Abstract
Background
Autism Spectrum Disorder (ASD) is characterized by social communication deficits and restricted, repetitive patterns of behavior. Varied immunological findings have been reported in children with ASD. To address the question of heterogeneity in immune responses, we sought to examine the diversity of immune profiles within a representative cohort of boys with ASD.
Methods
Peripheral blood mononuclear cells (PBMC) from male children with ASD (n=50) and from typically developing (TD) age-matched male controls (n=16) were stimulated with either lipopolysaccharide (LPS) or phytohaemagglutinin (PHA). Cytokine production was assessed after stimulation. The ASD study population was clustered into subgroups based on immune responses and assessed for behavioral outcomes.
Results
Children with ASD who had a pro-inflammatory profile based on LPS stimulation were more developmentally impaired as assessed by the Mullen's Scale of Early Learning (MSEL). They also had greater impairments in social affect as measured by the Autism Diagnostic Observation Schedule (ADOS). These children also displayed more frequent sleep disturbances and episodes of aggression. Similarly, children with ASD and a more activated T cell cytokine profile after PHA stimulation were more developmentally impaired as measured by the MSEL.
Conclusions
Children with ASD may be phenotypically characterized based upon their immune profile. Those showing either a pro-inflammatory response or increased T cell activation/skewing display a more impaired behavioral profile than children with non-inflamed or non-T cell activated immune profiles. These data suggest that there may be several possible immune subphenotypes within the ASD population that correlate with more severe behavioral impairments.
Keywords: Autism Spectrum Disorder, behavior, cytokine, immune, cluster analysis, children, endophenotyping
Introduction
Autism spectrum disorder (ASD) is a heterogeneous neurodevelopmental disorder characterized by stereotyped interests, repetitive behaviors and impairments in social communication. Currently 1 in 68 children have been identified as having ASD (1, 2). Despite the high prevalence of ASD, the etiology(ies) and pathogenesis remain poorly understood. Numerous published findings have identified widespread differences in the immune systems of children with ASD, both at the systemic and cellular levels (3). These differences have been associated with impairments in the core features of ASD as well as other aberrant behaviors, decreased adaptability and more impaired cognition in children with ASD (4, 5). Neuroimmune interactions begin during early neurodevelopment and continue throughout life, with the immune system supporting many aspects of neuronal function. Dysregulation in the immune system could increase sensitivity to altered neurologic function from a variety of sources, particularly during early development (6). Importantly, altered immune function in the brain of individuals with ASD is suggested by evidence of neuroglial activation and neuroinflammation in the CNS (7), as well as the presence of antibodies reactive to neuronal tissue (8-12) in a subset of children with ASD. These immune differences may be related to numerous genetic associations linked with immune functions in ASD, such as linkages to the major histocompatibility complex (HLA) genes (13-15).Consequently, research that identifies the biological basis of the immune dysfunction in children with autism may facilitate early detection, prevention and/or treatment.
Among the immunological findings in ASD, reports of increased pro-inflammatory markers remain the most consistently observed. These include increased levels of pro-inflammatory cytokines such as IL-1β, TNFα and IL-6, and chemokines such as MCP-1, in plasma, cerebral spinal fluid (CSF) and post mortem brain tissue (4, 16-19). As noted, many of these studies highlight a connection between immune dysregulation and more impaired behaviors (5). These findings suggest a pro-inflammatory immune profile is prevalent within the ASD population, or at least in a subgroup of the population, where immune activation may be linked to more impaired behavioral symptomology.
In this study we utilize a cohort of children which consists of children primarily from Northern California with a broad presentation of ASD severity and extent of comorbid features along with age- and geographically matched typically developing (TD) controls. We hypothesized that within this well-defined cohort of children, a subgroup could be identified based on immune function that would be associated with a characteristic behavioral profile. To determine this relationship, children in the study received a battery of cognitive and behavioral assessments along with a number of physiological measures including neurological and immunological evaluations. Immune measures were used to cluster children into subgroups and behaviors were assessed between resulting groups.
Material and Methods
Subjects
Participants were enrolled through the Autism Phenome Project (APP) study conducted at UC Davis M.I.N.D. institute. The study protocols including recruitment and behavioral assessments have been previously described in detail (20, 21). Because of the gender disparities in ASD, we choose to focus on males to eliminate issues of gender differences. In brief, following clinical evaluation for diagnostic confirmation, participants were placed in one of two groups: (1) children with a confirmed diagnosis of ASD [N=50, median age 3.21 (interquartile range 2.80–3.52)] years; or (2) children who were confirmed as typically developing (TD) controls [N=16, 2.80 (2.47-3.14)] years. Final diagnosis of ASD was confirmed by the Autism Diagnostic Interview-Revised (ADI-R) (22) and the Autism Diagnostic Observation Schedule (ADOS) (23). The ADOS and ADI-R consists of a standardized, semi-structured interview and a diagnostic algorithm from the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition Text Revision (DSM-IVTR) (24), with definitions of autism from the International Classification of Diseases, Tenth Revision (ICD-10) (25). Inclusion criteria for the TD children included scores on the Lifetime Edition of the Social Communication Questionnaire (26), a screening tool for ASD, below the recommended screening cutoff for young children of 11 and developmental scores within 2 standard deviations of the mean on all subscales of the Mullen Scale of Early Learning (MSEL) (27). Exclusion criteria for TD controls included a diagnosis of intellectual disability, pervasive developmental disorder, or specific language impairment, or any known developmental, neurological, or genetic disorder. The TD controls were recruited through interactions with school districts and other outreach mechanisms. The exclusion criteria for all subjects consisted of the presence of Fragile X syndrome or other serious neurological (e.g., seizures), psychiatric (e.g., bipolar disorder), or known medical conditions including autoimmune disease, inflammatory bowel disease or celiac disease. The administration of all diagnostic instruments was carried out by experienced clinicians at the MIND Institute.
