Abstract
Metformin, which is a drug commonly prescribed to treat type 2 diabetes, has anti-proliferative effects in cancer cells; however, the molecular mechanisms underlying this effect remain largely unknown. The aim is to investigate the role of tristetraprolin (TTP), an AU-rich element-binding protein, in anti-proliferative effects of metformin in cancer cells. p53 wild-type and p53 mutant breast cancer cells were treated with metformin, and expression of TTP and c-Myc was analyzed by semi-quantitative RT-PCR, Western blots, and promoter activity assay. Breast cancer cells were transfected with siRNA against TTP to inhibit TTP expression or c-Myc and, after metformin treatment, analyzed for cell proliferation by MTS assay. Metformin induces the expression of tristetraprolin (TTP) in breast cancer cells in a p53-independent manner. Importantly, inhibition of TTP abrogated the anti-proliferation effect of metformin. We observed that metformin decreased c-Myc levels, and ectopic expression of c-Myc blocked the effect of metformin on TTP expression and cell proliferation. Our data indicate that metformin induces TTP expression by reducing the expression of c-Myc, suggesting a new model whereby TTP acts as a mediator of metformin’s anti-proliferative activity in cancer cells.
Keywords: Metformin, Tristetraprolin, p53, c-Myc, Anti-proliferation
Introduction
Metformin is a first-line hypoglycemizing agent used for the treatment of type 2 diabetes (T2D) [1]. Recently, metformin has received increased attention because of its potential anti-tumorigenic effects [2–4]. Metformin exerts its effects by disrupting mitochondrial respiratory chain 1, which leads to decreased ATP synthesis and increased AMP associated with AMPK, ultimately leading to AMPK activation [5]. This regulation of AMPK by metformin leads to its anti-proliferative effects due to subsequent modification of the activity of mammalian target of rapamycin (mTOR) [6, 7] and p53 [8]. On the other hand, synthetic AMPK agonists provide a proliferative advantage to the cells [9], and there is emerging evidence to suggest that metformin can arrest cell proliferation in an AMPK-independent manner [9–15]. Specifically, metformin has been reported to affect several other intracellular pathways in tumor cells including HER1/HER2, Src, S6K1, c-Myc, and STAT3 [8, 16–19] and is also able to overcome dietary restriction resistance in cancer cells [20]. Overall, however, the mechanistic aspects of metformin action with respect to its anti-proliferative functions remain ill-defined.
AU-rich elements (AREs) post-transcriptionally regulate the expression of a variety of short-lived mRNAs such as cytokines and proto-oncogenes [21]. The stability of ARE-containing mRNAs is regulated by ARE-binding proteins [22]. One of the best-characterized ARE-binding proteins is tristetraprolin (TTP, ZFP36), which promotes the degradation of ARE-containing transcripts [23, 24]. TTP expression is significantly decreased in various cancers [25], which correlates with increased expression of proto-oncogenes and may contribute to cancer processes. Likewise, re-expression of TTP has a growth inhibitory effect [26–28]. The expression of TTP in cancer cells is induced by p53 [29] but inhibited by Myc [30]. However, nearly all types of cancers have abnormalities in the p53 pathway [31]. Furthermore, c-Myc is often activated in human cancers [32]. Together, these features may lead to a widespread decrease in the expression of TTP in human cancers.
We show here for the first time that metformin induces the expression of TTP in a p53-independent manner, and also that TTP mediates the anti-proliferative effect of metformin in both p53 wild-type and p53 mutant cancer cells. Specifically, metformin decreased the expression of c-Myc and increased the expression of TTP in both p53 wild-type and p53 mutant cells. Ectopic expression of c-Myc abrogated the effects of metformin with respect to TTP induction, while siRNA-mediated inhibition of TTP attenuated the anti-proliferative effects of metformin. Together, these studies identify a novel signaling pathway by which metformin induces TTP expression in a p53-independent manner, representing a possible novel pharmacological approach to treat p53 mutant cancer cells.
Methods and materials
Cell culture
The human MCF7 and MDA-MB-231 breast cancer cell lines were purchased from the Korean Cell Line Bank (Seoul, Korea). Cells were cultured in RPMI 1640 media supplemented with 10 % heat-inactivated fetal bovine serum (FBS) (Welgene, Korea) and were maintained at 37 °C in a humidified 5 % CO2 atmosphere. To investigate the induction of TTP, cells were treated with metformin (D150959 Sigma) in the presence or absence of 20 μM compound C (P5499 Sigma). Cells were harvested at the indicated length of time and analyzed for mRNA by RT-PCR, protein by Western blotting, and cell viability by MTS assay.
Cell viability/proliferation
For the MTS cell proliferation assay, cells were plated in triplicate at 1.0 × 104 cells/well in 96-well culture plates in culture media. At 24 h after plating, CellTiter 96® AQueous One Solution reagent (Promega) was added to each well according to the manufacturer’s instructions, and absorbance at 490 nm was determined for each well using a Victor 1420 Multilabel Counter (EG&G Wallac, Turku, Finland).
