Abstract
We aimed to develop a user-centered, web-based, decision support tool for breast cancer risk assessment and personalized risk management. Using a novel model choice algorithm, iPrevent® selects one of two validated breast cancer risk estimation models (IBIS or BOADICEA), based on risk factor data entered by the user. Resulting risk estimates are presented in simple language and graphic formats for easy comprehension. iPrevent® then presents risk-adapted, evidence-based, guideline-endorsed management options. Development was an iterative process with regular feedback from multidisciplinary experts and consumers. To verify iPrevent®, risk factor data for 127 cases derived from the Australian Breast Cancer Family Study were entered into iPrevent®, IBIS (v7.02), and BOADICEA (v3.0). Consistency of the model chosen by iPrevent® (i.e., IBIS or BOADICEA) with the programmed iPrevent® model choice algorithm was assessed. Estimated breast cancer risks from iPrevent® were compared with those attained directly from the chosen risk assessment model (IBIS or BOADICEA). Risk management interventions displayed by iPrevent® were assessed for appropriateness. Risk estimation model choice was 100 % consistent with the programmed iPrevent® logic. Discrepant 10-year and residual lifetime risk estimates of >1 % were found for 1 and 4 cases, respectively, none was clinically significant (maximal variation 1.4 %). Risk management interventions suggested by iPrevent® were 100 % appropriate. iPrevent® successfully integrates the IBIS and BOADICEA risk assessment models into a decision support tool that provides evidence-based, risk-adapted risk management advice. This may help to facilitate precision breast cancer prevention discussions between women and their healthcare providers.
Electronic supplementary material
The online version of this article (doi:10.1007/s10549-016-3726-y) contains supplementary material, which is available to authorized users.
Keywords: Breast cancer, Risk, Decision support, BRCA1, Chemoprevention
Introduction
A woman’s risk of breast cancer is due to a complex interplay between genetic, environmental, and lifestyle factors [1]. Major risk factors include having a family history of the disease and/or a mutation in a breast cancer predisposition gene, history of therapeutic chest irradiation, and history of a breast biopsy showing atypical hyperplasia or lobular carcinoma in situ (LCIS). Other risk factors include early menarche, late menopause, prolonged use of combined hormone replacement therapy, obesity, and alcohol consumption, while child bearing and breast feeding are protective. With medical practice moving toward precision prevention [2], it is now possible to estimate the risk of breast cancer for an individual woman. Knowledge of an individual’s breast cancer risk facilitates use of evidence-based management strategies [3] appropriate for that risk level, and allows calculation of the absolute benefit, in terms of risk reduction, for each strategy. Breast cancer risk management strategies include risk-reducing bilateral mastectomy [4], premenopausal bilateral salpingo-oophorectomy [5], medical prevention with selective estrogen receptor modulators or aromatase inhibitors [6, 7], breast cancer screening [3, 8], and lifestyle modifications such as maintaining a healthy weight and reducing alcohol intake [9].
Several mathematical models have been developed to estimate breast cancer risk [10]. Some are designed primarily for use by experienced clinicians or geneticists, others are aimed at specific risk groups, such as those at high risk [11]. To our knowledge, none integrates personalized, absolute risk estimates with comprehensive, risk-adapted, management options including personalized absolute risk reduction estimates for each option. Two well-validated breast cancer risk estimation models are the International Breast Cancer Intervention Study (IBIS) model [12] and the Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm (BOADICEA) model [13, 14]. These models have been validated in a prospective cohort showing good discrimination and accuracy [15, 16].
The IBIS and the BOADICEA tools vary in the extent of risk factor data they use to estimate risk. While both use family history data, IBIS limits family history input to first- and second-degree relatives or third-degree female relatives with breast or ovarian cancer only. BOADICEA also incorporates breast cancer pathology characteristics, family history of prostate or pancreatic cancer as well as the input of data from relatives of any degree of relatedness. IBIS also uses other risk factor data, including body mass index, reproductive factors, and personal history of high-risk breast lesions such as atypical hyperplasia and LCIS. BOADICEA does not currently consider these factors. Neither model integrates risk-adapted management information in its output.
