Abstract
Background
The oxytocin (OT) system, including receptor epigenetic mechanisms, has been shown to influence emotion processing, especially in females. Whether OT receptor (OXTR) epigenetic alterations occur across psychotic disorders in relation to illness-related disturbances in social cognition and brain anatomy is unknown.
Methods
Participants with affective and nonaffective psychotic disorders (92 women, 75 men) and healthy controls (38 women, 37 men) from the Chicago site of the BSNIP study completed the Penn Emotion Recognition Test (ER-40), a facial emotion recognition task. We measured cytosine methylation at site -934 upstream of the OXTR start codon in DNA from whole blood, and for the first time their relationship with plasma OT levels assessed by enzyme-immunoassay. Volumes of brain regions supporting social cognition were measured from MRI scans using FreeSurfer.
Results
Patients with prototypic schizophrenia features showed higher levels of DNA methylation than those with prototypic bipolar features. Methylation was higher in women than men, and was associated with poorer emotion recognition only in female patients and controls. Greater methylation was associated with smaller volumes in temporal-limbic and prefrontal regions associated previously with social cognition, but only in healthy women and females with schizophrenia.
Conclusion
DNA methylation of the OXTR site -934 was higher in schizophrenia spectrum than bipolar patients. Among patients, it was linked to behavioral deficits in social cognition and neuroanatomic structures known to support emotion processing only in schizophrenia spectrum individuals.
Keywords: oxytocin, epigenetics, psychosis, emotion recognition, structural imaging, sex differences
Individuals with psychotic disorders often have persistent impairments in social cognition (1, 2), especially in schizophrenia (2, 3). Multiple lines of clinical and preclinical evidence indicate that social cognition abilities are influenced by the neuropeptide oxytocin (OT)(4-6). Epigenetic alteration of genes regulating OT receptor (OXTR) expression may be one factor influencing social cognition deficits in psychotic disorders. This effect may be greater in schizophrenia than in other psychotic disorders because of their more severe social cognition deficits (2), and in females because of the more prominent role of the OT system in women in regulating social cognition (7-9).
Multiple converging lines of evidence suggest alterations in the OT system are involved in the social cognitive impairment in schizophrenia. OT peptide levels are altered in psychotic disorders (9-14) and are related to social cognition deficits with more pronounced associations in female patients (7-9). Adjunctive exogenous OT administration can improve social cognition in schizophrenia (15-19). Preliminary data suggests that social cognitive deficits in schizophrenia have been linked to OXTR polymorphisms (20, 21). However to date, no studies have examined epigenetic regulation of the OXTR and its relation to OT levels in psychotic disorders, social cognition or neuroanatomical structures important for social cognition including temporal-limbic regions where OXTR concentrations are high (22).
Examination of OT levels may be important for understanding epigenetic effects because epigenetic changes in OXTR might represent a response to variations in OT levels (23). Given the pronounced social cognition deficits in schizophrenia, it is plausible that OT system alterations might be greater than in affective psychotic disorders in which social cognition deficits are typically less severe (2), but this has not yet been investigated. Predictions that OT modulatory effects might be greater in females are supported by several lines of preclinical and clinical evidence (24). For example, in adult prairie voles acute OT administration has a greater impact in females than in males (25, 26). In female, but not male prairie voles, neonatal OT administration influences adult social behavior (27). In healthy humans, some opposite sex-related effects have been seen, as intranasal OT reduces neural activation in the amygdala in response to emotional face processing in men (28, 29) but increases amygdala activity in women (30, 31).
There are multiple possible OT system alterations that could disrupt social cognition. In autism, there is a defined epigenetic change that leads to decreased expression of the OXTR gene (32). This change occurs at a cytosine nucleotide that is 934 base pairs upstream of the OXTR translation start site, and resides within a region of the gene important for DNA methylation-mediated repression of transcription (33). Alleles isolated from cells in temporal cortex and blood show increased DNA methylation at this site in autism compared to matched controls. Methylation at this same OXTR region (site -934) assessed from blood has been correlated with temporal, limbic, and prefrontal activity measured by fMRI in healthy individuals performing social cognition tasks (34, 35). In addition, methylation of site -934 has been associated with high callous-unemotional traits in children with conduct problems (23, 36) and enhanced risk of postpartum depression in women (37).
In the present study, we assessed OXTR DNA methylation from whole blood in individuals with psychotic disorders and matched healthy controls. We hypothesized that individuals with psychotic disorders would demonstrate greater OXTR methylation compared to healthy controls, that this effect would be greatest in schizophrenia patients and in women, and that it would be related to social cognition deficits. Given that epigenetic processes have been associated with brain measures in healthy individuals (38), our second aim was to test whether higher levels of OXTR DNA methylation were related to volume reductions in brain regions known to be associated with social cognition deficits in schizophrenia (prefrontal, temporal, and limbic cortex) (39).
Methods and Materials
Participants
Subjects were participants from the Chicago site of the Bipolar and Schizophrenia Network on Intermediate Phenotypes (B-SNIP) consortium. Written informed consent was provided by all participants (participant assent and parental consent for those <18 years). A full description of subject recruitment and clinical assessment for the B-SNIP study is available (40). Participants included 167 individuals with a psychotic disorder (schizophrenia, schizoaffective disorder, psychotic bipolar disorder; 92 women, 75 men) and 75 healthy controls (38 women, 37 men) (Table 1). A consensus DSM-IV diagnosis was made using the Structured Clinical Interview for DSM-IV Axis I Disorders (41). Patients were both clinically stable and receiving consistent drug treatment for the month preceding testing. Exclusion criteria included: history of head injury with loss of consciousness >10 minutes; pregnancy; positive urine toxicology for common drugs of abuse on the day of testing; diagnosis of substance abuse in the past 30 days or substance dependence in the past 6 months; history of systemic medical or neurological disorder affecting mood or cognition; and Wide-Range Achievement Test reading test standard score <65.
Table 1. Demographics and clinical characteristics of study participants.