Plasmids, small interfering RNAs, transfections, and dual-luciferase assay
The pcDNA6/V5-TTP containing full-length ORF of human TTP [33] and the pGL3/TTPp-1343 containing human TTP promoter [29] were described previously. The pcDNA3-cMyc vector was purchased from Addgene.
For luciferase assays, cells were co-transfected with a pGL3/TTPp-1343-luciferase reporter construct and pRL-SV40 Renilla luciferase construct using TurboFectTM in vitro transfection reagent (Fermentas). Transfected cells were lysed with lysis buffer and mixed with luciferase assay reagent (Promega). The chemiluminescent signal was measured using a SpectraMax L Microplate (Molecular Devices, Sunnyvale, CA, USA). Firefly luciferase was normalized to Renilla luciferase in each sample. All luciferase assays reported in this study represent at least three independent experiments, each consisting of three wells per transfection.
Small interfering RNAs (siRNAs) against human TTP (TTP-siRNA, sc-36761), human c-Myc (c-Myc-siRNA, sc-29226), and control siRNA [scrambled siRNA (scRNA), sc-37007] were purchased from Santa Cruz Biotechnology (Santa Cruz). Cells were transfected 24 h after plating using LipofectamineTM RNAiMAX (Invitrogen) and were harvested at 48 h after transfection. The expression levels of TTP or c-Myc mRNA and protein were analyzed by RT-PCR and Western blotting, respectively.
SDS–PAGE analysis and immunoblotting
Proteins were resolved by SDS–PAGE, transferred onto Hybond-P membranes (Amersham Biosciences Inc.), and probed with appropriate dilutions of the following antibodies: rabbit anti-human TTP (T5327, Sigma), anti-human c-Myc (sc-40, Santa Cruz), anti-p53 (1026-1, Epitomics), anti-phospho-p53 (#9284, Cell Signaling), anti-AMPK (#2603, Cell Signaling), anti-phospho-AMPK (#2535, Cell Signaling), anti-STAT3 (#12640, Cell Signaling), anti-phospho-STAT3 (#9134, Cell Signaling), and anti-β-actin (A2228, Sigma). Immunoreactivity was detected using an ECL detection system (Amersham Biosciences Inc.). Films were exposed at multiple time points to ensure that the images were not saturated.
Quantitative real-time PCR and semi-qRT-PCR
DNase I-treated total RNA (3 mg) was reverse transcribed using oligo-dT and Superscript II reverse transcriptase (Invitrogen) according to the manufacturer’s instructions. qRT-PCR was performed by real-time monitoring of the increase in fluorescence of SYBR Green dye (QIAGEN, Hilden, Germany) using a StepOnePlusTM real-time PCR system (Applied Biosystems). Semi-qRT-PCR was performed using Taq polymerase (Solgent, Daejeon, Korea). The PCR primer pairs were as follows: TTP: 5′-CGCTACAAGACTGAGCTAT-3′, 5′-GAGGTAGAACTTGTGACAGA-3′; c-Myc: 5′- ACAGCATACATCCTGTCCGTCCAA-3′, 5′-TGTTCTCGTCGTTTCCGCAACAAG-3′; GAPDH: 5′-ACATCAAGAAGGTGGTGAAG-3′, 5′-CTGTTGCTGTAGCCAAATTC-3′.
Statistical analysis
For statistical comparisons, p values were determined using Student’s t test.
Results
Metformin induces tristetraprolin expression in both p53 wild-type and p53 mutant breast cancer cells
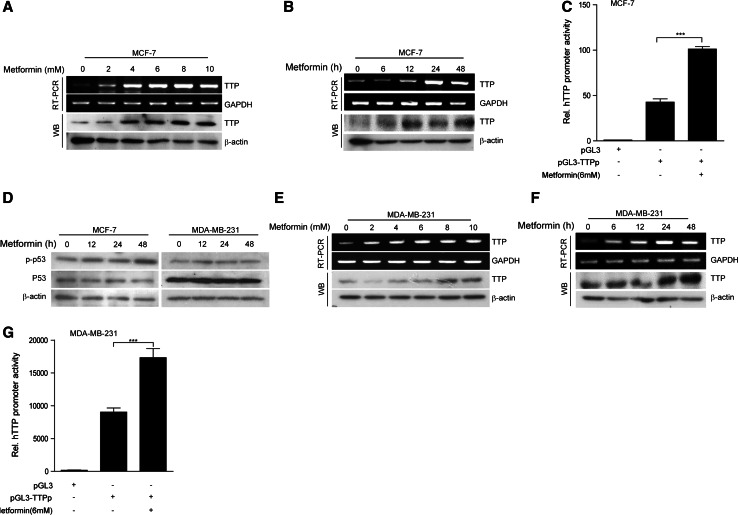
We previously reported that p53 is required for TTP induction in cancer cells [29]. In addition, metformin can enhance p53 activity [8], suggesting the possibility that metformin can induce TTP expression in p53 wild-type cancer cells. To evaluate this possibility, we treated p53 wild-type MCF7 breast cancer cells with metformin. As expected, treatment with metformin increased the mRNA and protein levels of TTP in MCF7 cells in a dose- (Fig. 1a) and time-dependent (Fig. 1b) manner. Metformin treatment enhanced TTP promoter activity (Fig. 1c), indicating that metformin enhances TTP gene expression in MCF7 cells. To test whether p53 activity is required for TTP induction by metformin, we treated MDA-MB-231 breast cancer cells, which have a mutant p53 status, with metformin and analyzed the induction of TTP. We confirmed that, while metformin increased phosphorylation of p53 in MCF7 cells, it did not do so in MDA-MB-231 cells (Fig. 1d). Unexpectedly, we found that metformin treatment also increased the mRNA and protein levels of TTP in MDA-MB-231 cells in a dose-(Fig. 1e) and time-(Fig. 1f) dependent manner., as well as promoter activity (Fig. 1g). Together, these data suggest that metformin can induce TTP expression in breast cancer cells in a p53-independent manner.
