Abstract
Objective
To estimate the association between duration of combination antiretroviral therapy (cART) during pregnancy and low infant birthweight (LBW), among women ≥37 weeks gestation.
Design
We conducted a retrospective cohort study of HIV-infected women who met eligibility criteria based on CD4 count ≤350 but had not started cART at entry into antenatal care (ANC). Our cohort was restricted to births that occurred ≥37 weeks gestation.
Methods
We used Poisson models with robust variance estimators to estimate risk ratios (RR) and 95% confidence intervals (CI).
Results
Of 50,765 HIV-infected women with antenatal visits between January 2009 and September 2013, 4,474 women met the inclusion criteria. LBW occurred in 302 pregnancies (7%). Nearly two-thirds of women (62%) eligible to initiate cART never started treatment. Overall, 14% were on cART for ≤8 weeks, 22% for 9-20 weeks, and 2% for 21-36 weeks. There was no evidence of an increased risk of LBW for women receiving cART for ≤8 weeks (RR 1.22, 95% CI: 0.77, 1.91), 9-20 weeks (RR 1.23, 95% CI: 0.82, 1.83), or 21-36 weeks (RR 0.87, 95% CI: 0.22, 3.46), compared to women who never initiated treatment. These findings were consistent across several sensitivity analyses.
Conclusions
Longer duration of cART was not associated with poor fetal growth among term pregnancies in our cohort. However, the relationship between cART and adverse pregnancy outcomes remains complicated. Continued work is required to investigate causality. An understanding cART's impact on adverse pregnancy outcomes is essential as cART becomes the cornerstone of PMTCT programs globally.
Keywords: antiretroviral therapy, fetal growth restriction, HIV, low birthweight, pregnancy outcomes
Introduction
In 2013, the World Health Organization (WHO) recommended lifetime combination antiretroviral therapy (cART) for all HIV-infected pregnant women in countries with a generalized HIV epidemic. Known as “Option B+,” this policy is currently being scaled up in several sub-Saharan African (SSA) countries, including Zambia.(1) Option B+ is expected to streamline treatment initiation for HIV-infected pregnant women by removing the need for CD4 count screening and simplifying treatment recommendations.(2) Improved access to treatment over time – and the resulting increase in women starting pregnancy already on cART – is expected to play a critical role in virtually eliminating new pediatric HIV infections and improving maternal health.(3)
However, cART use during pregnancy could negatively affect fetal growth. Adverse associations between cART during pregnancy and birthweight were first reported among HIV-infected pregnant women in Europe.(4, 5) These findings were not confirmed by studies in North and Latin America, where no adverse associations between cART and low birthweight (LBW; <2500 g) were observed.(6-9) cART with a protease inhibitor (PI) was associated with an increased odds of very low birthweight (VLBW; <1500 g) in a combined cohort study of 7 US sites, but the precision was poor, as very few outcomes were observed (n=16).(10) The relationship between cART and LBW among women in SSA is also unclear. cART has been associated with LBW among women in Cote d'Ivoire and small for gestational age among women Botswana.(11, 12) As cART access expands throughout SSA, the impact of longer durations of cART during pregnancy on LBW must be understood.
cART use during pregnancy could affect LBW through two different pathways: shortening gestation length (leading to preterm birth (PTB)) or restricting fetal growth. Duration of cART during pregnancy is directly related length of gestation, making it difficult to meaningfully assess the association between duration of treatment and PTB. We, therefore, limited our analysis cohort to those infants delivered at term. This approach allowed us to focus on the potential relationship between duration of cART and LBW among term infants, a proxy for intrauterine growth restriction (IUGR), in a population of HIV-infected women eligible to initiate cART for maternal health in Zambia.
Materials and Methods
Study Design and Setting
We conducted a retrospective cohort analysis of HIV-infected pregnant women attending public antenatal healthcare facilities in Lusaka, Zambia. Lusaka is an urban setting where antenatal care (ANC) coverage has been consistently reported at >95%.(13) Over the study period, HIV testing and CD4 screening were conducted within antenatal clinics. Among women attending ANC, HIV testing is near universal and CD4 count screening coverage is approximately 80%.(14) Pregnant women who meet local eligibility criteria for cART receive services either in integrated ART-ANC clinics or stand-alone HIV treatment departments, typically co-located at the same primary healthcare facility. Comprehensive medical information, including HIV treatment information, has been captured by the Zambia Electronic Perinatal Record System (ZEPRS) since 2007 in all 24 public antenatal clinics in Lusaka.(14) Ethical approval was obtained from the University of Zambia Biomedical Research Ethics Committee (Lusaka, Zambia) and the University of North Carolina, Chapel Hill (Chapel Hill, NC) and informed consent was not obtained for the analysis of routinely collected clinical data.