All children enrolled in the study were assessed for developmental performance using MSEL. The MSEL has components for visual reception, fine motor, receptive language and expressive language. Standardized developmental quotients (DQs) were calculated for each subscale of the MSEL by dividing each child's age-equivalent score by their chronological age at the time of testing and multiplying by 100 in order to provide a consistent metric for cognitive measures and to accommodate floor effects. The Child Behavior Checklist (CBCL) (28) was provided by parents of children in the study and consists of questions designed to measure behavioral issues in children, including sleep, hyperactivity, and aggression.
Participant groups did not differ significantly for age. All children were free of any major immune modifying medications and in good health and free of fever (temperature < 100.5°F) at the time of blood draw. All participants were native English speakers, ambulatory, and had no suspected vision or hearing problems. All subjects were screened via parental interview for current and past physical illness. This study was approved by the institutional review boards at the University of California, Davis. Informed consent was obtained prior to participation.
Cell isolation
Peripheral blood (8.5 ml) was collected in an acid-citrate-dextrose Vacutainer tube (BD Biosciences; San Jose, CA) and processed within 12 hours of collection. Blood was centrifuged for 10 min at 2300 rpm, and plasma was drawn off and stored at −80°C. The blood pellet was mixed 1:1 with Hanks Balanced Salt Solution (HBSS; Gibco, Gaithersburg, MD) without Ca2+ or Mg2+. The diluted blood was then layered, carefully, over a Ficoll-Paque gradient (Pharmacia Biotech, Piscataway, NJ) and centrifuged at 1,700 rpm for 30 minutes at room temperature. PBMC were then harvested from the interface layer and washed twice with HBSS. Viability was determined by trypan blue exclusion. Cells were then re-suspended at a final concentration of 0.5 × 106 cells/mL in tissue culture medium consisting of: RPMI 1640 (Gibco) supplemented with 10% low endotoxin heat inactivated fetal bovine serum (Omega Scientific; Tarzana, CA), 100 IU/ml penicillin and 100 IU/ml streptomycin (Sigma, St Louis, MO).
Cellular Stimulations
Isolated PBMC were stimulated for 48 hours in RPMI 1640 media with 10% fetal bovine serum (FBS, Gibco), 1% Penicillin and Streptomycin alone or with the addition of either 1.0 μg/mL lipopolysaccharide (LPS; Escherichia coli serotype 0111:B4, Sigma, St. Louis, MO) or 8 μg/mL Phytohaemagglutinin (PHA; Sigma) and cultured at 37°C with 5% CO2. After this period, cells were collected and spun at 2,000 rpm for 10 minutes and supernatants were collected and stored at −80°C until analyzed by Luminex multiplexing technology. 48 hours stims were used to relate this work to previous work by our group.
Luminex multiplex analysis
Supernatants from LPS stimulated cultures were quantified for GM-CSF, IL-1β, IL-6, IL-10, IL-12(p40), MCP-1(CCL2) and TNFα, and supernatants PHA stimulated cultures were quantified for IFNγ, IL-10, IL-13, and IL-17 using human multiplexing bead immunoassays (Millipore, Billerica, MA). Supernatants from media alone (unstimulated) cultures were analyzed as controls for the cytokines listed above. Samples were run per manufacturer recommendations. Specifically, 25μL of supernatant was incubated with antibody-coupled beads. After a series of washes, a biotinylated detection antibody was added to the beads, and the reaction mixture was detected by the addition of streptavidin–phycoerythrin. The bead sets were analyzed using a flow-based Luminex™ 100 suspension array system (Bio-Plex 200; Bio-Rad Laboratories, Inc.). Unknown sample cytokine concentrations were calculated by Bio-Plex Manager software using a standard curve derived from the known reference cytokine concentrations supplied by the manufacturer. A five-parameter model was used to calculate final concentrations and values are expressed in pg/ml. The sensitivity of this assay allowed the detection of cytokine concentrations with the following limits of detection: IFNγ (4.0 pg/mL), GM-CSF (2.3 pg/mL), IL-1β (0.7 pg/mL), IL-6 (0.4 pg/mL), IL-10 (0.3 pg/mL), IL-12p40 (12.3 pg/mL), IL-13 (0.3 pg/mL), IL-17 (0.4 pg/mL), MCP-1 (1.2 pg/mL) and TNFα (0.2 pg/mL). Values below the limit of detection (LOD) were replaced with one-half the LOD. Supernatant aliquots were thawed only once.