Fig. 1.
Metformin induces expression of TTP in both p53 wild-type and p53 mutant breast cancer cells. a, b Metformin increases TTP levels in p53 wild-type human breast cancer MCF7 cells. MCF7 cells were treated a with the indicated concentrations of metformin for 24 h or b with 6 mM metformin for the indicated length of time. The levels of TTP were measured by semi-qRT-PCR (a, b, top) and Western blotting (a, b, bottom). c Metformin induces TTP promoter activity in p53 wild-type MCF7 cells. MCF7 cells were transfected with pGL3/TTPp-1343 containing the TTP promoter. After treatment with 6 mM metformin for 24 h, luciferase activity was measured. The expression levels obtained from pGL3-transfected cells without metformin treatment were set to 1. Data are presented as the mean ± SD (n = 3). ***p < 0.001. d Metformin induces p53 phosphorylation in p53 wild-type MCF7 cells but not p53 mutant MDA-MB-231 cells. MCF7 and MDA-MB-231 cells were treated with 6 mM metformin for the indicated length of time. The levels of p53 and phospho-p53 (p-p53) were measured by Western blotting. e, f Metformin increases TTP levels in p53 mutant human breast cancer MDA-MB-231 cells. MDA-MB-231 cells were treated e with the indicated concentrations of metformin for 24 h or f with 6 mM metformin for the indicated length of time. The levels of TTP were measured by semi-qRT-PCR (e, f; top) and Western blotting (e, f; bottom). g Metformin induces TTP promoter activity in MDA-MB-231 cells. MDA-MB-231 cells were transfected with pGL3/TTPp-1343 containing the TTP promoter. After treatment with 6 mM metformin for 24 h, luciferase activity was measured. The expression levels obtained from pGL3-transfected cells without metformin treatment were set to 1. Data are presented as the mean ± SD (n = 3). ***p < 0.001
TTP mediates metformin’s anti-proliferative function
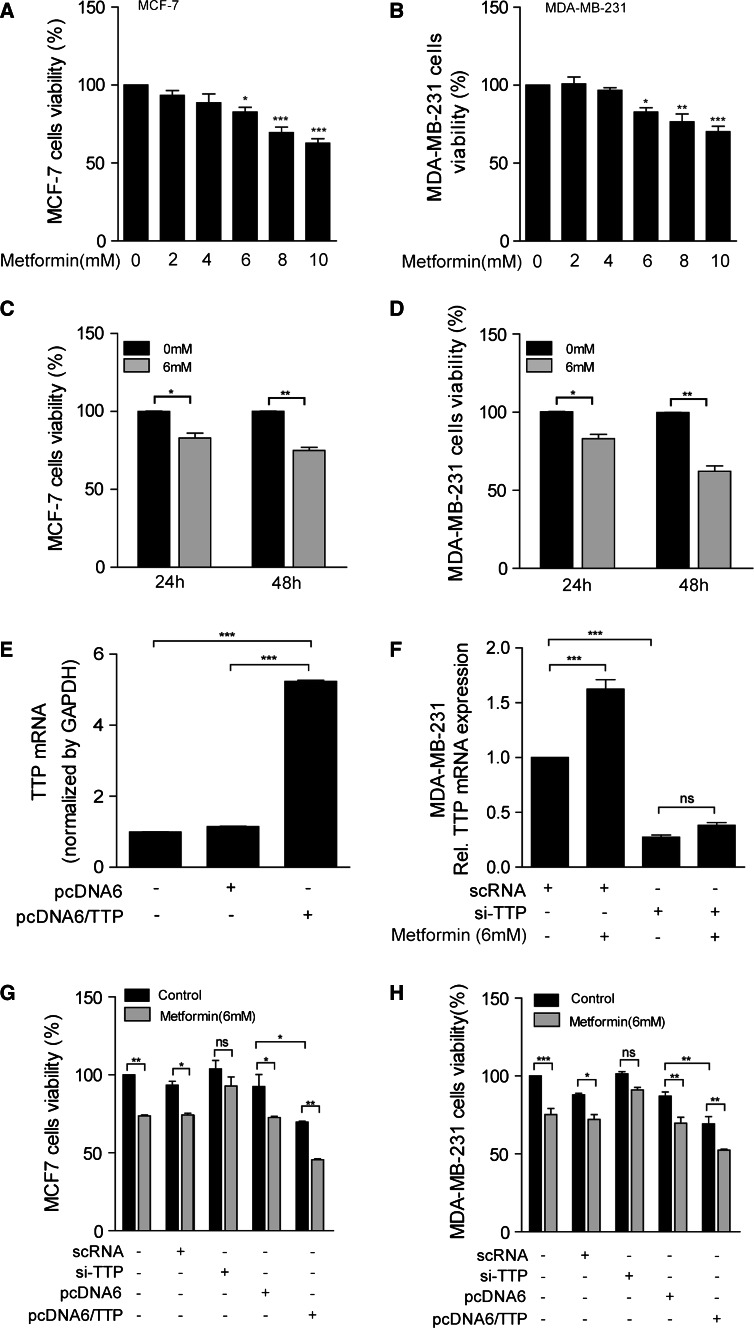
Previously metformin has been reported to exert anti-tumorigenic effect [2–4]. To confirm whether metformin shows anti-proliferative effect on MCF7 and MDA-MB-231 cells, we incubated these cells in the presence of different concentration of metformin for 24 and 48 h and analyzed the cell proliferation using MTS assay. Consistent with previous reports [2–4], metformin treatment significantly inhibited the growth of both MCF7 and MDA-MB-231 cells in a dose-dependent (Fig. 2a, b) and time-dependent manner (Fig. 2c, d). Since incubation of both MCF7 and MDA-MB-231 cells with 6 mM metformin for 24 h induced TTP expression (Fig. 1a, e, f) and showed anti-proliferative effect (Fig. 2a–d), we conducted further experiment under conditions of incubating cells with 6 mM metformin for 24 h. Ectopic expression of TTP has been reported to inhibit cancer cell growth [26–28], and we confirmed that overexpression of TTP (Fig. 2e) inhibited cell proliferation in both MCF7 (Fig. 2g) and MDA-MB-231 cells (Fig. 2h). We next tested whether TTP is required for the anti-proliferative activity of metformin. To this end, we inhibited the expression of TTP using siRNA (Fig. 2f) and examined the effects of TTP knock-down on the anti-proliferative activity of metformin in MCF7 and MDA-MB-231 cells. Importantly, treatment of cells with siRNA against TTP (TTP-siRNA) but not scRNA attenuated the inhibitory effects of metformin on the growth of both MCF7 (Fig. 2g) and MDA-MB-231 cells (Fig. 2h) These results strongly suggested that TTP mediates the anti-proliferative functions of metformin in breast cancer cells.