| Variables | Group | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Schizophrenia | Schizoaffective | Bipolar | Controls | |||||
|
| ||||||||
| Women (n=22) | Men (n=35) | Women (n=19) | Men (n=15) | Women (n=51) | Men (n=25) | Women (n=38) | Men (n=37) | |
| M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | |
| Age (years)G×S | 39 (14) | 33 (12) | 37 (11) | 38 (18) | 36 (14) | 26 (10) | 36 (13) | 39 (13) |
| Education (years) G, S | 13 (2) | 13 (3) | 14 (3) | 13 (3) | 14 (2) | 13 (3) | 15 (3) | 15 (3) |
| WRATG | 96 (18) | 93 (14) | 101 (16) | 98 (18) | 102 (14) | 108 (18) | 104 (13) | 102 (13) |
| PENN ER-40(% correct)G, S=0.08 | -0.6 (1.2) | -0.9 (1.6) | -0.4 (1.3) | -0.6 (1.5) | -0.3 (1.0) | -0.1 (1.3) | 0.3 (0.7) | -0.31 (1.2) |
| SFSG | 123 (21) | 128 (19) | 124 (20) | 127 (20) | 141 (22) | 138 (17) | 163 (14) | 162 (16) |
| Race/ethnicity, n(%)G, G×S | ||||||||
| White | 8 (36) | 17 (49) | 11 (58) | 7 (47) | 40 (78) | 19 (76) | 23 (60) | 21 (57) |
| Black | 10 (46) | 14 (40) | 7 (37) | 8 (53) | 10 (20) | 1 (4) | 9 (24) | 12 (32) |
| Other | 4 (18) | 4 (11) | 1 (5) | - | 1 (2) | 5 (20) | 6 (16) | 4 (11) |
| PANSS | ||||||||
| Positive subscaleG | 19 (6) | 19 (6) | 19 (4) | 20 (6) | 13 (4) | 12 (4) | ||
| Negative subscaleG | 20 (7) | 20 (7) | 18 (5) | 17 (8) | 14 (4) | 13 (5) | ||
| YMRS | 8 (5) | 8 (7) | 8 (6) | 8 (5) | 6 (6) | 5 (6) | ||
| MADRSG | 10 (8) | 10 (7) | 16 (11) | 15 (9) | 12 (10) | 8 (6) | ||
| Schizo-Bipolar Scale G, S | 7 (2) | 8 (1) | 5 (2) | 4 (1) | 0.4 (1) | 0.3 (1) | ||
| Suicide lethality, n (%) | 5 (23) | 7 (20) | 6 (31) | 2 (13) | 12 (23) | 6 (24) | ||
| Medication Class†, n (%) | ||||||||
| Antipsychotic G, S | 19 (86) | 32 (91) | 14 (74) | 14 (93) | 35 (69) | 20 (80) | ||
| MoodstabilizerG, G×S | 1 (4) | 9 (26) | 10 (53) | 12 (80) | 35 (69) | 19 (76) | ||
| AntidepressantG, G×S | 8 (36) | 12 (34) | 10 (53) | 11 (73) | 24 (47) | 11 (44) | ||
| Sedative/anxiolytic/hypnotic | 7 (32) | 8 (23) | 7 (37) | 6 (40) | 17 (33) | 5 (20) | ||
| Stimulant | 2 (9) | 3 (8) | - | 2 (13) | 6 (12) | 5 (20) | ||
| Anticholinergic | 1 (4) | 4 (11) | 1 (5) | - | 1 (2) | 3 (12) | ||
| Chlorpromazine equivalent | 592 (724) | 667 (599) | 400 (437) | 420 (297) | 254 (304) | 380 (332) | ||
| Age, onset psychiatric illnessG | 18 (5) | 14 (9) | 14 (6) | 15 (10) | 15 (6) | 14 (9) | ||
| Hospitalizations | 6 (6) | 4 (4) | 8 (7) | 7 (7) | 6 (8) | 4 (4) | ||
Note.
Group difference at p<0.05.
Sex difference at p<0.05.
Group × Sex interaction at p<0.05.
WRAT=Wide Range Achievement Test-IV: Reading test; PENN-ER40=Penn Emotion Recognition-40 Test; SFS=Social Functioning Scale (higher = better functioning); PANSS=Positive and Negative Syndrome Scale; YMRS=Young Mania Rating Scale; MADRS=Montgomery Asberg Depression Rating Scale; Schizo-Bipolar Scale (higher = schizophrenia).
Medication class Consistent with Hill et al. (2013) and Tamminga et al. (2013).
Measures
Emotion processing
The Penn Emotion Recognition-40 Test (ER-40)(42) assesses the ability to accurately recognize specific emotions conveyed in pictures of faces. Stimuli are 40 faces (half female) expressing one of four emotions or a neutral expression. The primary outcome measure was the overall accuracy of affect identification. A report of ER-40 performance in the full BSNIP sample is available that includes methods for deriving performance measures (2).
Epigenotyping procedures
Genomic DNA was isolated from EDTA-treated whole blood using the Gentra Puregene extraction kit (Qiagen Sciences, Germantown, MD), quantified, standardized to 50ng/ul, and quality checked with Picogreen (Invitrogen, Eugene, OR) and Nanodrop assays (Thermo Scientific, Wilmington, DE). Cytosine methylation was measured at site -934 upstream of the OXTR start codon by bisulfite pyrosequencing as reported in Jack et al. (35) in Dr. Connelly's laboratory at the University of Virginia. Samples were amplified in triplicate and randomized for pyrosequencing to account for plate and run variability, and averaged. On average, samples deviated from the mean ±1.4%.
Plasma hormone assays
An enzyme immunoassay was used to measure OT in unextracted plasma samples. Blood samples were drawn in the morning when possible (90% blood draws before noon). The percentage of participants drawn before noon was similar across groups and sex (p's>0.45). OT levels from these participants have been reported previously (43), and are presented here to inform the analysis of OXTR methylation. For assay details see Rubin et al. (44).
Structural magnetic resonance imaging procedure and measurement
MRI scans were obtained using a 3-Tesla GE Signa HDx scanner. Structural imaging was acquired using a T1-weighted 3-dimensional inversion recovered fast spoiled gradient echo sequence [time of repetition (TR)/echo time (TE)/inversion recovery (IR)] = 6.988 ms/2.848 ms/700 ms, flip angle = 8 degrees, 166 slices). FreeSurfer (45, 46) software was used to extract volumetric data (mm3) in a priori regions of interest (ROI) implicated in facial emotion processing (47, 48) including four prefrontal regions (superior frontal, middle frontal, inferior frontal, orbital frontal gyri), and six temporal-limbic regions (parahippocampal, middle temporal, and fusiform gyri, hippocampus, amygdala, and insula). A full presentation of the medial temporal lobe data from the full BSNIP sample (49, 50) and the whole-brain grey matter data (51) are available. 79% of the sample (n=190) had MRI scans due to additional exclusion factors related to patient safety and scheduling difficulties.
Statistical analyses
Prior to analysis, emotion recognition scores were standardized (mean = 0, SD = 1) relative to healthy controls. Extremely low scores were truncated to z-score=-4 to reduce the effects of outliers. Multivariable linear regression modeling was used to assess group differences in methylation levels and to examine the association of methylation levels with emotion processing and regional brain volumes (primary outcomes) as well as clinical symptoms and global functional status (secondary outcomes). Only when the omnibus test statistic was significant for the main effect of group or group by methylation interactions did we further probe for pairwise group differences using a Bonferroni correction for multiple comparisons. Patients were assigned a rating on the Schizobipolar Scale (52) depending on their similarity to prototypic schizophrenia and bipolar disorder, which was used to examine the relation between diagnostic features and OT measures. Stratification by sex was used in models examining the degree to which methylation levels were associated with emotion processing, regional brain volumes and secondary outcomes. Analyses were adjusted for age and race; intracranial volume was an additional covariate for brain volume analyses. While we examined methylation level by race interactions as a covariate, adding in this interaction did not alter the pattern of results presented here and thus for parsimony was not included in the final models. No adjustments were made for multiple testing for secondary exploratory analyses performed for heuristic purposes to illustrate the potential clinical significance of group differences. Associations of epigenetic data with medications were examined; none were significant except lithium (Table S1 in Supplement 1).
Results
Group differences in behavioral, plasma hormone levels, and MRI measures
Primary analyses of OT levels, MRI data, and PENN ER-40 data are available for the BSNIP study (2, 40, 44, 49, 50). Analyses with the subset of participants here yielded similar findings (Table 1; Table S2 in Supplement 1).
Group differences in DNA methylation and associations with plasma oxytocin levels
Although controls and the combined group of individuals with psychotic disorders did not differ in DNA methylation levels (p=0.70; Figure 1; Figure S1 in Supplement 1), overall diagnostic group differences in methylation levels were significant only at a trend level (p=0.08). Only individuals with schizophrenia showed higher levels of methylation compared to controls (B=2.29, SE=1.15, p=0.04; Cohen's d=0.33). On the Schizo-Bipolar Scale, patients with greater similarity to prototypic schizophrenia compared to those with bipolar features (B=0.36, SE=0.15, p=0.02) had higher levels of methylation; this association remained in the subset of participants not taking lithium. Although the magnitude of the methylation-Schizo-Bipolar Scale association was similar in male and female patients (p=0.76), greater methylation at OXTR site -934 was observed in females patients overall compared to male patients (p=0.005) as seen in controls.
