Abstract
Muscular weakness and muscle wasting may often be observed in critically ill patients on intensive care units (ICUs) and may present as failure to wean from mechanical ventilation. Importantly, mounting data demonstrate that mechanical ventilation itself may induce progressive dysfunction of the main respiratory muscle, i.e. the diaphragm. The respective condition was termed ‘ventilator‐induced diaphragmatic dysfunction’ (VIDD) and should be distinguished from peripheral muscular weakness as observed in ‘ICU‐acquired weakness (ICU‐AW)’.
Interestingly, VIDD and ICU‐AW may often be observed in critically ill patients with, e.g. severe sepsis or septic shock, and recent data demonstrate that the pathophysiology of these conditions may overlap. VIDD may mainly be characterized on a histopathological level as disuse muscular atrophy, and data demonstrate increased proteolysis and decreased protein synthesis as important underlying pathomechanisms. However, atrophy alone does not explain the observed loss of muscular force. When, e.g. isolated muscle strips are examined and force is normalized for cross‐sectional fibre area, the loss is disproportionally larger than would be expected by atrophy alone. Nevertheless, although the exact molecular pathways for the induction of proteolytic systems remain incompletely understood, data now suggest that VIDD may also be triggered by mechanisms including decreased diaphragmatic blood flow or increased oxidative stress. Here we provide a concise review on the available literature on respiratory muscle weakness and VIDD in the critically ill. Potential underlying pathomechanisms will be discussed before the background of current diagnostic options. Furthermore, we will elucidate and speculate on potential novel future therapeutic avenues.
Keywords: VIDD, Diaphragm, Weakness, Cachexia, Sepsis, Mechanical ventilation, ICU‐acquired weakness, weaning failure
Introduction
Historically, respiratory muscle weakness during mechanical ventilation was recognized a state of muscular fatigue. It was thought to be caused by prolonged increased work of breathing. Although first reports of diminished myofibre cross‐sectional area in diaphragms because of long term mechanical ventilation date back to the 1980s,1 direct harmful effects of mechanical ventilation on respiratory muscles were only thoroughly studied, e.g. in animal models in the last two decades. Currently, respective findings from animal models can partially be reproduced in human experiments and find their way to the clinical bedside.2 Within the complex of critical illness‐related weakness,3, 4, 5 the dysfunction of respiratory muscles, particularly of the diaphragm, represents a highly relevant and distinct clinical problem in intensive care units (ICUs). From a clinical perspective, diaphragmatic dysfunction contributes to difficult weaning or even failure to wean from mechanical ventilation in ICU patients. Overall, data indicate that weaning failure may affect up to 25% of mechanically ventilated patients in ICUs today.6
Ventilator‐induced diaphragmatic dysfunction (VIDD) was previously defined as loss of diaphragmatic force‐generating capacity specifically related to the use of mechanical ventilation.7 VIDD may typically be observed after variable periods of controlled mechanical ventilation (i.e. ventilation without spontaneous breathing)7, 8 while assisted ventilation modes (i.e. ventilation with preserved diaphragmatic contractions) attenuate the detrimental effects of controlled mechanical ventilation on the diaphragm and seem protective in animal models9, 10, 11, 12 unless high levels of support are used.8 As preserved muscle activity is protective, it seems conceivable that diaphragmatic contractile inactivity may be the cause for the development of VIDD.13 Despite the terminology ‘VIDD’, the condition does not exclusively affect the major respiratory muscle (i.e. the diaphragm), but may also involve the intercostal musculature to a minor degree.14, 15 The diaphragm, however, seems particularly prone to dysfunction under mechanical ventilation while auxiliary respiratory muscles, e.g. pectorales muscles,16 or skeletal limb muscles (e.g. soleus or extensor digitorum muscles) are typically spared.17, 18, 19, 20
While many of the pathophysiological aspects of VIDD that were found in animals have also been successfully reproduced in humans, the proof of decreased force generation was so far only performed indirectly in mechanically ventilated patients.21 Only very recently, direct force generation of isolated human muscle fibres was studied with ambiguous results.22, 23, 24
Pathophysiology of respiratory muscles dysfunction and VIDD: histological and structural changes
The pathophysiology of VIDD was to a large extent elucidated in models of healthy animals. Under continuous controlled mechanical ventilation, the diaphragm partially loses its force‐ and pressure‐generating capacity, classically assessed in intact animal models with trans‐diaphragmatic pressure under maximal phrenic nerve stimulation.25, 26, 27 Data demonstrate that loss of respective force may reach up to 50% within a few days. Importantly, the onset and time course of VIDD may vary between the respective species studied.25, 26, 27 This makes transfer of respective data to the human bedside particularly difficult. However, loss of force seems neither linked to age28 nor to changes in lung volumes26 nor to positive end‐expiratory pressure (PEEP).27, 29 In addition, phrenic nerve signal transmission and signal transduction at the neuromuscular endplate in VIDD appear normal. This is a distinct difference between VIDD and polyneuropathic forms of ICU‐acquired weakness (ICU‐AW),5 as the latter often shows conduction abnormalities in electrophysiological studies. Moreover, loss of force is reproducible in isolated muscle specimens,18, 29 rendering the pathophysiological changes of VIDD to a cellular level. Besides ultrastructural muscle fibre injury,14, 27, 30 the most common histopathological correlate is muscle atrophy.14, 18, 26, 31, 32, 33 This is especially the case in regard to type II (fast twitch) muscle fibres within the early course of the disease.14, 19 Remodelling processes with a histopathological increase of hybrid fibres at the expense of (slow twitch) type I fibres can be identified at later stages.31 However, it must be noted that atrophy alone does not explain the observed loss of force. When force is normalized for cross‐sectional area of isolated muscle strips (so‐called specific force or tension), the loss is disproportionally larger than would be expected by atrophy alone.18, 27, 34
Overall, protein synthesis in the diaphragm in established VIDD seems dramatically decreased.35 In murine models, proteolysis is activated as early as 6 h after initiation of controlled mechanical ventilation36 and loss in myosin heavy chain synthesis of up to 65% may be observed.19, 35 A decrease in anabolic signalling including pathways via insulin like growth factor‐1 (IGF‐1)32 and myogenin (MyoD) are found.37 Proteolysis is significantly increased.19, 38 All four proteolytic systems of the mammalian cell are activated in animal models of VIDD and later found induced in mechanically ventilated humans.39 Proteolysis is mediated by the calpain 19 and caspase system,40, 41 as well as the ubiquitin–proteasome complex33, 38 and the autophagy–lysosomal system.42 The biochemical changes of VIDD, mainly examined in healthy murine models, may be augmented or even triggered by oxidative stress pathways,19, 43, 44, 45 nuclear factor kB (NFkB),46, 47 or JAK–Stat signalling pathways.48 Similar to patients with severe sepsis and septic shock, distinct cytokine cascades are upregulated. This includes interleukin (IL)‐6, tumour necrosis factor‐α, and IL‐1β in rat diaphragmatic tissue exposed to mechanical ventilation47 and may further amplify major inflammatory signalling pathways including NFkB.47, 49 This ‘humoral’ response is well known to promote systemic immune cellular consequences and may also result in immune phenotypic changes.50, 51, 52 There is experimental proof of diaphragmatic dysfunction directly induced by sepsis.53, 54, 55, 56, 57 This includes free radicals, mitochondrial dysfunction,54 calpain,56 and caspase activation.57 In addition, clinical evidence points to severe sepsis/septic shock as an important risk factor for diaphragmatic dysfunction.58, 59 Interestingly, Maes and colleagues have developed a lipopolysaccharide rat model of controlled mechanical ventilation. They could now show that controlled mechanical ventilation potentiates sepsis‐induced diaphragm dysfunction, possibly because of increased pro‐inflammatory cytokine production, autophagy, and worsening of oxidative stress.60 To our knowledge, this is the first study of VIDD in a sepsis model that may indicate an important pathophysiological overlap. It should be clearly noticed that VIDD and sepsis induced diaphragmatic dysfunction are distinctive pathological entities13 and the findings of overlapping pathophysiology are controversial, even more so as mechanical ventilation was shown to protect rat diaphragms in sepsis.61 Nevertheless, this shared pathophysiology may offer therapeutic options in the future. Strategies to modulate the systemic cytokine and/or immune response by, e.g. blocking respective cascades or by improving host defence, may in theory be beneficial.62, 63, 64 However, because of current lack of respective trials, this remains speculative at this point in time.65
Recent data indicate a profound reduction in diaphragmatic blood flow in VIDD.66 It is therefore speculated that reduced oxygen delivery may lead to formation of reactive oxygen species with consecutive triggering of proteolytic cascades and enhanced oxidative stress.67 Angiogenetic factors change their expression profile under mechanical ventilation [downregulation of vascular endothelial growth factor (VEGF), upregulation of hypoxia‐inducible factor (HIF)‐1α], but their role in VIDD remains incompletely understood.47, 68
Exercise training or a genetically high aerobic capacity—interesting in the context of decreased blood supply—may protect the diaphragm from VIDD. Up‐regulation of heat shock proteins and improved antioxidant properties of a ‘trained diaphragm’ with decreased activation of proteases and respective mitochondrial ‘protection’ are among the postulated mechanisms.69, 70 Nevertheless, mechanical ventilation may also enhance anti‐oxidative pathways, potentially as a counter‐regulatory measure.68, 71 Overall, this aspect of VIDD may open new therapeutic avenues as the redox state of a cell might theoretically be influenced positively.71 Recent data indicate protection from diaphragmatic weakness with N‐acetylcysteine via augmented autophagosome formation in mice.72
Data from animal VIDD models demonstrate a quick and complete recovery within hours if spontaneous breathing is resumed early. However, this needs to be interpreted before the background of limited times of mechanical ventilation (12 to 27 h.).73, 74, 75 Moreover, the respective kinetics and time course of VIDD are species dependent,7 and data may therefore not be easily transferrable between species. As pathophysiology of VIDD was investigated mainly in models of healthy animals, it must be kept in mind that in critically ill patients, underlying comorbidities may contribute to diaphragmatic dysfunction.