Fig. 2.
TTP mediates the anti-proliferative function of metformin in both p53 wild-type and p53 mutant breast cancer cells. a–d Metformin inhibits proliferation of both p53 wild-type MCF7 cells and p53 mutant MDA-MB-231 cells. a, c MCF7 and b, d MDA-MB-231 cells were treated (a, b) with the indicated concentrations of metformin for 24 h and (c, d) with 6 mM metformin for 24 and 48 h. Cell viability was assessed by measuring absorbance at 490 nm using an MTS cell proliferation assay. The values obtained with mock-treated cells were set to 100. Values are the mean ± SD (n = 3). *p < 0.05, **p < 0.01, ***p < 0.001. e–h Inhibition of TTP attenuates the anti-proliferative effects of metformin in both MCF7 and MDA-MB-231 cells. MCF7 and MDA-MB-231 cells were transfected with e, g, h pcDNA6/TTP or f, g, h TTP-specific siRNA (TTP-siRNA). scRNA and pcDNA6 were used as negative controls. After treatment with 6 mM metformin for 24 h, cell viability was assessed by measuring the absorbance at 490 nm using an MTS cell proliferation assay. The values obtained with mock-treated cells were set to 100. Values are the mean ± SD (n = 3). *p < 0.05, **p < 0.01, ***p < 0.001. ns not significant
Metformin induces TTP expression in a c-Myc-dependent manner
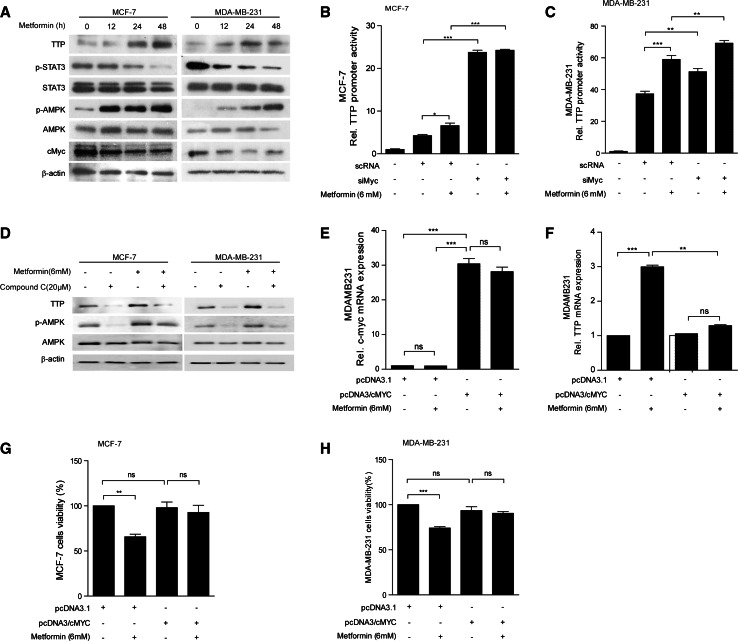
We previously reported that TTP expression is induced by STAT3 in LPS-stimulated macrophages [34]. However, it is unlikely that STAT3 mediates the induction of TTP expression by metformin in breast cancer cells, since metformin decreased STAT3 phosphorylation (Fig. 3a). It has been reported that metformin decreases c-Myc levels in an AMPK-dependent manner [18] and also that c-Myc suppresses TTP expression [30]. Consistently, we found that metformin treatment increased phosphorylation of AMPK and decreased c-Myc levels in a time-dependent manner in both MCF7 (Fig. 3a, left) and MDA-MB-231 cells (Fig. 3a, right). In addition, siRNA-mediated inhibition of c-Myc increased TTP promoter activity (Fig. 3b, c) in the absence of metformin in both MCF7 and MDA-MB-231 cells (Fig. 3b, c). These results suggest that down-regulation of c-Myc level by metformin induces TTP expression. However, it is not likely that, in MDA-MB-231 cells, down-regulation of c-Myc is the only mechanism for the metformin-induced TTP expression, since metformin further increased the TTP promoter activity in c-Myc-depleted MDA-MB-231 cells (Fig. 3c). These results indicate that while metformin induces TTP expression in c-Myc-dependent manner in MCF7 cells, it induces TTP expression through both c-Myc-dependent and c-Myc-independent manner in MDA-MB-231 cells.
Fig. 3.
