Abstract
Objectives
To evaluate 64Cu-TP3805 as a novel biomolecule, to PET image prostate cancer (PC), at the onset of which VPAC1, the superfamily of G-protein coupled receptors, is expressed in high density on PC cells, but not on normal cells.
Methods
25 patients undergoing radical prostatectomy were PET/CT imaged preoperatively with 64Cu-TP3805. Standardized uptake values (SUVmax) were determined, malignant lesions (SUV > 1.0) counted, and compared with histologic findings. Whole mount pathology slides from 6 VPAC1 PET imaged patients, 3 BPH patients, one malignant and one benign lymph node underwent digital autoradiography (DAR) after 64Cu-TP3805 incubation and compared to H&E stained slides.
Results
In 25 patient PET imaging, 212 prostate gland lesions had SUVmax > 1.0 vs.127 lesions identified by histology of biopsy tissues. The status of the additional 85 PET identified prostate lesions remains to be determined. In 68 histological slides from 6 PET imaged patients, DAR identified 105/107 PC foci, 19/19 HGPIN, and ejaculatory ducts and verumontanum involved with cancer.
Additionally, DAR found 9 PC lesions not previously identified histologically. The positive and negative lymph nodes were correctly identified and in 3/3 BPH patients and 5/5 cysts, DAR was negative.
Conclusion
This feasibility study demonstrated that 64Cu-TP3805 delineates PC in vivo and ex vivo, provided normal images for benign masses, and is worthy of further studies.
Keywords: PET Imaging, Prostate Cancer, VPAC1 Receptors, Cu-64-TP3805, Diagnosis
Introduction
Prostate cancer (PC) is the most common non-skin cancer in men. In 2015, in North America alone, there will be 220,800 new cases of PC and 27,540 men will die of it1. PC is also increasing worldwide2. There is considerable controversy over PSA based screening for PC, with no consistent recommendations from major medical organizations on the best approach to screening 3,4. While many biomarkers are in development to identify PC, non-invasively using blood and urine assays, the definitive diagnosis of PC relies on the histologic identification of cancer cells on invasive prostate tissue biopsy5. The standard biopsy procedure, transrectal ultrasound (TRUS) with 10 to 12 needle cores of the prostate gland, can be associated with morbidity, and over 2/3 of the time may not identify all malignant lesions6,7.
Radiologic examination such as TRUS, computerized x-ray tomography (CT), multiparametric magnetic resonance imaging (MRI), and nuclear scans such as single photon emission computerized tomography (SPECT) and positron emission tomography (PET), using such radiopharmaceuticals as, In-111-ProstaScint™, F-18-FDG and C-11-Choline, are available, but suffer from limitations8. Therefore, there is a compelling need for continuation of the development of a biomolecule, which will detect PC and its metastatic lesions with high sensitivity and specificity.
Recent approaches to imaging PC have generally been directed to targeting prostate-specific membrane antigen (PSMA)9–12. We have chosen to target VPAC1, which belongs to the superfamily of G-protein coupled surface receptors that are expressed in high density on certain cancer cells, including PC cells at the onset of oncogenesis and prior to the alterations in cell morphology13,14. VPAC1 receptors (combined for vasoactive intestinal and pituitary adenylate cyclase activating peptide, PACAP) are involved in cell proliferation, cell differentiations, and survival of PC cells, and are overexpressed in cancer of the prostate, breast, bladder, and lungs13. On stroma, normal cells and benign masses, VPAC1 receptors are minimally present13–19.
We hypothesized that a radiolabeled biomolecule with a high affinity for VPAC1 would not only image PC, but also distinguish malignant lesions from benign prostatic hyperplasia (BPH) and contribute to the management of patients on surveillance. To validate this hypothesis, a large body of preclinical data has been generated in our laboratory20–24. This included PET imaging of spontaneously grown PC in TRAMP (Transgenic adenocarcinoma of the mouse prostate) mice that mimicked the pathophysiology of human PC23 and a feasibility study of PET and PEM (positron emission mammography) imaging of breast cancer (BC) in humans, on which the VPAC1 receptors are also expressed in high density24.
The biomolecule we chose to target VPAC1 receptors was chosen out of four such compounds designed and evaluated extensively in our laboratory20–24. It consists of 28 amino acid PACAP analogue that is conjugated to a N2S2 (diaminedithiol(N2S2-Benzoyl)2) chelating agent at the C terminus of the peptide and labeled with 64Copper (t ½ = 12.8 hrs.), a positron emitting (β+, 19%, 656 KeV) radionuclide produced using a cyclotron. The agent was named 64Cu-TP3805. Our preclinical evaluation demonstrated that 64Cu-TP3805 has not only a strong affinity (Kd = 3.1×10−9M) for VPAC1, is receptor specific, and is stable in vivo, but also has <2% urinary excretion, a virtue favorable for imaging PC23. In this article, we describe our preliminary findings in PET imaging of 25 males known to have PC and that were scheduled for radical prostatectomy. Our in vivo data were substantiated by using digital autoradiography (DAR) on whole mount histologic slides from 6 PET imaged patients. In addition, DAR was also performed on slides obtained from three BPH patients not imaged by PET and two excised lymph nodes from institutional tissue bank.