Study Population
Women were included in the present analysis if they entered ANC after January 1, 2009 and delivered before September 1, 2013, were HIV-infected, had a CD4 count of ≤350 cells/uL (the threshold for ART eligibility during the study period), were not on cART at the time of presentation and delivered a singleton pregnancy at a public healthcare facility at ≥37 weeks gestation.(15, 16) Women who conceived while on cART were excluded since women who initiate cART and survive long enough to get pregnant may not be comparable to women initiating cART during pregnancy.(17) Therefore our interest was in a population of women newly initiating cART. Duration of cART is directly related to length of gestation; with each week that a woman remains pregnant and on cART, the probability of having a preterm birth diminishes. This could result in a spurious association between duration of treatment and preterm birth, where women on cART longer appear less likely to have a preterm birth. For this reason, we limited our analysis cohort to those infants delivered at term and focused on an outcome of LBW. In resource-limited settings such as Zambia, where ultrasound is not routinely available, LBW among infants delivered at term can be considered a proxy for IUGR.(18) We assessed LBW among term infants, rather than small for gestational age, as an outcome due to concerns about measurement error in gestational age for deliveries born prior to term.(19) Women with the following chronic conditions: heart disease, hypertension, and Type 2 diabetes have poorer pregnancy outcomes (20, 21) and may be more likely to seek ANC earlier due to their preexisting conditions. To minimize confounding, this group was also excluded.
Definitions
The exposure of interest was duration of cART before delivery, measured in completed weeks (e.g. 12 weeks and 4 days was considered 12 completed weeks) and categorized as never initiated (referent), ≤8 weeks, 9-20 weeks, and 21-36 weeks. Category cut-points were based on the functional form of the relationship between the exposure and outcome, as well as clinical considerations to approximately correspond with cART initiation in early, mid and late pregnancy. We also considered duration of cART before delivery as a continuous measure of weeks on cART. The primary outcome was a binary measure of LBW, defined as <2,500 grams.(22) Mean birthweight as a continuous measure was used as a secondary outcome. Birthweight is measured in ZEPRS in kilograms (rounded to the nearest hundredth) and was converted to grams for this analysis.
Confounding variables were identified using directed acyclic graphs(23, 24) and included age, baseline body mass index (BMI), baseline CD4 count, baseline hemoglobin, education, parity, previous PTB (<37 weeks gestation), number of ANC visits and gestational age at birth. Number of ANC visits and education were used as proxies for health seeking behavior.(25-27) The functional form of the relationship between continuous confounders and outcome was assessed and confounders were modeled either as linear terms (age, CD4 count and gestational age at birth) or using restricted quadratic splines (BMI and hemoglobin).(28) All potential confounders were included in multivariable models, unless otherwise noted. Information on viral load, WHO clinical stage, antiretroviral adherence and drug regimen was not available in our electronic medical record. However, recommended first line treatment in Zambia is a non-nucleoside reverse transcriptase inhibitor (NNRTI)-based regimen.(29-31)
Gestational age was measured in completed weeks and calculated following standard clinical guidelines by last menstrual period (LMP) for pregnancies <20 weeks at time of enrollment into ANC. For those ≥20 weeks at enrollment, both LMP and symphysis-fundal height were used. If these two methods yielded gestational ages within 3 weeks of each other, the date based on the LMP was used. If not, the fundal height–derived gestational age was used. LMP was available for all women. Gestational age dating based on LMP is known to contain error.(32, 33) To assess the validity of gestational age dating in our data, we compared mean birthweight for each week of gestational age at birth to a reference growth curve adjusted to a Zambian population.(34) Comparisons with a reference growth curve values suggested substantial measurement error in gestational dating for women delivering before 35 weeks. For women delivering at ≥35 weeks gestation, mean birthweights were comparable to reference curve values, indicating relatively unbiased gestational age dating (Web Appendix Figure 1).
Figure 1.
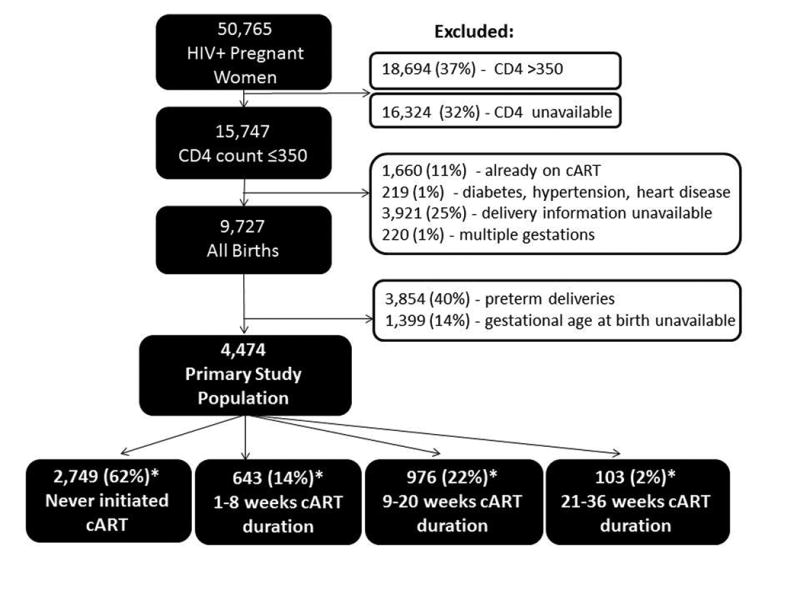
Inclusion criteria for 4,747 term births among HIV-infected pregnant women included in the study population. *Duration of cART could not be calculated for 3 women who had no delivery date available.