Figure 1. Level of OXTR DNA methylation in women and men with psychotic disorders and healthy controls.
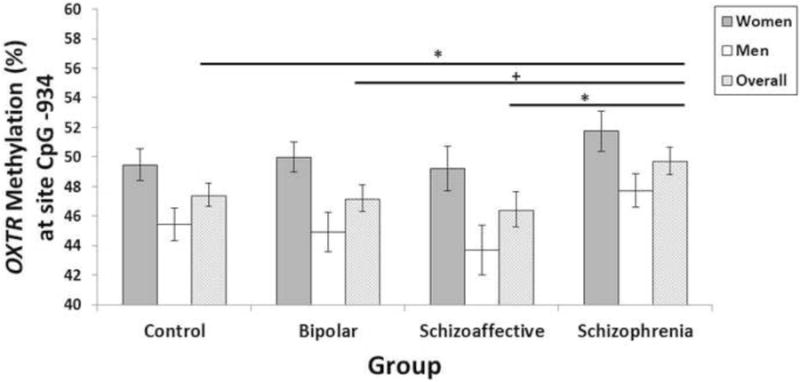
Note. *p<0.05, +p=0.06. Estimated means and standard errors from the statistical models that included age and race as covariates. Although controls and the combined group of individuals with psychotic disorders did not differ in DNA methylation, overall diagnostic group differences in methylation levels just missed statistical significance (p=0.08). Specifically, only individuals with schizophrenia showed higher levels of methylation compared to controls (B=2.29, SE=1.15, p=0.04; Cohen's d=0.33) and schizoaffective patients (B=3.27, SE= 1.41, p=0.02, Cohen's d=0.38). There was a trend for a similar increase in individuals with schizophrenia relative to psychotic bipolar patients (B=2.29, SE=1.21, p=0.06, Cohen's d=0.35). There were no significant differences between controls and either schizoaffective (B=-0.98, SE=1.34, p=0.46; Cohen's d=0.11) or psychotic bipolar patients (B=-0.01, SE=1.10, p=0.99; Cohen's d=0.03). Women had significantly higher methylation levels compared to men (p<0.001). The sex × group interaction was not significant (p=0.92).
Associations between DNA methylation and peripheral OT levels were not significant in controls or in any diagnostic group (Table S3 in Supplement 1). However, associations between DNA methylation levels and peripheral OT levels were in opposite directions for male (r=-0.24, p=0.04) and female (r=0.23, p=0.03) patients.
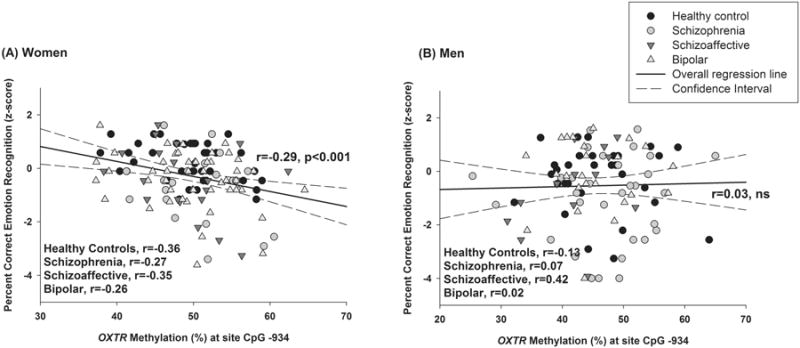
DNA methylation-behavior associations
Among all women, but not men, greater methylation was associated with poorer recognition of emotional expressions (p<0.001; Table 2, Figure 2). The association did not differ between patient and control females. Follow-up analyses in women indicated that higher methylation was associated with greater difficulties identifying angry (p<0.001), sad (p=0.006), happy (p=0.02) as well as low- (p<0.001) and high-intensity facial expressions (p=0.004) [trend on fear p=0.07] (Table 2). These associations did not statistically differ across groups–that is, methylation × group interactions among women were not significant. OXTR methylation also was not significantly associated with any of the secondary outcome measures including general social functioning or with positive, negative, depression, or mania symptom levels in our clinically stable sample.
Table 2. Partial correlations (r) between OXTR DNA methylation and primary and secondary outcomes including facial emotion processing, social functioning, and clinical symptoms.
| Outcomes | Women | Men | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Controls | Scz | SczA | BP | Controls | Scz | SczA | BP | |
| PENN-ER40 | ||||||||
| Overall | -0.38* | -0.26 | -0.49T | -0.29* | -0.05 | 0.08 | 0.45 | 0.01 |
| Neutral | -0.20 | -0.36 | -0.09 | 0.09 | 0.19 | 0.06 | 0.16 | 0.07 |
| Angry | -0.21 | -0.67** | -0.29 | -0.18 | 0.17 | 0.22 | 0.01 | 0.17 |
| Fear | 0.01 | -0.27 | -0.55* | -0.02 | -0.14 | 0.05 | -0.02 | -0.03 |
| Happy | -0.01 | -0.38 | -0.22 | -0.14 | 0.09 | 0.20 | 0.02 | -0.12 |
| Sad | -0.27 | -0.52* | -0.35 | -0.08 | 0.05 | -0.02 | -0.10 | 0.19 |
| Low intensity | -0.38* | -0.42T | -0.49T | -0.22 | 0.13 | 0.18 | 0.29 | 0.03 |
| High intensity | -0.09 | -0.57* | -0.40 | -0.09 | -0.18 | 0.02 | -0.23 | 0.20 |
| SFS | -0.20 | 0.09 | 0.25 | 0.05 | -0.21 | 0.00 | -0.06 | 0.15 |
| PANSS | ||||||||
| Positive subscale | - | 0.16 | -0.09 | 0.11 | - | -0.17 | -0.16 | -0.13 |
| Negative subscale | - | 0.05 | -0.14 | 0.03 | - | 0.09 | 0.07 | 0.18 |
| YMRS | - | -0.06 | 0.04 | -0.09 | - | -0.35T | -0.04 | 0.13 |
| MADRS | - | 0.01 | -0.22 | -0.10 | - | -0.03 | -0.38 | -0.22 |
Note.
p<0.001;
p<0.01.
p<0.05.
p>0.05 and p<0.10.
Scz=schizophrenia; SczA=schizoaffective; BP=psychotic bipolar; PENN-ER40=Penn Emotion Recognition-40 Test % Correct; SFS=Social Functioning Scale (higher = better functioning); PANSS=Positive and Negative Syndrome Scale; YMRS=Young Mania Rating Scale; MADRS=Montgomery Asberg Depression Rating Scale. Adjusted for age and race.
Figure 2. Raw associations between levels of OXTR DNA methylation and emotion recognition.
DNA methylation-regional brain volume associations
There were significant methylation × group interactions in some regional brain volumes in females but not males (Table 3), including right hippocampus (p=0.006), left middle temporal gyrus (p=0.007), left superior frontal gyrus (p=0.04), and right inferior frontal gyrus (p=0.02). In healthy females, greater methylation was associated with smaller volumes in the right hippocampus (β=-0.29, p=0.04), left middle temporal gyrus (β=-0.28, p=0.04), and right inferior frontal gyrus (β=-0.34, p=0.008). In females with schizophrenia, higher methylation was also associated with smaller volumes of right hippocampus (β=-0.29, p=0.04). Conversely, in females with schizoaffective disorder, greater methylation was associated with larger volumes in the left middle temporal gyrus (β=0.61, p=0.007) and left superior frontal gyrus (β=0.47, p=0.01). In men (combined patients and controls), DNA methylation was positively associated with volumes of left middle frontal gyrus (p=0.04) and left insula (p=0.04). Associations between peripheral OT levels and brain regions are presented in Table S4 in Supplement 1.
