Abstract
Inhibition of cochlear amplifier gain by the medial olivocochlear (MOC) efferent system has several putative roles: aiding listening in noise, protection against damage from acoustic overexposure, and slowing age-induced hearing loss. The human MOC reflex has been studied almost exclusively by measuring changes in otoacoustic emissions. However, to help understand how the MOC system influences what we hear, it is important to have measurements of the MOC effect on the total output of the organ of Corti, i.e., on cochlear nerve responses that couple sounds to the brain. In this work we measured the inhibition produced by the MOC reflex on the amplitude of cochlear nerve compound action potentials (CAPs) in response to moderate level (52–60 dB peSPL) clicks from five, young, normal hearing, awake, alert, human adults. MOC activity was elicited by 65 dB SPL, contralateral broadband noise (CAS). Using tympanic membrane electrodes, approximately 10 hours of data collection were needed from each subject to yield reliable measurements of the MOC reflex inhibition on CAP amplitudes from one click level. The CAS produced a 16% reduction of CAP amplitude, equivalent to a 1.98 dB effective attenuation (averaged over five subjects). Based on previous reports of efferent effects as functions of level and frequency, it is possible that much larger effective attenuations would be observed at lower sound levels or with clicks of higher frequency content. For a preliminary comparison, we also measured MOC reflex inhibition of DPOAEs evoked from the same ears with f2’s near 4 kHz. The resulting effective attenuations on DPOAEs were, on average, less than half the effective attenuations on CAPs.
Keywords: medial olivocochlear reflex, olivocochlear efferents, compound action potential, otoacoustic emissions, cochlear amplifier
INTRODUCTION
To enhance the sensitivity and frequency selectivity of hearing, sound-evoked cochlear mechanical vibrations are amplified within the cochlea. This “cochlear amplification” is under central control via the medial olivocochlear (MOC) efferent system. MOC efferent fibers synapse on, and inhibit, outer hair cell responses thereby reducing cochlear-amplifier gain. In humans, MOC inhibition has mostly been studied with measurements of otoacoustic emissions (OAEs) (see Guinan 2006, for review). OAEs provide an indirect measure of MOC effects on cochlear mechanics but do not reveal the MOC effects on the cochlear neural responses that mediate hearing. Several reports presented measurements of MOC effects on cochlear neural responses in humans (Folsom & Owsley 1987; Kawase & Takasaka 1995; Chabert et al. 2002). However, each of these reports has issues that prevent it from giving a clear picture of MOC effects on cochlear neural output in awake, alert, normal-hearing humans. Indirect measurements of MOC inhibition have been made psychophysically (e.g., Kawase et al. 2000; Aguilar et al. 2013; Wicher & Moore 2014; Strickland 2001, 2004, 2008; Wojtczak et al. 2014; Jennings et al. 2009; Roverud & Strickland 2010; Yasin et al. 2014). But, psychophysical measurements are confounded by the possibility that the MOC reflex, the sound used to elicit MOC reflex, or the attention required for the psychophysical measurements, may change signal processing in the brain as well as in the cochlea (Keefe et al. 2009; Wittekindt et al. 2014). To understand the roles of the MOC reflex in human hearing, it is necessary to know the extent to which the MOC reflex inhibits responses from the cochlear nerve and to do this direct measurements of cochlear-nerve responses are needed.
Here we set out to obtain statistically significant measurements of human cochlear-nerve compound action potentials (CAP) responses without and with MOC activity elicited by contralateral noise. CAP responses from a single click level were measured from auditory-brainstem response (ABR) wave I recorded with a tympanic membrane electrode. To achieve adequate accuracy, this required extensive measurements done over five two-hour sessions for each subject. After the CAP data had been obtained, we did a brief set of measurements of MOC effects on distortion-product OAEs (DPOAEs) from the same ears as a preliminary step in a secondary goal of comparing MOC effects on CAPs and OAEs.
METHODS
Methods Overview
CAP measurements were made from the first negative peak in the ABR waveform recorded with an electrode placed on the tympanic membrane (Lichtenhan & Chertoff 2008; Chertoff et al. 2010). The MOC reflex was activated by contralateral acoustic stimulation (CAS) that was alternated on and off across runs. In five subjects, the MOC-induced change on CAPs was measured at one sound level as the percentage change in CAP amplitude from the no-CAS to the with-CAS condition. We refer to these five subjects as “CAS-on-CAP subjects”. In each of these subjects CAP measurements were averaged over multiple (five) two-hour visits to the lab to achieve an adequate signal-to-noise ratio. The percent change in the CAP amplitude was translated into effective attenuation using the slope of the change in CAP amplitude with sound level averaged from 19 subjects and, in three of the five CAS-on-CAP subjects, using the slope from the individual ears. Effective attenuation is the amount that the probe sound level would have to be raised with MOC inhibition to obtain the same response amplitude that was obtained without MOC inhibition. Effective attenuation is a constant-response metric that allows changes in different measured quantities to be compared along the same sound-level axis. In contrast, the percentage change metric is greatly affected by nonlinearities such as those in the inner hair cell to auditory-nerve synapse. Thus, for comparing changes in CAPs and OAEs, effective attenuation is the preferred metric.
During the CAS-on-CAP subjects’ sixth visit to the lab, we measured their middle-ear-muscle reflex (MEMR) thresholds and made DPOAE measurements with and without CAS from which we calculated MOC-reflex-induced DPOAE effective attenuations.
Stimulus calibration, stimulus generation, and data acquisition were performed with a National Instruments PXI-1031 chassis and the Eaton-Peabody Laboratories Cochlear Function Test Suite (http://www.masseyeandear.org/research/otolaryngology/investigators/laboratories/eaton-peabody-laboratories/epl-engineering-resources/). Custom written software in MATLAB (MathWorks) was used for offline analyses. All procedures were approved by the Institutional Review Board of Washington University in St. Louis.
CAP Measurements with and without CAS
Hearing sensitivity of prospective subjects was assessed with a 20 dB HL hearing screening at 0.5, 1, 2, and 4 kHz using a calibrated audiometer (Earscan, Micro Audiometrics Corp.). Subjects were accepted only if they could hear the 20 dB HL tones. CAP amplitudes measurements were made with an electrode placed on the right tympanic membrane (noninverting; Lilly TM-Wick Electrode, Intelligent Hearing Systems), and surface electrodes placed on their high forehead (inverting) and contralateral mastoid (ground). Following an extensive pilot study, we learned that our subject recruitment must screen for an ability to remain exceptionally still with the tympanic membrane electrode in place, an ability to stay awake and mentally alert while comfortably reclined in a dark room, and the discipline to return for multiple two-hour sessions. Only five subjects (four females and one male, 18–25 years old) were able to complete these experiments. Since sleep has been reported to reduce MOC effects (Froehlich et al. 1993), the experimenter kept subjects awake and aroused by asking questions between runs, instructing the subject to silently formulate answers during the run, and then asking the subject to speak their answers to the experimenter at the end of each run (communicating using a battery powered baby monitoring system). This is referred to as “tasking”. Subjects sat reclined in a double-walled sound-treated room for all measurements.