Statistical analyses
In the primary analysis, we used multivariable Poisson models with robust variance estimators to estimate risk ratios (RRs) and 95% confidence intervals (CIs)(35) for the association between categories of duration of cART before delivery and LBW. In secondary analysis, we used multivariable linear regression to estimate the association between duration of cART before delivery and birthweight, treating duration of cART first as a continuous measure (weeks on cART) and then as a categorical measure.
We additionally performed several sensitivity analyses. First, to minimize the impact of length of time in care, we compared women in each category of cART duration to a referent of women who never initiated cART, but were enrolled in ANC for the same duration of time. Second, we stratified the association between cART duration and LBW by baseline CD4 count to see if women with greater immunosuppression were at a higher risk of LBW. Third, we assessed women's duration of cART at 37 weeks gestation (instead of at delivery) so that treatment duration was assessed before women reached term. Finally, we included all women who initiated cART with a CD4 count>350 (n=214) to make results as generalizable as possible to a setting where Option B+ has been implemented.
As with many sources of routinely collected clinical information, missing data was a concern. To address possible bias from missing data, in a sensitivity analysis we assessed predictors of missing 1) CD4 counts among all HIV-infected pregnant women, 2) cART initiation dates 3) confounders and 4) missing data 1-3 combined (all missing data imputed) and performed multiple imputation (n=50 imputations) for the missing data. All statistical analyses were performed using SAS version 9.2 (SAS Institute Inc. Cary, NC).
Results
Among 50,765 HIV-infected pregnant women enrolled in ANC, 4,474 women met inclusion criteria for our study cohort (Figure 1). LBW occurred in 302 of pregnancies (7%). Nulliparity was more common among women who had a LBW infant, than among women with normal birthweight infants (29% versus 18%, p-value 0.08). Median baseline BMI and CD4 count values were similar between those delivering LBW and normal birthweight infants. Only 10% of women attended ≥4 ANC visits, as recommended by the WHO (Table 1).(36)
Table 1. Sociodemographic and obstetric characteristics of 4,474 HIV-infected women eligible for cART initiation during pregnancy in Lusaka, Zambia 2009-2013.
| Characteristic | Low Birthweight (<2,500g) N(%) or Median (IQR) |
Normal Birthweight (≥2,500g) N(%) or Median (IQR) |
|---|---|---|
| N=302 (6.9) | N=4,070 (93.1) | |
| Age | 27 (23, 32) | 28 (24, 32) |
| Education | ||
| Primary or None | 117 (45.5) | 1,599 (44.7) |
| Secondary or Tertiary | 140 (54.5) | 1,979 (55.3) |
| Parity | ||
| 0 | 79 (29.4) | 669 (18.2) |
| 1 | 68 (25.3) | 930 (25.2) |
| 2 | 50 (18.6) | 919 (24.9) |
| 2+ | 72 (26.8) | 1167 (31.7) |
| Body mass index (kg/m2) | 22.3 (20.5, 24.7) | 23.7 (21.8, 26.2) |
| CD4 count (cells/mm3) | 237 (171, 286) | 243 (174, 300) |
| Hemoglobin (g/L) | 10.6 (9.7, 11.8) | 10.8 (9.9, 11.7) |
| Number of ANC visits | ||
| 1 | 137 (45.4) | 1920 (47.2) |
| 2 | 81 (26.8) | 1038 (25.5) |
| 3 | 59 (19.5) | 715 (17.6) |
| ≥4 | 25 (8.3) | 397 (9.8) |
| Previous preterm birtha | ||
| No/unknown | 292 (96.7) | 3949 (97.0) |
| Yes | 10 (3.3) | 12 (3.0) |
| Gestational age at birth | 39 (37, 40) | 39 (38, 40) |
| 37 weeks | 80 (26.5) | 758 (18.6) |
| 38 weeks | 65 (21.5) | 818 (20.1) |
| 39 weeks | 68 (22.5) | 897 (22.0) |
| 40 weeks | 49 (16.2) | 666 (16.4) |
| 41 weeks | 40 (13.2) | 931 (22.9) |
Previous preterm birth: delivery <37 weeks gestation. Missing data: LBW (2.3%), age (0.1%), education (12.6%), parity (9.8%), BMI (32.2%), CD4 (0%), hemoglobin (8.4%), number of ANC visits (0%), previous preterm birth (0%).
Of the 4,474 cART-eligible women in our cohort, 2,749 (62%) never initiated treatment, 643 (14%) received ≤8 weeks of cART, 976 (22%) received 9-20 weeks of cART and 103 (2%) received 21-36 weeks of cART (Table 2). Of the 2,749 women who never initiated cART, 81% received zidovudine (with or without single-dose nevirapine) to prevent vertical HIV transmission, 13% received only single-dose nevirapine and 6% had no reliable information recorded. Among the 1,722 women who initiated cART, the median time from 1st ANC visit to cART initiation was 36 days (IQR 24, 64 days).