Cluster Analysis
Cluster analysis was performed using a k-medians algorithm using a Euclidean dissimilarity measure on all children, including both children with ASD and TD, in the study. Based on previous observations in the literature (Reviewed in 5), a number of cytokines associated with inflammatory or T cell processes, previously reported as altered in ASD, were used to discriminate subgroups in ASD based on cytokine production after immune challenge. For analysis of the LPS data, the inflammatory markers IL-1β, IL-6, IL-10, MCP-1, and TNFα were used as discriminating variables; for PHA-treated samples, IFNγ (TH1) and IL-13 (TH2) were used. Two groups were assumed for the LPS data, “pro-inflammatory” verses “non-inflammatory”, and three groups for the PHA data, “TH1-skewed”, “TH2-skewed”, or “non-skewed”. Biplots for LPS data were generated using IL-1β, IL-6, IL-10, MCP-1, and TNFα as variables. For the PHA data, biplots were generated using IFNγ and IL-13 as variables. In both cases variables were standardized.
Statistical Analysis
Data analysis was performed using STATA 12 software. Data was determined to be non-parametric using the Shapiro-Wilks test for normality. The Wilcoxon-rank sum test was used for between subject group comparisons. A probability value (p) of less than 0.05 was considered to be significant.
Results
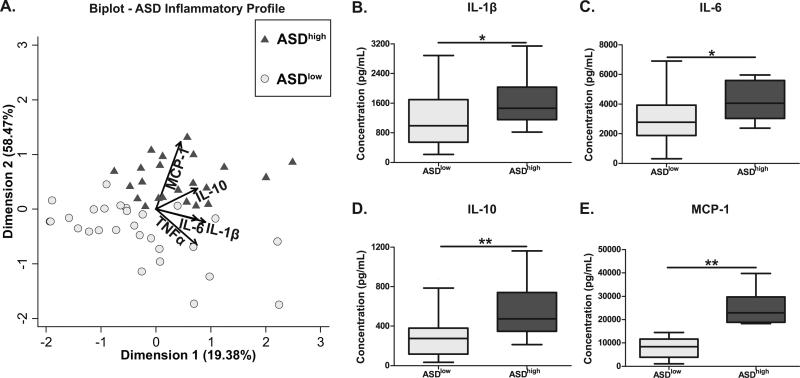
We stratified children in this study based on immunological measures using a k-median cluster analysis algorithm. Children with ASD were grouped into two clusters. The first cluster (ASDhigh; n=22) consisted of subjects whose PBMC displayed a pro-inflammatory response profile based on cytokine production after stimulation with LPS. The second cluster (ASDlow; n=28) consisted of subjects who displayed a less robust response to LPS (Figure 1A). Children in the ASDhigh group displayed significantly increased production of IL-1β [1,465.7 (1,115.9-2,028.2) pg/mL vs. 994.3 (558.1-1,668.1) pg/mL; p = 0.04], IL-6 [4,060.5 (3,061.7-5,552.3) pg/mL vs. 2,768.1 (1917.2-3916.5) pg/mL; p = 0.01], IL-10 [474.6 (358.8-734.5) pg/mL vs. 275.2 (119.0-376.0) pg/mL; p < 0.01], and MCP-1 [22,891.9 (18,903.5-29,395.6) pg/mL vs. 8,414.1 (3,945.4-11,500.0) pg/mL; p < 0.01] when compared with the ASDlow group (Figure 1B-F).
Figure 1. Cluster analysis of PBMC cytokine response to LPS.
Children with ASD were clustered into two groups based on a k-medians cluster analysis using the inflammatory markers IL-1β, IL-6, IL-10, MCP-1, and TNFα as measured in PBMC cell culture supernatants after LPS stimulation. The distribution of subjects based on this clustering are shown using a (A) biplot to indicate the vectors each particular cytokine has in discriminating the groups. Vectors points in the direction which is most like the variable represented by the vector. Axis represent principal complements 1 and 2. Post clustering analysis of cytokines production between the groups shows the ASDhigh groups produces significantly more (B) IL-1β (p=0.04), (C) IL-6 (p=0.01), (D) IL-10 (p<0.01), and (E) MCP-1 (p<0.01) than the ASDlow group. * p<0.05, ** p<0.01.
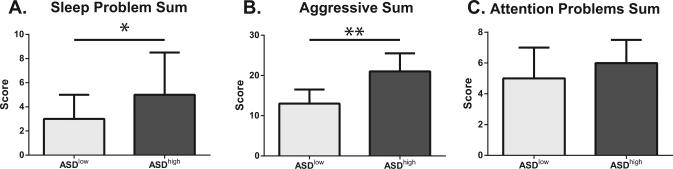
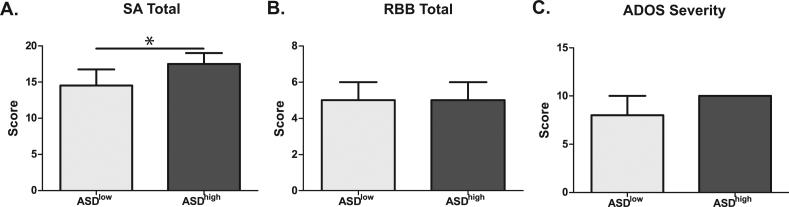
Using this cluster analysis we next assessed differences in behavioral measures between the ASDhigh and ASDlow groups. Children in the ASDhigh group displayed more impaired developmental scores as measured by the MSEL including differences in verbal and non-verbal development (Figure 2). ASD children with the more inflammatory immune profile also displayed more impaired social affect as measured by the social affect domain of the ADOS [17.5 (13.0-19.0) vs. 14.5 (12.0-16.5); p = 0.03] and a trend for increased ADOS severity scores, with 64% (14/22) obtaining the maximum severity score of 10 in the ASDhigh but only 31% (9/28) in the ASDlow group attaining that score, although this did not reach statistical significance [10.0 (8.0-10.0) vs. 8.0 (7.0-10.0); p = 0.07] (Figure 3). A greater number of sleep issues [5.0 (3.0-8.0) vs. 3.0 (1.0-5.0); p = 0.04] and increased level of aggression [21.0 (17.0-25.0) vs. 13.0 (7.0-16.0); p < 0.01] was also reported by caregivers as measured by the CBCL in the ASDhigh group (Figure 4).