Materials and Methods
TP3805 Synthesis and Kit Preparation
The peptide was synthesized and kits were prepared as described previously24. The 28-amino acid peptide, the amino acid sequence of which is published previously, was synthesized by American Peptide Co (Sunnyvale, CA) on a Wang resin, purified and characterized by electrospray mass spectroscopy20. The peptide stock (M.W. 3805) was stored at −80°C, 20μg peptide kits under sterile N2 were prepared aseptically, and stored at −10°C until use24. Stability of the kits was checked by their ability to label them with 64Cu as measured by high-pressure liquid chromatography (HPLC).
Preparation of 64Cu-TP3805
On the day of preparation, a kit vial was removed, brought to room temperature, and a required quantity of 64Cu solution (Washington University, St. Louis, MO) was added to the vial (usually 222 MBq, 6 mCi in 30μl of 0.1 M HCl), followed by the addition of 220 μl of sterile water. The vial was incubated at 50°C for ninety minutes. The solution was then diluted for I.V. injection by the addition of 3 ml sterile 0.9% NaCl.
The radiochemical purity was determined by HPLC, with reverse phase Zorbax 300SB.C18, 4.6 mm × 250mm column (Agilent), eluted with a linear 23 min. gradient from 0% to 100% acetonitrile in 0.1% aqueous TFA in 7 min and then to 90% in 15 min; ran for 23 min. (Labeling efficiency of ≥95% was considered as the criterion for kit stability). This procedure rendered a 64Cu-TP3805 specific activity of 44.4 GBq (1.2 Ci/μmol). Sterility of each preparation was determined as described previously24.
Patient Inclusion
Exploratory investigational new drug (eIND) number 101550 was assigned by the Food and Drug Administration (FDA). Approvals were also obtained, prospectively, from the Institutional Review Board (IRB), Clinical Cancer Research Review Committee (CCRRC), and Radioactive Drug Research Committee (RDRC). Patients (n=25, 6 African Americans, 19 Caucasian, age ranges: 44–77 yrs., mean 63.4±7.6 yrs.) known to have PC and scheduled for radical prostatectomy (Gleason score 6 to 8), and who signed informed consent form, were recruited in consecutive order and imaged 1–3 weeks prior to the surgery. Prior to imaging, each patient underwent US guided, 12 core needle biopsy, 4 each from the apex, midsection, and base, each subdivided into four subsections: L lateral, L medial, R medial, and R lateral. Standard H&E staining was performed and Gleason score was assigned. Statistical analysis assumed that there will be three PC lesions per patient and 64Cu-TP3805 will correctly detect 80% of the lesions, with 87% power.
PET/CT Imaging
PET/CT images were obtained in supine position with a 4 min. bed time using a Biograph-6 PET/CT scanner (Siemens, Inc.). For 64Cu-TP3805 imaging, patients neither fasted nor had their blood glycemic levels determined. 64Cu-TP3805 was injected I.V. (148±10% MBq, 4±10% mCi) through an indwelling catheter. The quantity of 64Cu was determined by the dose escalation study performed previously24 and was approved by the FDA. Whole body scans were then obtained at 30 min. and 2 hr. after injection. The 30 min. time was based upon our breast cancer imaging study, in which optimal uptake was observed in 30 min. post injection24. Following I.V. administration, each patient was carefully observed, during and after the imaging procedure, by monitoring and recording their vital signs. Twenty-four hours later, each patient was also contacted for any delayed adverse events.
Image Analysis
All PET/CT images were read by two board certified nuclear medicine physicians (SK,CI) who determined standardized (maximum) uptake values (SUVmax) in 12 sectors (4 each of the apex, middle, and base) of each prostate as seen on the PET image. These sections approximated the standard biopsy sites.
Digital Autoradiography (DAR)
From all PET imaged patients, prostatectomy specimens received fresh from the operating room were fixed in neutral-buffered 10% formalin solution for 24 hours. Post fixation, the glands were serially sectioned at 4 mm from apex to base. Tissue sections were processed for paraffin infiltration and embedded to construct whole mount tissue slices. The paraffin blocks were sectioned at 3μm and mounted on large format glass slides, dried at 60°C, de-paraffinized and stained with hematoxylin and eosin. The stained slides were examined microscopically and areas of tumor were mapped. Using paraffin blocks from six of the PET imaged patients, slides were cut in duplicate. From each of these six PET imaged patients, 9–15 deparaffinized 3μm thick histological sections (n=66) were incubated at room temperature with 64Cu-TP3805 solution, washed thoroughly with phosphate buffered saline (PBS), dried and subjected to 15 sec. digital autoradiography (DAR, Imaging 4000, Kodak/Bruker Inc.). These slides were then H&E stained and foci were marked by a urologic pathologist (PM, RB) as PC, benign, cystic, or prostatic intraepithelial neoplasia (PIN). In addition, three histological slices each from 3 BPH patients (n=9), from one malignant lymph node (n=3) and from one benign lymph node (n=3) were also examined with DAR followed by H&E staining. The histologic findings were then analyzed with the autoradiographic findings for match or mismatch.