Clinical management decisions in medicine, and oncology in particular, are becoming more data dependent but for many decisions the relative benefits and harms are uncertain, suggesting a need for greater shared decision making. While scientific advances enable a more tailored approach to patients, this requires greater specialist knowledge which may not be widely available. Indeed, qualitative studies undertaken to inform the development of iPrevent®, revealed that healthcare providers often have difficulty accurately and easily assessing and communicating breast cancer risk and the absolute benefits and disadvantages of risk management interventions [17]. They seek a tool that is evidence based, accessible, provides 10-year and residual lifetime risk estimates, and displays absolute rather than relative risks and risk reductions in multiple formats to account for patients with differing information needs [17].
We aimed to develop and verify a tool for healthcare providers and women to use collaboratively, that integrates accurate and personalized breast cancer risk assessment (using the IBIS and BOADICEA models) and that displays risk-adapted, personalized, risk management information.
Methods
A user-centered approach was employed with all aspects of iPrevent® design. We assessed user needs by conducting focus groups with primary care doctors and nurses, breast surgeons, consumers, breast cancer screening program staff, and clinicians in genetics clinics [17, 18]. This identified potential barriers to implementation of the tool in everyday clinical practice, as well as the concerns of prospective users. Where possible, these issues were addressed in the software design phase. During iPrevent® development, the wording, format, and layout of the output pages was reviewed and optimized by a prototype design committee comprising: an academic general practitioner (with a special interest in the development of clinical decision support tools for cancer), a breast surgeon, a clinical geneticist, a psycho-oncologist (with a special interest in risk presentation), an epidemiologist, two consumer advocates, two medical oncologists (with a special interest in breast cancer risk management), and the software developers. The aim of this inclusive approach to the iPrevent® design was to maximize clinical utility by building a tool that satisfies user requirements.
iPrevent® starts with a disclaimer page outlining the limitations of its use. The software comprises three main modules: (i) data input, (ii) risk evaluation, and (iii) results output including personalized risk estimation and risk management options.
Data input
The data input module is presented as a series of pages with related questions through which the user can easily navigate backwards and forwards (Fig. 1). iPrevent® requires the user to enter the data required by either tool, where available, but reduces the data input burden for family history when compared to BOADICEA. The program minimizes the number of questions required to be completed by hiding those questions that become unnecessary, for example, detailed family history data are not collected for users who answer a screening question by stating that they have no family history of breast, ovarian, pancreatic, or prostate cancer. Conversely, some questions must always be answered as they are necessary for accurate risk estimation by IBIS and/or BOADICEA, e.g., BOADICEA requires the year of birth and age at diagnosis of relatives affected by cancer.
Fig. 1.
Screenshot of the iPrevent® reproductive history data entry page
At the conclusion of the data input stage, iPrevent® displays a plain English summary of the entered family history. Should the user identify errors at this time, she can navigate back to the family history pages and correct data.
Risk evaluation
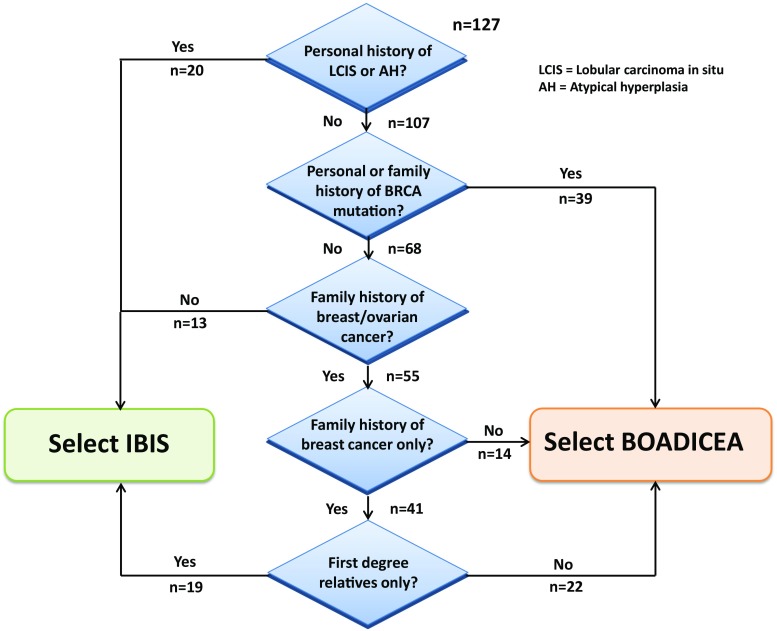
The iPrevent® model choice algorithm (Fig. 2) was adapted from Amir et al. [10] and selects the risk assessment model, IBIS, or BOADICEA, which will be used to calculate the individualized risk estimate for each user. The risk factor data entered into iPrevent® are used to interface with the relevant breast cancer risk estimation model, via the Internet. Interfacing with BOADICEA is done through the online version of the tool (version 3.0) hosted at Cambridge, UK [19]. Interfacing with IBIS (version 7.02) is achieved via the Harvard Risk Service.