Table 2. Results for the association between duration of cART before delivery and low birthweight.
| Weeks on cART before delivery | Low Birthweight (<2,500 g) N(%) |
Normal Birthweight a (≥2,500 g) N(%) |
Unadjusted RR (95% CI) | Adjusted RR (95% CI)b |
|---|---|---|---|---|
| N=302(6.9) | N=4070 (93.1) | |||
| 21-36 | 4 (1.3) | 95 (2.3) | 0.64 (0.24, 1.69) | 0.87 (0.22, 3.46) |
| 9-20 | 83 (27.5) | 872 (21.4) | 1.38 (1.07, 1.77) | 1.23 (0.82, 1.83) |
| ≤8 | 46 (15.2) | 590 (14.5) | 1.15 (0.84, 1.57) | 1.22 (0.77, 1.91) |
| Never initiated | 169 (56.0) | 2,512 (61.7) | 1.00 | 1.00 |
1 woman is missing duration of cART exposure information.
Adjusted for: number of ANC visits, age, BMI, CD4 count, education, hemoglobin, parity, previous preterm birth and gestational age at birth.
Women who deliver in a facility may differ from those who deliver at home. To assess possible selection bias, women included in the study population were compared with eligible women who presented to ANC, but did not deliver in a ZEPRS-supported facility (n=3,921), and may have delivered at home. Women with no delivery information were similar to women included in the study, with a few exceptions (Web Appendix Table 1). Women without delivery information were more likely to have only 1 ANC visit (75% vs. 47%, p-value <0.01) and less likely to have a cART initiation date recorded (22% vs. 41%, p-value <0.01).
Main analyses
In the primary analysis, compared to women who never initiated treatment, there was no evidence that receiving cART for ≤8 weeks (RR 1.22, 95% CI: 0.77, 1.91), 9-20 weeks (RR 1.23, 95% CI: 0.82, 1.83), or 21-36 weeks (RR 0.87, 95% CI: 0.22, 3.46) was associated with an increased risk for LBW among infants born at term, after adjustment for multiple confounders (Table 2).
Similarly, there was no evidence of an association when birthweight among term infants was considered as a continuous outcome. Compared to women who never initiated cART, the mean birthweight for women receiving ≤8 weeks of cART was lower by 2.35 grams (95 % CI: -53.69, 49.98), lower by 44.19 grams for women receiving 9-20 weeks of cART (95% CI: -89.55, 1.17) and lower by 65.77 grams for women receiving 21-36 weeks of cART (95% CI: -194.92, 63.38). The distribution of birthweights between exposure categories, stratified by gestational age, did not differ meaningfully (Web Appendix Figure 2). When weeks on cART before delivery was considered as a continuous measure, a one-week increase in duration on cART before delivery was associated with a decrease in mean birthweight of 2.53 grams (95% CI -5.40, 0.35) (Table 3).
Figure 2.
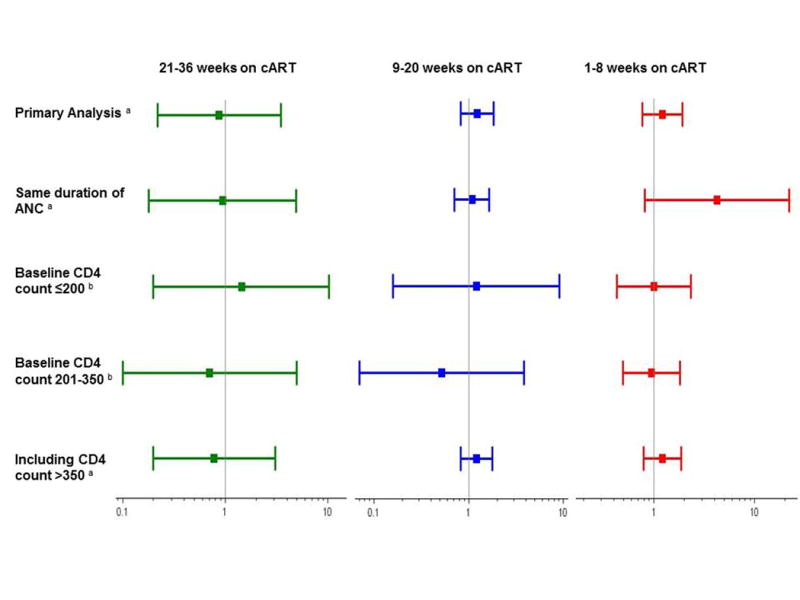
Risk ratios and 95% confidence intervals for the association between duration of cART and LBW, among term infants. a Adjusted for: number of ANC visits, age, BMI, CD4 count, education, hemoglobin, parity, previous preterm birth and gestational age at birth. b Adjusted for: number of ANC visits, age, BMI, education, hemoglobin, parity, previous preterm birth and gestational age at birth.
Table 3.
Results for the association between duration of cART before delivery and birthweight.
| Exposure Specification | Mean Birthweight (g) (SD) | Unadjusted Mean Change in Birthweight (95% CI) | Adjusted Mean Change in Birthweight a (95% CI) |
|---|---|---|---|
| Weeks on cART before delivery, continuous | 3040.00 (428.19) | -3.23 (-5.20, -1.26) | -2.53 (-5.40, 0.35) |
| Weeks on cART before delivery | |||
| 21-36 | 3059.90 (421.46) | 1.89 (-83.85, 87.62) | -65.77 (-194.92, 63.38) |
| 9-20 | 2995.79 (418.38) | -62.22 (-93.79, -30.65) | -44.19 (-89.55, 1.17) |
| ≤8 | 3027.44 (439.57) | -30.57 (-67.52, 6.37) | -2.35 (-54.69, 49.98) |
| Never initiated | 3058.01 (428.22) | 0.00 | 0.00 |
Adjusted for: number of ANC visits, age, BMI, CD4 count, education, hemoglobin, parity, previous preterm birth and gestational age at birth.