Results
Peptide and Kit Stability
As determined by mass spectrometric analysis, TP3805 was stable for more than 3 years. The kits were stable for longer than 18 months and labeled 64Cu with >95% efficiency20–24. All preparations were sterile. The total 64Cu dose remaining in the syringe and the intravenous line averaged less than 5.5 MBq (150 μCi). Following administration of 64Cu-TP3805, none of the patients had any adverse events of any kind.
PET Image Analysis
PET images acquired both at 30 min. and 2 hours post injection of 64Cu-TP3805 revealed liver uptake and mild GI uptake on 2 hour images. Although none of the organ distribution data were quantified in humans, in animal tissue distribution data, liver uptake was 25.4 ± 1.74% at 4 hours post injection, and that 7% of radioactivity was eliminated in 24 hours via feces22. During 24 hours post injection, urinary uptake in mice was <2%22. Consistent with this, no bladder uptake of 64Cu was noted in any image in any of the patients (Fig. 1). For malignant PC lesions, generally, no significantly or consistently greater SUVs were noted at 2hr images than those acquired at 30 min. post injection.
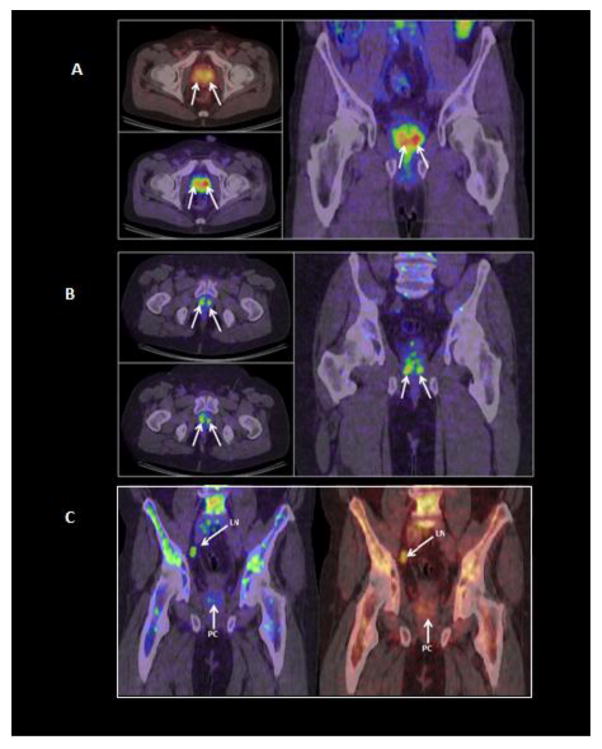
Fig. 1.
A composite of cross-sectional and coronal PET/CT images of two patients (1A: 68 yrs, Gleason score 7. Fig. 1B: 51 yrs, Gleason score 6) obtained 30 min. post-injection of 4±10% 64Cu-TP3805. Multiple PC lesions are delineated (arrow). Radioactivity in the bladder is negligible. Some bone marrow uptake is visible. A Tc-99m-MDP bone scan was negative. Fig. 1C: A 58 yr old male with PC (Gleason score 7) received 4mCi 64Cu-TP3805, 30 min. prior to PET/CT imaging. The coronal images in different colors demonstrated a lymph node (LN) and prostate cancer (PC). The lymph node was dissected and found to be malignant by histology. Bone marrow uptake of 64Cu-TP3805 is apparent. A standard of care Tc-99m-MDP bone scan was negative for metastatic bone lesions.
PET images in all patients revealed more than one malignant lesion in the prostate. In one patient, a lymph node was delineated by the 64Cu-TP3805 PET scan (Fig. 1C).
The lymph node was dissected and was found to be malignant by histology. There were no known distant metastatic bony lesions in any of the patients, and none were detected by the PET scan. In two patient (Fig. 1B & 1C), 64Cu activity was noted in iliac crest. The bone scans, performed, 2 weeks before were negative.
SUVmax for lesions in each of the 12 biopsy sectors, for each patient, were determined, the range for which was 0.7–8.8. These were corroborated with the sectional histology results. Lesions with SUVmax ≤1.0 were considered normal (n=88). In 25 patients, PET images identified more lesions (n=212) with SUVmax >1.0, than the lesions histologically considered to be malignant (n=127, Table 1).