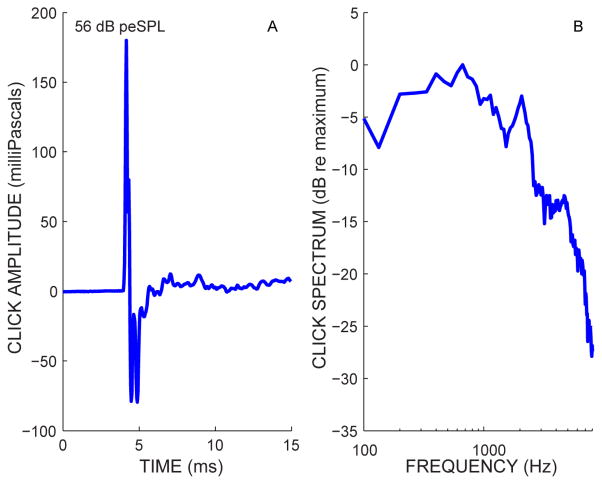
Alternating condensation and rarefaction clicks were presented at 11.1/sec through a free-field loudspeaker (A’Diva Ti, Anthony Gallo Acoustics) powered by an amplifier (CT475 Drivecore, Crown). We avoided high-rate clicks because they produce CAP adaptation. If MOC inhibition reduces the firing rate of an adapted synapse, the adaptation will decrease which increases the CAP amplitude and partially erases the MOC-induced reduction in the response. Thus, the click repetition rate was kept low to avoid CAP adaptation and allow accurate measurement of the MOC effect. A measuring device ensured that the distance between the loudspeaker and ears did not vary. The loudspeaker was placed such that the click arrived at the ear canal 4 ms after the electric pulse to the loudspeaker, which resulted in a 4 ms baseline measurement before any possible CAP response. The clicks were monitored with a ½″ microphone (Larson-Davis model 378B02) strapped to the subject’s head and positioned near the entrance of the ipsilateral ear canal. The loudspeaker produced clicks with a quick decay and very little ringing (Fig. 1).
Fig. 1.
The average waveform (A) and spectrum (B) of the click stimulus.
CAPs and associated acoustic click sound pressure waveforms were simultaneously measured over response epochs of 15 ms, sampled at 25 kHz, and saved to disk. CAPs were band pass filtered at 0.1–3 kHz (GRASS P511, Astro-Med, Inc.) and amplified 10,000 times. CAP artifact rejection thresholds on individual traces ranged from 15–45 μV and were adjusted according to each subject’s noise level. Separate averages were kept for positive and negative clicks; averaging the two together yielded the CAP, and subtracting the negative-click average from the positive-click average yielded an estimate of the noise. The level of the clicks used here (~15 dB SL) did not evoke noticeable deflections in the response waveform that would be attributable to cochlear microphonic or summating potential. A screening procedure identified the lowest click level that yielded a reasonable response from averages of approximately 1024 responses (i.e., identifiable ABR waves 1 & 5 with latencies consistent with laboratory normative data). The resulting click levels were 55, 52, 55, 60, and 55 dB peSPL for subjects 2, 3, 5, 6 and 7, respectively. These click levels were used for the lengthy CAP measurements without and with CAS to obtain the MOC-induced percentage change in the CAP amplitude.
CAPs measurements were made from interleaved runs without and with CAS. The CAS was 65 dB SPL, 0.1–10 kHz broadband, non-frozen noise presented through an ER-10C acoustic assembly (Etymotic Research, Inc.) sealed into the ear canal by a foam plug (which minimized acoustic crosstalk to the ipsilateral ear) and driven by a TDT SA-1 amplifier (Tucker-Davis Technologies). A response waveform from 2048–4096 averages without CAS was obtained, and then a response from the exact same number of averages was immediately obtained with CAS. The 2048–4096 range of response averaging was long enough to acquire substantial data, but short enough to provide breaks that the experimenter could use to keep the subject alert. The criterion for determining whether to collect 2048 or 4096 averages was whether the experimenter sensed from a subject’s verbal responses to tasking that help was needed to maintain alertness. This process was repeated throughout each subject’s five visits to the lab. Each subject’s responses were separately averaged for the without and with CAS conditions (see below).
Post-acquisition processing of the CAP data was done to further reject noisy waveforms and to estimate the uncertainty in the resulting averages. The initial data from each subject consisted of N=18 to 26 waveform averages without CAS and the same number with CAS, each including 2048–4096 traces. Individual waveforms were rejected (e.g., if a with-CAS CAP was rejected, its paired without-CAS CAP was not rejected unless it too met the rejection criteria) if the noise estimate for that waveform was greater than a fixed value (e.g., 0.25 μV) set separately for each subject (see below). All waveforms from a subject and CAS condition that passed the criterion were averaged, and from each averaged waveform the CAP amplitude was measured: CAP amplitude was calculated as the difference between the average waveform from 0–4 ms (i.e., no physiologic response) and the maximum negative of the CAP response occurring up to 1.5 ms thereafter. The CAS-induced percentage change in the CAP was 100 times (the CAP amplitude without CAS) minus (the CAP amplitude with CAS) divided by (the CAP amplitude without CAS).
To estimate the statistical significance of the CAS-induced percentage change in the CAPs, a bootstrap procedure was done using the unrejected waveforms from each subject: (a) the null hypothesis was that there was no difference in the CAPs from the N with-CAS runs versus from the M without-CAS runs, so the N+M waveforms were pooled. (b) N waveforms randomly selected from this pool were averaged and a pseudo-with-CAS CAP was calculated from this average waveform. The remaining M waveforms were averaged and a pseudo-without-CAS CAP was calculated from this average waveform. (c) a pseudo-CAS-induced-CAP-percentage-change was calculated as 100 times (the pseudo-without-CAS CAP) minus (the pseudo-with-CAS CAP) divided by (the pseudo-without-CAS CAP). (d) Steps b and c were done 100,000 times which formed a distribution of 100,000 pseudo-CAP-percentage-changes. The absolute value of the real CAP change was compared with the absolute values of this distribution and the probability “p” that the real CAP change could be due to chance was taken as the fraction of values in the null distribution that were equal or greater than the real CAP change. In addition, 95 percent confidence intervals were obtained as the points that excluded the upper and lower 2.5 percent of the null-distribution values.
In the CAP data processing described above, individual CAP waveform averages were rejected if the noise estimate for that waveform was above a criterion set for each subject. To determine the optimum noise rejection criteria, on each subject the criterion was varied over a large range and for each criterion value the 95% confidence interval (CI) of the CAP change was calculated by the bootstrap method described above. For all subjects except subject #2, as the rejection level was lowered and more waveforms were rejected, the 95% CI became larger. This indicates that each waveform was contributing more signal than noise to the average, and none should be rejected. For subject 2 (who had the lowest CAP amplitude and for whom the most averages were obtained) as the rejection criterion was lowered and the noisiest waveforms were rejected, the 95% CI of the resulting average was reduced until the rejection criteria was 0.25 μV, after which the 95% CI increased. This indicates that waveforms with noise over 0.25 μV contributed more noise than signal and made the result worse (made it have a larger 95% CI) whereas the reverse was true for waveforms with noise less than 0.25 μV. We therefore only rejected CAP waveforms on subject 2 and only those with noise over 0.25 μV. For each subject, the average of the non-rejected waveforms was used to obtain the MOC-induced change in CAP amplitude (a percent change).