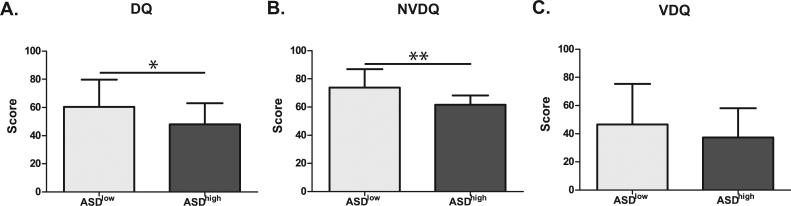
Figure 2. Cognitive scores in ASDhigh vs. ASDlow children.
Children in the ASDhigh group displayed more cognitive impairment than children in the ASDlow group. This was evidenced by significantly lower T-scores on the developmental quotient (DQ) (p=0.04) section and the non-verbal development quotient (NVDQ) (p<0.01) section of the MSEL. The verbal developmental quotient (VDQ) section also showed decreased T-scores, but this failed to reach significance (p=0.15). * p<0.05, ** p<0.01.
Figure 3. ADOS subscale scores in ASD high vs. ASDlow children.
Children in the ASDhigh group showed increased social deficits as measured by ADOS compared with children in the ASDlow group. In particular, children in the ASDhigh group had significantly increased (A) social affect issues (p=0.03). There was no evidence for increased (B) repetitive behaviors in this group. Overall severity (C) was higher in the ASDhigh group, but did not reach significance (p=0.06). * p<0.05.
Figure 4. CBCL subscale scores in ASDhigh vs. ASDlow children.
Children in the ASDhigh group had significantly increased (A) sleep (p<0.05) and (B) aggression (p<0.01) as measured by the CBCL compared with children in the ASDlow group. No difference in (C) attention problems were seen between these two groups. * p<0.05, ** p<0.01.
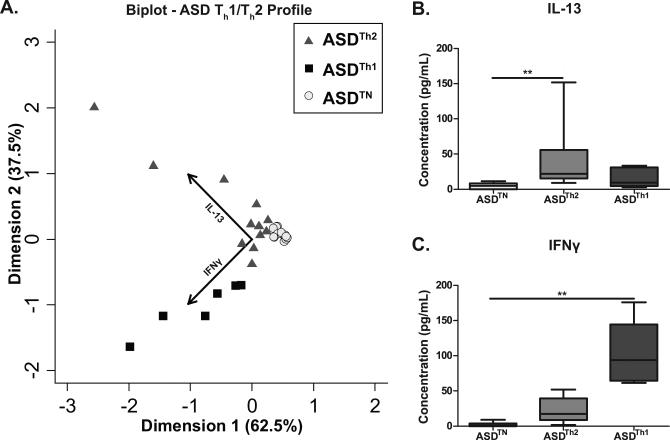
As the various immune cells work as a coordinated network, we sought to determine if T helper cell cytokines profiles could also help to elucidate immune endophenotypes in the ASD group. Children with ASD were placed into groups using a k-median cluster algorithm, with the assumption of three groups (“TH1 skewed”, “TH2 skewed”, and a “non-skewed” group based on cytokine responses following challenge with PHA) (Figure 5A). The TH2 skewed group (ASDTH2) (n=12) displayed significantly more IL-13 [21.9 (15.7-48.5) pg/mL vs. 4.9 (0.2-8.2) pg/mL; p < 0.01], a prototypical TH2 cytokine, than the non-skewed group (ASDTN) (n=19), which showed neither increased production of IL-13 or IFNγ (Figure 5B). Similarly, The TH1 skewed group (ASDTH1) (n=6) had significantly higher production of IFNγ, the prototypical TH1 cytokine, than the non-skewed group [93.8 (65.5-133.9) pg/mL vs. 1.0 (0.1-3.7) pg/mL; p <0.01] (Figure 5C).
Figure 5. Cluster analysis of PBMC cytokine response to PHA.
Children with ASD were clustered into three groups based on a k-medians cluster analysis using the T-helper associated cytokines IL-13 and IFNγ as measured in PBMC cell culture supernatants after PHA stimulation. The distribution of subjects based on this clustering are shown using a (A) biplot to indicate the vectors each particular cytokines has in discriminating the groups. Axis represent principal complements 1 and 2. Vectors points in the direction which is most like the variable represented by the vector. Post analysis of cytokine production between groups shows the ASDTh2 group produces significantly more (B) IL-13 (p<0.01) and ASDTH1 groups produces significantly more (C) IFNγ (p<0.01) than the ASDTH non-skewed group. ** p<0.01.