DAR for histological tissue examination is a well-established technique13. The DAR data from 6 patients using 66 histological slides, each of which were H&E stained post-DAR, read by a pathologist, marked with a color code, and compared visually, provided better insight into the greater number of malignant lesions seen on PET images with >1.0 SUVmax than determined by histology of core biopsy (Table 2).
Table 2 shows that, in 66 slices, there were a total of 107 PC foci, as determined by histology (Fig. 2). Out of these, 105 (98%) were identified by DAR. DAR missed 2 (1.8%) PC lesions due to technical artifact. For 3 BPH patients without PC (Fig. 3A and B), DAR was negative. The malignant lymph node and benign lymph node (Fig. 3C and D) were correctly identified by DAR. Additionally, 9 (8.5%) small PC lesions not previously noted by histology were identified by DAR. Furthermore, DAR identified 19 lesions corresponding to high grade prostatic intraepithelial neoplasia (HGPIN) (Fig. 4A and B), 2 ejaculatory duct and 5 urethra verumontanum not previously noted by histology. For 5 intraprostatic cysts, DAR was negative (Fig. 4C)
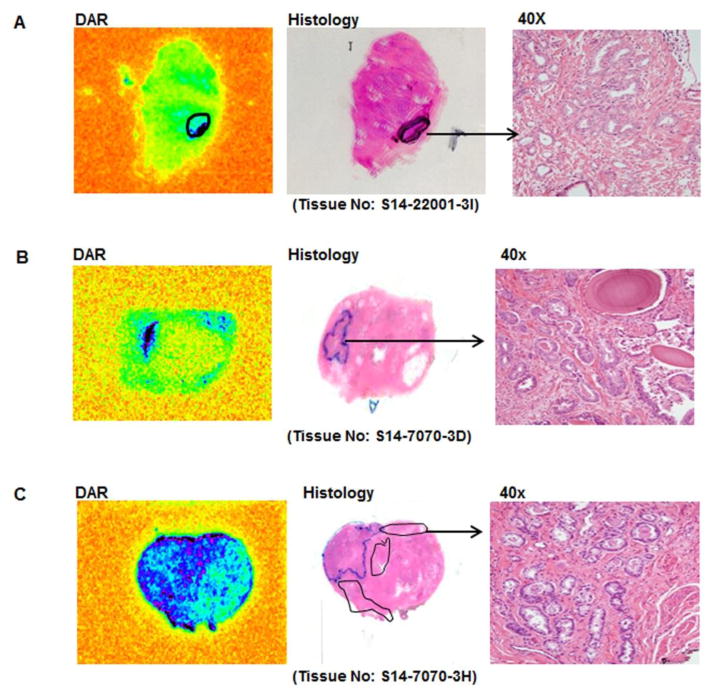
Fig. 2.
Examples of digital audioradiography (DAR) and H&E stained of the same slide (histology as marked by the pathologist) are shown for two different patients (2A is patient #1, and 2B & 2C are patient #2) who received 64Cu-TP3805 prior to radical prostatectomy. The malignant lesions are marked on histology slides and corroborate well with DAR.
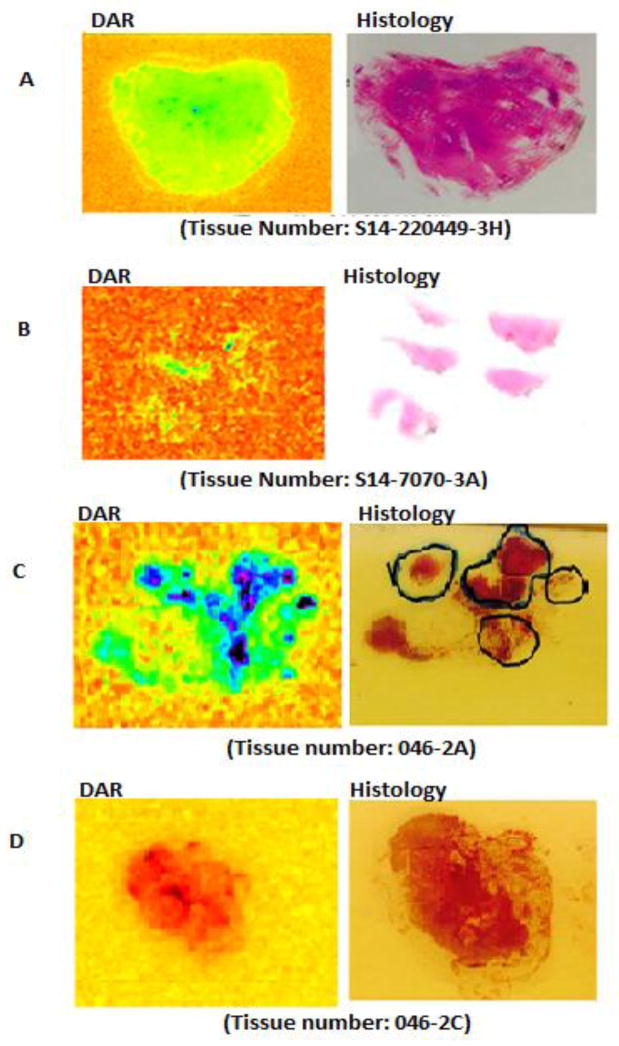
Fig. 3.