CAP level functions
To convert the MOC-induced percentage change in CAP amplitude into the MOC-induced effective attenuation, we needed to know how much the CAP amplitude decreases as sound level decreased. To determine this, we measured the slope of CAP amplitudes as a function of click level with no CAS (not to be confused with the measurements of MOC inhibition on CAPs that were completed over ~10 hours at a single click level). Two of the 5 original CAS-on-CAP subjects were not available for a seventh visit to the lab to make these measurements, so we made the conversion of CAP percent change to effective attenuation using the slope of the CAP amplitude versus sound level averaged across a group of subjects. Originally we recruited eight subjects (including 3 of the 5 CAS-on-CAP subjects) for a single visit to record CAP level functions using 2048 response averaging. On each subject, individual CAP measurements at a sound level were accepted if the CAP amplitude was greater than twice the noise estimate for that waveform. Noise was estimated as the rms value of the waveform from an average formed after reversing the polarity of alternate CAP waveforms so that the CAP was cancelled. For each subject, a least-squares straight-line fit was made to the accepted CAP data from sound levels 47–65 dB peSPL. These sound levels were chosen to be from 5 dB below the lowest level, to 5 dB above the highest level at which the CAS-on-CAP percent amplitude changes were measured across the 5 CAS-on-CAP subjects. The average of the 8 slopes was 9.1 ± 5.4 percent/dB (mean ± SD). In response to the criticism that this method ignores individual differences and that the 8 subjects may not be representative, we obtained CAP level functions on 11 new subjects and we repeated the measurements on the 3 CAS-on-CAP subjects who were still available. These measurements were done at 50, 55 and 60 dB SPL with multiple averages of 1024 responses at each level. Individual measurements were accepted if their noise floor was less than a criterion (range: 0.06 to 0.205 μV) chosen for each subject to minimize the signal-to-noise ratio without reducing to one the number of accepted waveforms. On each subject the accepted waveforms at each sound level were averaged into a single waveform from which the CAP amplitude was determined. CAPs that were less than twice the noise level were discarded and the slope of the CAP versus tone SPL was calculated for each subject from a least-squares straight-line fit to the accepted CAP data. The average of the 11 slopes was 7.0 ± 3.6 percent/dB (mean ± SD). Combining the two sets of data, the average of the 19 slopes was 7.9 ± 4.4 percent/dB (mean ± SD). This average was used to convert the CAP percent changes to effective attenuations on the 5 CAS-on-CAP subjects. For the 3 of the 5 CAS-on-CAP subjects for whom we had the slope of their individual CAP level functions, we also converted the CAP percent changes to effective attenuations with their individual slopes (averaged from the good values of both data sets). The MOC-induced effective attenuations were obtained by dividing the subjects’ percent CAP change by the mean (and individual, when available) CAP percent/dB slope. An estimate of the 95% CI of this effective attenuation was obtained by combining the bootstrap of the null distribution of the CAP changes (described above) with a bootstrap average of 19 CAP-percent/dB slope measurements chosen with replacement from the pool of the 19 CAP-percent/dB slope measurements. This yields an overestimate (i.e. a conservative estimate) of the 95% confidence interval.
DPOAE Measurements
An ER-10C acoustic assembly driven by a TDT SA-1 amplifier was used for all DPOAE measurements. We measured the 2f1-f2 DPOAE, where f1 and f2 are the frequencies of the primary tones, f2/f1=1.2 and L1=10 dB re: L2, where L1 and L2 are the levels of the f1 and f2 tones. The 2f1-f2 DPOAE amplitude fine structure was measured in each of the five CAS-on-CAP subjects by stepping f2 in 6 or 12 Hz increments from around 3.7 to 4.4 kHz with L2 3 dB below the click level used for each of the five CAS-on-CAP subject’s CAP measurements. The f2 at the broad maximum of each subject’s DPOAE amplitude fine structure was used for the DPOAE level function measurements. At each L2 level, 10 DPOAE measurements without CAS were made (which took 16 seconds), followed by 20 measurements with a CAS of 65 dB SPL broadband noise (0.1–10 kHz) (32 seconds), and finally 10 additional measurements without CAS. L2 was generally varied from 40–70 dB SPL, but on one subject L2 was varied from 20–70 dB SPL.
As sound level was increased, the DPOAE amplitudes grew in nearly straight lines at low levels and flattened or dipped at levels above 54 dB SPL. In a region of flat growth or dips, the concept of a MOC-induced effective attenuation does not apply, so we only considered DPOAEs from levels of 54 dB and below. DPOAE data from a single average at a single sound level were rejected if the signal-to-noise ratio was less than 10 dB or the noise from that average was greater than zero dB SPL. The remaining 2f1-f2 DPOAE amplitudes in dB SPL, as functions of their L2 levels in dB SPL, were fitted with a straight line (via MATLAB polyfit), one line each for the without-CAS and with-CAS conditions. The distances (in dB) along the sound-level (L2) axis between the fitted lines for without-CAS and with-CAS conditions quantified the MOC-induced DPOAE effective attenuation. Since the DPOAE measurements were done with tones at frequencies near 4 kHz whereas the CAP measurements were done with clicks, it was unclear what sound level of the DPOAE measurements should be used for the comparison. Changes in the sound level, at which the DPOAE effective attenuation was computed changed the effective attenuation very little for subjects 5 and 7, but for the other subjects it could produce large changes (e.g. +/−1½ dB). To capture the DPOAE effective attenuation for different choices of the comparison sound level, we measured effective attenuation with L2 levels at 0, −5 and −10 dB relative to the click level used for the CAS-on-CAP measurement on that subject. Each subject’s DPOAE effective attenuation was the average of these three resulting effective attenuations. An estimate of the 95% CI was obtained from a bootstrap that included the effective attenuations at all three L2 levels. The bootstrap was done by (a) for each subject, CAS condition (without or with CAS), and L2 level, randomly selecting N DPOAE amplitudes, with replacement, from the pooled original N values at that level, (b) for each subject and CAS condition, fitting a straight line to the selected DPOAE amplitudes versus L2 level, (c) randomly choosing a comparison level (from 0, −5, −10) and calculating the effective attenuation between the without-CAS and with-CAS data at that level, (d) doing steps (a) to (c) 10,000 times and estimating the 95% CI of the resulting distribution. With this method, the uncertainty in the choice of the criterion sound level is included in the size of the 95% CI.
Middle Ear Muscle Reflex Measurements
MEMR thresholds were measured from the CAS-elicited change in the amplitude of a probe tone presented to the same ear used for CAP and DPOAE measurements (the right ear). The CAS was the same broadband noise as that used for measurements of MOC inhibition on CAPs and DPOAEs. A “suppressor” tone was used to suppress the stimulus frequency OAE (SFOAE) produced by the probe tone (Lilaonitkul & Guinan 2009a; Francis & Guinan 2010; Knudson et al. 2014). Suppressing the SFOAE insures that any CAS-induced change in the ear-canal sound pressure level of the probe is due only to the MEMR and not to a MOC effect on the SFOAE. A 40 dB SPL probe tone was presented with a 60 dB SPL suppressor tone that was 50 Hz lower in frequency than the probe and measurements were made with and without our standard 65 dB SPL CAS. Since the SFOAE originates near the peak of the traveling wave (Lichtenhan 2012), a suppressor tone that is 20 dB higher in level than the probe tone and near in frequency to the probe frequency is expected to remove the SFOAE. The CAS broadband noise was varied in level from 50–85 dB SPL while the probe and suppressor levels were constant. At each CAS level the ear-canal sound pressure at the probe frequency was measured without and with CAS. We measured the MEMR threshold using two different probe-tone frequencies: a low-frequency probe of 678 Hz (a frequency that is sometimes used in clinical protocols) and a high-frequency probe equal to the f2 used in the individual ears for our DPOAE measurements. Ten measurements of the sound level at the probe frequency were made without CAS, followed by 20 measurements with CAS, and finally 10 measurements without CAS during a recovery state. This process was repeated across CAS levels.
RESULTS
CAP Measurements
Averaging 2048–4096 responses yielded without-CAS CAP amplitude values that were more variable across runs than the change caused by the MOC reflex. To overcome this, we had to average responses from one click level over five two-hour sessions from each subject. Our initial screen for the lowest click level needed to get a suitable CAP with a modest number of response averages (e.g., 2048) identified the click levels to be used in each subject. These click levels ranged from 52 to 60 dB peSPL across subjects and, relative to behavioral click thresholds, averaged 15.4 dB SL ± 3.6 SD. With clicks set to the level determined for a particular subject, CAPs without and with CAS were measured over approximately 10 hours on each subject. The precise number of averages for each subject was 122,880 (#2), 73,730 (#3), 49,840 (#5), 38,244 (#6), and 46,430 (#7). The total number of averages was different across subjects because of variability in the time needed to prepare subjects for each session and in keeping them alert and comfortable throughout the visit, and because some responses were rejected online due to artifacts.