Metformin induces TTP expression through down-regulation of c-Myc. a Metformin treatment increases phospho-AMPK (pAMPK) but decreases c-Myc and phospho-STAT3 (pSTAT3) in MCF7 and MDA-MB-231 cells. MCF7 and MDA-MB-231 cells were treated with 6 mM metformin for the indicated length of time, and the levels of TTP, STAT3, pSTAT3, AMPK, pAMPK, and c-Myc were measured by Western blotting. b, c inhibition of c-Myc by siRNA enhances TTP promoter activity in MCF7 and MDA-MB-231 cells. b MCF7 and c MDA-MB-231 cells were transfected with pGL3/TTPp-1343 containing the TTP promoter. After treatment with 6 mM metformin for 24 h, luciferase activity was measured. The expression levels obtained from pGL3-transfected cells without metformin treatment were set to 1. Data are presented as the mean ± SD (n = 3). *p < 0.05, **p < 0.01, ***p < 0.001. d Metformin increases TTP expression in an AMPK-dependent manner. MCF7 and MDA-MB-231 cells were treated with 6 mM metformin with or without 20 μM Compound C for 24 h. The levels of TTP, AMPK, and pAMPK were measured by Western blotting. e, f Overexpression of c-Myc blocks the effect of metformin on TTP induction. MDA-MB-231 cells were transfected with pcDNA3/c-Myc or control pcDNA3. Cells were treated with 6 mM metformin for 24 h. e c-Myc and f TTP levels were measured by quantitative RT-PCR. The values obtained with pcDNA-transfected and mock-treated cells were set to 1. Data are presented as the mean ± SD (n = 3). ***p < 0.001. ns not significant. g, h Overexpression of c-Myc attenuates the anti-proliferative effect of metformin in MCF7 and MDA-MB-231 cells. g MCF7 and h MDA-MB-231 cells were transfected with pcDNA3/c-Myc or control pcDNA3. Cells were treated with 6 mM metformin for 24 h. Cell viability was assessed by measuring the absorbance at 490 nm using an MTS cell proliferation assay. The values obtained with mock-treated cells were set to 100. Data are presented as the mean ± SD (n = 3). **p < 0.01, ***p < 0.001. ns not significant
We next tested whether metformin induces TTP expression in an AMPK-dependent manner. We treated MCF7 and MDA-MB-231 cells with 6 mM metformin in the presence or absence of AMPK inhibitor compound C for 24 h and analyzed the effect of compound C on the metformin-induced TTP expression. The compound C inhibited AMPK phosphorylation and blocked metformin-mediated induction of TTP in both MCF7 and MDA-MB-231 cells (Fig. 3d), indicating that AMPK phosphorylation is required for metformin-induced TTP expression. We next tested whether overexpression of c-Myc could block the effect of metformin on the induction of TTP expression in cancer cells. Specifically, we transfected MCF7 and MDA-MB-231 cells with c-Myc (Fig. 3e) and analyzed the extent of TTP induction by real-time PCR (Fig. 3f) and cell proliferation by MTS assay (Fig. 3g, h) after treatment of the cells with metformin. Cells transfected with pcDNA3 empty vector were used as controls. Metformin treatment increased TTP expression (Fig. 3f) and inhibited cell proliferation in both pcDNA3-transfected MCF7 (Fig. 3g) and MDA-MB-231 cells (Fig. 3h). However, in cells over-expressing c-Myc, metformin failed to induce TTP expression (Fig. 3f) and did not show anti-proliferative effect in both MCF7 (Fig. 3g) and MDA-MB-231 cells (Fig. 3h). Taken together, these data suggested that metformin induces TTP expression by down-regulating c-Myc and also that TTP mediates metformin’s anti-proliferative function in breast cancer cells.