A and B show the absence of 64Cu radioactivity on the slides examined from three separate patients with benign prostatic hyperplasia (BPH) as determined by Histology of the same three slides. Examples of histologically, delineated malignant lymph node (3C on the right) and benign lymph node (3D on the right). Note the uptake of 64Cu-TP3805 in the malignant lesions (3C on the left) but not in the benign node (3D on the left).
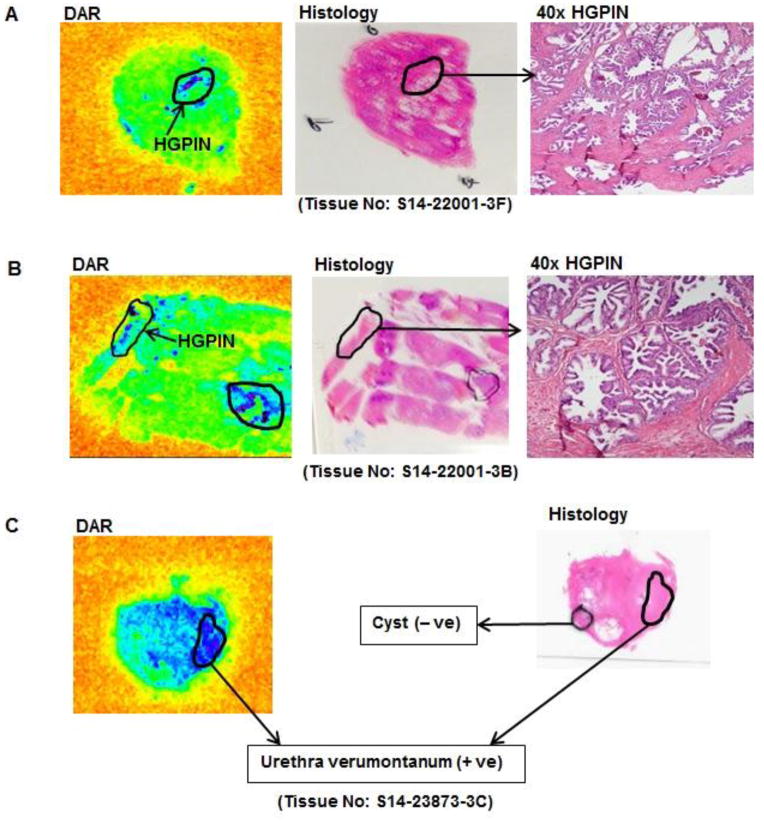
Fig. 4.
Digital audioradiography (DAR) of histological tissues from three separate patients demonstrates the 64Cu-TP3805 uptake on the lesions of HGPIN (4A and 4B) and on urethra verumontanum (4C), but not on the cyst (4C) where VPAC1 receptors are not expressed (all lesions were marked by the pathologist).
Comment
Although great strides have been made over the past few years to diagnose PC with minimally invasive procedures, ultrasound guided transrectal prostate biopsy for histologic examination of prostate tissue is considered the gold standard approach7.
As the controversy due, primarily to, the non-specificity of the PSA screening for early detection of PC continues, the need is even greater to have reliable tools that can provide information about the patient’s disease. The anatomical diffusion MR reliably images PC with Gleason score 7 and when combined with T2 weighted imaging, PC lesions with Gleason score 6 can also be imaged with high sensitivity. In the era of molecular imaging, novel blood and urine markers and a large number of novel radiopharmaceuticals have been developed8–12,25–29. ProstaScint™ (111In-capromab pendetide) is a radiolabeled murine monoclonal antibody against intracellular epitope of PSMA, initially approved in 1996, has the largest published clinical data to date. ProstaScint™ binds to an intracellular portion of PSMA, reducing its ability to image living tumor cells8. A recent study concluded that ProstaScint™ can be used to detect lymph node metastases from PC but the test had limited utility in diagnosing tumors in the prostate gland8. Another agent is ionic 64CuCl2 shown to be taken up in human PC grown in experimental mice25. The uptake is thought to be mediated by human Copper Transporter 1 (hCtr1). A newer agent 68Ga labeled Prostate Specific Membrane Antigen (PSMA) ligand, in humans, demonstrated high sensitivity, but low specificity, and presents both false positive and false negative results26,27. Anti-3-[(18)F]FACBC is a relatively new tracer primarily studied in the setting of recurrent disease. In comparison, the detection for extraprostatic recurrence with this agent was superior to ProstaScint™ but at present, studies of this F-18 agent in the primary detection setting are limited30. Although results of these investigations have generally been encouraging, much remains unknown about their applicability in routine clinical practice.