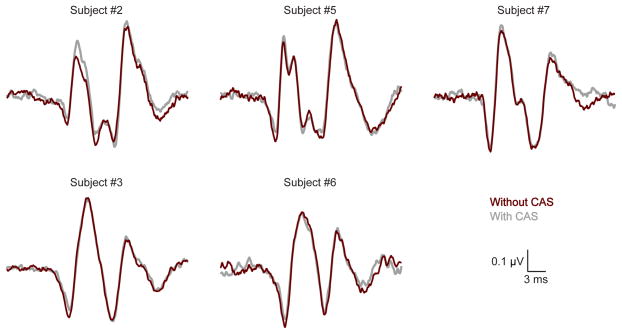
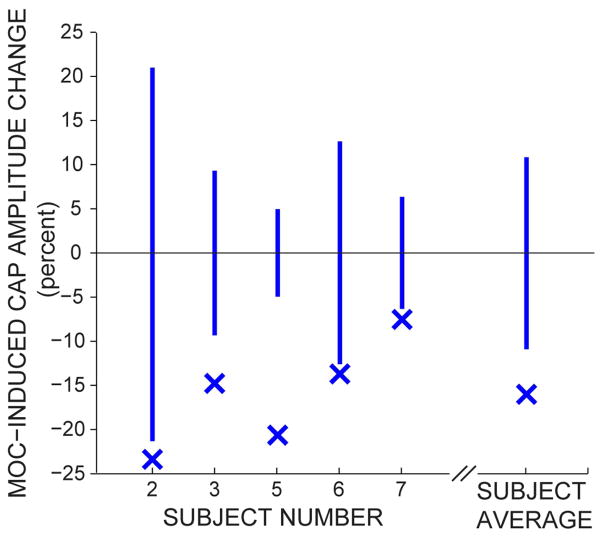
The response waveforms, without- and with-CAS, averaged over all of the individual measurements are shown in Fig. 2. All ABR waveform peaks are deflected downward in Fig. 2 because the tympanic membrane electrode was non-inverting and the high forehead was inverting. The click from the loudspeaker took 4 ms to reach the ear, so in Fig. 2 the CAPs (ABR wave 1) are at ~5 ms and ABR wave 5 at ~8.5 ms in the recorded waveforms. In three subjects (#2, #5, #7), ABR wave 3 is at ~7 ms. Fig. 2 shows that the amplitude of first downward deflection (the CAP) was always less with CAS than without CAS for all CAS-on-CAP subjects, i.e., the MOC reflex inhibited the CAP response in all subjects. This is shown more clearly in Fig. 3, which quantifies the percent reduction of the CAP for each CAS-on-CAP subject and for the average across these five subjects. In all five subjects the MOC reduction of the CAP amplitude was statistically significant (p=0.029, =0.0013, <0.0001, =0.033, and =0.019 in subjects 2, 3, 5, 6, and 7, respectively).
Fig. 2.
Auditory brainstem response (ABR) waveforms recorded from a tympanic-membrane electrode, without (red) and with (gray) the contralateral acoustic stimulus (CAS). All ABR peaks are deflected downward. The cochlear-nerve compound action potential (CAP) is the first negative deflection. Each waveform is the result of approximately 10 hours of response averaging. CAS with broadband noise reduced CAP amplitudes in all subjects.
Fig. 3.
For each subject and for the average across all subjects, the Xs identify the percent reduction in cochlear-nerve compound action potential (CAP) amplitudes produced by broadband noise contralateral acoustic stimulation (CAS). Negative values indicate that CAS reduced the CAP amplitudes. The vertical bars show the 95% confidence intervals for the null distribution, i.e., for the assumption that the without-CAS and with-CAS runs produced no difference in CAP amplitude.
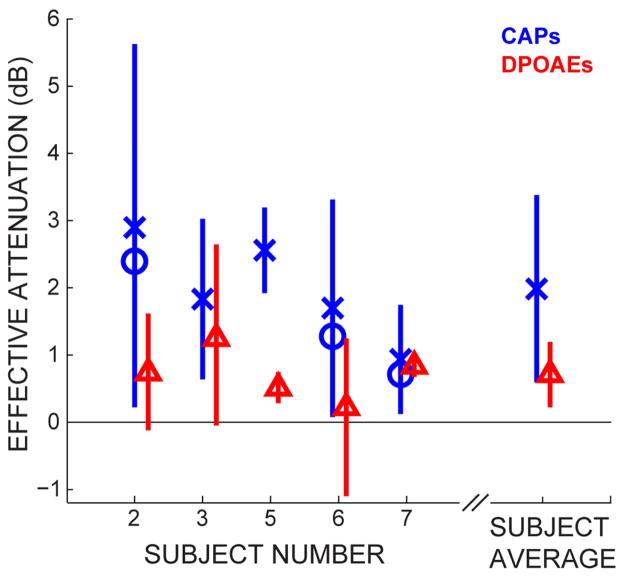
Measurements of CAP amplitudes as a function of click level obtained from 19 subjects were used to calculate the slope of CAP amplitude growth. The average slope was 7.9 (± 4.4 SD by a bootstrap) percent growth for a 1 dB increase in click level. The CAP percent reductions of Fig. 3 were converted to effective attenuations by dividing them by 7.9 percent per dB. For the three CAS-on-CAP subjects for whom we had individual slope measurements, the conversion was also done with the subject’s own slope (Fig. 4).
Fig. 4.
Effective attenuations induced by the medial olivocochlear (MOC) efferent reflex elicited with broadband noise contralateral acoustic stimulation. Effective attenuations of compound action potentials (CAPs) were calculated using average CAP-vs-sound-level growth (X’s) or from individual CAP-vs-sound-level growth (circles). CAP bars are 95% confidence intervals estimated by a bootstrap using the CAP null distribution and the distribution of the CAP growth functions (see Methods). Effective attenuations of distortion product otoacoustic emissions (DPOAEs) are shown as triangles with bootstrapped 95% confidence intervals (see Methods).
DPOAE Measurements
DPOAE responses arise mainly from the f2 region of the cochlea. We targeted the 4 kHz frequency region for DPOAE measurements because 4 kHz tone-evoked behavioral thresholds relate well to click-evoked ABR threshold (Jerger & Mauldin 1978; Gorga et al. 1985). Thus the DPOAEs were evoked with an f2≈4 kHz. In each subject, an f2 was chosen that was near the maximum of the subject’s DPOAE fine structure as determined from DPOAE amplitude measurements with close f2 steps: 3.856 kHz (#2), 4.132 kHz (#3), 4.012 kHz (#5), 4.068 kHz (#6), and 4.18 kHz (#7).
On each CAS-on-CAP subject, DPOAE level functions were obtained with and without the same CAS used in the CAP measurements. From these level functions, the DPOAE effective attenuations were calculated (see Methods). The DPOAE effective attenuations for each subject, and the average across subjects, are shown in Fig. 4.
The CAS-evoked MOC activity produced effective attenuations of DPOAEs and CAPs that varied across subjects (Fig. 4). The MOC-induced effective attenuation on DPOAEs was, on average, less than half the effective attenuation on CAPs. But, the difference was not significant.
Middle Ear Muscle Reflex Thresholds
CAS-evoked MEMR thresholds were measured on each of the five CAS-on-CAP subjects to determine if the middle-ear-muscle reflex influenced the measurements of CAS on CAPs and DPOAEs. The CAS level that yielded a 0.1 dB change in probe level was defined as the MEMR threshold (a stricter criterion than the 1.4%, or 0.12 dB, change used by Abdala et al. 2013). MEMR thresholds were almost always greater than the 65 dB SPL CAS used in our measurements of DPOAEs and CAPs (Table 1). Subject #7 had a MEMR threshold that was 65 dB SPL when measured with a 678 Hz probe tone. However, the MEMR threshold for Subject #7 measured with a ~4 kHz probe tone – the f2 used for DPOAE level functions in individual ears and the frequency that associates best to the click-evoked CAPs – was greater than 65 dB SPL. This indicates that MEMRs had a negligible influence on our measures of MOC-induced inhibition of CAPs and DPOAEs.