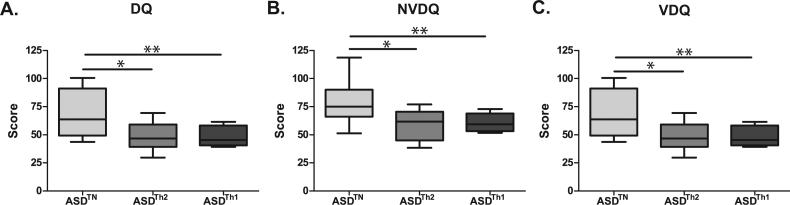
When assessing behavioral correlates in the clustered groups based on cytokine responses to PHA, the ASDTH1 group displayed more developmental impairment compared to the ASDTN group as measured on the MSEL for developmental quotient (DQ) [45.4 (40.9-57.1) vs. 637 (49.3-91.2); p = 0.02], verbal developmental quotient (VDQ) [32.8 (26.7-41.4) vs. 59.5 (35.9-80.3); p = 0.03], and non-verbal developmental quotient (NVDQ) [59.3 (53.7-67.6) vs. 75.0 (66.1-90.0); p = 0.04] (Figure 6). The ASDTH2 group was similarly cognitively impaired relative to the ASDTN group and showed significantly more impaired cognitive scores on the MSEL, DQ [46.6 (39.4-59.1) vs. 63.7 (49.3-91.2); p < 0.01], VDQ [35.0 (25.0-45.5) vs. 59.5 (35.9-80.3); p = 0.02], NVDQ [61.7 (47.9-68.1) vs. 75.0 (66.1-90.0); p < 0.01] (Figure 6).
Figure 6. Cognitive scores in children with ASD with an activated T Cell profile.
Children in both the ASDTH2 and ASDTH1 groups displayed more cognitive impairment than children in the ASDTN group. This was evidenced by significantly lower T-scores on the (A) developmental quotient (DQ) (ASDTH2; p<0.01; ASDTH1; p=0.02), the (B) non-verbal development quotient (NVDQ) (ASDTH2; p<0.01; ASDTH1; p=0.04), and the (C) verbal developmental quotient (VDQ) (ASDTH2; p=0.02; ASDTH1; p=0.03) section of the MSEL. * p<0.05, ** p<0.01.
Discussion
We have found that when children with ASD were categorized into pro-inflammatory and non-inflammatory groups based on the production of inflammatory related cytokines by their PBMC stimulated with LPS in culture, those with pro-inflammatory profiles showed more impaired developmental and behavioral scores, as well as increased problems with sleep and aggression. These children also displayed more impaired social affect, and a trend towards increased severity of core autism symptoms. Although the ADOS severity scores did not reach significance, two thirds of the children in the ASDhigh group had a maximum score of 10, with the others in that group near the upper limits of the scoring range, whereas less than a third had a maximum score in the ASDlow group. These findings are in agreement with previously reported findings in cultured monocytes (29) and plasma (4) from children with ASD that found increased production of pro-inflammatory cytokines were associated with more severe behavioral impairments.
Cytokines, pro-inflammatory ones in particular, play a number of roles in regulating behaviors. IL-1β and TNFα are both key messengers involved in sickness behavior during illness and infection, and their cross-communication with the neuroendocrine system can lead to social withdrawal (30, 31). In addition, regulation of IL-1β is important for proper neuronal function, as sustained expression of IL-1β in the hippocampus has been shown to impair spatial memory in mice (32), and blockage of IL-1β can alter synaptic plasticity (33). Regulation of TNFα is also important, as it can induce cell death in neurons and plays a role in synaptic pruning (34). All three of the major inflammatory cytokines, IL-1β, TNFα, and IL-6, have a complex relationship with sleep and sleep regulation. All three are considered somnogenic, but are themselves also regulated by circadian rhythms through a complex biofeedback mechanism (35).
In addition to production of innate inflammatory cytokines in response to LPS, a number of studies have reported increased levels of T-cell-associated cytokines in children with ASD, which are associated with more impaired behaviors (36). The cytokines reported are often suggestive of an immune skewing towards either an allergy/asthma, TH2 (4, 16, 37), or an anti-microbial, TH1 (18, 19, 38, 39) profile. We did not find a clear dominance of either immune profile, therefore we sought to determine if T helper cell skewing to either TH1 or TH2 predicted differences in behavioral phenotype. For the analysis presented in this study, only the last 37 ASD subjects receiving the same batch of PHA were used. Subjects in the earliest part of the study received a separate batch of PHA and had significantly different T cell responses in both subjects with ASD and TD controls. We found that children with ASD and a TH2 immune profile showed significantly decreased developmental scores compared to non-cytokine skewed children; however, this disparity in developmental rate was also present in children with a TH1 profile when compared to non-cytokine skewed children. This suggests that there is perhaps a general lack of T cell regulation in some children with ASD, which leads to either a TH1 or TH2 skewing, and immune cell activation associated with alterations in behavior, such as increased developmental impairment. This would be consistent with an increased pro-inflammatory profile in general, rather than a specific allergy/asthma or cell mediated pattern of immune responses. In our previous studies (4), we also noted that increased inflammatory T cell cytokine profiles (e.g. TNFα) in children with ASD were related to more impaired behaviors.
Similar to inflammatory cytokines, TH1 and TH2 associated cytokines have a number of effects on behaviors. IFNγ, the archetypal TH1 cytokine, is associated with increased “emotionality” in mice (40) and has been shown to be elevated in patients with depression (41). TH2 cytokines appear to have a more beneficial function with IL-4 KO mice showing cognitive impairments that can be rescued by transfer of bone marrow from wild type mice (42). However, IL-4 is also increased in response to stress and may reflect allergic inflammation resulting in the increase of other pro-inflammatory cytokines (43). It should be noted that in this study we did not look at IL-4 cytokine production as our previous studies in similarly aged pediatric populations found that the production of IL-13 was a better proxy for TH2 skewing from PHA stimulated cultures and was more reliably and more frequently detected above the minimum readable levels of the Luminex assay.