Pathophysiology of respiratory muscle dysfunction and VIDD: translation to the bedside
Human data are basically available from two groups of patients. The bulk of evidence derives from mechanically ventilated brain dead organ donors. Only very recently, studies were expanded to mixed ICU populations with underlying diseases. Levine and colleagues could show in a seminal study in 14 brain death organ donors that disuse atrophy may occur shortly after start of controlled mechanical ventilation.16 When compared to controls (i.e. patients with thoracic surgery on 3 h of CMV), muscle biopsies of the costal diaphragm in organ donors (mechanically ventilated for 18 to 69 h) exhibit strongly decreased cross‐sectional areas of slow‐twitch and fast‐twitch fibres (57% vs. 53%, respectively). Atrophy was associated with reduced glutathione levels and up‐regulation of caspase‐3 and ubiquitin ligases. These changes were limited to the diaphragm and not reproducible, e.g. in pectorales muscles.16 Findings were later confirmed and linked to up‐regulation of autophagic systems via transcription factors (e.g. FOXO‐1), activation of the ubiquitin–proteasome complex, NFkB activity, and induction of the calpain system in mechanically ventilated human muscles and diaphragms.76, 77, 78, 79, 80 Hooijman lately documented the activation of the ubiquitin–proteasome complex in a mixed population of critically ill patients.22 While direct force loss (i.e. loss of performance of isolated muscle strips) is extensively documented in animal models, the condition in humans was until recently only indirectly characterized by airway occlusion pressure responses to phrenic nerve stimulation.81, 82 In the last year, first reports of human muscle fibres appeared and finally documented a loss of specific force in mechanically ventilated human muscles.22, 23, 24 But a clear distinction of the studied populations should be noted. Hooijman initially found no evidence for diminished contractile performance in sarcomeres when studying specimens from brain death organ donors (mean duration of mechanical ventilation of 26 ± 5 h),23 whereas the group later published data from mixed ICU populations suffering from comorbidities like sepsis or trauma (duration of mechanical ventilation 14 to 607 h) with contractile weakness of human diaphragmatic muscle fibres.22, 24 It is unclear to what extent these underlying conditions have contributed to the documented weakness. So, speaking in strict terms, the formal proof of contractile weakness caused by mechanical ventilation is not yet established.
VIDD appears to be associated with mitochondrial dysfunction. Mechanical ventilation may alter mitochondrial respiratory chain enzyme function (e.g. cytochrome‐c oxidase), may induce micro‐deletions within mitochondrial DNA, and may decrease levels of mitochondrial scavengers for reactive oxygen species (ROS) culminating in diaphragmatic lipid overload. This metabolic oversupply of resting diaphragms could again trigger ROS production. However, these findings could not be reproduced in biceps muscle specimens.83 A causative link between lipid overload and mitochondrial dysfunction is not firmly established.84 Nevertheless, oxidative stress has been linked to activation of apoptic, proteasomal, and autophagocytic pathways,85 and expression of angiogenetic factors varies with the ventilation mode used.86
In conclusion, histopathological and biochemical changes of respiratory muscle dysfunction/VIDD in animal models could now be reproduced to some extent in human experiments.2 Loss of specific force in isolated human muscle fibres has finally been documented22, 24 in mixed ICU populations with underlying comorbidities (but not in brain death organ donors23). With VIDD animal models available, this may open up new options for development of specific therapeutic approaches in order to treat or prevent respiratory muscle dysfunction/VIDD.
Clinical approaches to respiratory muscle dysfunction/VIDD
VIDD is diagnosed by exclusion of other causes of weaning failure (e.g. decompensated congestive heart failure) and other specific causes of diaphragmatic weakness such as electrolyte disturbances, malnutrition, drugs, central nervous system disorders, and distinct neuromuscular disorders.7 Global tests of respiratory muscle strength like measurement of maximum inspiratory pressures87 or the rapid shallow breathing index (respiratory rate divided by tidal volume)88 serve as screening tools. However, they lack specificity. Assessment of trans‐diaphragmatic pressure in combination with transdermal phrenical nerve (magnetic) stimulation may be considered the gold standard.82, 89, 90 However, this approach is invasive with the need for an oesophageal and gastric balloon. Alternatively, twitch tracheal airway pressure avoids balloon placement,81, 82, 91 but the accuracy of this approach is debated.92 The electrophysiological assessment of diaphragmatic electrical activity via a modified nasogastric tube (EAdi)93, 94 is a new promising but almost unexplored technique for VIDD. The electromyographic signal describes the summation amplitude of electrical activity and allows assessment of neuronal respiratory drive and estimation of neuro‐ventilatory efficiency (tidal volume divided by electrical activity).94, 95, 96, 97 Although there is lack of specific VIDD studies, this approach may hold potential for respiratory monitoring as it incorporates the neuronal feedback loop of the respiratory drive.93, 94, 97 Further studies are warranted.
A non‐invasive modality for patients with suspected diaphragmatic weakness is ultrasound, readily available at the bedside and most likely free from adverse effects (Figures 1 and 2). With reference values established, ultrasound allows both reproducible assessment98 of diaphragmatic function and structure and may exclude alternative causes of weaning failure.94, 99, 100, 101, 102 The inspiratory downward motion of the diaphragm should be greater than one centimetre (Figure 1). This was evaluated in ventilated patients during spontaneous breathing trials and predicts successful weaning with an accuracy similar to the rapid shallow breathing index.103 A thickening fraction (inspiratory minus expiratory thickness divided by expiratory thickness) of ≥30% was shown to exert a positive predictive value of 91% for weaning success104 (Figure 2A and 2B). A sustained loss of diaphragmatic thickness was reported for patients undergoing prolonged mechanical ventilation, most prominent in the first three days.105 Changes in thickness may be associated with diaphragmatic weakness.106
Figure 1.