Fig. 2.
iPrevent® algorithm for the choice of risk estimation model and verification
Results output: risk estimation
The results module is designed to provide a risk output style that is the same regardless of the background model used to calculate the risk.
The 10-year and residual lifetime breast cancer risks, as estimated by either IBIS or BOADICEA, are presented to the woman along with the age-matched population 10-year and residual lifetime risks. While IBIS provides age-matched population risks, the online BOADICEA tool currently provides age-matched country-specific population risks in graphical form only. We used population-based data from Australian Cancer Incidence and Mortality (ACIM), an Australian dataset for 2009, to estimate the age-matched population breast cancer risks [20]. iPrevent® displays this population risk estimate when BOADICEA is the nominated risk estimation model.
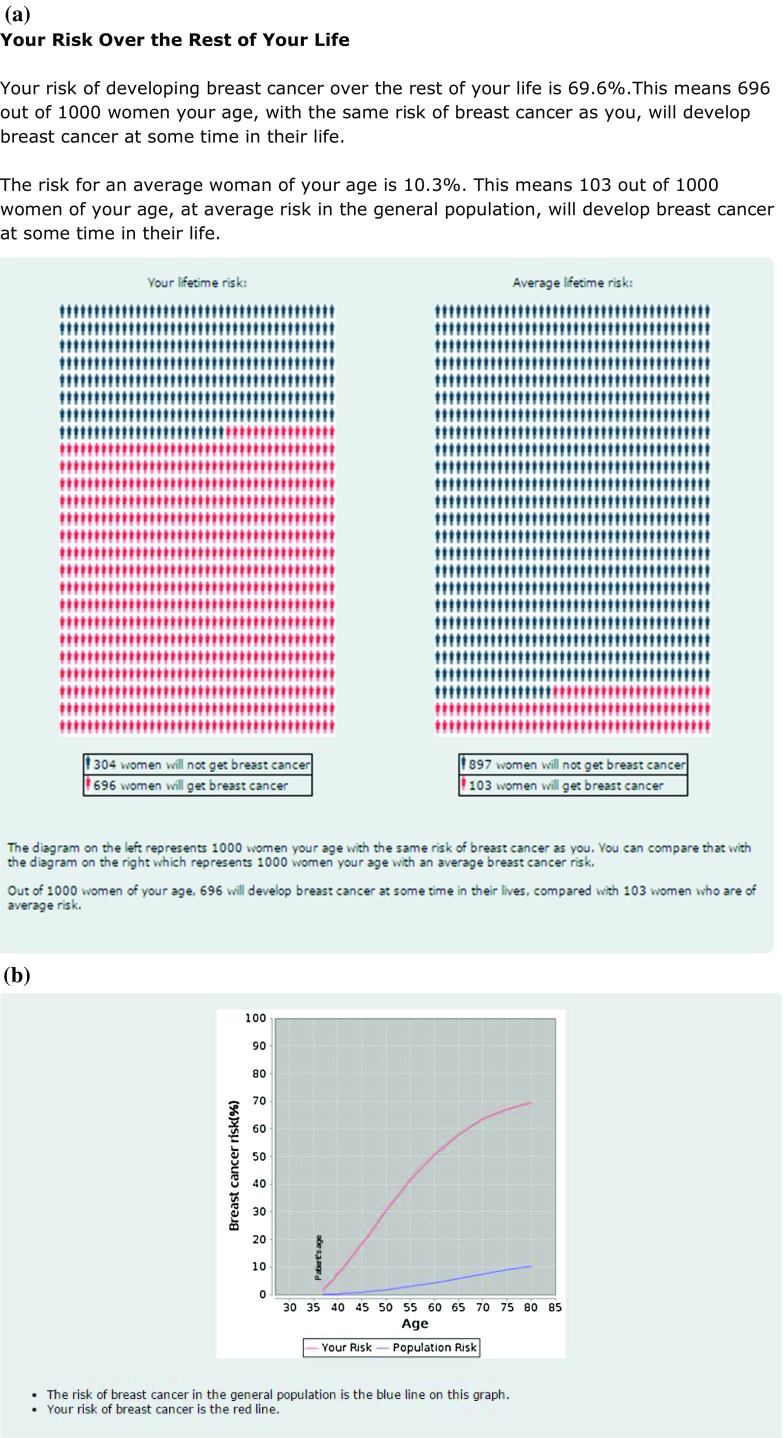