While several studies have assessed immunological findings as they relate to behaviors in ASD, to our knowledge this is the first study to attempt an assessment of behavioral phenotypes in children with ASD based on functionally determined immune response profiles. This method of identifying subgroups within ASD, based on functional cytokine clustering, is less prone to technical errors than with a single analyte approach as it does no rely solely on the measure of a single cytokine. Assessing subjects based on an immune profile is also more in line with the complex nature of the immune network of interactions following perturbation, were individual cytokines serve multiple interconnected roles. Thus, a profile of multiple cytokines in response to stimulation offers a more physiologically relevant method to assess immune function in individuals and between groups.
Within the ASD group, there appears to be multiple immune endophenotypes. Children with a more pronounced response to immune stimulation, be it LPS (innate) or PHA (T cell), demonstrate the greatest developmental and behavioral impairments and show differences in core and comorbid behaviors similar to that previously described in a variety of immunological studies in ASD (4, 5, 44). It remains unclear if these immunological abnormalities are directly related to developmental and behavioral characteristics or are an epiphenomenon; However, the ability to describe subgroups of children with ASD based upon their reactivity cluster suggests that their involvement in the pathology of ASD is more than coincidental.
Although immune abnormalities were first described in ASD over forty years ago, no consensus has been reached as to what constitutes clinically significant immune dysfunction in ASD. Many factors such as study design, age of cases, use of siblings who often display a broader immune phenotype as controls, the use of adults as controls in studies of childhood ASD and less stringent diagnosis of ASD could contribute to a lack of accord among the studies of immune dysfunction in ASD. In addition, clinic based studies may introduce certain biases not observed in population based case-control studies. Issues with study design notwithstanding, the heterogeneity of ASD has been suggested to be a major contributor to the disparity in immune findings described to date, and presents a major issue in all ASD studies. Trials attempting to modulate the immune system in children with ASD have met with mixed results. The use of intravenous gamma globulin (IVIG) has resulted in varied results. Most children see no benefit whereas a small number reportedly display remarkable behavioral improvements (45, 46). The use of antibiotics to alter gut flora and systemic immune responses has met with similar results, with a minority of children experiencing benefit (47). Studies involving immune therapies in conjunction with anti-psychotic medications, such as cox-2 inhibitors and Risperidone, have had better results; children receiving both therapies showed marked improvement relative to Risperidone alone (48). Collectively these studies suggest that immune therapies can benefit some children with ASD, but more stringent methodological studies are needed. Given the heterogeneity of ASD, there remains a great need to further elucidate immune dysfunction in children with the disorder, and to work towards establishing immune endophenotypes within the broader spectrum in order to better target current and future therapies.
We demonstrate in this study that is it is possible to characterize developmental/behavioral differences in children with ASD based on immune profiles. This novel method for categorizing children with ASD may have beneficial roles in behavioral and/or pharmaceutical therapy trials that may lead to the development of more individualized treatment recommendations based on both biological and behavioral characteristics.
Acknowledgements
This study was funded by the National Institute of Health grants RO1MH089626, R21HD065269, U24 MH081810, PO1ES011269 Autism Speaks Foundation, the Jane Botsford Johnson Foundation, National Alliance for Research on Schizophrenia and Depression, and, the Peter Emch Foundation. We would like to thank the staff of both the UC Davis M.I.N.D. Institute and the APP study for their technical support. The commitment of the families who took part in these studies, at both the M.I.N.D Institute and as part of the APP study, is also gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Autism, Developmental Disabilities Monitoring Network Surveillance Year Principal I, Centers for Disease C, Prevention Prevalence of autism spectrum disorders--Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2008. Morbidity and mortality weekly report Surveillance summaries. 2012;61:1–19. [PubMed] [Google Scholar]
- 2.Blumberg SJ, Bramlett MD, Kogan MD, Schieve LA, Jones JR, Lu MC. Changes in prevalence of parent-reported autism spectrum disorder in school-aged US children: 2007 to 2011–2012. 2013 [PubMed] [Google Scholar]
- 3.Ashwood P, Wills S, Van de Water J. The immune response in autism: a new frontier for autism research. J Leukoc Biol. 2006;80:1–15. doi: 10.1189/jlb.1205707. [DOI] [PubMed] [Google Scholar]