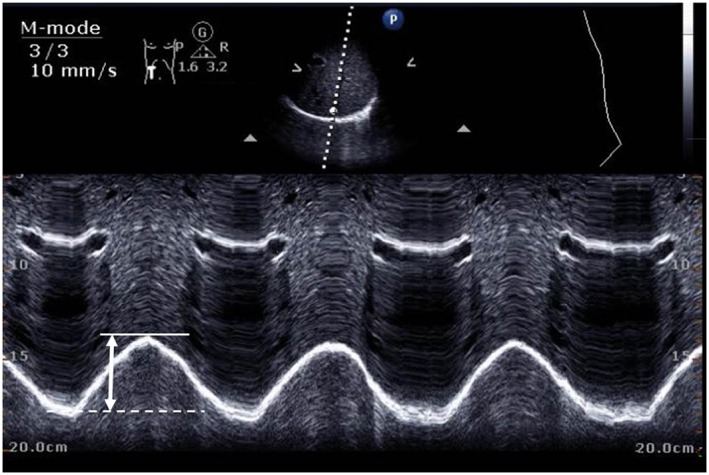
Assessment of diaphragmatic motion (motion mode, phased array 3.5–5 Mhz transducer). Sub‐costal position (mid‐clavicular line, angle of >70° in supine subjects) with visualization of the posterior diaphragmatic third. The diaphragmatic dome should be hit perpendicularly. Liver/spleen may be used as sound windows for right/left hemi‐diaphragm. Diaphragmatic dome excursion at rest should be ≥1 cm (lower limit of normal in healthy controls).101, 108 Inspiratory diaphragmatic position (solid line), expiratory position (dotted line), and excursion (arrow) are indicated.
Figure 2.
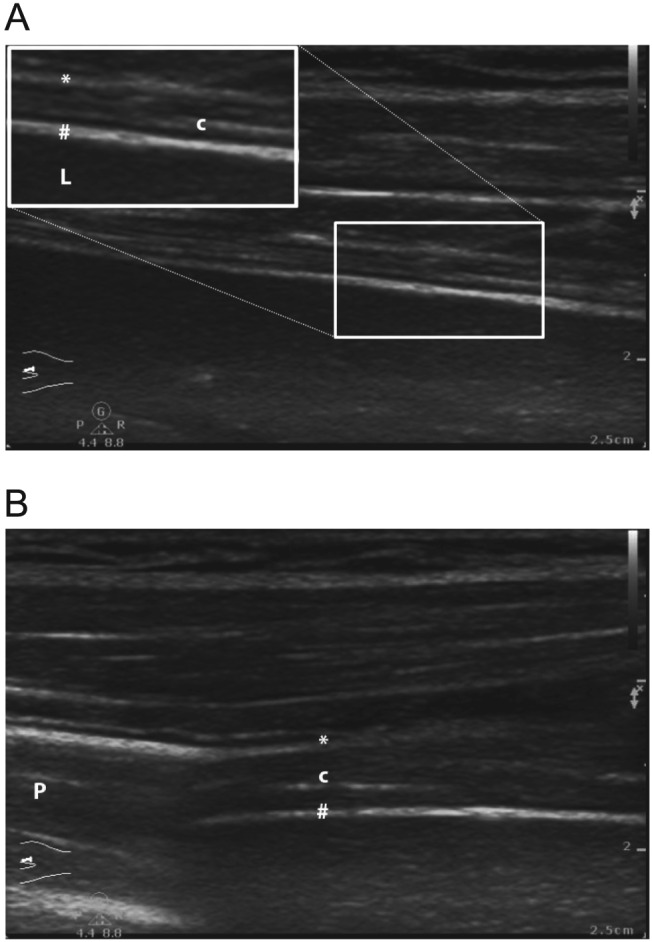
Assessment of (A) expiratory and (B) inspiratory diaphragmatic thickness (brightness mode, linear array high frequency transducer of >10 Mhz, zone of apposition: mid‐axillary line). Note in‐ and expiratory variation (limits of normal may vary).102, 108 The thickening fraction [i.e. (inspiratory–expiratory diameter) / expiratory diameter] can be assessed.143 Maximum diameters are measured between diaphragmatic pleural line (*) and peritoneal line (#). Central diaphragmatic tendon (c). Liver (L), Lungs (P) are indicated.
The consequences of application of PEEP (inducing caudal diaphragm displacement) remain unknown.107 As ultrasound allows dynamic assessment of the diaphragm, it will mainly provide useful insight if performed during unassisted breathing, i.e. during spontaneous breathing trials.108 Recent data indicate that the thickening fraction is also of use under mechanical ventilation, as thickening is caused by muscular contraction, but not by passive inhalation.98 Despite a growing body of evidence for the use of ultrasound, it remains a pure imaging technique without direct assessment of ventilation or diaphragmatic force. Ultrasound may also be of limited value in the early course of critical illness, when controlled ventilation needs to be applied.94 For technical details, please refer to figure legends (Figures 1 and 2).
VIDD management: a role for ventilatory and pharmacological interventions?
Human data on interventions for VIDD are sparse. When choosing ventilatory modes, spontaneous breathing efforts during mechanical ventilation seem protective for VIDD in healthy animals.12, 109, 110 As most studies on VIDD were performed in healthy animals, questions remain about the applicability in the acute phase of critical illness.39 The complex and dynamic pathophysiology of respiratory support during critical illness asks for an individually tailored approach. Early in acute critical illness, controlled mechanical ventilation may often be mandatory, but an early switch to an assisted mode seems clearly desirable. Diaphragmatic inactivity is considered to initiate VIDD.13 Interventions like intermittent spontaneous breathing or respiratory muscle training might be of benefit. Diaphragmatic contractions via phrenic nerve pacing have been successfully used in tetraplegic patients111, 112 and during cardiothoracic surgery113 with documented increases in mitochondrial respiration.114 While short periods of intermittent spontaneous breathing had little effect in a rodent model,12 adding a resistive inspiratory load in order to train the inspiratory muscles has shown promising results for patients in small series115, 116, 117 and improved weaning outcome in a randomized trial.118
The period of controlled mechanical ventilation should be kept short.82, 119 Ventilation modes with sustained patient effort should be introduced early,39 as can be inferred from various animal experiments. Assist Control Ventilation modes (controlled mechanical ventilation with the possibility to trigger additional breaths) and pressure support ventilation (patient's breathing efforts supported by a preset pressure) may attenuate VIDD in animals.9, 109 Nevertheless, partial supportive modes can still cause VIDD in the setting of over‐assist,8 so mode per se is not protective. On the contrary, in septic rabbits, controlled mechanical ventilation may protect from VIDD61 and the use of modes that allow spontaneous breathing is contradictory to the documented benefit of neuromuscular blockers in the early course of severe acute respiratory distress syndrome (ARDS).120
The preferred ventilation mode, the optimal level of support (‘unloading’), or the rate of support reduction for patients is currently unknown.39 Spontaneous breathing is well maintained with so‐called effort adapted modes 121 like neurally adjusted ventilatory assist (NAVA) or adaptive support ventilation (ASV). ASV seems to mitigate deleterious effects of mechanical ventilation on the diaphragm of piglets.10 NAVA delivers assist in unison with the patient's inspiratory neural effort.93 As the support level is titrated against the patient's respiratory demand, patients are protected against over‐assist.122, 123 This approach was successfully used in various clinical ICU settings97, 122, 124, 125 but most importantly in patients with critical illness myo‐neuropathy.97
Currently, no medical treatment for VIDD is available.126 Anti‐oxidants show benefits in animal models40, 71, 72, 127 and in a randomized trial in critically ill surgical patients.128 However, human data remain sparse. Targeting of proteolytic (especially proteasome) pathways in rodent models may further elucidate the pathophysiology and theoretically open novel therapeutic avenues. Inhibition of lysosomal proteases and calpain with leupeptin129 or the proteasome using bortezomib130 may be of interest as data indicate potential to completely or partially prevent VIDD. However, results could not be reproduced with epoxomicin.131 Overall, effects may be time dependent.129, 130, 131, 132 Moreover, R548, a JAK/STAT inhibitor, maintained normal diaphragmatic contractility,133 and a recent FOXO (forkhead BoxO) animal knockout model suggests new potential therapeutic targets.134
While the effects of corticosteroids on VIDD seem dose dependent conflicting,135, 136 propofol was accused as a causative agent in animal models.137 Neuromuscular blockers do not to have additive effects on diaphragmatic dysfunction.136 Moreover, moderate hypercapnia exerts protective effects on diaphragmatic muscular strength,138, 139 which fits to the overall concept of lung protective ventilation.140 In addition, the calcium sensitizer Levosimendan may improve diaphragmatic neuro‐mechanical efficiency in healthy volunteers, while the fast skeletal troponin activator CK‐2066260 improved diaphragmatic fibre strength ex vivo in an experimental human trial.24 Further studies in critically ill patients with respiratory failure are warranted.141
In conclusion, prolonged periods of complete diaphragmatic rest should be avoided and diaphragmatic contractions preserved whenever possible. Respiratory muscle training may lead to improved weaning success.