Table 3. Partial Correlations (r) between OXTR DNA methylation and regional brain volumes for a priori regions of interest based on their previously established relation to social cognition.
| Region | OXTR DNA methylation | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Women | Men | |||||||
|
|
|
|||||||
| Controls (n=35) | Scz (n=17) | SczA (n=13) | BP (n=41) | Controls (n=33) | Scz (n=22) | SczA (n=13) | BP (n=16) | |
| Left Hemisphere | ||||||||
| Temporal and limbic regions | ||||||||
| Hippocampus | -0.23 | -0.54T | -0.15 | 0.12 | 0.25 | 0.10 | 0.05 | -0.12 |
| Parahippocampal gyrus | -0.23 | 0.14 | 0.11 | 0.26 | 0.16 | -0.16 | 0.31 | -0.07 |
| Fusiform gyrus | 0.24 | 0.17 | 0.20 | -0.08 | 0.14 | 0.17 | 0.05 | -0.01 |
| Middle temporal gyrus | -0.32T | 0.25 | 0.50 | -0.09 | 0.05 | -0.35 | -0.22 | 0.24 |
| Amygdala | -0.41* | -0.41 | 0.10 | -0.01 | -0.05 | 0.08 | -0.33 | 0.10 |
| Insula | -0.02 | 0.28 | -0.04 | 0.01 | 0.11 | 0.21 | 0.61T | 0.29 |
| Prefrontal Cortex | ||||||||
| Superior frontal gyrus | -0.22 | 0.19 | 0.16 | -0.08 | -0.15 | 0.10 | -0.02 | 0.41 |
| Middle frontal gyrus | -0.24 | 0.08 | 0.20 | -0.15 | 0.05 | -0.31 | -0.65* | -0.41 |
| Inferior frontal gyrus | -0.20 | -0.14 | -0.01 | -0.09 | 0.21 | 0.12 | 0.20 | -0.22 |
| Orbital frontal cortex | -0.21 | 0.62* | -0.57 | 0.01 | -0.15 | -0.11 | 0.27 | 0.08 |
| Right Hemisphere | ||||||||
| Temporal and limbic regions | ||||||||
| Hippocampus | -0.34T | -0.69** | 0.15 | 0.29T | 0.25 | -0.07 | 0.21 | -0.13 |
| Parahippocampal gyrus | -0.02 | -0.20 | 0.52 | 0.36* | 0.17 | -0.01 | -0.40 | -0.20 |
| Fusiform gyrus | 0.29 | 0.68* | 0.03 | -0.05 | -0.16 | 0.03 | -0.42 | -0.15 |
| Middle temporal gyrus | -0.33T | 0.44 | 0.38 | 0.10 | 0.17 | -0.30 | 0.23 | 0.25 |
| Amygdala | -0.34T | -0.55T | 0.32 | 0.02 | -0.27 | -0.08 | -0.42 | -0.04 |
| Insula | 0.09 | 0.29 | 0.00 | -0.30T | 0.13 | -0.05 | 0.44 | 0.06 |
| Prefrontal Cortex | ||||||||
| Superior frontal gyrus | -0.11 | 0.11 | 0.31 | 0.11 | -0.06 | 0.31 | -0.02 | 0.06 |
| Middle frontal gyrus | -0.39* | 0.10 | -0.27 | -0.19 | -0.07 | -0.22 | -0.20 | -0.47 |
| Inferior frontal gyrus | -0.42* | 0.46 | 0.08 | 0.03 | -0.01 | 0.05 | -0.34 | -0.21 |
| Orbital frontal cortex | -0.24 | 0.45 | -0.64T | -0.05 | 0.17 | 0.09 | -0.01 | -0.16 |
Note.
p<0.01;
p<0.05;
p>0.05 and p<0.09.
Scz=schizophrenia; SczA=schizoaffective; BP=psychotic bipolar. Adjusted for age, race, and total intracranial volume.
Discussion
This is the first study examining the impact of epigenetic modification of OXTR, indexed here in blood, in individuals with psychotic disorders, and its relation to social cognition abilities and their neuroanatomical substrate. Greater methylation of OXTR was observed in individuals with prototypic schizophrenia versus psychotic bipolar disorder, but only in females (patients and controls) was methylation related to performance on a test of social cognition and to volumetric measures of neuroanatomic regions subserving social cognition. These effects were observed despite the absence of altered peripheral OT levels in any psychotic disorder to which greater methylation might have been a homeostatic compensation. These findings suggest that the OT pathway may be disrupted at the level of the receptor and not at the level of the neuropeptide synthesis, and that in psychotic disorders, this effect is relatively specific to women with schizophrenia. This notion that disruptions in the OT system in schizophrenia are downstream from plasma levels is consistent with our previous report of a dissociation of peptide levels and social cognition (43). While these methylation changes were observed in blood, not brain, the findings from our in vivo studies of DNA methylation in blood appear clinically and brain-relevant because they were disorder specific and related to behavioral measures of social cognition and to volumetric measures of its neuroanatomic substrate.
Sex differences in methylation occurred across patients and controls, with women showing higher levels of OXTR CpG site -934 DNA methylation, as previously reported in healthy individuals (34). Level of methylation was also related to social cognition measures in females across all subject groups. Thus, our data suggest that increased DNA methylation at the OXTR site -934 is a general sex-specific effect, but one that appears to amplify social cognition deficits specifically in females with schizophrenia.
The observation that a general change in OXTR DNA methylation in women may be a sex-specific contributor to a reduced ability to perceive emotional cues in faces in women with schizophrenia is novel. One reason differences in DNA methylation could disrupt emotion processing in women, but not men, might be related to sex differences in OXTR distributions in frontal, hippocampal, and limbic regions, as indicated by studies of mice and prairie voles (53, 54). In adulthood, female prairie voles are more sensitive than males to the social effects of OT administration (26). Consistent with this idea, intranasal OT has sex-divergent effects on amygdala activity in response to emotion face processing (28, 30). Although women with psychotic disorders generally have less severe emotion processing deficits than men (55-57), DNA methylation of OXTR in site -934 may lead to greater social cognition deficits in female schizophrenia patients and thus could represent a potential sexually-dimorphic target for therapeutic intervention.
Although the mechanisms through which greater OXTR methylation in women with schizophrenia influences the severity of social cognition deficits and its neuroanatomic substrate are unknown, greater methylation may lead to transcriptional down-regulation of OXTR in the brain (32, 33). It is also unclear whether greater methylation in schizophrenia represents illness-related pathology, a failed compensation, or a compensation that is partially successful, without which social cognitive dysfunction could be more severe. Additional research is needed to determine whether DNA methylation represents an effort to restore an altered biological system to homeostasis in schizophrenia, or whether it is a disease or treatment effect. Our data in female patients show a positive association between OXTR methylation and peripheral levels of OT, whereas a negative association was seen in male patients. Although the interpretation of this difference is unclear, it suggests that homeostatic processes or homeostatic demand may be quite different in male and female patients. While such demand does not appear to involve a response to altered OT plasma levels, it may represent a response to impaired signal transduction mechanisms or deficit in widely distributed neural systems supporting social cognition. This sex difference in the pattern of association between OXTR methylation and peripheral OT levels might help explain why beneficial effects of intranasal OT have been reported predominantly in male patients (16,18, 58). In males, exogenous OT might increase signaling at the OXTR to enhance social and emotional functioning. However, it should be noted that studies of the consequences of exogenous OT have been less common in female patients, and little is known regarding sex differences in response to OT therapies or their variability in relation to genotype.