- 4.Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, Van de Water J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun. 2011;25:40–45. doi: 10.1016/j.bbi.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Onore C, Careaga M, Ashwood P. The role of immune dysfunction in the pathophysiology of autism. Brain Behav Immun. 2012;26:383–392. doi: 10.1016/j.bbi.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goines PE, Ashwood P. Cytokine dysregulation in autism spectrum disorders (ASD): possible role of the environment. Neurotoxicol Teratol. 2013;36:67–81. doi: 10.1016/j.ntt.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pardo CA, Vargas DL, Zimmerman AW. Immunity, neuroglia and neuroinflammation in autism. Int Rev Psychiatry. 2005;17:485–495. doi: 10.1080/02646830500381930. [DOI] [PubMed] [Google Scholar]
- 8.Cabanlit M, Wills S, Goines P, Ashwood P, Van de Water J. Brain-specific autoantibodies in the plasma of subjects with autistic spectrum disorder. Ann N Y Acad Sci. 2007;1107:92–103. doi: 10.1196/annals.1381.010. [DOI] [PubMed] [Google Scholar]
- 9.Connolly AM, Chez M, Streif EM, Keeling RM, Golumbek PT, Kwon JM, et al. Brain-derived neurotrophic factor and autoantibodies to neural antigens in sera of children with autistic spectrum disorders, Landau-Kleffner syndrome, and epilepsy. Biol Psychiatry. 2006;59:354–363. doi: 10.1016/j.biopsych.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Singer HS, Morris CM, Williams PN, Yoon DY, Hong JJ, Zimmerman AW. Antibrain antibodies in children with autism and their unaffected siblings. Journal of Neuroimmunology. 2006;178:149–155. doi: 10.1016/j.jneuroim.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 11.Todd RD, Hickok JM, Anderson GM, Cohen DJ. Antibrain antibodies in infantile autism. Biol Psychiatry. 1988;23:644–647. doi: 10.1016/0006-3223(88)90012-1. [DOI] [PubMed] [Google Scholar]
- 12.Wills S, Cabanlit M, Bennett J, Ashwood P, Amaral DG, Van de Water J. Detection of autoantibodies to neural cells of the cerebellum in the plasma of subjects with autism spectrum disorders. Brain Behav Immun. 2009;23:64–74. doi: 10.1016/j.bbi.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torres AR, Sweeten TL, Cutler A, Bedke BJ, Fillmore M, Stubbs EG, et al. The association and linkage of the HLA-A2 class I allele with autism. Hum Immunol. 2006;67:346–351. doi: 10.1016/j.humimm.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Torres AR, Maciulis A, Odell D. The association of MHC genes with autism. Front Biosci. 2001;6:D936–943. doi: 10.2741/torres. [DOI] [PubMed] [Google Scholar]
- 15.Warren RP, Odell JD, Warren WL, Burger RA, Maciulis A, Daniels WW, et al. Strong association of the third hypervariable region of HLA-DR beta 1 with autism. J Neuroimmunol. 1996;67:97–102. doi: 10.1016/0165-5728(96)00052-5. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki K, Matsuzaki H, Iwata K, Kameno Y, Shimmura C, Kawai S, et al. Plasma cytokine profiles in subjects with high-functioning autism spectrum disorders. PLoS One. 2011;6:e20470. doi: 10.1371/journal.pone.0020470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Emanuele E, Orsi P, Boso M, Broglia D, Brondino N, Barale F, et al. Low-grade endotoxemia in patients with severe autism. Neurosci Lett. 2010;471:162–165. doi: 10.1016/j.neulet.2010.01.033. [DOI] [PubMed] [Google Scholar]
- 18.Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57:67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- 19.Li X, Chauhan A, Sheikh AM, Patil S, Chauhan V, Li XM, et al. Elevated immune response in the brain of autistic patients. J Neuroimmunol. 2009;207:111–116. doi: 10.1016/j.jneuroim.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Onore CE, Nordahl CW, Young GS, Van de Water JA, Rogers SJ, Ashwood P. Levels of soluble platelet endothelial cell adhesion molecule-1 and P-selectin are decreased in children with autism spectrum disorder. Biol Psychiatry. 2012;72:1020–1025. doi: 10.1016/j.biopsych.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nordahl CW, Lange N, Li DD, Barnett LA, Lee A, Buonocore MH, et al. Brain enlargement is associated with regression in preschool-age boys with autism spectrum disorders. Proc Natl Acad Sci U S A. 2011;108:20195–20200. doi: 10.1073/pnas.1107560108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lord C, Pickles A, McLennan J, Rutter M, Bregman J, Folstein S, et al. Diagnosing autism: analyses of data from the Autism Diagnostic Interview. J Autism Dev Disord. 1997;27:501–517. doi: 10.1023/a:1025873925661. [DOI] [PubMed] [Google Scholar]
- 23.Joseph RM, Tager-Flusberg H, Lord C. Cognitive profiles and social-communicative functioning in children with autism spectrum disorder. J Child Psychol Psychiatry. 2002;43:807–821. doi: 10.1111/1469-7610.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Association AP diagnostic criteria from dsM-iV-tr. Amer Psychiatric Pub Incorporated. 2000 [Google Scholar]
- 25.Steinhausen HC, Erdin A. Abnormal psychosocial situations and ICD-10 diagnoses in children and adolescents attending a psychiatric service. J Child Psychol Psychiatry. 1992;33:731–740. doi: 10.1111/j.1469-7610.1992.tb00908.x. [DOI] [PubMed] [Google Scholar]