We have chosen to target VPAC1 receptors with 64Cu-TP3805, the early evaluation of which demonstrated that it has the ability to target only malignant, but not normal or benign lesions, indicating a virtue of much needed specificity. 64Cu-TP3805 has a high affinity (Kd=3.1×10−9M) for VPAC1, which is over-expressed in high density on PC cells at the onset of oncogenesis. Furthermore, 64Cu-TP3805 has excellent (97%) stability in mouse and human serum. Urine collected in metabolic cages, at 4 hrs. and 24 hrs. post-injection, 98±1.8% and 85±3% of 64Cu, respectively, was associated with TP3805. Its firm stability in vivo was also evident by receptor blocking studies (tumor uptake, unblocked 5.78±0.66% ID/g vs. blocked 1.84±0.44% ID/g, n=25, P<0.01)21. These characteristics, together with its repeated ability to correctly image only malignant breast cancer, but not benign, lesions in MMTV neu transgenic mice and in humans, as well as spontaneously grown PC in TRAMP mice, and the lack of its urinary excretion, prompted us to hypothesize that 64Cu-TP3805 would permit us to image PC early and accurately, and perhaps allow one to distinguish PC from other non-malignant conditions21–24. The objective here was to perform a feasibility study to determine if 64Cu-TP3805 shall image known PC, a multifocal disease, with high efficacy.
The SUVmax, determined from PET images, indicated that 64Cu-TP3805 imaged more lesions in vivo than those observed by histology of biopsy tissues. Since the routine histological examinations were performed using 3μm thick slices, cut from a few of the 4 mm thick interval sections, it is conceivable that some of the PET positive PC foci may not have been detectable by histology. This observation needs to be confirmed using further histologic examination of several slices obtained from whole mount excised prostate tissues. However, the in vitro data from six of the PET imaged patients, in whom we performed DAR and corroborated its findings with whole mount histologic examinations of the same slides ex-vivo, lead us, in part, to the validation of our in vivo imaging data, in that, not only did 64Cu-TP3805 imaging identify all malignant lesions, but it also identified the HGPINs, the ejaculatory ducts, and urethra verumontanum, but not the BPH or cysts (Fig. 2–4). If such a trend persisted in the remaining 19 patients, it at least in part, may explain the additional number of malignant lesions observed in the prostate by 64Cu-TP3805 PET imaging. Detection of HGPIN is consistent with early expression of VPAC1 prior to the modulation of cell morphology. Although this probable sensitivity could be a problem in the patient management, HGPIN has a high predicative value as a marker for adeno carcinoma. Similarly, ejaculatory ducts and urethra verumontanum (UV), in which PC cells are present, were also seen by DAR, but not cysts in which VPAC1 expression does not exist (Fig. 4C). DAR was positive for malignant lymph node, but was negative for normal lymph node (Fig. 3C&D). However, the reasons for the bone marrow uptake seen in a patient (Fig. 1) with negative bone scan are not yet clear.
These data consistently demonstrated positive results for malignant lesions and negative results for benign tissues indicating high specificity of 64Cu-TP3805 to image malignant lesions, but not benign masses. However, these observations need to be evaluated on a large number of patients. The data are also consistent with our previous observations in which we have targeted VPAC1 biomarker to image breast cancer in humans using 64Cu-TP380524. Although preliminary and need to be substantiated in a large number of cases with different prostatic conditions, 64Cu-TP3805 identified more lesions by PET imaging than seen by histology (212/127, Table 1) in 25 patients and by DAR than the corresponding H&E staining (43+32=75/43 Table 2). These data exceeded our goal of 80% positivity and provided us with real insight into imaging many malignant and precancerous PC lesions and rendered 64Cu-TP3805 worthy of further evaluation in imaging PC early and accurately.
Conclusion
There is a compelling need for a biomolecule that will image PC, early and accurately, but not benign prostate conditions, and that will minimize the number of unnecessary biopsies, reduce patient anxiety, and reduce health care cost. Although our data are preliminary, they demonstrated that 64Cu-TP3805 delineated PC and HGPIN lesions, with excellent specificity for VPAC1, and call for further studies.
Supplementary Material
Acknowledgments
Financial Support: NCI R01CA157372 and NuView Life Sciences (MLT)
The principal author, M.L. Thakur (MLT), thanks all of his colleagues for their enthusiasm, support, and collaboration, Ms. Nancy Pedano and Colleen Dascenzo for the patient care, Jessica Shell, Brian Schulli, and Virginia Vargara for their technical contributions, Nicole DiIenno and Kelly Mosley for preparing the manuscript, and our patients for their participation. MLT also thanks NIH for NCI R01CA157372 and NuView Life Sciences, in part, for financial support.