The sexually-dimorphic pattern of associations between OXTR methylation and peripheral OT levels in patients is consistent with the hypothesis that the regulation of OT pathways, as well as the broader physiological and behavioral consequences of activity in this system, is different in males and females. Thus, sex differences may need to be systematically examined in the development of therapeutics that directly or indirectly influence OT pathways and in developing models of disease-related alterations. One important sex-related factor might be steroid regulation of OT and OXTR production, as estrogen is known in rats to modulate the density of OXTR within the ventromedial nucleus of the hypothalamus (59-62) and OXTR gene expression (63-65). There is some evidence for a protective effect of estrogen in schizophrenia, but the absence of data regarding steroids in the present study limits our capacity to speculate on the role of estrogen in the present outcomes.
DNA methylation of OXTR had sex-specific associations not only with behavior, but with morphometric characteristics of brain regions known to be important for emotion recognition and social cognition. In healthy women, greater DNA methylation in the blood was associated with smaller volumes in the hippocampus, middle temporal gyrus, and right inferior frontal gyrus–all regions previously implicated in processing emotional faces (47) and regions showing sex differences when processing emotions (47, 57, 66-68). This inverse pattern of OXTR methylation-volume associations, particularly in the hippocampus, was also noteworthy in females with schizophrenia. These findings suggest that in healthy women and women with schizophrenia, DNA methylation of OXTR, possibly associated with less receptor-mediated activity, may have longer term effects on synaptic density, dendritic arborization, and/or other factors that determine grey matter volume (69-71). Interestingly, these associations appear to be relatively specific to schizophrenia as they were not significant in individuals with bipolar disorder.
The observation of diagnosis-specific associations of DNA methylation in blood with behavioral and neuroanatomical measures of the social cognition system suggest that in vivo clinical studies of DNA methylation in peripheral cells are associated with changes in the brain, and thus may be a promising direction for future research. Yet, more research is needed to determine the mechanisms of effects reported here. For example, the associations between OT level and epigenetic changes remain an important target of investigation in order to clarify what alterations are disease consequences and which are efforts at compensation. However, since OT levels themselves do not appear to be abnormal, if changes in methylation do represent a compensatory process, they would appear to be a response to receptor or other downstream changes in OT signal transduction. The sex differences are another important issue because of their potential importance for therapeutic intervention. For example, female patients may be especially sensitive to the consequences of methylation and presumed reductions in OT receptors. Also, it is important to note that DNA methylation of other OXTR CpG dinucleotides sites (i.e., -924, -860) may also be elevated in schizophrenia and impact emotion processing via effects on OXTR expression. Last, given the multiple biological overlaps across psychotic disorders, the observation of schizophrenia-specific findings is important to investigate and confirm since among those studied here, schizophrenia is the psychotic disorder with the greatest persistent disability in social cognition.
Our sex-specific findings highlight the complex pathophysiology of schizophrenia, with female-specific epigenetic factors in OT pathways contributing to and perhaps amplifying social cognition deficits in this disorder. Our findings complement other data highlighting the importance of sex differences in schizophrenia which have been reported in cognition (72-76), clinical symptoms and disease course (77-82), and treatment response (76, 80, 83). The factors causing more severe illness in males or that preserve social cognition in female schizophrenia patients remain to be identified. The observations that methylation levels were similar in male and female patients and that these changes only impacted social cognition in healthy women and women with schizophrenia provide a clue that mechanisms for sex differences in schizophrenia at least in terms of social cognition may include sex-specific changes in OT pathways.
Limitations in this study include a cross-sectional study design and small sample sizes by diagnostic group and sex thus limiting statistical power. Secondly, we did not formally assess high callous-unemotional traits or autism features that prior work has shown to be related to OT levels and its effects in some individuals (23, 32, 36). Third, OT measurements were conducted at one time point. Fourth, the EIA measurement of peripheral OT was done in unextracted plasma samples which is associated with higher levels of the peptide (84, 85). Nevertheless, findings from our previous studies focusing on peripheral OT levels and social cognition, which have used the present assay methodologies (7), have been replicated by researchers using different assay methods (9). Fifth, we focused on methylation levels of a single CpG site in the promoter region of OXTR and assayed peripherally derived DNA from blood which are imperfect markers of brain DNA methylation (86). Although we do not know the exact association between blood and brain methylation at CpG site -934, a number of studies to date reflect the utility of this peripheral assay in predicting brain endophenotypes related to social cognition (34, 35). The degree to which methylation and its effects are dynamic (87) or static, the relative importance of other promotor regions, and the similarity of epigenetic changes in blood and brain remain to be determined. If methylation of OXTR is dynamic, then it may contribute to the varying levels of social cognition deficits seen clinically over the course of illness. Sixth, it is important to note that our patients were being treated with diverse medications and dosages which might influence emotion perception, blood levels of OT, or methylation. Although medications did not have a statistical relation to methylation levels, since the majority of patients were treated with medications, we are unable to fully determine the contribution of treatment effects. Finally, we focused here on OT and methylation of the OXTR. However, OT functions in the context of other neuropeptide such as vasopressin and steroid hormones, as well as the broader neuroendocrine milieu that might influence the OT system and social cognition deficits (43, 44).
To date, the field does not provide a basis for mechanistic understanding of how OT levels and methylation impact social cognition and brain anatomy and function. As in many areas of clinical psychiatry research, especially in early developing areas such as epigenetics, this study does not establish mechanism but takes steps forward by showing sex and disorder selectivity, and the clinical relevance of epigenetic factors to social cognition deficits and relevant brain anatomy. As to biological mechanism, this is the first study to determine whether methylation may represent a response to increased OT levels. As peripheral OT levels were not altered or related to methylation levels, epigenetic changes do not appear to be a direct response to increased or decreased OT levels, and thus appear to involve a disease-related change in post-synaptic mechanisms. Taken together, our findings provide a significant step forward in understanding OT system abnormalities in schizophrenia by showing greater sex-specific epigenetic effects in schizophrenia but not bipolar disorder, and that while similar to sex specific effects in controls they had clinical relevance in schizophrenia as seen in associations with behavioral measures of social cognition and the neuroanatomic substrate of that neurobehavioral dimension.
Supplementary Material
Acknowledgments
This work was supported in part by a 2012 NARSAD Young Investigator Grant from the Brain and Behavior Research Foundation to Dr. Rubin, and by the National Institute of Health (K12HD055892, K08MH083888, MH083126, MH077851, MH078113, MH077945, MH077852, and MH077862). The funding agencies had no role in the design and conduct of the study collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript. We would like to thank Katie Lancaster, Meghan Puglia, and Kayla Chase for their critical review of the manuscript.
Dr. Bishop has served as an advisory board member for Physician Choice Laboratory Services (PCLS). Dr. Sweeney has consulted to Eli Lilly, Roche, and Takeda and with Dr. Bishop received grant support from Janssen. Dr. Keshavan received research support from Sunovion and GlaxoSmithKline. Dr. Connelly received grant support from the Fetzer Institute. Dr. Tamminga has served on a drug development advisory board for Intracellular Therapies, as an expert witness for Kaye Scholer LLP, and has served as a consultant for Astellas, Eli Lilly, Lundbeck, and Puretech Ventures/Karuna.