Discussion
Metformin, a first-line drug for type 2 diabetes, has recently received increased attention because of its anti-proliferative effects in cancer cells [2–4]. However, the mechanisms underlying the anti-proliferative effects of metformin remain unclear. Here, we describe a role for TTP in mediating metformin’s anti-proliferative effect in cancer cells. Specifically, we found that metformin increased expression level of TTP in cancer cells in an AMPK-dependent manner and also that down-regulation of TTP by siRNA attenuated the anti-proliferative effect of metformin. Thus, our data indicate that TTP induction is required for the anti-proliferative activity of metformin in cancer cells.
TTP can inhibit the growth of cancer cells by down-regulating expression of oncogenes [26, 35, 36]. However, a significant decrease in the expression of TTP has been observed in many cancer cells [25, 26, 33]. Thus, we hypothesized that induction of TTP in cancer cells may lead to an inhibition of growth. In this study, we found that TTP expression was induced by metformin in breast cancer cells. Metformin enhanced TTP promoter activity, indicating that this regulation occurs at the level of transcription.
We previously reported that activation of p53 increases TTP transcription in cancer cells [29]. Furthermore, Metformin can activate p53 in an AMPK-dependent manner [8, 37]. Thus, we considered the possibility that metformin may induce the expression of TTP through p53. Consistently, we found that metformin increased TTP expression level in an AMPK-dependent manner. However, considering the abnormalities in the p53 pathway in nearly all types of cancers [31], if metformin induces TTP expression through p53 pathway, TTP induction by metformin would be limited to only a small portion of cancer cells containing with an intact p53 signaling pathway. Indeed, it is unlikely that p53 is essential for TTP induction by metformin, since metformin induced the expression of TTP in p53 mutant cells as well as p53 wild-type cancer cells as indicated by MDA-MB-231 and MCF7 cells in this study, respectively. However, we found that, in p53 mutant MDA-MB-231 cells, TTP protein increased at high concentration of metformin and to less extend compared with p53 wild-type MCF7 cells. These results suggest the possibility that p53 may be involved in the induction of TTP expression by metformin.
What would be the p53-independent mechanism of TTP induction by metformin in cancer cells? It has been reported that c-Myc acts as a negative regulator of TTP expression [30]. The induction of TTP by metformin is likely the result of inhibition of c-Myc. In this study, we obtained strong evidence in support of this hypothesis: inhibition of c-Myc increased TTP expression level; metformin decreased the expression of c-Myc; and ectopic expression of c-Myc abrogated the effects of metformin with respect to induction of TTP. Metformin has been reported to decrease c-Myc expression via the AMPK pathway [18]. If metformin induces TTP expression through inhibition of c-Myc, TTP induction by metformin would depend on AMPK activity. Consistently, we found that metformin induced TTP expression in an AMPK-dependent manner. However, the transcription factors acting as positive regulators for TTP induction by metformin remain elusive. Thus, further investigation into the specific transcription factors required for TTP induction will reveal the mechanisms underlying how metformin induces TTP expression.
In conclusion, we identified TTP as a down-stream target of metformin and a mediator of the anti-proliferative effects of metformin in cancer cells. Specifically, metformin induced TTP expression by down-regulating c-Myc, a negative regulator of TTP expression [30]. Importantly, our study provides a molecular basis for the anti-proliferative effects of metformin in cancer cells. Our finding that TTP is induced by metformin in both p53 wild-type and p53 mutant cancer cells and mediates the anti-proliferative effect of metformin further highlights the important role of TTP in human cancer cells. Metformin showed only modest anti-proliferative effect on cancer cells used in this study. However, since the mechanism of action of metformin is unique compared with that of other chemotherapeutic agents, metformin in combination with other chemotherapeutic agents may trigger significant tumor growth inhibition in vivo. Given the emerging evidence supporting the anti-proliferative effects of metformin in various types of cancer cells [2–4], it will be of interest to explore whether the regulatory mechanisms described here are relevant to other types of cancers.
Acknowledgments
This work was supported by Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2014R1A6A1030318) and NRF-2014R1A1A2007525.
Compliance with ethical standard
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Indira Pandiri and Yingqing Chen authors have been contributed equally to this work.