Table 1.
Middle ear muscle reflex (MEMR) thresholds for the five CAS-on-CAP subjects.
| Middle Ear Muscle Reflex Thresholds (dB SPL): Probe Frequency | ||
|---|---|---|
| Subject | 678 Hz | ~4 kHz |
| #2 | 80 | 85 |
| #3 | 75 | 85 |
| #5 | >85 | 75 |
| #6 | 80 | 80 |
| #7 | 65 | 70 |
DISCUSSION
MOC inhibition of CAP responses
Using five humans with normal hearing we found that the MOC activity elicited by a 65 dB SPL broadband-noise CAS produced an average reduction of 16% in cochlear-nerve CAP responses to 52–60 dB peSPL (~15 dB SL) clicks. The 16% reduction of CAP amplitude was equivalent to an effective attenuation of 1.98 dB. This is not very large but the stimulus and recording conditions of this experiment were not conditions that would be expected to produce large MOC inhibitions. In choosing the sound level, we had to balance the tradeoff between using a low click level to get a large MOC inhibition and using a high click level to obtain good CAP waveform morphology. It would be erroneous to conclude from these data that peripheral MOC inhibition is less than 2 dB in general. A wide range of data indicates that the MOC inhibition would have been larger if it had been measured at lower sound levels and at a higher-frequency cochlear location. Since the earliest measurements of efferent inhibition, the data have consistently shown that MOC inhibition of CAP responses is largest at sound levels near threshold and decreases as sound level is increased (Desmedt 1962; Gifford & Guinan 1987; Guinan 1996, 2006). In addition, MOC inhibition of auditory-nerve responses in animals parallels the density of MOC innervation (Guinan & Gifford 1988; Maison et al. 2003). Assuming that human MOC innervation is densest in the mid-to-upper basal turn, as it is in animals, the peak MOC density in humans would be at about the 4 kHz place. Our click stimuli had their highest spectral strength at frequencies below about 3 kHz and perhaps they may not have excited MOC fibers in the region of their densest innervation. Another factor is that, at least in animals, half as many MOC fibers are excited by contralateral sound as by ipsilateral sound (Liberman 1988), so our CAS may not have activated as many MOC fibers as ipsilateral or bilateral sound. Finally, the CAS sound level was only 65 dB SPL (which is only a little louder than a normal conversation) and MOC activation increases as sound level increases (Guinan et al. 2003). All of these factors indicate that the measurement of an average MOC effective attenuation of 1.98 dB does not apply to all circumstances. That is to say, for many of the conditions in which MOC inhibition is expected to be the most functionally significant (e.g. the inhibition of near-threshold, high-frequency sounds by MOC activity evoked in a crowded restaurant), MOC inhibition can be expected to be much larger than 1.98 dB.
Another thing to consider in the interpretation of our results is that they were obtained from only five subjects and there was considerable variation in the MOC inhibition from one subject to the next. This is consistent with previous reports of MOC inhibition from human OAE measurements (Backus & Guinan 2007; Marshall et al. 2014). The coefficient of variation of the MOC inhibition across our five subjects was 35% which compares with the coefficient of variation of 32% for the MOC inhibition of the 25 subjects of Backus and Guinan. We can use our measured coefficient of variation to get an estimate of how well the average MOC inhibition from 5 subjects reveals the MOC inhibition that would have been obtained from a large number of subjects. Assuming that MOC inhibition in individual subjects is normally distributed about a mean inhibition of 16%, and using the coefficient of variation we found for our five subjects of 0.35, then a random sample of 5 subjects from this distribution would yield an average MOC inhibition that 95% of the time is in the range 11% to 21% (effective attenuations of 1.4 to 2.6 dB). Thus, our average MOC effect of 1.98 dB is probably not too far from the actual average MOC effect for the conditions of our experiment.
The large variation in MOC inhibition across subjects suggests that whatever benefits MOC inhibition confers (see Guinan 2006), these benefits vary considerably from one subject to the next, at least for passive listening. With this being said, for active listening (i.e. when doing an auditory task or actively trying to hear a signal in noise) it is possible that the differences in MOC activation across subjects are reduced (see de Boer & Thornton 2008).
This is the first report of MOC effects on cochlear-nerve CAP amplitudes from awake humans with what we believe are reliable measurements. Three previous reports measured the effect of contralateral sound on CAPs in humans. Folsom & Owsley (1987) evoked CAPs with filtered clicks, used averages of 4096 responses from an ear-canal electrode (not a tympanic-membrane electrode), and reported that CAS usually produced CAP inhibition, but in one case produced CAP enhancement. We found that averaging 4096 responses was not enough for sufficient noise reduction. Thus, Folsom & Owsley’s conclusions may have been influenced by signal averaging that did not achieve an adequate signal-to-noise ratio (i.e., their report of CAP enhancement may have originated from noise). Using a 4 kHz CAS tone at 45 dB SL, Folsom & Owsley reported CAS-induced CAP reductions of 22% for 30 dB SL clicks and 15% for 40 dB SL clicks and said these produced CAP effective attenuation of 15–20 dB. Their percent CAP reductions are similar to ours, but their effective attenuations are much higher than what we found and are surprisingly high considering that 15–20 dB effective attenuations can be obtained with electric-shock excitation of MOC fibers that is thought to be far more potent than Folsom & Owsley’s pure-tone CAS. Folsom & Owsley’s exemplar response waveforms appear to have uncancelled cochlear microphonic and summating potentials likely resulting from non-alternating stimuli, which would have influenced their measures of CAP amplitude measures – a conclusion also drawn by Puria et al. (1996) and Guinan (2006). Chabert et al. (2002) measured CAS effects on CAPs from 3 of 12 people who maintained normal ABR thresholds during an operation that permitted placement of an electrode on the cochlear nerve. For CAPs from 30 dB HL clicks and using a 50 dB HL white-noise CAS, they found a CAP amplitude reduction of 27% which they converted to a 10 dB equivalent attenuation. Both of these are higher than the values we obtained. Chabert et al.’s larger values are surprising considering that they were achieved in the presence of the depressive influence of anesthesia on MOC reflex inhibition (e.g., Boyev et al. 2002; Guitton et al. 2004; Chambers et al. 2012) and in an operating room environment that can be acoustically and electrically noisy. Finally, Kawase & Takasaka (1995) measured CAPs with a tympanic membrane electrode in awake humans and quantified the CAS-induced release of ipsilaterally-masked CAPs. They did not report CAS effects on un-masked CAPs, leaving us with no comparisons to make with the data we report here.
Psychophysical methods are another approach to quantify MOC effects on total cochlear output (e.g., Kawase et al. 2000; Aguilar et al. 2013; Wicher & Moore 2014; Strickland 2001, 2004, 2008; Wojtczak et al. 2014; Jennings et al. 2009; Roverud & Strickland 2010; Yasin et al. 2014). Perhaps the most suitable measurements for comparison with our data are those of Yasin et al. (2014), although an exact comparison is not possible because we examined the effect of the contralateral MOC reflex on click-evoked CAPs (an objective measure) and they examined the effect of the ipsilateral MOC reflex effects on psychophysical measurements. Yasin et al. (2014) measured noise-band-elicited effects on psychophysical responses to 4 kHz tones for a wide range of conditions. For high-level elicitors and low-level probes, they found MOC-induced effective attenuations of cochlear-amplifier gain of over 20 dB. However, at moderate probe levels (i.e., 55–60 dB SPL) the MOC attenuations were only a few dB. Considering the data from Yasin et al., it seems possible, even likely, that even though we found MOC inhibitions of only a few dB at moderate probe levels, there are much larger MOC inhibitions at low sound levels.