Footnotes
Financial Disclosures: All other authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Savla GN, Vella L, Armstrong CC, Penn DL, Twamley EW. Deficits in domains of social cognition in schizophrenia: a meta-analysis of the empirical evidence. Schizophr Bull. 2013;39:979–992. doi: 10.1093/schbul/sbs080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruocco AC, Reilly JL, Rubin LH, Daros AR, Gershon ES, Tamminga CA, et al. Emotion recognition deficits in schizophrenia-spectrum disorders and psychotic bipolar disorder: Findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. Schizophr Res. 2014;158:105–112. doi: 10.1016/j.schres.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daros AR, Ruocco AC, Reilly JL, Harris MS, Sweeney JA. Facial emotion recognition in first-episode schizophrenia and bipolar disorder with psychosis. Schizophr Res. 2014;153:32–37. doi: 10.1016/j.schres.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heinrichs M, von Dawans B, Domes G. Oxytocin, vasopressin, and human social behavior. Front Neuroendocrinal. 2009;30:548–557. doi: 10.1016/j.yfrne.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Kanat M, Heinrichs M, Domes G. Oxytocin and the social brain: neural mechanisms and perspectives in human research. Brain Res. 2014;1580:160–171. doi: 10.1016/j.brainres.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci. 2011;12:524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- 7.Rubin LH, Carter CS, Drogos L, Jamadar R, Pournajafi-Nazarloo H, Sweeney JA, et al. Sex-specific associations between peripheral oxytocin and emotion perception in schizophrenia. Schizophr Res. 2011;130:266–270. doi: 10.1016/j.schres.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strauss GP, Keller WR, Koenig Jl, Gold JM, Frost KH, Buchanan RW. Plasma oxytocin levels predict social cue recognition in individuals with schizophrenia. Schizophr Res. 2015;162:47–51. doi: 10.1016/j.schres.2015.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strauss GP, Keller WR, Koenig Jl, Sullivan SK, Gold JM, Buchanan RW. Endogenous oxytocin levels are associated with the perception of emotion in dynamic body expressions in schizophrenia. Schizophr Res. 2015;162:52–56. doi: 10.1016/j.schres.2015.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldman M, Marlow-O'Connor M, Torres I, Carter CS. Diminished plasma oxytocin in schizophrenic patients with neuroendocrine dysfunction and emotional deficits. Schizophr Res. 2008;98:247–255. doi: 10.1016/j.schres.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Linkowski P, Geenen V, Kerkhofs M, Mendlewicz J, Legros JJ. Cerebrospinal fluid neurophysins in affective illness and in schizophrenia. Eur Arch Psychiatry Neurol Sci. 1984;234:162–165. doi: 10.1007/BF00461555. [DOI] [PubMed] [Google Scholar]
- 12.Beckmann H, Lang RE, Gattaz WF. Vasopressin--oxytocin in cerebrospinal fluid of schizophrenic patients and normal controls. Psychoneuroendocrinology. 1985;10:187–191. doi: 10.1016/0306-4530(85)90056-3. [DOI] [PubMed] [Google Scholar]
- 13.Legros JJ, Gazzotti C, Carvelli T, Franchimont P, Timsit-Berthier M, von Frenckell R, et al. Apomorphine stimulation of vasopressin- and oxytocin-neurophysins. Evidence for increased oxytocinergic and decreased vasopressinergic function in schizophrenics. Psychoneuroendocrinology. 1992;17:611–617. doi: 10.1016/0306-4530(92)90019-4. [DOI] [PubMed] [Google Scholar]
- 14.Turan T, Uysal C, Asdemir A, Kiliç E. May oxytocin be a trait marker for bipolar disorder? Psychoneuroendocrinology. 2013 doi: 10.1016/j.psyneuen.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Averbeck BB, Bobin T, Evans S, Shergill SS. Emotion recognition and oxytocin in patients with schizophrenia. Psychol Med. 2011:1–8. doi: 10.1017/S0033291711001413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pedersen CA, Gibson CM, Rau SW, Salimi K, Smedley KL, Casey RL, et al. Intranasal oxytocin reduces psychotic symptoms and improves Theory of Mind and social perception in schizophrenia. Schizophr Res. 2011;132:50–53. doi: 10.1016/j.schres.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 17.Woolley JD, Chuang B, Lam O, Lai W, O'Donovan A, Rankin KP, et al. Oxytocin administration enhances controlled social cognition in patients with schizophrenia. Psychoneuroendocrinology. 2014;47:116–125. doi: 10.1016/j.psyneuen.2014.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gibson CM, Penn DL, Smedley KL, Leserman J, Elliott T, Pedersen CA. A pilot six-week randomized controlled trial of oxytocin on social cognition and social skills in schizophrenia. Schizophr Res. 2014;156:261–265. doi: 10.1016/j.schres.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Fischer-Shofty M, Shamay-Tsoory SG, Levkovitz Y. Characterization of the effects of oxytocin on fear recognition in patients with schizophrenia and in healthy controls. Frontiers in neuroscience. 2013;7:127. doi: 10.3389/fnins.2013.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis MC, Horan WP, Nurmi EL, Rizzo S, Li W, Sugar CA, et al. Associations between oxytocin receptor genotypes and social cognitive performance in individuals with schizophrenia. Schizophr Res. 2014 doi: 10.1016/j.schres.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montag C, Brockmann EM, Lehmann A, Muller DJ, Rujescu D, Gallinat J. Association between oxytocin receptor gene polymorphisms and self-rated ‘empathic concern’ in schizophrenia. PLoS One. 2012;7:e51882. doi: 10.1371/journal.pone.0051882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Argiolas A, Gessa GL. Central functions of oxytocin. Neurosci Biobehav Rev. 1991;15:217–231. doi: 10.1016/s0149-7634(05)80002-8. [DOI] [PubMed] [Google Scholar]
- 23.Dadds MR, Moul C, Cauchi A, Dobson-Stone C, Hawes DJ, Brennan J, et al. Methylation of the oxytocin receptor gene and oxytocin blood levels in the development of psychopathy. Development and psychopathology. 2014;26:33–40. doi: 10.1017/S0954579413000497. [DOI] [PubMed] [Google Scholar]
- 24.Dumais KM, Veenema AH. Vasopressin and oxytocin receptor systems in the brain: Sex differences and sex-specific regulation of social behavior. Front Neuroendocrinal. 2015 doi: 10.1016/j.yfrne.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cushing BS, Carter CS. Peripheral pulses of oxytocin increase partner preferences in female, but not male, prairie voles. Horm Behav. 2000;37:49–56. doi: 10.1006/hbeh.1999.1558. [DOI] [PubMed] [Google Scholar]
- 26.Insel TR, Hulihan TJ. A gender-specific mechanism for pair bonding: oxytocin and partner preference formation in monogamous voles. Behav Neurosci. 1995;109:782–789. doi: 10.1037//0735-7044.109.4.782. [DOI] [PubMed] [Google Scholar]
- 27.Bales KL, Carter CS. Sex differences and developmental effects of oxytocin on aggression and social behavior in prairie voles (Microtus ochrogaster) Horm Behav. 2003;44:178–184. doi: 10.1016/s0018-506x(03)00154-5. [DOI] [PubMed] [Google Scholar]