Sensitivity analyses and imputation of missing data
Our results were consistent across several sensitivity analyses, although sample size was limited in some analyses. When women on cART were compared with non-initiators with the same duration of ANC, the RR was 4.20 (95% CI: 0.81, 21.82) for ≤8 weeks of cART, 1.08 (95%CI: 0.71, 1.64) for 9-20 weeks of cART and 0.95 (95% CI: 0.18, 4.92) for 21-36 weeks of cART. Similar findings were observed when we stratified by baseline CD4 count, when cART duration was assessed at 37 weeks gestation and when women who initiated cART with a CD4 >350 were included (Figure 2).
A high proportion of CD4 counts, cART initiation dates and confounder data were missing. Among 50,765 HIV-infected pregnant women, 32% (n=16,324) were missing CD4 counts. Of the 4,474 women included in the study, 62% (n=2,773) had ≥1 missing confounder. An additional 1,002 (18%) women, not included in the primary analysis, were on cART at delivery but were missing a cART initiation date. In general, performing multiple imputation improved precision but resulted in similar point estimates. When all missing data were imputed, point estimates were similar to the primary analysis; receiving cART ≤8 weeks was associated with RR 1.15 (95% CI: 0.87, 1.54), 9-20 weeks RR 1.35 (95% CI: 1.07, 1.70) and 21-36 weeks RR 0.96 (95% CI: 0.52, 1.79).
Discussion
Main findings
Among HIV-infected women with CD4 count ≤350 who were treatment-naïve at entry into ANC and who delivered at ≥37 weeks, longer duration of cART during pregnancy did not result in increased risk of LBW or decreased mean birthweight. These findings did not change meaningfully across numerous sensitivity analyses. Modest decreases in birthweight were observed with increasing cART duration when cART duration was considered as a continuous measure. However, this small decrease must be weighed against the substantial benefits of cART in preventing mother-to-child (PMTCT) transmission. Our analysis included only term births, and therefore our findings are focused on LBW, a proxy for IUGR in this population.
Limitations and Strengths
We note several limitations of this work. First, birthweight measurements were considered more reliable than gestational age dating in our data. However, there is some evidence of digit-preference in birthweight around 3,000 grams (Web Appendix Figure 2), which may lead to underestimates in the proportion of LBW infants in our cohort. Second, we did not have access to information on important confounders, including infant HIV status, markers of maternal HIV disease (e.g., viral load, WHO clinical stage), antiretroviral regimens or adherence. Second, as with all analyses of routinely collected clinical data, selection bias may arise since not all women present for care. However, clinical findings are applicable only in clinical settings, so associations observed among those who present for care may be generalizable to other populations in care. In addition, our analysis cohort did not include pregnant women who miscarried or delivered prior to seeking institutional healthcare, which could limit the external validity of our results. Third, if duration of cART is associated with preterm birth, restricting to a population of infants born at term may bias analyses aimed at generalizing to a population of infants born preterm and at term.(37) In our analysis, we limited external validity (e.g. generalizability) in an effort to provide internally valid estimates for a population of infants born at term. Fourth, despite limiting our analyses to term births, measurement error in gestational age may still be present (Web Appendix Figure 1). Finally, reported effect estimates for women on cART for 21-36 weeks and ≤ 8 weeks were imprecise due to the small numbers of women in these categories.
Strengths of our study included the use of data from an electronic medical record system covering 24 public health clinics in Lusaka, the use of a study population with uniform eligibility for cART initiation and the consistency of results across multiple sensitivity analyses. In addition, our analysis provides some of the first evidence about the relationship between duration of cART and the risk of LBW due to fetal growth restriction among HIV-infected women in SSA.
Interpretation
The aim of this analysis was to determine the association between cART duration during pregnancy and LBW among infants born at term. Our findings are consistent with other work looking at treatment duration and LBW among term and preterm births. cART initiated either early during pregnancy (≤25 or <28 weeks gestation) or late (≥28 or 32 weeks gestation) was not associated with LBW in studies in South Africa and the US.(6, 38) However, since term and preterm births were combined in these studies – without further stratification as in this report – it is difficult to distinguish whether late initiators had no increased risk of LBW, or were simply less likely to have a PTB (and therefore LBW) by virtue of starting treatment later during pregnancy. Birthweight Z-scores adjusted for gestational age were considered in a French study and no association with duration of cART was found.(39) An analysis from Malawi and Mozambique attempted to stratify by gestational age at birth, but was unable to investigate the association between duration of cART and LBW among term infants due to the small sample size (n=496).(40) Our study, therefore, provides some of the first evidence from SSA on associations between duration of cART and LBW due to fetal growth restriction.
