte Biology. 2015:97. doi: 10.1189/jlb.4A0714-345RR. [DOI] [PMC free article] [PubMed] [Google Scholar]