- 28.Domes G, Heinrichs M, Glascher J, Buchel C, Braus DF, Herpertz SC. Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biol Psychiatry. 2007;62:1187–1190. doi: 10.1016/j.biopsych.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 29.Petrovic P, Kalisch R, Singer T, Dolan RJ. Oxytocin attenuates affective evaluations of conditioned faces and amygdala activity. J Neurosci. 2008;28:6607–6615. doi: 10.1523/JNEUROSCI.4572-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Domes G, Lischke A, Berger C, Grossmann A, Hauenstein K, Heinrichs M, et al. Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology. 2010;35:83–93. doi: 10.1016/j.psyneuen.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 31.Lischke A, Gamer M, Berger C, Grossmann A, Hauenstein K, Heinrichs M, et al. Oxytocin increases amygdala reactivity to threatening scenes in females. Psychoneuroendocrinology. 2012;37:1431–1438. doi: 10.1016/j.psyneuen.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 32.Gregory SG, Connelly JJ, Towers AJ, Johnson J, Biscocho D, Markunas CA, et al. Genomic and epigenetic evidence for oxytocin receptor deficiency in autism. BMC Med. 2009;7:62. doi: 10.1186/1741-7015-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kusui C, Kimura T, Ogita K, Nakamura H, Matsumura Y, Koyama M, et al. DNA methylation of the human oxytocin receptor gene promoter regulates tissue-specific gene suppression. Biochem Biophys Res Commun. 2001;289:681–686. doi: 10.1006/bbrc.2001.6024. [DOI] [PubMed] [Google Scholar]
- 34.Puglia MH, Lillard TS, Morris JP, Connelly JJ. Epigenetic modification of the oxytocin receptor gene influences the perception of anger and fear in the human brain. Proc Natl Acad Sci USA. 2015;112:3308–3313. doi: 10.1073/pnas.1422096112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jack A, Connelly JJ, Morris JP. DNA methylation of the oxytocin receptor gene predicts neural response to ambiguous social stimuli. Frontiers in human neuroscience. 2012;6:280. doi: 10.3389/fnhum.2012.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cecil CAM, Lysenko U, Jaffee SR, Pingault JB, Smith RG, Relton CL, et al. Environmental risk, Oxytocin Receptor Gene (OXTR) methylation and youth callous-unemotional traits: a 13-year longitudinal study. Mol Psychiatr. 2014;19:1071–1077. doi: 10.1038/mp.2014.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bell AF, Carter SC, Steer CD, Golding J, Davis JM, Steffen AD, et al. Interaction between oxytocin receptor DNA methylation and genotype is associated with risk of postpartum depression in women without depression in pregnancy. Frontiers in Genetics. doi: 10.3389/fgene.2015.00243. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dannlowski U, Kugel H, Redlich R, Halik A, Schneider I, Opel N, et al. Serotonin transporter gene methylation is associated with hippocampal gray matter volume. Hum Brain Mapp. 2014;35:5356–5367. doi: 10.1002/hbm.22555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li H, Chan RC, McAlonan GM, Gong QY. Facial emotion processing in schizophrenia: a meta-analysis of functional neuroimaging data. Schizophr Bull. 2010;36:1029–1039. doi: 10.1093/schbul/sbn190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tamminga CA, Ivleva El, Keshavan MS, Pearlson GD, Clementz BA, Witte B, et al. Clinical Phenotypes of Psychosis in the Bipolar and Schizophrenia Network on Intermediate Phenotypes (B-SNIP) Am J Psychiatry. 2013 doi: 10.1176/appi.ajp.2013.12101339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.First M, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IVAxis I Disorders, Patient Edition (SCID-P) New York: New York State Psychiatric Institute; 1995. [Google Scholar]
- 42.Gur RC, Sara R, Hagendoorn M, Marom O, Hughett P, Macy L, et al. A method for obtaining 3-dimensional facial expressions and its standardization for use in neurocognitive studies. J Neurosci Methods. 2002;115:137–143. doi: 10.1016/s0165-0270(02)00006-7. [DOI] [PubMed] [Google Scholar]
- 43.Rubin LH, Carter CS, Bishop JR, Pournajafi-Nazarloo H, Harris MS, Hill SK, et al. Peripheral vasopressin but not oxytocin relates to severity of acute psychosis in women with acutely-ill untreated first-episode psychosis. Schizophr Res. 2013;146:138–143. doi: 10.1016/j.schres.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rubin LH, Carter CS, Bishop JR, Pournajafi-Nazarloo H, Drogos LL, Hill SK, et al. Reduced levels of vasopressin and reduced behavioral modulation of oxytocin in psychotic disorders. Schizophr Bull. 2014;40:1374–1384. doi: 10.1093/schbul/sbu027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 46.Fischl B, Salat DH, van der Kouwe Al, Makris N, Ségonne F, Quinn BT, et al. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23:S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 47.Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, et al. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci. 2009;34:418–432. [PMC free article] [PubMed] [Google Scholar]
- 48.Murphy FC, Nimmo-Smith I, Lawrence AD. Functional neuroanatomy of emotions: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3:207–233. doi: 10.3758/cabn.3.3.207. [DOI] [PubMed] [Google Scholar]
- 49.Mathew I, Gardin TM, Tandon N, Eack S, Francis AN, Seidman U, et al. Medial temporal lobe structures and hippocampal subfields in psychotic disorders: findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. JAMA Psychiatry. 2014;71:769–777. doi: 10.1001/jamapsychiatry.2014.453. [DOI] [PubMed] [Google Scholar]
- 50.Arnold SJ, Ivleva El, Gopal TA, Reddy AP, Jeon-Slaughter H, Sacco CB, et al. Hippocampal volume is reduced in schizophrenia and schizoaffective disorder but not in psychotic bipolar I disorder demonstrated by both manual tracing and automated panellation (FreeSurfer) Schizophr Bull. 2015;41:233–249. doi: 10.1093/schbul/sbu009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ivleva El, Bidesi AS, Keshavan MS, Pearlson GD, Meda SA, Dodig D, et al. Gray matter volume as an intermediate phenotype for psychosis: Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) Am J Psychiatry. 2013;170:1285–1296. doi: 10.1176/appi.ajp.2013.13010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Keshavan MS, Morris DW, Sweeney JA, Pearlson G, Thaker G, Seidman LJ, et al. A dimensional approach to the psychosis spectrum between bipolar disorder and schizophrenia: the Schizo-Bipolar Scale. Schizophr Res. 2011;133:250–254. doi: 10.1016/j.schres.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Insel TR, Gelhard R, Shapiro LE. The comparative distribution of forebrain receptors for neurohypophyseal peptides in monogamous and polygamous mice. Neuroscience. 1991;43:623–630. doi: 10.1016/0306-4522(91)90321-e. [DOI] [PubMed] [Google Scholar]
- 54.Smeltzer MD, Curtis JT, Aragona BJ, Wang Z. Dopamine, oxytocin, and vasopressin receptor binding in the medial prefrontal cortex of monogamous and promiscuous voles. Neurosci Lett. 2006;394:146–151. doi: 10.1016/j.neulet.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 55.Gur RC, Richard J, Calkins ME, Chiavacci R, Hansen JA, Bilker WB, et al. Age group and sex differences in performance on a computerized neurocognitive battery in children age 8-21. Neuropsychology. 2012;26:251–265. doi: 10.1037/a0026712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoffmann H, Kessler H, Eppel T, Rukavina S, Traue HC. Expression intensity, gender and facial emotion recognition: Women recognize only subtle facial emotions better than men. Acta Psychol (Amst) 2010;135:278–283. doi: 10.1016/j.actpsy.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 57.Goldstein JM. Sex, hormones and affective arousal circuitry dysfunction in schizophrenia. Horm Behav. 2006;50:612–622. doi: 10.1016/j.yhbeh.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 58.Feifel D, Macdonald K, Nguyen A, Cobb P, Warlan H, Galangue B, et al. Adjunctive intranasal oxytocin reduces symptoms in schizophrenia patients. Biol Psychiatry. 2010;68:678–680. doi: 10.1016/j.biopsych.2010.04.039. [DOI] [PubMed] [Google Scholar]