Respiratory muscle dysfunction in critically ill patients: summary and outlook
Ventilator‐induced diaphragmatic dysfunction is an established and specific ‘side effect’ of prolonged mechanical ventilation. The clinical hallmark is respiratory muscle weakness which contributes to weaning failure and thus implies a significant health care burden. Major pathophysiological changes are disuse atrophy and microstructural changes including decreased protein synthesis, increased proteolysis, and oxidative stress, possibly linked to mitochondrial dysfunction.
For the clinician, bedside ultrasound evaluation and assessment of the electrical diaphragmatic activity are promising tools for the diagnosis and monitoring of respiratory muscle dysfunction/VIDD. Respiratory muscle training may have beneficial effects. Questions remain about the optimal strategy and mode of mechanical ventilation for affected patients. Effort adapted ventilation modes may offer advantages. Whether this may lead to improved clinical outcomes needs to be established.
Acknowledgements
The Department of Intensive Care Medicine has/had research contracts with Orion Corporation, Abbott Nutrition International, B. Braun Medical AG, CSEM SA, Edwards Lifesciences Services GmbH, Kenta Biotech Ltd, Maquet Critical Care AB, Omnicare Clinical Research AG and research and development/consulting contracts with Edwards Lifesciences SA, Maquet Critical Care AB, and Nestlé. The money was paid into a departmental fund; the authors received no personal financial gain. Unrestricted educational grants from the following organizations for organizing a quarterly postgraduate educational symposium (Berner Forum for Intensive Care) were provided by: Fresenius Kabi; gsk; MSD; Lilly; Baxter; astellas; AstraZeneca; B.Braun; CSL Behring; Maquet; Novartis; Covidien; Nycomed; Pierre Fabre Pharma (RobaPharma); Pfizer, Orion Pharma. The additional authors declare that there is no conflict of interest. All authors certify compliance with the Ethical guidelines for authorship and publishing established by the Journal of Cachexia, Sarcopenia, and Muscle.142
Berger, D. , Bloechlinger, S. , von Haehling, S. , Doehner, W. , Takala, J. , Z'Graggen, W. J. , and Schefold, J. C. (2016) Dysfunction of respiratory muscles in critically ill patients on the intensive care unit. Journal of Cachexia, Sarcopenia and Muscle, 7: 403–412. doi: 10.1002/jcsm.12108.
References
- 1. Knisely AS, Leal SM, Singer DB. Abnormalities of diaphragmatic muscle in neonates with ventilated lungs. J Pediatr 1988;113:1074–1077. [DOI] [PubMed] [Google Scholar]
- 2. Jaber S, Jung B, Matecki S, Petrof BJ. Clinical review: ventilator‐induced diaphragmatic dysfunction—human studies confirm animal model findings!. Critical care (London, England) 2011;15:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anker SD, Coats AJ, Morley JE, Rosano G, Bernabei R, von Haehling S. Muscle wasting disease: a proposal for a new disease classification. J Cachexia Sarcopenia Muscle 2014;5:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Palus S, von Haehling S, Springer J. Muscle wasting: an overview of recent developments in basic research. J Cachexia Sarcopenia Muscle 2014;5:193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schefold JC, Bierbrauer J, Weber‐Carstens S. Intensive care unit‐acquired weakness (ICUAW) and muscle wasting in critically ill patients with severe sepsis and septic shock. J Cachexia Sarcopenia Muscle 2010;1:147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Esteban A, Frutos F, Tobin MJ, Alia I, Solsona JF, Valverdu I, et al. A comparison of four methods of weaning patients from mechanical ventilation. Spanish Lung Failure Collaborative Group. N Engl J Med 1995;332:345–350. [DOI] [PubMed] [Google Scholar]
- 7. Vassilakopoulos T, Petrof BJ. Ventilator‐induced diaphragmatic dysfunction. Am J Respir Crit Care Med 2004;169:336–341. [DOI] [PubMed] [Google Scholar]
- 8. Hudson MB, Smuder AJ, Nelson WB, Bruells CS, Levine S, Powers SK. Both high level pressure support ventilation and controlled mechanical ventilation induce diaphragm dysfunction and atrophy. Crit Care Med 2012;40:1254–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sassoon C, Zhu E, Caiozzo V. Assist‐control mechanical ventilation attenuates ventilator‐induced diaphragmatic dysfunction. Am J Respir Crit Care Med 2004;170:626–632. [DOI] [PubMed] [Google Scholar]
- 10. Jung B, Constantin J, Rossel N, Le Goff C, Sebbane M, Coisel Y, et al. Adaptive support ventilation prevents ventilator‐induced diaphragmatic dysfunction in piglet: an in vivo and in vitro study. Anesthesiology 2010;112:1435–1443. [DOI] [PubMed] [Google Scholar]
- 11. Futier E, Constantin J, Combaret L, Mosoni L, Roszyk L, Sapin V. Pressure support ventilation attenuates ventilator‐induced protein modifications in the diaphragm. Critical care (London, England) 2008;12:R116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gayan‐Ramirez G, Testelmans D, Maes K, Racz G, Cadot P, Zador E. Intermittent spontaneous breathing protects the rat diaphragm from mechanical ventilation effects. Crit Care Med 2005;33:2804–2809. [DOI] [PubMed] [Google Scholar]
- 13. Powers SK, Smuder AJ, Fuller D, Levine S. CrossTalk proposal: mechanical ventilation‐induced diaphragm atrophy is primarily due to inactivity. J Physiol 2013;591:5255–5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bernard N, Matecki S, Py G, Lopez S, Mercier J, Capdevila X. Effects of prolonged mechanical ventilation on respiratory muscle ultrastructure and mitochondrial respiration in rabbits. Intensive Care Med 2003;29:111–118. [DOI] [PubMed] [Google Scholar]
- 15. Capdevila X, Lopez S, Bernard N, Rabischong E, Ramonatxo M, Martinazzo G. Effects of controlled mechanical ventilation on respiratory muscle contractile properties in rabbits. Intensive Care Med 2003;29:103–110. [DOI] [PubMed] [Google Scholar]
- 16. Levine S, Nguyen T, Taylor N, Friscia M, Budak M, Rothenberg P, et al. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med 2008;358:1327–1335. [DOI] [PubMed] [Google Scholar]
- 17. Yang L, Luo J, Bourdon J, Lin M, Gottfried S, Petrof B. Controlled mechanical ventilation leads to remodeling of the rat diaphragm. Am J Respir Crit Care Med 2002;166:1135–1140. [DOI] [PubMed] [Google Scholar]
- 18. Le Bourdelles G, Viires N, Boczkowski J, Seta N, Pavlovic D, Aubier M. Effects of mechanical ventilation on diaphragmatic contractile properties in rats. Am J Respir Crit Care Med 1994;149:1539–1544. [DOI] [PubMed] [Google Scholar]
- 19. Shanely RA, Zergeroglu MA, Lennon SL, Sugiura T, Yimlamai T, Enns D. Mechanical ventilation‐induced diaphragmatic atrophy is associated with oxidative injury and increased proteolytic activity. Am J Respir Crit Care Med 2002;166:1369–1374. [DOI] [PubMed] [Google Scholar]
- 20. van Hees HW, Schellekens WJ, Andrade Acuna GL, Linkels M, Hafmans T, Ottenheijm CA, et al. Titin and diaphragm dysfunction in mechanically ventilated rats. Intensive Care Med 2012;38:702–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jaber S, Petrof B, Jung B, Chanques G, Berthet J, Rabuel C, et al. Rapidly progressive diaphragmatic weakness and injury during mechanical ventilation in humans. Am J Respir Crit Care Med 2011;183:364–371. [DOI] [PubMed] [Google Scholar]