Glossary
- PET
Positron Emission Tomography
- CT
X-Ray Computerized Tomography
- SUV
Standardized Uptake Value
- BPH
Benign Prostatic Hyperplasia
- DAR
Digital Autoradiography
- HGPIN
High Grade Prostatic Intraepithelial Neoplasia
- PSA
Prostate Specific Antigen
- ED
Ejaculatory Duct
- PSMA
Prostate Specific Membrane Antigen
- UV
Urethra Verumontanum
- H&E
Hematoxylin and Eosin
- GI
Gastrointestinal Tract
- HPLC
High Pressure Liquid Chromatography
- CCRRC
Clinical Cancer Research Review Committee
- IRB
Institutional Review Board
- FDA
Food and Drug Administration
- RDRC
Radioactive Drug Research Committee
- TRUS
Transrectal Ultrasound
- MRI
Magnetic Resonance Imaging
- SPECT
Single Photon Emission Computerized Tomography
- PC
Prostate Cancer
- BC
Breast Cancer
- TRAMP
Transgenic Adrenocarcinoma of the Mouse Prostate
- PEM
Positron Emission Mammography
References
- 1.American Cancer Society. Cancer Facts & Figures. Atlanta: American Cancer Society; 2015. http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2015/index. Updated 2015. [Google Scholar]
- 2.Cancer Research UK. 2015 www.cancerresearchuk.org.
- 3.Bell N, Gorber SC, Shane A, et al. Recommendations on screening for prostate cancer with the prostate-specific antigen test. Canadian Medical Association Journal. 2014 Nov 4;186(16) doi: 10.1503/cmaj.140703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loeb S. Guideline of guidelines: prostate cancer screening. BJU Int. 2014 Sep;114(3):323–5. doi: 10.1111/bju.12854. [DOI] [PubMed] [Google Scholar]
- 5.Bryant RJ, Lilja H. Emerging PSA-based tests to improve screening. Urol Clin North Am. 2014 May;41(2):267–76. doi: 10.1016/j.ucl.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bjurlin MA, Carter HB, Schellhammer P, et al. Optimization of initial prostate biopsy in clinical practice: sampling, labeling and specimen processing. J Urol. 2013 Jun;189(6):2039–46. doi: 10.1016/j.juro.2013.02.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loeb S, Roehl K, Antenor JA, et al. Baseline prostate-specific antigen compared with medical prostate-specific antigen for age group as predictor of prostate cancer risk in men younger than 60 years old. Urology. 2006;67(2):316–320. doi: 10.1016/j.urology.2005.08.040. [DOI] [PubMed] [Google Scholar]
- 8.Jadvar H. Molecular imaging of prostate cancer: a concise synopsis. Molecular Imaging. 2009;8(2):56–64. [PMC free article] [PubMed] [Google Scholar]
- 9.Chakraborty PS, Tripathi M, Agarwal KK, et al. Metastatic poorly differentiated prostatic carcinoma with neuroendocrine differentiation: negative on 68Ga-PSMA PET/CT. Clin Nucl Med. 2015;46:163–166. doi: 10.1097/RLU.0000000000000594. [DOI] [PubMed] [Google Scholar]
- 10.Castanares MA, Mukherjee A, Chowdhury WH, et al. Evaluation of prostate-specific membrane antigen as an imaging reporter. J Nucl Med. 2014 May;55(5):805–811. doi: 10.2967/jnumed.113.134031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vallabhajosula S, Nikolopoulou A, Babich JW, et al. 99mTC-labeled small-molecule inhibitors of prostate-specific membrane antigen: pharmacokinetics and biodistribution studies in healthy subjects and patients with metastatic prostate cancer. J Nucl Med. 2014;55(11):1791–1798. doi: 10.2967/jnumed.114.140426. [DOI] [PubMed] [Google Scholar]
- 12.Pandit-Taskar N, O’Donoghue JA, Beylergil V, et al. Zr-huJ591 immuno-PET imaging in patients with advanced metastatic prostate cancer. Eur J Nucl Med Mol Imaging. 2014 Nov;41(11):2093–2105. doi: 10.1007/s00259-014-2830-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reubi JC, Laderach U, Waser B, et al. Vasoactive intestinal peptide/pituitary adenylate cyclase-activating peptide receptor subtypes in human tumors and their tissues of origin. Cancer Res. 2000;60:3105–3112. [PubMed] [Google Scholar]
- 14.Lelievre V, Pineau N, Wasche JA. The biological significance of PACAP and PACAP receptors in human tumors: from cell lines to cancers. In: Vaudry H, Arimura A, editors. Pituitary Adenylate Cyclase-Activating Polypeptide. 20. Vol. 17. Springer Publishing Co; 2003. pp. 361–399. Endocrine Updates. [Google Scholar]