- 59.de Kloet ER, Voorhuis DA, Boschma Y, Elands J. Estradiol modulates density of putative ‘oxytocin receptors’ in discrete rat brain regions. Neuroendocrinology. 1986;44:415–421. doi: 10.1159/000124680. [DOI] [PubMed] [Google Scholar]
- 60.De Kloet ER, Voorhuis TA, Elands J. Estradiol induces oxytocin binding sites in rat hypothalamic ventromedial nucleus. European journal of pharmacology. 1985;118:185–186. doi: 10.1016/0014-2999(85)90679-x. [DOI] [PubMed] [Google Scholar]
- 61.Johnson AE, Ball GF, Coirini H, Harbaugh CR, McEwen BS, Insel TR. Time course of the estradiol-dependent induction of oxytocin receptor binding in the ventromedial hypothalamic nucleus of the rat. Endocrinology. 1989;125:1414–1419. doi: 10.1210/endo-125-3-1414. [DOI] [PubMed] [Google Scholar]
- 62.Johnson AE, Coirini H, Ball GF, McEwen BS. Anatomical localization of the effects of 17 beta-estradiol on oxytocin receptor binding in the ventromedial hypothalamic nucleus. Endocrinology. 1989;124:207–211. doi: 10.1210/endo-124-1-207. [DOI] [PubMed] [Google Scholar]
- 63.Harony-Nicolas H, Mamrut S, Brodsky L, Shahar-Gold H, Barki-Harrington L, Wagner S. Brain region-specific methylation in the promoter of the murine oxytocin receptor gene is involved in its expression regulation. Psychoneuroendocrinology. 2014;39:121–131. doi: 10.1016/j.psyneuen.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 64.Dellovade TL, Zhu YS, Pfaff DW. Thyroid hormones and estrogen affect oxytocin gene expression in hypothalamic neurons. J Neuroendocrinal. 1999;11:1–10. doi: 10.1046/j.1365-2826.1999.00250.x. [DOI] [PubMed] [Google Scholar]
- 65.Richard S, Zingg HH. The human oxytocin gene promoter is regulated by estrogens. J Biol Chem. 1990;265:6098–6103. [PubMed] [Google Scholar]
- 66.McClure EB, Monk CS, Nelson EE, Zarahn E, Leibenluft E, Bilder RM, et al. A developmental examination of gender differences in brain engagement during evaluation of threat. Biol Psychiatry. 2004;55:1047–1055. doi: 10.1016/j.biopsych.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 67.Stevens JS, Hamann S. Sex differences in brain activation to emotional stimuli: a metaanalysis of neuroimaging studies. Neuropsychologia. 2012;50:1578–1593. doi: 10.1016/j.neuropsychologia.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 68.Whittle S, Yucel M, Yap MB, Allen NB. Sex differences in the neural correlates of emotion: evidence from neuroimaging. Biol Psychol. 2011;87:319–333. doi: 10.1016/j.biopsycho.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 69.Terry RD, DeTeresa R, Hansen LA. Neocortical cell counts in normal human adult aging. Ann Neurol. 1987;21:530–539. doi: 10.1002/ana.410210603. [DOI] [PubMed] [Google Scholar]
- 70.Jacobs B, Driscoll L, Schall M. Life-span dendritic and spine changes in areas 10 and 18 of human cortex: a quantitative Golgi study. J Comp Neurol. 1997;386:661–680. [PubMed] [Google Scholar]
- 71.Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: Inverse relationships during postadolescent brain maturation. J Neurosci. 2001;21:8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gur RC, Ragland JD, Moberg PJ, Bilker WB, Kohler C, Siegel SJ, et al. Computerized neurocognitive scanning: II. The profile of schizophrenia. Neuropsychopharmacology. 2001;25:777–788. doi: 10.1016/S0893-133X(01)00279-2. [DOI] [PubMed] [Google Scholar]
- 73.Sota TL, Heinrichs RW. Sex differences in verbal memory in schizophrenia patients treated with “typical” neuroleptics. Schizophr Res. 2003;62:175–182. doi: 10.1016/s0920-9964(02)00373-0. [DOI] [PubMed] [Google Scholar]
- 74.Fiszdon JM, Silverstein SM, Buchwald J, Hull JW, Smith TE. Verbal memory in schizophrenia: sex differences over repeated assessments. Schizophr Res. 2003;61:235–243. doi: 10.1016/s0920-9964(02)00285-2. [DOI] [PubMed] [Google Scholar]
- 75.Halari R, Mehrotra R, Sharma T, Ng V, Kumari V. Cognitive impariment but preservation of sexual dimorphism in cognitive abilities in chronic schizophrenia. Psychiatry Research. 2006;141:129–139. doi: 10.1016/j.psychres.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 76.Rubin LH, Haas GL, Keshavan MS, Sweeney JA, Maki PM. Sex difference in cognitive response to antipsychotic treatment in first episode schizophrenia. Neuropsychopharmacology. 2008;33:290–297. doi: 10.1038/sj.npp.1301395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang XY, Chen da C, Xiu MH, Yang FD, Haile CN, Kosten TA, et al. Gender differences in never-medicated first-episode schizophrenia and medicated chronic schizophrenia patients. J Clin Psychiatry. 2012;73:1025–1033. doi: 10.4088/JCP.11m07422. [DOI] [PubMed] [Google Scholar]
- 78.Tang YL, Gillespie CF, Epstein MP, Mao PX, Jiang F, Chen Q, et al. Gender differences in 542 Chinese inpatients with schizophrenia. Schizophr Res. 2007;97:88–96. doi: 10.1016/j.schres.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 79.Lindamer LA, Lohr JB, Harris MJ, McAdams LA, Jeste DV. Gender-related clinical differences in older patients with schizophrenia. J Clin Psychiatry. 1999;60:61–67. doi: 10.4088/jcp.v60n0114. quiz 68-69. [DOI] [PubMed] [Google Scholar]
- 80.Szymanski S, Lieberman JA, Alvir JM, Mayerhoff D, Loebel A, Geisler S, et al. Gender differences in onset of illness, treatment response, course, and biologic indexes in first-episode schizophrenic patients. Am J Psychiatry. 1995;152:698–703. doi: 10.1176/ajp.152.5.698. [DOI] [PubMed] [Google Scholar]
- 81.Seeman MV. Gender differences in schizophrenia. Can J Psychiatry. 1982;27:107–112. doi: 10.1177/070674378202700204. [DOI] [PubMed] [Google Scholar]
- 82.Goldstein JM. Gender differences in the course of schizophrenia. Am J Psychiatry. 1988;145:684–689. doi: 10.1176/ajp.145.6.684. [DOI] [PubMed] [Google Scholar]
- 83.Tamminga CA. Gender and schizophrenia. J Clin Psychiatry. 1997;58(Suppl 15):33–37. [PubMed] [Google Scholar]
- 84.McCullough ME, Churchland PS, Mendez AJ. Problems with measuring peripheral oxytocin: can the data on oxytocin and human behavior be trusted? Neurosci Biobehav Rev. 2013;37:1485–1492. doi: 10.1016/j.neubiorev.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 85.Szeto A, McCabe PM, Nation DA, Tabak BA, Rossetti MA, McCullough ME, et al. Evaluation of enzyme immunoassay and radioimmunoassay methods for the measurement of plasma oxytocin. Psychosom Med. 2011;73:393–400. doi: 10.1097/PSY.0b013e31821df0c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Walton E, Hass J, Liu J, Roffman JL, Bernardoni F, Roessner V, et al. Correspondence of DNA Methylation Between Blood and Brain Tissue and its Application to Schizophrenia Research. Schizophr Bull. 2015 doi: 10.1093/schbul/sbv074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Unternaehrer E, Luers P, Mill J, Dempster E, Meyer AH, Staehli S, et al. Dynamic changes in DNA methylation of stress-associated genes (OXTR, BDNF) after acute psychosocial stress. Translational psychiatry. 2012;2:el50. doi: 10.1038/tp.2012.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