Contributor Information
Hun Taeg Chung, Email: chung@ulsan.ac.kr.
Jeong Woo Park, Email: jwpark@ulsan.ac.kr.
References
- 1.Bailey CJ, Turner RC. Metformin. N Engl J Med. 1996;334:574–579. doi: 10.1056/NEJM199602293340906. [DOI] [PubMed] [Google Scholar]
- 2.Kourelis TV, Siegel RD. Metformin and cancer: new applications for an old drug. Med Oncol. 2012;29:1314–1327. doi: 10.1007/s12032-011-9846-7. [DOI] [PubMed] [Google Scholar]
- 3.Jiralerspong S, Palla SL, Giordano SH, et al. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J Clin Oncol. 2009;27:3297–3302. doi: 10.1200/JCO.2009.19.6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quinn BJ, Kitagawa H, Memmott RM, Gills JJ, Dennis PA. Repositioning metformin for cancer prevention and treatment. Trends Endocrinol Metab. 2013;24:469–480. doi: 10.1016/j.tem.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Dowling RJ, Goodwin PJ, Stambolic V. Understanding the benefit of metformin use in cancer treatment. BMC Med. 2011;9:33. doi: 10.1186/1741-7015-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Javeshghani S, Zakikhani M, Austin S, et al. Carbon source and myc expression influence the antiproliferative actions of metformin. Cancer Res. 2012;72:6257–6267. doi: 10.1158/0008-5472.CAN-12-2907. [DOI] [PubMed] [Google Scholar]
- 7.Russo GL, Russo M, Ungaro P. AMP-activated protein kinase: a target for old drugs against diabetes and cancer. Biochem Pharmacol. 2013;86:339–350. doi: 10.1016/j.bcp.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 8.Vazquez-Martin A, Oliveras-Ferraros C, Del Barco S, Martin-Castillo B, Menendez JA. If mammalian target of metformin indirectly is mammalian target of rapamycin, then the insulin-like growth factor-1 receptor axis will audit the efficacy of metformin in cancer clinical trials. J Clin Oncol. 2009;27:e207–209. doi: 10.1200/JCO.2009.24.5456. [DOI] [PubMed] [Google Scholar]
- 9.Vincent EE, Coelho PP, Blagih J, Griss T, Viollet B, Jones RG. Differential effects of AMPK agonists on cell growth and metabolism. Oncogene. 2015;34:3627–3639. doi: 10.1038/onc.2014.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cufi S, Corominas-Faja B, Vazquez-Martin A, et al. Metformin-induced preferential killing of breast cancer initiating CD44+ CD24−/low cells is sufficient to overcome primary resistance to trastuzumab in HER2+ human breast cancer xenografts. Oncotarget. 2012;3:395–398. doi: 10.18632/oncotarget.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirsch HA, Iliopoulos D, Tsichlis PN, Struhl K. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res. 2009;69:7507–7511. doi: 10.1158/0008-5472.CAN-09-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wurth R, Pattarozzi A, Gatti M, et al. Metformin selectively affects human glioblastoma tumor-initiating cell viability: a role for metformin-induced inhibition of Akt. Cell Cycle. 2013;12:145–156. doi: 10.4161/cc.23050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sato A, Sunayama J, Okada M, et al. Glioma-initiating cell elimination by metformin activation of FOXO3 via AMPK. Stem Cells Transl Med. 2012;1:811–824. doi: 10.5966/sctm.2012-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Memmott RM, Mercado JR, Maier CR, Kawabata S, Fox SD, Dennis PA. Metformin prevents tobacco carcinogen–induced lung tumorigenesis. Cancer Prev Res (Phila) 2010;3:1066–1076. doi: 10.1158/1940-6207.CAPR-10-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu X, Chhipa RR, Pooya S, et al. Discrete mechanisms of mTOR and cell cycle regulation by AMPK agonists independent of AMPK. Proc Natl Acad Sci USA. 2014;111:E435–444. doi: 10.1073/pnas.1311121111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu B, Fan Z, Edgerton SM, Yang X, Lind SE, Thor AD. Potent anti-proliferative effects of metformin on trastuzumab-resistant breast cancer cells via inhibition of erbB2/IGF-1 receptor interactions. Cell Cycle. 2011;10:2959–2966. doi: 10.4161/cc.10.17.16359. [DOI] [PubMed] [Google Scholar]
- 17.Martin-Castillo B, Vazquez-Martin A, Oliveras-Ferraros C, Menendez JA. Metformin and cancer: doses, mechanisms and the dandelion and hormetic phenomena. Cell Cycle. 2010;9:1057–1064. doi: 10.4161/cc.9.6.10994. [DOI] [PubMed] [Google Scholar]
- 18.Akinyeke T, Matsumura S, Wang X, et al. Metformin targets c-MYC oncogene to prevent prostate cancer. Carcinogenesis. 2013;34:2823–2832. doi: 10.1093/carcin/bgt307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng XS, Wang S, Deng A, et al. Metformin targets Stat3 to inhibit cell growth and induce apoptosis in triple-negative breast cancers. Cell Cycle. 2012;11:367–376. doi: 10.4161/cc.11.2.18813. [DOI] [PubMed] [Google Scholar]