- 15.Zia H, Hida T, Jakowlew S, et al. Breast cancer growth is inhibited by vasoactive intestinal peptide, (VIP) hybrid, a synthetic VIP receptor antagonist. Cancer Res. 1996;56:3486–3489. [PubMed] [Google Scholar]
- 16.Leyton J, Gozes Y, Pisegna J, et al. PACAP(6–38) is a PACAP receptor antagonist for breast cancer cells. Breast Cancer Res Treat. 1999;56:177–186. doi: 10.1023/a:1006262611290. [DOI] [PubMed] [Google Scholar]
- 17.Moody TW, Gozes I. Vasoactive intestinal peptide receptors: a molecular target in breast and lung cancer. Curr Pharm Des. 2007;13:1099–1104. doi: 10.2174/138161207780619000. [DOI] [PubMed] [Google Scholar]
- 18.Valdehita A, Bajo AM, Fernández-Martínez AB, et al. Nuclear localization of vasoactive intestinal peptide (VIP) receptors in human breast cancer. Peptides. 2010;31:2035–2045. doi: 10.1016/j.peptides.2010.07.024. [DOI] [PubMed] [Google Scholar]
- 19.Valdehita A, Carmena MJ, Bajo AM, et al. RNA interference-directed silencing of VPAC1 receptor inhibits VIP effects on both EGFR and HER2 transactivation and VEGF secretion in human breast cancer cells. Mol Cell Endocrinol. 2012;348:241–246. doi: 10.1016/j.mce.2011.08.031. [DOI] [PubMed] [Google Scholar]
- 20.Thakur ML, Aruva M, Gariepy J, et al. Imaging oncogene overexpression using 64Cu-VIP analog: comparison with 99mTc-VIP analog. J Nucl Med. 2004;45:1381–1389. [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang K, Aruva M, Shanthly N, et al. Vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase activating peptide (PACAP) receptor specific peptide analogues for PET imaging of breast cancer: in vitro/in vivo evaluation. Regul Pept. 2007;144:91–100. doi: 10.1016/j.regpep.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thakur ML, Devadhas D, Zhang K, et al. Imaging spontaneous MMTVneu transgenic murine mammary tumors: targeting metabolic activity versus genetic products. J Nucl Med. 2010;51:106–111. doi: 10.2967/jnumed.109.069542. [DOI] [PubMed] [Google Scholar]
- 23.Zhang K, Aruva MR, Shanthly N, et al. PET imaging of VPAC1 expression in experimental and spontaneous prostate cancer. J Nucl Med. 2008;49:112–121. doi: 10.2967/jnumed.107.043703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thakur ML, Zhang K, Berger A, et al. VPAC1 receptors for imaging breast cancer: a feasibility study. J Nucl Med. 2013;54(7):1019–1025. doi: 10.2967/jnumed.112.114876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cai H, Wu JS, Muzik O, et al. Reduced 64Cu uptake and tumor growth inhibition by knockdown of human copper transporter 1 in xenograft mouse model of prostate cancer. J Nucl Med. 2014 Apr;55(4):622–629. doi: 10.2967/jnumed.113.126979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sah BR, Burger IA, Schibli R, et al. Dosimetry and first clinical evaluation of the new 18F-radiolabeled bombesin analogue BAY 864367 in patients with prostate cancer. J Nucl Med. 2015 Mar;56(3):372–378. doi: 10.2967/jnumed.114.147116. [DOI] [PubMed] [Google Scholar]
- 27.Afshar-Oromieh A, Malcher A, Eder M, et al. PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: biodistribution in humans and first evaluation of tumour lesions. Eur J Nucl Med Mol Imag. 2013 Apr;40(4):486–495. doi: 10.1007/s00259-012-2298-2. [DOI] [PubMed] [Google Scholar]
- 28.Szabo Z, Mena E, Rowe SP, et al. Initial evaluation of 18F-DCFPyL for prostate-specific membrane antigen (PSMA)-targeted PET imaging of prostate cancer. Mol Imaging Biol. 2015 Apr 21; doi: 10.1007/s11307-015-0850-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eiber M, Maurer T, Souvatzoglou M, et al. Evaluation of hybrid 68Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med. 2015 May;56(5):668–674. doi: 10.2967/jnumed.115.154153. [DOI] [PubMed] [Google Scholar]
- 30.Schuster DM, Nieh PT, Jani AB, et al. Anti-3-[(18)F]FACBC positron emission tomography-computerized tomography and (111)In-capromab pendetide single photon emission computerized tomography-computerized tomography for recurrent prostate carcinoma: results of a prospective clinical trial. J Urol. 2014 May;191(5):1446–1453. doi: 10.1016/j.juro.2013.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.