PI-based cART has been hypothesized to impact fetal growth by inhibiting progesterone production during pregnancy.(41) Among HIV-uninfected women, low progesterone levels have been associated with lower birthweights.(42, 43) In animal models, PI-based cART regimens have been associated with decreased progesterone levels, which correlated with lower fetal weight.(41) Recent findings from the IMPAACT PROMISE study suggest that PI-based cART increases the risk of both LBW and PTB.(44) In Zambia, women initiating treatment during pregnancy start NNRTI-based cART.(29-31) PI-based cART is reserved for second line therapy, which may be one reason we did not observe an association between duration of cART and LBW among infants born at term.
Although the association between cART duration and PTB is of significant interest, we were unable to examine this specific research question in the current context. Studying the relationship between duration of cART and PTB is difficult because duration of treatment is directly related to length of gestation and timing of delivery. Women on cART longer are, by definition, closer to term and therefore less likely to have a PTB. In populations at high risk for PTB, this could result in longer durations of cART looking protective against PTB. Because of these constraints, we restricted our analysis to term births and focused on an outcome of LBW due growth restriction. Given our focus on LBW among infants born at term, as a proxy for IUGR, our results may be helpful to clinicians caring for patients at low-risk for PTB. Additional work is needed to understand how duration of cART may affect preterm delivery.
Finally, this cannot establish causality and we caution against over interpretation in this regard. Prospective randomized studies investigating the effect of timing of cART initiation on LBW could help to establish causality. However, such a randomized study would pose serious ethical challenges, and might itself leave questions unanswered since women included in randomized controlled trials are often a highly selected group, and may not be generalizable to a real-world clinical population of HIV-infected pregnant women. Analysis of retrospective routinely collected clinical data can only establish causality under critical (and empirically unverifiable) assumptions including no uncontrolled confounding and treatment variation irrelevance, also known as the consistency assumption.(45) However, given the challenges of understanding how timing of treatment may impact LBW, analysis of routinely collected clinical data still provides important information about LBW trends among women initiating cART during pregnancy.
In our cohort, there was no evidence that longer duration of cART was associated with poor fetal growth among term pregnancies. Despite slight decreases in birthweight with increasing cART duration, the benefits of cART during pregnancy for PMTCT continue to outweigh the risks. Our findings remained consistent across numerous sensitivities analyses, providing some level of reassurance about our primary findings. However, the relationship between cART use and adverse pregnancy outcomes – particularly those associated with PTB – remain complicated and continued work is required to investigate causality. While beyond the scope of the current analysis, future research should also investigate whether cART use from conception increases the risk of LBW or PTB. An understanding of the relationship between cART and adverse pregnancy outcomes is of particular importance, as maternal combination regimens become the cornerstone of PMTCT programs globally.
Supplementary Material
Acknowledgments
AMB contributed to the study conception and design, conducted the analysis and drafted the manuscript. DW, CJW, WCM, AP and BHC contributed to the study conception and design, acquisition and interpretation of the data and critically reviewed the manuscript for important intellectual content. MMM, BV, MM and PM contributed to the interpretation of the data and critically reviewed the manuscript for important intellectual content. The authors also thank Dr. Allen Wilcox for his assistance in the development of this manuscript. Trainee support was provided through grants from the National Institutes of Health (T32 AI007001) and the Doris Duke Charitable Foundation (2009060).
Funding: National Institutes of Health (T32 AI007001) and Doris Duke Charitable Foundation (2009060).
Footnotes
Conflicts of Interest: The authors report no conflicts of interest.
Presentations at conferences: A portion of this work was presented as a poster at the 17th International Workshop for HIV Observational Databases in Barcelona, Spain, March 27-29, 2014 and as an oral presentation at the 8th International Workshop on HIV Treatment, Pathogenesis and Prevention Research in Resource-Poor settings in Lusaka, Zambia, May 5-9, 2014.