- 22. Hooijman PE, Beishuizen A, Witt CC, de Waard MC, Girbes ARJ, Spoelstra‐de Man AME, et al. Diaphragm muscle fiber weakness and ubiquitin–proteasome activation in critically Ill patients. Am J Respir Crit Care Med 2015;191:1126–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hooijman PE, Paul MA, Stienen GJ, Beishuizen A, Van Hees HW, Singhal S, et al. Unaffected contractility of diaphragm muscle fibers in humans on mechanical ventilation. Am J Physiol Lung Cell Mol Physiol 2014;307:L460–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hooijman PE, Beishuizen A, de Waard MC, de Man FS, Vermeijden JW, Steenvoorde P, et al. Diaphragm fiber strength is reduced in critically ill patients and restored by a troponin activator. Am J Respir Crit Care Med 2014;189:863–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Radell PJ, Remahl S, Nichols DG, Eriksson LI. Effects of prolonged mechanical ventilation and inactivity on piglet diaphragm function. Intensive Care Med 2002;28:358–364. [DOI] [PubMed] [Google Scholar]
- 26. Anzueto A, Peters JI, Tobin MJ, de los Santos R, Seidenfeld JJ, Moore G. Effects of prolonged controlled mechanical ventilation on diaphragmatic function in healthy adult baboons. Crit Care Med 1997;25:1187–1190. [DOI] [PubMed] [Google Scholar]
- 27. Sassoon CS, Caiozzo VJ, Manka A, Sieck GC. Altered diaphragm contractile properties with controlled mechanical ventilation. Journal of applied physiology (Bethesda, Md: 1985) 2002;92:2585–2595. [DOI] [PubMed] [Google Scholar]
- 28. Criswell DS, Shanely RA, Betters JJ, McKenzie MJ, Sellman JE, Van Gammeren DL. Cumulative effects of aging and mechanical ventilation on in vitro diaphragm function. Chest 2003;124:2302–2308. [DOI] [PubMed] [Google Scholar]
- 29. Sassoon C, Zhu E, Fang L, Sieck GC, Powers SK. Positive end‐expiratory airway pressure does not aggravate ventilator‐induced diaphragmatic dysfunction in rabbits. Critical care (London, England) 2014;18:494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Radell P, Edstrom L, Stibler H, Eriksson LI, Ansved T. Changes in diaphragm structure following prolonged mechanical ventilation in piglets. Acta Anaesthesiol Scand 2004;48:430–437. [DOI] [PubMed] [Google Scholar]
- 31. Yang L, Luo J, Bourdon J, Lin MC, Gottfried SB, Petrof BJ. Controlled mechanical ventilation leads to remodeling of the rat diaphragm. Am J Respir Crit Care Med 2002;166:1135–1140. [DOI] [PubMed] [Google Scholar]
- 32. Gayan‐Ramirez G, de Paepe K, Cadot P, Decramer M. Detrimental effects of short‐term mechanical ventilation on diaphragm function and IGF‐I mRNA in rats. Intensive Care Med 2003;29:825–833. [DOI] [PubMed] [Google Scholar]
- 33. Tang H, Lee M, Khuong A, Wright E, Shrager JB. Diaphragm muscle atrophy in the mouse after long‐term mechanical ventilation. Muscle Nerve 2013;48:272–278. [DOI] [PubMed] [Google Scholar]
- 34. Powers SK, Shanely RA, Coombes JS, Koesterer TJ, McKenzie M, Van Gammeren D. Mechanical ventilation results in progressive contractile dysfunction in the diaphragm. Journal of applied physiology (Bethesda, Md: 1985) 2002;92:1851–1858. [DOI] [PubMed] [Google Scholar]
- 35. Shanely RA, Van Gammeren D, Deruisseau KC, Zergeroglu AM, McKenzie MJ, Yarasheski KE. Mechanical ventilation depresses protein synthesis in the rat diaphragm. Am J Respir Crit Care Med 2004;170:994–999. [DOI] [PubMed] [Google Scholar]
- 36. Mrozek S, Jung B, Petrof BJ, Pauly M, Roberge S, Lacampagne A, et al. Rapid onset of specific diaphragm weakness in a healthy murine model of ventilator‐induced diaphragmatic dysfunction. Anesthesiology 2012;117:560–567. [DOI] [PubMed] [Google Scholar]
- 37. Racz GZ, Gayan‐Ramirez G, Testelmans D, Cadot P, De Paepe K, Zador E. Early changes in rat diaphragm biology with mechanical ventilation. Am J Respir Crit Care Med 2003;168:297–304. [DOI] [PubMed] [Google Scholar]
- 38. DeRuisseau KC, Kavazis AN, Deering MA, Falk DJ, Van Gammeren D, Yimlamai T. Mechanical ventilation induces alterations of the ubiquitin–proteasome pathway in the diaphragm. Journal of applied physiology (Bethesda, Md: 1985) 2005;98:1314–1321. [DOI] [PubMed] [Google Scholar]
- 39. Vassilakopoulos T. Ventilator‐induced diaphragmatic dysfunction In Tobin M, ed. Principles and Practice of Mechanical Ventilation, 3rd ed. New York, USA; McGraw Hill; 2013. [Google Scholar]
- 40. McClung JM, Van Gammeren D, Whidden MA, Falk DJ, Kavazis AN, Hudson MB. Apocynin attenuates diaphragm oxidative stress and protease activation during prolonged mechanical ventilation. Crit Care Med 2009;37:1373–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McClung JM, Kavazis AN, DeRuisseau KC, Falk DJ, Deering MA, Lee Y. Caspase‐3 regulation of diaphragm myonuclear domain during mechanical ventilation‐induced atrophy. Am J Respir Crit Care Med 2007;175:150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hussain SN, Mofarrahi M, Sigala I, Kim HC, Vassilakopoulos T, Maltais F, et al. Mechanical ventilation‐induced diaphragm disuse in humans triggers autophagy. Am J Respir Crit Care Med 2010;182:1377–1386. [DOI] [PubMed] [Google Scholar]
- 43. Jaber S, Sebbane M, Koechlin C, Hayot M, Capdevila X, Eledjam JJ. Effects of short vs. prolonged mechanical ventilation on antioxidant systems in piglet diaphragm. Intensive Care Med 2005;31:1427–1433. [DOI] [PubMed] [Google Scholar]
- 44. Falk DJ, Deruisseau KC, Van Gammeren DL, Deering MA, Kavazis AN, Powers SK. Mechanical ventilation promotes redox status alterations in the diaphragm. Journal of applied physiology (Bethesda, Md: 1985) 2006;101:1017–1024. [DOI] [PubMed] [Google Scholar]
- 45. Zergeroglu MA, McKenzie MJ, Shanely RA, Van Gammeren D, DeRuisseau KC, Powers SK. Mechanical ventilation‐induced oxidative stress in the diaphragm. Journal of applied physiology (Bethesda, Md: 1985) 2003;95:1116–1124. [DOI] [PubMed] [Google Scholar]
- 46. Smuder AJ, Hudson MB, Nelson WB, Kavazis AN, Powers SK. Nuclear factor‐kappaB signaling contributes to mechanical ventilation‐induced diaphragm weakness*. Crit Care Med 2012;40:927–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bruells CS, Maes K, Rossaint R, Thomas D, Cielen N, Bleilevens C, et al. Prolonged mechanical ventilation alters the expression pattern of angio‐neogenetic factors in a pre‐clinical rat model. PLoS One 2013;8:e70524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tang H, Smith IJ, Hussain SN, Goldberg P, Lee M, Sugiarto S, et al. The JAK–STAT pathway is critical in ventilator‐induced diaphragm dysfunction. Mol Med 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dodd SL, Gagnon BJ, Senf SM, Hain BA, Judge AR. Ros‐mediated activation of NF‐kappaB and Foxo during muscle disuse. Muscle Nerve 2010;41:110–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hotchkiss RS, Monneret G, Payen D. Sepsis‐induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol 2013;13:862–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schefold JC, Hasper D, Reinke P, Monneret G, Volk HD. Consider delayed immunosuppression into the concept of sepsis. Crit Care Med 2008;36:3118. [DOI] [PubMed] [Google Scholar]