A possibility that needs to be considered is whether the CAS-induced CAP amplitude reductions that we measured were due, or were partly due, to effects of the lateral olivocochlear (LOC) efferent system. LOC fibers synapses on the type I afferent fibers that give rise to the CAP. Previous measurements in lightly anesthetized cats found that CAS-induced inhibition of single-auditory-nerve fiber responses was greatest at the characteristic frequency of each fiber and less at the edges of the tuning curves (Warren & Liberman 1989). This pattern is consistent with MOC reduction of cochlear amplifier gain and is not consistent with direct LOC inhibition of afferent fiber responses (Warren & Liberman 1989). In anesthetized guinea pigs, Larsen & Liberman (2009) presented five minutes of sustained 75 dB SPL contralateral broadband and found suppressions of ipsilateral CAPs, DPOAEs, and round-window noise as well as increases in cochlear microphonic. Based on the constellation of findings, e.g., CAS-induced effects on CAPs paralleled the effects on DPOAEs, Larsen & Liberman argued that all of their findings were mediated by the MOC, and were not due to the LOC efferent system, MEMRs, or acoustic crosstalk. A key observation of Larsen & Liberman was that the effects strongly varied with ipsilateral sound level (larger at low levels) which is an attribute of MOC effects, whereas, in contrast, LOC effects are a constant fraction of baseline amplitudes (Groff & Liberman 2003; Le Prell et al. 2003; Darrow et al. 2007). Finally, LOC neural circuitry is almost entirely ipsilateral, and there is no known contralateral excitatory input to LOC neurons (Brown et al. 2013). In fact, if LOC neurons in the brainstem receive inputs similar to the neurons that surround them in the brainstem, they would receive strong contralateral inhibition (Guinan 1996). Although, a role of LOC efferent system in the CAS-induced CAP amplitude reductions cannot be ruled out, the bulk of available evidence argues against this possibility.
MOC inhibition of DPOAE responses
The effect of a CAS (the same CAS as was used on the CAP responses) on 2f1-f2 DPOAEs elicited with a 42–54 dB SPL f2 near 4 kHz was an average effective attenuation of 0.7 dB. Thus, on average, the effective attenuation on these DPOAEs was less than half the effective attenuation on click-evoked CAPs. This is consistent with Puria et al. (1996) who used lightly anesthetized cats and found effective attenuations to be generally less on DPOAEs than on CAPs evoked by tone pips at the f2 frequency. Larsen & Liberman (2009) also found that CAS produced smaller changes in DPOAEs than in CAPs. One limitation of our comparisons of human MOC inhibition on DPOAEs and CAPs is that the CAP data were obtained with clicks while our DPOAE data were obtained with f2’s near 4 kHz. Our choice to use clicks to evoke CAPs instead of tone bursts near the f2 frequency was a compromise brought about by the long averaging times needed to get accurate CAP measurements. Clicks elicit single-auditory-nerve-fiber responses throughout the cochlea, but only responses from mid-to-high frequency regions align in time and produce the first peak of the CAP response (Liberman et al. 2010). In experiments where both were measured, the thresholds of click-evoked CAP measurements covaried most closely with behavioral tone thresholds at 2–4 kHz (Jerger & Mauldin 1978; Gorga et al. 1985). Our clicks had marked low frequency energy (Fig. 1) so the resulting CAPs may have originated from more apical regions than would have been true for clicks with flatter spectra. Nonetheless, MOC effects at 4 kHz are of special interest because the 4 kHz region is particularly vulnerable to damage from acoustic overexposure that MOC reflex might protect against. Although our data suggest that human MOC inhibition is larger on click-evoked CAP responses than on DPOAEs from f2’s near 4 kHz, at frequencies below 4 kHz, MOC effects on stimulus frequency OAEs (SFOAEs) are larger at 0.5 and 1 kHz than at 4 kHz (Lilaonitkul & Guinan 2009b). This may mean that at low frequencies (e.g., 1 kHz) MOC effects on OAEs are larger than on CAPs. However, in anesthetized cats where an electrode can be put directly on the cochlea to measure tone-burst evoked CAPs and DPOAEs from an f2 equivalent in frequency to the CAP-evoking tone burst, there was little difference across frequency in the relationship between the MOC effects on CAPs and DPOAEs (Puria et al. 1996). CAP responses come from the peak of the traveling wave (Kiang 1965; Dolan et al. 1985; Cheatham et al. 2010; Cody et al. 1980) and receive the full benefit of cochlear amplification, whereas DPOAEs arise primarily just basal to the f2 cochlear place (Shera & Guinan 2007) and in this region the responses to f1 and f2 are not fully cochlear amplified, thus it is not surprising that MOC effects on click-evoked CAP responses are larger than on DPOAE responses. It should be kept in mind that, in individual ears, our CAS-on-CAP measurements were a result of approximately 10 hours of response averaging while our CAS-on-DPOAE measurements were from a mere 32 seconds of averaging at each sound level. While the primary goal of our work was to obtain excellent CAP measurements and rigorously analyze the MOC inhibition of CAP amplitude, the comparison with DPOAE data was a secondary goal and only the general comparison, and not detailed comparisons on individual subjects, is warranted. Nevertheless, our first-ever results from humans are consistent with what has been previously found in animals.
The Middle Ear Muscle Reflex and MOC effective attenuation
We measured MEMR thresholds by using ear-canal sound pressure measurements of a tone while suppressing its SFOAEs so that any change in sound pressure must be from MEMR contractions. We used both a clinically-conventional low-frequency probe tone, and a higher-frequency probe equal to the f2 for the individual-ear DPOAEs, to test if the MEMR influenced transmission of our DPOAE- and CAP-evoking stimuli. Consistent with other reports, we found that MEMR thresholds can be lower than clinical audiology norms, and that higher frequency probe tones such as that used for our DPOAE and CAP measurements are affected less by MEMRs (Goodman & Keefe 2006; Zhao & Dhar 2010). Our results indicate that CAS-elicited changes in middle ear transmission did not significantly affect our DPOAE and CAP stimuli. Thus, our measured changes in CAPs and DPOAEs are attributable to MOC effects.
Clinical uses and the importance of understanding MOC effects
To understand what the MOC system does in normal ears, it is important to study MOC effects on cochlear nerve responses. With this knowledge we can begin to illuminate how the MOC reflex influences hearing in diseased ears. While we quantified MOC reflex inhibition on click-evoked cochlear nerve responses using an electrode on the human tympanic membrane, the amount of time needed for adequate response averaging will limit its clinical utility. Measurements with a trans-tympanic electrode (e.g., Verschooten & Joris 2014) would likely require fewer averages, but nonetheless this has limitations for clinical utility: the procedure does not avoid the requirement that patients remain awake and mentally alert, the hole in the tympanic membrane can cause problems for in situ measurement of sound pressure levels, and these measurements would certainly not be possible for challenging cases of, for example, children with Autism – in whom MOC measurements may be helpful (Danesh & Kaf 2012).
One final note regards the effect of CAS on ABR wave 5. Clinical uses of ABRs focus on wave 5 and our data show small effects of CAS on wave 5 (Fig. 1). We made no attempt to analyze this further because CAS effects on wave 5 may originate from complicated MOC effects in the cochlea, cochlear nucleus, or from other processes – both inhibitory and excitatory – throughout the brainstem (Bledsoe et al. 2009; Brown 2011; Brown et al. 2013).
CONCLUSIONS
MOC activity elicited by contralateral broadband noise was shown to reduce click-evoked CAP amplitudes in the first-ever reliable measurements with this paradigm in awake, alert, normal-hearing humans. A preliminary assessment using sparse DPOAE data found that the same contralateral broadband noise elicited MOC activity that evoked smaller changes in DPOAEs from the same ears, a result consistent with previous findings in laboratory animals.
Highlights.