References
- 1.WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva, Switzerland: World Health Organization; 2013. [PubMed] [Google Scholar]
- 2.Ahmed S, Kim MH, Abrams EJ. Risks and benefits of lifelong antiretroviral treatment for pregnant and breastfeeding women: a review of the evidence for the Option B+ approach. Curr Opin HIV AIDS. 2013 Sep;8(5):474–89. doi: 10.1097/COH.0b013e328363a8f2. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants. Recommendations for a public health approach. 2010 [PubMed] [Google Scholar]
- 4.Thorne C, Patel D, Newell ML. Increased risk of adverse pregnancy outcomes in HIV-infected women treated with highly active antiretroviral therapy in Europe. AIDS (London, England) 2004;18(17):2337–9. doi: 10.1097/00002030-200411190-00019. [DOI] [PubMed] [Google Scholar]
- 5.Townsend CL, Cortina-Borja M, Peckham CS, Tookey PA. Antiretroviral therapy and premature delivery in diagnosed HIV-infected women in the United Kingdom and Ireland. Aids. 2007 May 11;21(8):1019–26. doi: 10.1097/QAD.0b013e328133884b. [DOI] [PubMed] [Google Scholar]
- 6.Tuomala RE, Watts DH, Li D, et al. Improved obstetric outcomes and few maternal toxicities are associated with antiretroviral therapy, including highly active antiretroviral therapy during pregnancy. Journal of acquired immune deficiency syndromes (1999) 2005;38(4):449–73. doi: 10.1097/01.qai.0000139398.38236.4d. [DOI] [PubMed] [Google Scholar]
- 7.Schulte J, Dominguez K, Sukalac T, Bohannon B, Fowler MG, Pediatric Spectrum of HIVDC Declines in low birth weight and preterm birth among infants who were born to HIV-infected women during an era of increased use of maternal antiretroviral drugs: Pediatric Spectrum of HIV Disease, 1989-2004. Pediatrics. 2007;119(4):e900–6. doi: 10.1542/peds.2006-1123. [DOI] [PubMed] [Google Scholar]
- 8.Cotter AM, Garcia AG, Duthely ML, Luke B, O'Sullivan MJ. Is antiretroviral therapy during pregnancy associated with an increased risk of preterm delivery, low birth weight, or stillbirth? The Journal of infectious diseases. 2006;193(9):1195–201. doi: 10.1086/503045. [DOI] [PubMed] [Google Scholar]
- 9.Szyld EG, Warley EM, Freimanis L, et al. Maternal antiretroviral drugs during pregnancy and infant low birth weight and preterm birth. AIDS (London, England) 2006;20(18):2345–53. doi: 10.1097/01.aids.0000253362.01696.9d. [DOI] [PubMed] [Google Scholar]
- 10.Tuomala RE, Shapiro DE, Mofenson LM, et al. Antiretroviral therapy during pregnancy and the risk of an adverse outcome. The New England journal of medicine. 2002;346(24):1863–70. doi: 10.1056/NEJMoa991159. [DOI] [PubMed] [Google Scholar]
- 11.Ekouevi DK, Coffie PA, Becquet R, et al. Antiretroviral therapy in pregnant women with advanced HIV disease and pregnancy outcomes in Abidjan, Cote d'Ivoire. AIDS (London, England) 2008;22(14):1815–20. doi: 10.1097/QAD.0b013e32830b8ab9. [DOI] [PubMed] [Google Scholar]
- 12.Chen JY, Ribaudo HJ, Souda S, et al. Highly active antiretroviral therapy and adverse birth outcomes among HIV-infected women in Botswana. J Infect Dis. 2012 Dec 1;206(11):1695–705. doi: 10.1093/infdis/jis553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Central Statistical Office (CSO) MoHM, Tropical Diseases Research Centre (TDRC), University of Zambia, and Macro International Inc. Zambia Demographic and Health Survey. Calverton, Maryland, USA: 2007. 2009. [Google Scholar]
- 14.Chi BH, Vwalika B, Killam WP, et al. Implementation of the Zambia electronic perinatal record system for comprehensive prenatal and delivery care. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2011;113(2):131–6. doi: 10.1016/j.ijgo.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Council ZNA. National Protocol Guidelines - Zambia. Lusaka, Zambia: Zambian Ministry of Health; 2007. Integrated Prevention of Mother-to-Child Transmission of HIV/AIDS. [Google Scholar]
- 16.Health ZMo. National Protocol Guidelines 2010. Lusaka Zambia: 2010. Integrated Prevention of Mother-to-Child Transmission of HIV. [Google Scholar]
- 17.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. American Journal of Epidemiology. 2003;158(9):915–20. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- 18.Rayco-Solon P, Fulford AJ, Prentice AM. Differential effects of seasonality on preterm birth and intrauterine growth restriction in rural Africans. Am J Clin Nutr. 2005 Jan;81(1):134–9. doi: 10.1093/ajcn/81.1.134. [DOI] [PubMed] [Google Scholar]
- 19.Bengtson AM, Westreich D, Musonda P, et al. Multiple overimputation to address missing data and measurement error in gestational age: application to duration of HIV treatment before delivery and pregnancy outcomes. In Press, Epidemiology. 2015 doi: 10.1097/EDE.0000000000000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livingston JC, Maxwell BD, Sibai BM. Chronic hypertension in pregnancy. Minerva ginecologica. 2003 Feb;55(1):1–13. [PubMed] [Google Scholar]
- 21.Svare JA, Hansen BB, Molsted-Pedersen L. Perinatal complications in women with gestational diabetes mellitus. Acta Obstet Gynecol Scand. 2001 Oct;80(10):899–904. doi: 10.1034/j.1600-0412.2001.801006.x. [DOI] [PubMed] [Google Scholar]
- 22.United Nations Children's Fund, World Health Organization. Low Birthweight: Country,regional and global estimates. 2004 [Google Scholar]
- 23.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology (Cambridge, Mass) 1999;10(1):37–48. [PubMed] [Google Scholar]