- 52. Schefold JC. Measurement of monocytic HLA‐DR (mHLA‐DR) expression in patients with severe sepsis and septic shock: assessment of immune organ failure. Intensive Care Med 2010;36:1810–1812. [DOI] [PubMed] [Google Scholar]
- 53. Boczkowski J, Lanone S, Ungureanu‐Longrois D, Danialou G, Fournier T, Aubier M. Induction of diaphragmatic nitric oxide synthase after endotoxin administration in rats: role on diaphragmatic contractile dysfunction. J Clin Invest 1996;98:1550–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Callahan LA, Supinski GS. Sepsis induces diaphragm electron transport chain dysfunction and protein depletion. Am J Respir Crit Care Med 2005;172:861–868. [DOI] [PubMed] [Google Scholar]
- 55. Supinski G, Vanags J, Callahan L. Eicosapentaenoic acid preserves diaphragm force generation following endotoxin administration. Critical care (London, England) 2010;14:R35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Supinski GS, Callahan LA. Calpain activation contributes to endotoxin‐induced diaphragmatic dysfunction. Am J Respir Cell Mol Biol 2010;42:80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Supinski GS, Callahan LA. Caspase activation contributes to endotoxin‐induced diaphragm weakness. Journal of applied physiology (Bethesda, Md: 1985) 2006;100:1770–1777. [DOI] [PubMed] [Google Scholar]
- 58. Demoule A, Jung B, Prodanovic H, Molinari N, Chanques G, Coirault C. Diaphragm dysfunction on admission to the intensive care unit. Prevalence, risk factors, and prognostic impact—a prospective study. Am J Respir Crit Care Med 2013;188:213–219. [DOI] [PubMed] [Google Scholar]
- 59. Supinski GS, Callahan LA. Diaphragm weakness in mechanically ventilated critically ill patients. Critical care (London, England) 2013;17:R120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Maes K, Stamiris A, Thomas D, Cielen N, Smuder A, Powers SK, et al. Effects of controlled mechanical ventilation on sepsis‐induced diaphragm dysfunction in rats. Crit Care Med 2014;42:e772–782. [DOI] [PubMed] [Google Scholar]
- 61. Ebihara S, Hussain SN, Danialou G, Cho WK, Gottfried SB, Petrof BJ. Mechanical ventilation protects against diaphragm injury in sepsis: interaction of oxidative and mechanical stresses. Am J Respir Crit Care Med 2002;165:221–228. [DOI] [PubMed] [Google Scholar]
- 62. Schefold JC, von Haehling S, Corsepius M, Pohle C, Kruschke P, Zuckermann H. A novel selective extracorporeal intervention in sepsis: immunoadsorption of endotoxin, interleukin 6, and complement‐activating product 5a. Shock 2007;28:418–425. [DOI] [PubMed] [Google Scholar]
- 63. Cruz DN, de Cal M, Piccinni P, Ronco C. Polymyxin‐B hemoperfusion and endotoxin removal: lessons from a review of the literature. Contrib Nephrol 2010;167:77–82. [DOI] [PubMed] [Google Scholar]
- 64. Meisel C, Schefold JC, Pschowski R, Baumann T, Hetzger K, Gregor J, et al Am J Respir Crit Care Med. 2009. Oct 1;180(7):640-8. doi: 10.1164/rccm.200903-0363OC. Epub 2009 Jul 9. [DOI] [PubMed] [Google Scholar]
- 65. Schefold JC. Immunostimulation using granulocyte‐ and granulocyte‐macrophage colony stimulating factor in patients with severe sepsis and septic shock. Critical care (London, England) 2011;15:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Davis RT 3rd, Bruells CS, Stabley JN, McCullough DJ, Powers SK, Behnke BJ. Mechanical ventilation reduces rat diaphragm blood flow and impairs oxygen delivery and uptake. Crit Care Med 2012;40:2858–2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhu E, Sassoon CS. Ventilator‐induced diaphragmatic vascular dysfunction. Crit Care Med 2012;40:2914–2915. [DOI] [PubMed] [Google Scholar]
- 68. DeRuisseau KC, Shanely RA, Akunuri N, Hamilton MT, Van Gammeren D, Zergeroglu AM. Diaphragm unloading via controlled mechanical ventilation alters the gene expression profile. Am J Respir Crit Care Med 2005;172:1267–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Smuder AJ, Min K, Hudson MB, Kavazis AN, Kwon OS, Nelson WB. Endurance exercise attenuates ventilator‐induced diaphragm dysfunction. Journal of applied physiology (Bethesda, Md: 1985) 2012;112:501–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sollanek KJ, Smuder AJ, Wiggs MP, Morton AB, Koch LG, Britton SL, Powers SK. Role of Intrinsic Aerobic Capacity and Ventilator‐Induced Diaphragm Dysfunction; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Agten A, Maes K, Smuder A, Powers SK, Decramer M, Gayan‐Ramirez G. N‐Acetylcysteine protects the rat diaphragm from the decreased contractility associated with controlled mechanical ventilation. Crit Care Med 2011;39:777–782. [DOI] [PubMed] [Google Scholar]
- 72. Azuelos I, Jung B, Picard M, Liang F, Li T, Lemaire C. Relationship between autophagy and ventilator‐induced diaphragmatic dysfunction. Anesthesiology 2015;122:1349–1361. [DOI] [PubMed] [Google Scholar]
- 73. Thomas D, Maes K, Agten A, Heunks L, Dekhuijzen R, Decramer M. Time course of diaphragm function recovery after controlled mechanical ventilation in rats. Journal of applied physiology (Bethesda, Md: 1985) 2013;115:775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bruells CS, Bergs I, Rossaint R, Du J, Bleilevens C, Goetzenich A. Recovery of diaphragm function following mechanical ventilation in a rodent model. PLoS One 2014;9:e87460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Callahan LA, Supinski GS. Rapid and complete recovery in ventilator‐induced diaphragm weakness—problem solved?, vol. 115; 2013. [DOI] [PMC free article] [PubMed]
- 76. Hussain S, Mofarrahi M, Sigala I, Kim H, Vassilakopoulos T, Maltais F, et al. Mechanical ventilation‐induced diaphragm disuse in humans triggers autophagy. Am J Respir Crit Care Med 2010;182:1377–1386. [DOI] [PubMed] [Google Scholar]
- 77. Levine S, Biswas C, Dierov J, Barsotti R, Shrager J, Nguyen T, et al. Increased proteolysis, myosin depletion and atrophic AKT‐FOXO signaling in human diaphragm disuse. Am J Respir Crit Care Med 2011;183:483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Elkina Y, von Haehling S, Anker SD, Springer J. The role of myostatin in muscle wasting: an overview. J Cachexia Sarcopenia Muscle 2011;2:143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Fogelman DR, Holmes H, Mohammed K, Katz MH, Prado CM, Lieffers J, et al. Does IGFR1 inhibition result in increased muscle mass loss in patients undergoing treatment for pancreatic cancer? J Cachexia Sarcopenia Muscle 2014;5:307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Heymsfield SB, Adamek M, Gonzalez MC, Jia G, Thomas DM. Assessing skeletal muscle mass: historical overview and state of the art. J Cachexia Sarcopenia Muscle 2014;5:9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Jaber S, Petrof BJ, Jung B, Chanques G, Berthet JP, Rabuel C, et al Rapidly progressive diaphragmatic weakness and injury during mechanical ventilation in humans. Am J Respir Crit Care Med 2011;183:364–371. [DOI] [PubMed] [Google Scholar]