A tympanic-membrane electrode recorded human compound action potentials (CAPs)
Medial olivocochlear efferent (MOC) activity was elicited by contralateral noise
Reliable MOC reflex effects on human CAPs are reported for the first time
The reflex is stronger than predicted from common otoacoustic emissions measures
Acknowledgments
We thank Spencer B. Smith and Drs. Alec N. Salt and Magdalena Wojtczak for productively criticizing earlier versions of this work. Support was provided by the McDonnell Center for Systems Neuroscience, the American Otological Society, and the National Institutes of Health, National Institute on Deafness and Other Communication Disorders (R01 DC005977).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdala C, Mishra S, Garinis A. Maturation of the human medial efferent reflex revisited. J Acoust Soc Am. 2013;133:938–50. doi: 10.1121/1.4773265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar E, Eustaquio-Martin A, Lopez-Poveda EA. Contralateral efferent reflex effects on threshold and suprathreshold psychoacoustical tuning curves at low and high frequencies. J Assoc Res Otolaryngol. 2013;14(3):341–57. doi: 10.1007/s10162-013-0373-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backus BC, Guinan JJ., Jr Measurement of the Distribution of Medial Olivocochlear Acoustic Reflex Strengths Across Normal-Hearing Individuals via Otoacoustic Emissions. J Assoc Res Otolaryngol. 2007;8:484–496. doi: 10.1007/s10162-007-0100-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bledsoe SC, Jr, Koehler S, Tucci DL, Zhou J, Le Prell C, Shore SE. Ventral cochlear nucleus responses to contralateral sound are mediated by commissural and olivocochlear pathways. J Neurophysiol. 2009;102(2):886–900. doi: 10.1152/jn.91003.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyev KP, Liberman MC, Brown MC. Effects of anesthesia on efferent-mediated adaptation of the DPOAE. J Assoc Res Otolaryngol. 2002;3(3):362–73. doi: 10.1007/s101620020044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MC. Anatomy of olivocochlear neurons. In: Ryugo David K, Fay Richard R, Popper Arthur., editors. Springer Handbook of Auditory Research. Vol. 38. 2011. pp. 25–27. [Google Scholar]
- Brown MC, Mukerji S, Drottar M, Windsor AM, Lee DJ. Identification of inputs to olivocochlear neurons using transneuronal labeling with pseudorabies virus (PRV) J Assoc Res Otolaryngol. 2013;14(5):703–17. doi: 10.1007/s10162-013-0400-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabert R, Magnan J, Lallemant JG, Uziel A, Puel JL. Contralateral sound stimulation suppresses the compound action potential from the auditory nerve in humans. Otol Neurotol. 2002;23:784–8. doi: 10.1097/00129492-200209000-00029. [DOI] [PubMed] [Google Scholar]
- Chambers AR, Hancock KE, Maison SF, Liberman MC, Polley DB. Sound-evoked olivocochlear activation in unanesthetized mice. J Assoc Res Otolaryngol. 2012;13:209–17. doi: 10.1007/s10162-011-0306-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheatham MA, Naik K, Dallos P. Using the cochlear microphonic as a tool to evaluate cochlear function in mouse models of hearing. J Assoc Res Otolaryngol. 2010;12:113–125. doi: 10.1007/s10162-010-0240-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chertoff M, Lichtenhan J, Willis M. Click- and chirp-evoked human compound action potentials. J Acoust Soc Am. 2010;127(5):2992–6. doi: 10.1121/1.3372756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cody AR, Robertson D, Bredberg G, Johnston BM. Electrophysiological and morphological changes in the guinea pig cochlea following mechanical trauma to the organ of corti. Acta Otolaryngol. 1980;89:440–452. doi: 10.3109/00016488009127160. [DOI] [PubMed] [Google Scholar]
- Danesh AA, Kaf WA. DPOAEs and contralateral acoustic stimulation and their link to sound hypersensitivity in children with autism. Int J Audiol. 2012;51(4):345–52. doi: 10.3109/14992027.2011.626202. [DOI] [PubMed] [Google Scholar]
- Darrow KN, Maison SF, Liberman MC. Selective removal of lateral olivocochlear efferents increases vulnerability to acute acoustic injury. J Neurophysiol. 2007;97:1775–1785. doi: 10.1152/jn.00955.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer J, Thornton AR. Neural correlates of perceptual learning in the auditory brainstem: efferent activity predicts and reflects improvement at a speech-in-noise discrimination task. J Neurosci. 2008;28:4929–37. doi: 10.1523/JNEUROSCI.0902-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmedt JE. Auditory-evoked potentials from cochlea to cortex as influenced by activation of the efferent olivocochlear bundle. J Acous Soc Am. 1962;34:1478–1496. [Google Scholar]
- Dolan TG, Mills JH, Schmiedt RA. A comparison of brainstem, whole-nerve AP and single-fiber tuning curves in gerbil: Normative data. Hear Res. 1985;17:259–266. doi: 10.1016/0378-5955(85)90070-x. [DOI] [PubMed] [Google Scholar]
- Folsom RC, Owsley RM. N1 action potentials in humans. Influence of simultaneous contralateral stimulation. Acta Otolaryngol (Stockh) 1987;103:262–265. doi: 10.3109/00016488709107792. [DOI] [PubMed] [Google Scholar]
- Francis NA, Guinan JJ., Jr Acoustic stimulation of human medial olivocochlear efferents reduces stimulus-frequency and click-evoked otoacoustic emission delays: Implications for cochlear filter bandwidths. Hear Res. 2010;267(1–2):36–45. doi: 10.1016/j.heares.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich P, Collet L, Valatx JL, Morgon A. Sleep and active cochlear micromechanical properties in human subjects. Hear Res. 1993;66:1–7. doi: 10.1016/0378-5955(93)90254-x. [DOI] [PubMed] [Google Scholar]
- Gifford ML, Guinan JJ., Jr Effects of electrical stimulation of medial olivocochlear neurons on ipsilateral and contralateral cochlear responses. Hearing Res. 1987;29:179–194. doi: 10.1016/0378-5955(87)90166-3. [DOI] [PubMed] [Google Scholar]
- Goodman SS, Keefe DH. Simultaneous measurement of noise-activated middle-ear muscle reflex and stimulus frequency otoacoustic emissions. J Assoc Res Otolaryngol. 2006;7(2):125–39. doi: 10.1007/s10162-006-0028-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorga MP, Worthington DW, Reiland JK, Beauchaine KA, Goldgar DE. Some comparisons between auditory brain stem response thresholds, latencies, and the pure-tone audiogram. Ear & Hear. 1985;6(2):105–12. doi: 10.1097/00003446-198503000-00008. [DOI] [PubMed] [Google Scholar]
- Groff JA, Liberman MC. Modulation of cochlear afferent response by the lateral olivocochlear system: activation via electrical stimulation of the inferior colliculus. J Neurophysiol. 2003;90:3178–3200. doi: 10.1152/jn.00537.2003. [DOI] [PubMed] [Google Scholar]
- Guinan JJ., Jr . The Physiology of Olivocochlear Efferents. In: Dallos PJ, Popper AN, Fay RR, editors. The Cochlea. Springer Handbook of Auditory Research. Springer-Verlag; New York: 1996. pp. 435–502. [Google Scholar]
- Guinan JJ., Jr Olivocochlear efferents: anatomy, physiology, function, and the measurement of efferent effects in humans. Ear & Hear. 2006 Dec;27(6):589–607. doi: 10.1097/01.aud.0000240507.83072.e7. Review. Erratum in: Ear & Hear. 2007 Feb;28(1):129. [DOI] [PubMed] [Google Scholar]
- Guinan JJ, Backus BC, Lilaonitkul W, Aharonson V. Medial olivocochlear efferent reflex in humans: otoacoustic emission (OAE) measurement issues and the advantages of stimulus frequency OAEs. J Assoc Res Otolaryngol. 2003;4:521–540. doi: 10.1007/s10162-002-3037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guitton MJ, Avan P, Puel JL, Bonfils P. Medial olivocochlear efferent activity in awake guinea pigs. Neuroreport. 2004;15:1379–82. doi: 10.1097/01.wnr.0000131672.15566.64. [DOI] [PubMed] [Google Scholar]