- 26.Berument SK, Rutter M, Lord C, Pickles A, Bailey A. Autism screening questionnaire: diagnostic validity. Br J Psychiatry. 1999;175:444–451. doi: 10.1192/bjp.175.5.444. [DOI] [PubMed] [Google Scholar]
- 27.Mullen EM. Mullen Scales of Early Learning. American Guidance Service; 1995. [Google Scholar]
- 28.Achenbach TM. The Child Behavior Checklist manual. The University of Vermont; Burlington, VT: 1991. [Google Scholar]
- 29.Enstrom AM, Onore CE, Van de Water JA, Ashwood P. Differential monocyte responses to TLR ligands in children with autism spectrum disorders. Brain Behav Immun. 2010;24:64–71. doi: 10.1016/j.bbi.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dantzer R, Kelley KW. Autistic children: a neuroimmune perspective. Brain Behav Immun. 2008;22:804–805. doi: 10.1016/j.bbi.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 31.Harden LM, du Plessis I, Poole S, Laburn HP. Interleukin (IL)-6 and IL-1 beta act synergistically within the brain to induce sickness behavior and fever in rats. Brain Behav Immun. 2008;22:838–849. doi: 10.1016/j.bbi.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 32.Moore AH, Wu M, Shaftel SS, Graham KA, O'Banion MK. Sustained expression of interleukin-1beta in mouse hippocampus impairs spatial memory. Neuroscience. 2009;164:1484–1495. doi: 10.1016/j.neuroscience.2009.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schneider H, Pitossi F, Balschun D, Wagner A, del Rey A, Besedovsky HO. A neuromodulatory role of interleukin-1beta in the hippocampus. Proc Natl Acad Sci U S A. 1998;95:7778–7783. doi: 10.1073/pnas.95.13.7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cacci E, Claasen JH, Kokaia Z. Microglia-derived tumor necrosis factor-alpha exaggerates death of newborn hippocampal progenitor cells in vitro. J Neurosci Res. 2005;80:789–797. doi: 10.1002/jnr.20531. [DOI] [PubMed] [Google Scholar]
- 35.Krueger JM, Obal F, Jr., Opp M, Toth L, Johannsen L, Cady AB. Somnogenic cytokines and models concerning their effects on sleep. Yale J Biol Med. 1990;63:157–172. [PMC free article] [PubMed] [Google Scholar]
- 36.Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah IN, Van de Water J. Associations of impaired behaviors with elevated plasma chemokines in autism spectrum disorders. J Neuroimmunol. 2011;232:196–199. doi: 10.1016/j.jneuroim.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta S, Aggarwal S, Rashanravan B, Lee T. Th1- and Th2-like cytokines in CD4+ and CD8+ T cells in autism. J Neuroimmunol. 1998;85:106–109. doi: 10.1016/s0165-5728(98)00021-6. [DOI] [PubMed] [Google Scholar]
- 38.Singh VK. Plasma increase of interleukin-12 and interferon-gamma. Pathological significance in autism. J Neuroimmunol. 1996;66:143–145. doi: 10.1016/0165-5728(96)00014-8. [DOI] [PubMed] [Google Scholar]
- 39.Croonenberghs J, Bosmans E, Deboutte D, Kenis G, Maes M. Activation of the inflammatory response system in autism. Neuropsychobiology. 2002;45:1–6. doi: 10.1159/000048665. [DOI] [PubMed] [Google Scholar]
- 40.Kustova Y, Sei Y, Morse HC, Basile AS. The influence of a targeted deletion of the IFN[g534] gene on emotional behaviors. Brain, Behavior, and Immunity. 1998;12:308–324. doi: 10.1006/brbi.1998.0546. [DOI] [PubMed] [Google Scholar]
- 41.Maes M, Scharpe S, Meltzer HY, Okayli G, Bosmans E, Dhondt P, et al. Increased Neopterin and Interferon-Gamma Secretion and Lower Availability of L-Tryptophan in Major Depression - Further Evidence for an Immune-Response. Psychiatry Research. 1994;54:143–160. doi: 10.1016/0165-1781(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 42.Derecki NC, Cardani AN, Yang CH, Quinnies KM, Crihfield A, Lynch KR, et al. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J Exp Med. 2010;207:1067–1080. doi: 10.1084/jem.20091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peters EM, Liezmann C, Pavlovic S, Örsal A, Rose M, Kruse J. 157. HPA axis function is altered by cutaneous allergic inflammation in mice in a neuropeptide- and neurotrophin-dependent manner. Brain, Behavior, and Immunity. 2013;32:e45–e46. [Google Scholar]
- 44.Masi A, Quintana DS, Glozier N, Lloyd AR, Hickie IB, Guastella AJ. Cytokine aberrations in autism spectrum disorder: a systematic review and meta-analysis. Mol Psychiatry. 2014 doi: 10.1038/mp.2014.59. [DOI] [PubMed] [Google Scholar]
- 45.Gupta S, Samra D, Agrawal S. Adaptive and Innate Immune Responses in Autism: Rationale for Therapeutic Use of Intravenous Immunoglobulin. J Clin Immunol. 2010;30:90–96. doi: 10.1007/s10875-010-9402-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Plioplys AV. Intravenous immunoglobulin treatment of children with autism. J Child Neurol. 1998;13:79–82. doi: 10.1177/088307389801300207. [DOI] [PubMed] [Google Scholar]
- 47.Sandler RH, Finegold SM, Bolte ER, Buchanan CP, Maxwell AP, Vaisanen ML, et al. Short-term benefit from oral vancomycin treatment of regressive-onset autism. J Child Neurol. 2000;15:429–435. doi: 10.1177/088307380001500701. [DOI] [PubMed] [Google Scholar]
- 48.Asadabadi M, Mohammadi MR, Ghanizadeh A, Modabbernia A, Ashrafi M, Hassanzadeh E, et al. Celecoxib as adjunctive treatment to risperidone in children with autistic disorder: a randomized, double-blind, placebo-controlled trial. Psychopharmacology (Berl) 2013;225:51–59. doi: 10.1007/s00213-012-2796-8. [DOI] [PubMed] [Google Scholar]