- 82. Hermans G, Agten A, Testelmans D, Decramer M, Gayan‐Ramirez G. Increased duration of mechanical ventilation is associated with decreased diaphragmatic force: a prospective observational study. Critical care (London, England) 2010;14:R127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Picard M, Jung B, Liang F, Azuelos I, Hussain S, Goldberg P, et al. Mitochondrial dysfunction and lipid accumulation in the human diaphragm during mechanical ventilation. Am J Respir Crit Care Med 2012;186:1140–1149. [DOI] [PubMed] [Google Scholar]
- 84. Lecuona E, Sassoon CS, Barreiro E. Lipid overload: trigger or consequence of mitochondrial oxidative stress in ventilator‐induced diaphragmatic dysfunction? Am J Respir Crit Care Med 2012;186:1074–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Tang H, Lee M, Budak MT, Pietras N, Hittinger S, Vu M, et al Intrinsic apoptosis in mechanically ventilated human diaphragm: linkage to a novel Fos/FoxO1/Stat3‐Bim axis. FASEB J 2011;25:2921–2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Dermitzaki D, Tzortzaki E, Soulitzis N, Neofytou E, Prinianakis G, Matalliotakis I. Molecular response of the human diaphragm on different modes of mechanical ventilation. Respiration; international review of thoracic diseases 2013;85:228–235. [DOI] [PubMed] [Google Scholar]
- 87. ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med 2002;166:518–624. [DOI] [PubMed] [Google Scholar]
- 88. Yang KL, Tobin MJ. A prospective study of indexes predicting the outcome of trials of weaning from mechanical ventilation. N Engl J Med 1991;324:1445–1450. [DOI] [PubMed] [Google Scholar]
- 89. Similowski T, Fleury B, Launois S, Cathala HP, Bouche P, Derenne JP. Cervical magnetic stimulation. A new method of bilateral phrenic nerve stimulation for use in clinical practice. Rev Mal Respir 1988;5:609–614. [PubMed] [Google Scholar]
- 90. Laghi F, Cattapan SE, Jubran A, Parthasarathy S, Warshawsky P, Choi YS. Is weaning failure caused by low‐frequency fatigue of the diaphragm? Am J Respir Crit Care Med 2003;167:120–127. [DOI] [PubMed] [Google Scholar]
- 91. Cattapan SE, Laghi F, Tobin MJ. Can diaphragmatic contractility be assessed by airway twitch pressure in mechanically ventilated patients? Thorax 2003;58:58–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Watson AC, Hughes PD, Louise Harris M, Hart N, Ware RJ, Wendon J. Measurement of twitch transdiaphragmatic, esophageal, and endotracheal tube pressure with bilateral anterolateral magnetic phrenic nerve stimulation in patients in the intensive care unit. Crit Care Med 2001;29:1325–1331. [DOI] [PubMed] [Google Scholar]
- 93. Sinderby C, Navalesi P, Beck J, Skrobik Y, Comtois N, Friberg S. Neural control of mechanical ventilation in respiratory failure. Nat Med 1999;5:1433–1436. [DOI] [PubMed] [Google Scholar]
- 94. Doorduin J, van Hees HW, van der Hoeven JG, Heunks LM. Monitoring of the respiratory muscles in the critically ill. Am J Respir Crit Care Med 2013;187:20–27. [DOI] [PubMed] [Google Scholar]
- 95. Liu L, Liu H, Yang Y, Huang Y, Liu S, Beck J. Neuroventilatory efficiency and extubation readiness in critically ill patients. Critical care (London, England) 2012;16:R143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Roze H, Repusseau B, Perrier V, Germain A, Seramondi R, Dewitte A. Neuro‐ventilatory efficiency during weaning from mechanical ventilation using neurally adjusted ventilatory assist. Br J Anaesth 2013;111:955–960. [DOI] [PubMed] [Google Scholar]
- 97. Tuchscherer D, Z'Graggen WJ, Passath C, Takala J, Sinderby C, Brander L. Neurally adjusted ventilatory assist in patients with critical illness‐associated polyneuromyopathy. Intensive Care Med 2011;37:1951–1961. [DOI] [PubMed] [Google Scholar]
- 98. Goligher EC, Laghi F, Detsky ME, Farias P, Murray A, Brace D, et al. Measuring diaphragm thickness with ultrasound in mechanically ventilated patients: feasibility, reproducibility and validity. Intensive Care Med 2015;41:642–649. [DOI] [PubMed] [Google Scholar]
- 99. Kabitz HJ, Walterspacher S, Mellies U, Criee CP, Windisch W. Recommendations for respiratory muscle testing. Pneumologie 2014;68:307–314. [DOI] [PubMed] [Google Scholar]
- 100. Kabitz HJ, Windisch W, Schonhofer B. Understanding ventilator‐induced diaphragmatic dysfunction (VIDD): progress and advances. Pneumologie 2013;67:435–441. [DOI] [PubMed] [Google Scholar]
- 101. Boussuges A, Gole Y, Blanc P. Diaphragmatic motion studied by m‐mode ultrasonography: methods, reproducibility, and normal values. Chest 2009;135:391–400. [DOI] [PubMed] [Google Scholar]
- 102. Ueki J, De Bruin PF, Pride NB. In vivo assessment of diaphragm contraction by ultrasound in normal subjects. Thorax 1995;50:1157–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kim WY, Suh HJ, Hong SB, Koh Y, Lim CM. Diaphragm dysfunction assessed by ultrasonography: influence on weaning from mechanical ventilation. Crit Care Med 2011;39:2627–2630. [DOI] [PubMed] [Google Scholar]
- 104. DiNino E, Gartman EJ, Sethi JM, McCool FD. Diaphragm ultrasound as a predictor of successful extubation from mechanical ventilation. Thorax 2014;69:423–427. [DOI] [PubMed] [Google Scholar]
- 105. Schepens T, Verbrugghe W, Dams K, Corthouts B, Parizel P, Jorens P. The course of diaphragm atrophy in ventilated patients assessed with ultrasound: a longitudinal cohort study. Crit Care 2015;19:422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Goligher EC, Fan E, Herridge MS, Murray A, Vorona S, Brace D, et al. Evolution of diaphragm thickness during mechanical ventilation. Impact of inspiratory effort. Am J Respir Crit Care Med 2015;192:1080–1088. [DOI] [PubMed] [Google Scholar]
- 107. Zambon M, Cabrini L, Beccaria P, Zangrillo A, Colombo S. Ultrasound in critically ill patients: focus on diaphragm. Intensive Care Med 2013;39:986. [DOI] [PubMed] [Google Scholar]
- 108. Matamis D, Soilemezi E, Tsagourias M, Akoumianaki E, Dimassi S, Boroli F. Sonographic evaluation of the diaphragm in critically ill patients. Technique and clinical applications. Intensive Care Med 2013;39:801–810. [DOI] [PubMed] [Google Scholar]
- 109. Futier E, Constantin JM, Combaret L, Mosoni L, Roszyk L, Sapin V. Pressure support ventilation attenuates ventilator‐induced protein modifications in the diaphragm. Critical care (London, England) 2008;12:R116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Sassoon CS, Zhu E, Caiozzo VJ. Assist‐control mechanical ventilation attenuates ventilator‐induced diaphragmatic dysfunction. Am J Respir Crit Care Med 2004;170:626–632. [DOI] [PubMed] [Google Scholar]
- 111. Ayas NT, McCool FD, Gore R, Lieberman SL, Brown R. Prevention of human diaphragm atrophy with short periods of electrical stimulation. Am J Respir Crit Care Med 1999;159:2018–2020. [DOI] [PubMed] [Google Scholar]
- 112. Nochomovitz ML, Hopkins M, Brodkey J, Montenegro H, Mortimer JT, Cherniack NS. Conditioning of the diaphragm with phrenic nerve stimulation after prolonged disuse. Am Rev Respir Dis 1984;130:685–688. [DOI] [PubMed] [Google Scholar]