- Jennings SG, Strickland EA, Heinz MG. Precursor effects on behavioral estimates of frequency selectivity and gain in forward masking. J Acoust Soc Am. 2009;125(4):2172–81. doi: 10.1121/1.3081383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerger J, Mauldin L. Prediction of sensorineural hearing level from the brain stem evoked response. Arch Otolaryngol. 1978;104(8):456–61. doi: 10.1001/archotol.1978.00790080038010. [DOI] [PubMed] [Google Scholar]
- Kawase T, Ogura M, Hidaka H, Sasaki N, Suzuki Y, Takasaka T. Effects of contralateral noise on measurement of the psychophysical tuning curve. Hear Res. 2000;142(1–2):63–70. doi: 10.1016/s0378-5955(00)00010-1. [DOI] [PubMed] [Google Scholar]
- Kawase T, Takasaka T. The effects of contralateral noise on masked compound action potential in humans. Hear Res. 1995;91(1–2):1–6. doi: 10.1016/0378-5955(95)00145-x. [DOI] [PubMed] [Google Scholar]
- Keefe DH, Schairer KS, Ellison JC, Fitzpatrick DF, Jesteadt W. Use of stimulus-frequency otoacoustic emissions to investigate efferent and cochlear contributions to temporal overshoot. J Acoust Soc Am. 2009;125(3):1595–604. doi: 10.1121/1.3068443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiang NYS. MIT Research Monograph No. 35. Cambridge MA: M.I.T. Press; 1965. Discharge patterns of single-fibers in the cat’s auditory nerve. [Google Scholar]
- Knudson IM, Shera CA, Melcher JR. Increased contralateral suppression of otoacoustic emissions indicates a hyper-responsive medial olivocochlear system in humans with tinnitus and hyperacusis. J Neurophysiol. 2014;112(12):3197–208. doi: 10.1152/jn.00576.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen E, Liberman MC. Slow build-up of cochlear suppression during sustained contralateral noise: Central modulation of olivocochlear efferents? Hear Res. 2009;256:1–10. doi: 10.1016/j.heares.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Prell CG, Shore SE, Hughes LF, Bledsoe SC., Jr Disruption of lateral efferent pathways: functional changes in auditory evoked responses. J Assoc Res Otolaryngol. 2003;4:276–290. doi: 10.1007/s10162-002-3018-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC. Response properties of cochlear efferent neurons: monaural vs. binaural stimulation and the effects of noise. J Neurophysiol. 1988;60:1779–1798. doi: 10.1152/jn.1988.60.5.1779. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Rosowski JJ, Lewis RF. Physiology and Pathophysiology. In: Merchant SN, Nadol JB Jr, editors. Schuknecht’s Pathology of the Ear. 3. People’s Medical Publishing House; USA: 2010. pp. 98–136. [Google Scholar]
- Lichtenhan JT. Effects of low-frequency biasing on otoacoustic and neural measures suggest that stimulus-frequency otoacoustic emissions originate near the peak region of the traveling wave. J Assoc Res Otolaryngol. 2012;13(1):17–28. doi: 10.1007/s10162-011-0296-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenhan JT, Chertoff ME. Temporary hearing loss influences post-stimulus time histogram and single neuron action potential estimates from human compound action potentials. J Acoust Soc Am. 2008;123(4):2200–12. doi: 10.1121/1.2885748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilaonitkul W, Guinan JJ., Jr Reflex control of the human inner ear: a half-octave offset in medial efferent feedback that is consistent with an efferent role in the control of masking. J Neurophysiol. 2009a;101(3):1394–406. doi: 10.1152/jn.90925.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilaonitkul W, Guinan JJ., Jr Human Medial Olivocochlear Reflex: Effects as Functions of Contralateral, Ipsilateral, and Bilateral Elicitor Bandwidths. J Assoc Res Otolaryngol. 2009b;10:459–70. doi: 10.1007/s10162-009-0163-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maison SF, Adams JC, Liberman MC. Olivocochlear innervation in the mouse: immunocytochemical maps, crossed versus uncrossed contributions, and transmitter colocalization. J Comp Neurol. 2003;455:406–416. doi: 10.1002/cne.10490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall L, Lapsley Miller JA, Guinan JJ, Jr, Shera CA, Reed CM, Perez ZD, Delhorne LA, Boege P. Otoacoustic-emission-based medial-olivocochlear reflex assays for humans. J Acoust Soc Am. 2014;136:2697–713. doi: 10.1121/1.4896745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puria S, Guinan JJ, Jr, Liberman MC. Olivocochlear reflex assays: Effects of contralateral sound on compound action potentials vs. ear-canal distortion products. J Acoust Soc Am. 1996;99:500–507. doi: 10.1121/1.414508. [DOI] [PubMed] [Google Scholar]
- Roverud E, Strickland EA. The time course of cochlear gain reduction measured using a more efficient psychophysical technique. J Acoust Soc Am. 2010;128:1203–1214. doi: 10.1121/1.3473695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shera CA, Guinan JJ., Jr Evoked otoacoustic emissions arise by two fundamentally different mechanisms: a taxonomy for mammalian OAEs. J Acoust Soc Am. 1999;105(2 Pt 1):782–98. doi: 10.1121/1.426948. [DOI] [PubMed] [Google Scholar]
- Shera CA, Guinan JJ., Jr . Mechanisms of mammalian otoacoustic emission. In: Manley GA, Lonsbury-Martin BL, Popper AN, Fay R, editors. Active Processes and Otoacoustic Emissions. Springer; New York: 2007. pp. 305–342. [Google Scholar]
- Strickland EA. The relationship between frequency selectivity and overshoot. J Acoust Soc Am. 2001;109(5 Pt 1):2062–73. doi: 10.1121/1.1357811. [DOI] [PubMed] [Google Scholar]
- Strickland EA. The temporal effect with notched-noise maskers: analysis in terms of input-output functions. J Acoust Soc Am. 2004;115(5 Pt 1):2234–45. doi: 10.1121/1.1691036. [DOI] [PubMed] [Google Scholar]
- Strickland EA. The relationship between precursor level and the temporal effect. J Acoust Soc Am. 2008;123(2):946–54. doi: 10.1121/1.2821977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verschooten E, Joris PX. Estimation of neural phase locking from stimulus-evoked potentials. J Assoc Res Otolaryngol. 2014;15(5):767–87. doi: 10.1007/s10162-014-0465-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren EH, III, Liberman MC. Effects of contralateral sound on auditory-nerve responses. I. Contributions of cochlear efferents. Hear Res. 1989;37:89–104. doi: 10.1016/0378-5955(89)90032-4. [DOI] [PubMed] [Google Scholar]
- Wicher A, Moore BC. Effect of broadband and narrowband contralateral noise on psychophysical tuning curves and otoacoustic emissions. J Acoust Soc Am. 2014;135(5):2931–41. doi: 10.1121/1.4871358. [DOI] [PubMed] [Google Scholar]
- Wittekindt A, Kaiser J, Abel C. Attentional modulation of the inner ear: a combined otoacoustic emission and EEG study. J Neurosci. 2014;34(30):9995–10002. doi: 10.1523/JNEUROSCI.4861-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtczak M, Beim JA, Oxenham AJ. Exploring the role of feedback-based auditory reflexes in forward masking by Schroeder-phase complexes. J Assoc Res Otolaryngol. 2014;16(1):81–99. doi: 10.1007/s10162-014-0495-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasin I, Drga V, Plack CJ. Effect of human auditory efferent feedback on cochlear gain and compression. J Neurosci. 2014;34:15319–26. doi: 10.1523/JNEUROSCI.1043-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]