- 24.Hernan MA, Hernandez-Diaz S, Werler MM, Mitchell AA. Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology. American Journal of Epidemiology. 2002;155(2):176–84. doi: 10.1093/aje/155.2.176. [DOI] [PubMed] [Google Scholar]
- 25.Jain T, Garg S, Singh MM, et al. Antepartum morbidities and health seeking behaviour among women in an urban slum of Delhi. Journal of the Indian Medical Association. 2011;109(5):315–7. [PubMed] [Google Scholar]
- 26.Amin R, Shah NM, Becker S. Socioeconomic factors differentiating maternal and child health-seeking behavior in rural Bangladesh: A cross-sectional analysis. International journal for equity in health. 2010;9 doi: 10.1186/1475-9276-9-9. 9-9276-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halim N, Bohara AK, Ruan X. Healthy mothers, healthy children: does maternal demand for antenatal care matter for child health in Nepal? Health policy and planning. 2011;26(3):242–56. doi: 10.1093/heapol/czq040. [DOI] [PubMed] [Google Scholar]
- 28.Howe CJ, Cole SR, Westreich DJ, Greenland S, Napravnik S, Eron JJ., Jr Splines for trend analysis and continuous confounder control. Epidemiology (Cambridge, Mass) 2011;22(6):874–5. doi: 10.1097/EDE.0b013e31823029dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Republic of Zambia MoH. 2010 National Protocol Guidelines. Integrated Prevention of Mother-to-Child Transmission of HIV. 2010 [Google Scholar]
- 30.Chi BH, Sinkala M, Stringer EM, et al. Early clinical and immune response to NNRTI-based antiretroviral therapy among women with prior exposure to single-dose nevirapine. Aids. 2007 May 11;21(8):957–64. doi: 10.1097/QAD.0b013e32810996b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chintu N, Giganti MJ, Putta NB, et al. Peripartum nevirapine exposure and subsequent clinical outcomes among HIV-infected women receiving antiretroviral therapy for at least 12 months. Trop Med Int Health. 2010 Jul;15(7):842–7. doi: 10.1111/j.1365-3156.2010.02540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Howards PP, Hertz-Picciotto I, Weinberg CR, Poole C. Misclassification of gestational age in the study of spontaneous abortion. Am J Epidemiol. 2006 Dec 1;164(11):1126–36. doi: 10.1093/aje/kwj327. [DOI] [PubMed] [Google Scholar]
- 33.Lynch CD, Zhang J. The research implications of the selection of a gestational age estimation method. Paediatric and perinatal epidemiology. 2007;21(Suppl 2):86–96. doi: 10.1111/j.1365-3016.2007.00865.x. [DOI] [PubMed] [Google Scholar]
- 34.Mikolajczyk RT, Zhang J, Betran AP, et al. A global reference for fetal-weight and birthweight percentiles. Lancet. 2011;377(9780):1855–61. doi: 10.1016/S0140-6736(11)60364-4. [DOI] [PubMed] [Google Scholar]
- 35.Barros AJ, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC medical research methodology. 2003;3:21. doi: 10.1186/1471-2288-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Organization WH. WHO antenatal care randomized trial: manual for implementation of the new model. Geneva, Switzerland: World Health Organization; 2002. [Google Scholar]
- 37.Cole SR, Platt RW, Schisterman EF, et al. Illustrating bias due to conditioning on a collider. Int J Epidemiol. 2010 Apr;39(2):417–20. doi: 10.1093/ije/dyp334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Merwe K, Hoffman R, Black V, Chersich M, Coovadia A, Rees H. Birth outcomes in South African women receiving highly active antiretroviral therapy: a retrospective observational study. J Int AIDS Soc. 2011;14:42. doi: 10.1186/1758-2652-14-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Briand N, Mandelbrot L, Le Chenadec J, et al. No relation between in-utero exposure to HAART and intrauterine growth retardation. Aids. 2009 Jun 19;23(10):1235–43. doi: 10.1097/QAD.0b013e32832be0df. [DOI] [PubMed] [Google Scholar]
- 40.Marazzi MC, Palombi L, Nielsen-Saines K, et al. Extended antenatal use of triple antiretroviral therapy for prevention of mother-to-child transmission of HIV-1 correlates with favorable pregnancy outcomes. AIDS (London, England) 2011;25(13):1611–8. doi: 10.1097/QAD.0b013e3283493ed0. [DOI] [PubMed] [Google Scholar]
- 41.Papp E, Mohammadi H, Loutfy MR, et al. HIV protease inhibitor use during pregnancy is associated with decreased progesterone levels; a potential mechanism contributing to fetal growth restriction. J Infect Dis. 2014 Jul 16; doi: 10.1093/infdis/jiu393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mucci LA, Lagiou P, Tamimi RM, Hsieh CC, Adami HO, Trichopoulos D. Pregnancy estriol, estradiol, progesterone and prolactin in relation to birth weight and other birth size variables (United States) Cancer causes & control : CCC. 2003 May;14(4):311–8. doi: 10.1023/a:1023966813330. [DOI] [PubMed] [Google Scholar]
- 43.Hartwig IR, Pincus MK, Diemert A, Hecher K, Arck PC. Sex-specific effect of first-trimester maternal progesterone on birthweight. Hum Reprod. 2013 Jan;28(1):77–86. doi: 10.1093/humrep/des367. [DOI] [PubMed] [Google Scholar]
- 44.Fowler M, Qin M, Shapiro D, et al. CROI. Seattle, Washington: 2015. PROMISE: Efficacy and Safety of 2 Strategies to Prevent Perinatal HIV Transmission. [Google Scholar]
- 45.VanderWeele TJ. Concerning the consistency assumption in causal inference. Epidemiology. 2009 Nov;20(6):880–3. doi: 10.1097/EDE.0b013e3181bd5638. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