- 113. Ahn B, Beaver T, Martin T, Hess P, Brumback BA, Ahmed S. Phrenic nerve stimulation increases human diaphragm fiber force after cardiothoracic surgery. Am J Respir Crit Care Med 2014;190:837–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Martin AD, Joseph AM, Beaver TM, Smith BK, Martin TD, Berg K. Effect of intermittent phrenic nerve stimulation during cardiothoracic surgery on mitochondrial respiration in the human diaphragm. Crit Care Med 2014;42:e152–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Aldrich TK, Uhrlass RM. Weaning from mechanical ventilation: successful use of modified inspiratory resistive training in muscular dystrophy. Crit Care Med 1987;15:247–249. [PubMed] [Google Scholar]
- 116. Aldrich TK, Karpel JP. Inspiratory muscle resistive training in respiratory failure. Am Rev Respir Dis 1985;131:461–462. [DOI] [PubMed] [Google Scholar]
- 117. Caruso P, Denari SD, Ruiz SA, Bernal KG, Manfrin GM, Friedrich C. Inspiratory muscle training is ineffective in mechanically ventilated critically ill patients. Clinics (Sao Paulo) 2005;60:479–484. [DOI] [PubMed] [Google Scholar]
- 118. Martin AD, Smith BK, Davenport PD, Harman E, Gonzalez‐Rothi RJ, Baz M, et al. Inspiratory muscle strength training improves weaning outcome in failure to wean patients: a randomized trial. Critical care (London, England) 2011;15:R84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Jaber S, Petrof B, Jung B, Chanques G, Berthet J‐P, Rabuel C. Rapidly progressive diaphragmatic weakness and injury during mechanical ventilation in humans. Am J Respir Crit Care Med 2011;183:364–371. [DOI] [PubMed] [Google Scholar]
- 120. Papazian L, Forel JM, Gacouin A, Penot‐Ragon C, Perrin G, Loundou A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 2010;363:1107–1116. [DOI] [PubMed] [Google Scholar]
- 121. Moerer O. Effort‐adapted modes of assisted breathing. Curr Opin Crit Care 2012;18:61–69. [DOI] [PubMed] [Google Scholar]
- 122. Brander L, Leong‐Poi H, Beck J, Brunet F, Hutchison SJ, Slutsky AS. Titration and implementation of neurally adjusted ventilatory assist in critically ill patients. Chest 2009;135:695–703. [DOI] [PubMed] [Google Scholar]
- 123. Terzi N, Pelieu I, Guittet L, Ramakers M, Seguin A, Daubin C. Neurally adjusted ventilatory assist in patients recovering spontaneous breathing after acute respiratory distress syndrome: physiological evaluation. Crit Care Med 2010;38:1830–1837. [DOI] [PubMed] [Google Scholar]
- 124. Berger D, Bloechlinger S, Takala J, Sinderby C, Brander L. Heart–lung interactions during neurally adjusted ventilatory assist. Critical care (London, England) 2014;18:499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Passath C, Takala J, Tuchscherer D, Jakob SM, Sinderby C, Brander L. Physiologic response to changing positive end‐expiratory pressure during neurally adjusted ventilatory assist in sedated, critically ill adults. Chest 2010;138:578–587. [DOI] [PubMed] [Google Scholar]
- 126. Morley JE, von Haehling S, Anker SD. Are we closer to having drugs to treat muscle wasting disease? J Cachexia Sarcopenia Muscle 2014;5:83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. McClung J, Kavazis A, Whidden M, DeRuisseau K, Falk D, Criswell D. Antioxidant administration attenuates mechanical ventilation‐induced rat diaphragm muscle atrophy independent of protein kinase B (PKB Akt) signalling. J Physiol 2007;585:203–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Nathens AB, Neff MJ, Jurkovich GJ, Klotz P, Farver K, Ruzinski JT. Randomized, prospective trial of antioxidant supplementation in critically ill surgical patients. Ann Surg 2002;236:814–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Maes K, Testelmans D, Powers S, Decramer M, Gayan‐Ramirez G. Leupeptin inhibits ventilator‐induced diaphragm dysfunction in rats. Am J Respir Crit Care Med 2007;175:1134–1138. [DOI] [PubMed] [Google Scholar]
- 130. Agten A, Maes K, Thomas D, Cielen N, Van Hees HW, Dekhuijzen RP. Bortezomib partially protects the rat diaphragm from ventilator‐induced diaphragm dysfunction. Crit Care Med 2012;40:2449–2455. [DOI] [PubMed] [Google Scholar]
- 131. Smuder AJ, Nelson WB, Hudson MB, Kavazis AN, Powers SK. Inhibition of the ubiquitin–proteasome pathway does not protect against ventilator‐induced accelerated proteolysis or atrophy in the diaphragm. Anesthesiology 2014;121:115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Laghi F. Proteasome inhibition and ventilator‐induced diaphragmatic dysfunction: is the glass half full or half empty? Crit Care Med 2012;40:2525–2526. [DOI] [PubMed] [Google Scholar]
- 133. Smith IJ, Godinez GL, Singh BK, McCaughey KM, Alcantara RR, Gururaja T, et al. Inhibition of Janus kinase signaling during controlled mechanical ventilation prevents ventilation‐induced diaphragm dysfunction. FASEB J 2014;28:2790–2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Smuder AJ, Sollanek KJ, Min K, Nelson WB, Powers SK. Inhibition of forkhead boxO‐specific transcription prevents mechanical ventilation‐induced diaphragm dysfunction. Crit Care Med 2015;43:e133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Maes K, Testelmans D, Cadot P, Deruisseau K, Powers S, Decramer M. Effects of acute administration of corticosteroids during mechanical ventilation on rat diaphragm. Am J Respir Crit Care Med 2008;178:1219–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Ochala J, Renaud G, Llano Diez M, Banduseela VC, Aare S, Ahlbeck K. Diaphragm muscle weakness in an experimental porcine intensive care unit model. PLoS One 2011;6:e20558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Bruells CS, Maes K, Rossaint R, Thomas D, Cielen N, Bergs I. Sedation using propofol induces similar diaphragm dysfunction and atrophy during spontaneous breathing and mechanical ventilation in rats. Anesthesiology 2014;120:665–672. [DOI] [PubMed] [Google Scholar]
- 138. Jung B, Sebbane M, Goff C, Rossel N, Chanques G, Futier E. Moderate and prolonged hypercapnic acidosis may protect against ventilator‐induced diaphragmatic dysfunction in healthy piglet: an in vivo study. Crit Care 2013;17:R15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Schellekens WJ, van Hees HW, Kox M, Linkels M, Acuna GL, Dekhuijzen PN. Hypercapnia attenuates ventilator‐induced diaphragm atrophy and modulates dysfunction. Critical care (London, England) 2014;18:R28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 2000;342:1301–1308. [DOI] [PubMed] [Google Scholar]
- 141. Doorduin J, Sinderby CA, Beck J, Stegeman DF, van Hees HW, van der Hoeven JG. The calcium sensitizer levosimendan improves human diaphragm function. Am J Respir Crit Care Med 2012;185:90–95. [DOI] [PubMed] [Google Scholar]
- 142. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2015. J Cachexia Sarcopenia Muscle 2015;6:315–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Vivier E, Mekontso Dessap A, Dimassi S, Vargas F, Lyazidi A, Thille AW. Diaphragm ultrasonography to estimate the work of breathing during non‐invasive ventilation. Intensive Care Med 2012;38:796–803. [DOI] [PubMed] [Google Scholar]