- 20.Cufi S, Corominas-Faja B, Lopez-Bonet E, et al. Dietary restriction-resistant human tumors harboring the PIK3CA-activating mutation H1047R are sensitive to metformin. Oncotarget. 2013;4:1484–1495. doi: 10.18632/oncotarget.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaw G, Kamen R. A conserved AU sequence from the 3′ untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986;46:659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- 22.Shyu AB, Wilkinson MF. The double lives of shuttling mRNA binding proteins. Cell. 2000;102:135–138. doi: 10.1016/S0092-8674(00)00018-0. [DOI] [PubMed] [Google Scholar]
- 23.Carballo E, Lai WS, Blackshear PJ. Feedback inhibition of macrophage tumor necrosis factor-alpha production by tristetraprolin. Science. 1998;281:1001–1005. doi: 10.1126/science.281.5379.1001. [DOI] [PubMed] [Google Scholar]
- 24.Brooks SA, Blackshear PJ. Tristetraprolin (TTP): interactions with mRNA and proteins, and current thoughts on mechanisms of action. Biochim Biophys Acta. 2013;1829:666–679. doi: 10.1016/j.bbagrm.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brennan SE, Kuwano Y, Alkharouf N, Blackshear PJ, Gorospe M, Wilson GM. The mRNA-destabilizing protein tristetraprolin is suppressed in many cancers, altering tumorigenic phenotypes and patient prognosis. Cancer Res. 2009;69:5168–5176. doi: 10.1158/0008-5472.CAN-08-4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee HH, Son YJ, Lee WH, et al. Tristetraprolin regulates expression of VEGF and tumorigenesis in human colon cancer. Int J Cancer. 2010;126:1817–1827. doi: 10.1002/ijc.24847. [DOI] [PubMed] [Google Scholar]
- 27.Marderosian M, Sharma A, Funk AP, et al. Tristetraprolin regulates Cyclin D1 and c-Myc mRNA stability in response to rapamycin in an Akt-dependent manner via p38 MAPK signaling. Oncogene. 2006;25:6277–6290. doi: 10.1038/sj.onc.1209645. [DOI] [PubMed] [Google Scholar]
- 28.Young LE, Sanduja S, Bemis-Standoli K, Pena EA, Price RL, Dixon DA. The mRNA binding proteins HuR and tristetraprolin regulate cyclooxygenase 2 expression during colon carcinogenesis. Gastroenterology. 2009;136:1669–1679. doi: 10.1053/j.gastro.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JY, Kim HJ, Yoon NA, et al. Tumor suppressor p53 plays a key role in induction of both tristetraprolin and let-7 in human cancer cells. Nucleic Acids Res. 2013;41:5614–5625. doi: 10.1093/nar/gkt222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rounbehler RJ, Fallahi M, Yang C, et al. Tristetraprolin impairs myc-induced lymphoma and abolishes the malignant state. Cell. 2012;150:563–574. doi: 10.1016/j.cell.2012.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soussi T, Beroud C. Assessing TP53 status in human tumours to evaluate clinical outcome. Nat Rev Cancer. 2001;1:233–240. doi: 10.1038/35106009. [DOI] [PubMed] [Google Scholar]
- 32.Cole MD. The myc oncogene: its role in transformation and differentiation. Annu Rev Genet. 1986;20:361–384. doi: 10.1146/annurev.ge.20.120186.002045. [DOI] [PubMed] [Google Scholar]
- 33.Kim HK, Kim CW, Vo MT, et al. Expression of proviral integration site for Moloney murine leukemia virus 1 (Pim-1) is post-transcriptionally regulated by tristetraprolin in cancer cells. J Biol Chem. 2012;287:28770–28778. doi: 10.1074/jbc.M112.376483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joe Y, Kim HJ, Kim S, et al. Tristetraprolin mediates anti-inflammatory effects of nicotine in lipopolysaccharide-stimulated macrophages. J Biol Chem. 2011;286:24735–24742. doi: 10.1074/jbc.M110.204859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lykke-Andersen J, Wagner E. Recruitment and activation of mRNA decay enzymes by two ARE-mediated decay activation domains in the proteins TTP and BRF-1. Genes Dev. 2005;19:351–361. doi: 10.1101/gad.1282305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim CW, Vo MT, Kim HK, et al. Ectopic over-expression of tristetraprolin in human cancer cells promotes biogenesis of let-7 by down-regulation of Lin28. Nucleic Acids Res. 2012;40:3856–3869. doi: 10.1093/nar/gkr1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He G, Zhang YW, Lee JH, et al. AMP-activated protein kinase induces p53 by phosphorylating MDMX and inhibiting its activity. Mol Cell Biol. 2014;34:148–157. doi: 10.1128/MCB.00670-13. [DOI] [PMC free article] [PubMed] [Google Scholar]