iPrevent® initially conveys a qualitative outline of risk e.g., “Your risk of developing breast cancer is substantially increased for a woman of your age. However this does not necessarily mean that you will develop breast cancer.” It then allows a woman, with the support of her healthcare provider, to access the detailed, quantitative risk estimates only if they choose to. Users may elect whether to view any, or all of the specific risk estimation formats, i.e., text, pictograms, and/or graphs as shown in Fig. 3a and b. Figure 3c shows the comparable risk estimate outputs derived directly for IBIS and BOADICEA.
Fig. 3.
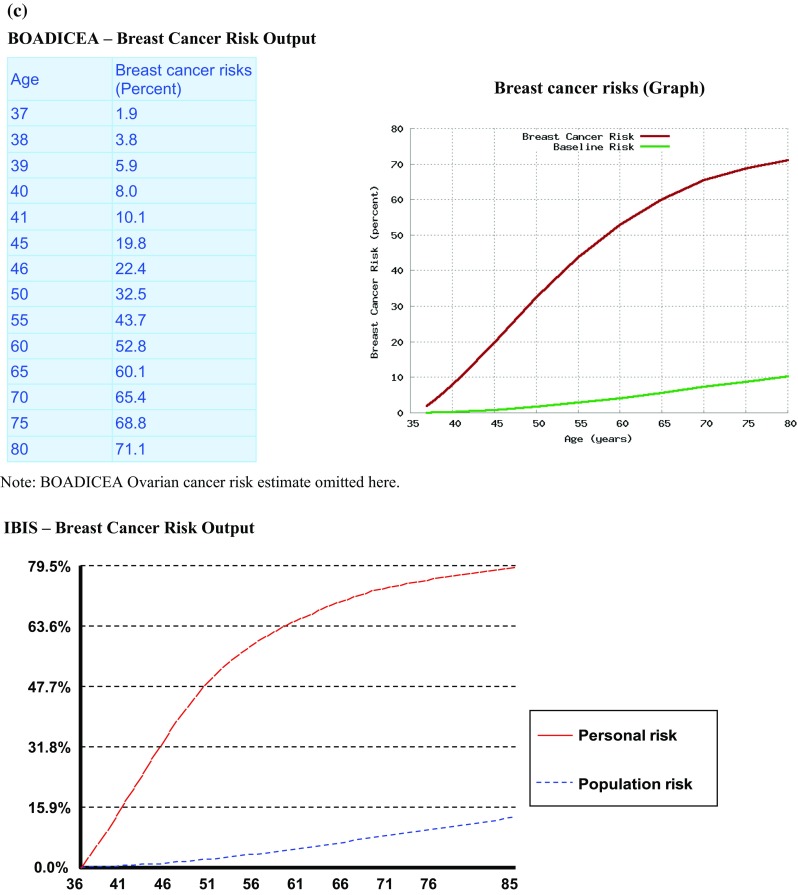
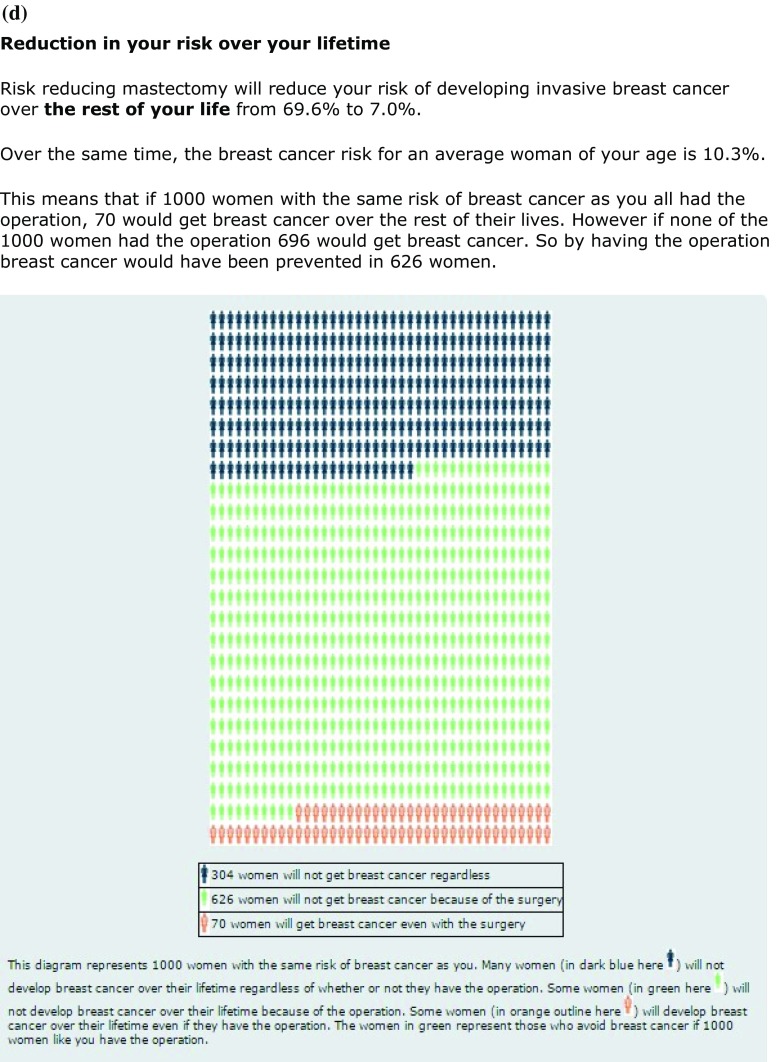
a iPrevent® Screenshot—text and pictogram of personalized risk and population risk for a 36-year-old BRCA1 mutation carrier. b iPrevent® Screenshot—graph of personalized risk and population risk for a 36-year-old BRCA1 mutation carrier. c Selected output derived directly from IBIS and BOADICEA for the same 36-year-old BRCA1 mutation carrier for comparison. d iPrevent® Screenshot—text and pictogram of risk reduction from bilateral prophylactic mastectomy for a 36-year-old BRCA1 mutation carrier
Results output: risk management
iPrevent® presents risk-adapted management options based on Australian guidelines [3]. Using the estimated residual lifetime risk, women are assigned a risk category: average risk (i.e., <1.5 times population risk), moderately increased risk (i.e., 1.5–3 times population risk), or high risk (i.e., >3 times population risk) [3]. Based on the assigned category, relevant risk management options are presented to the user (Table 1).
Table 1.
iPrevent® risk management options by breast cancer risk category
| Risk category | Category risk definition | Lifestyle modificationa | Radiological screeningb | Risk-reducing medicationc | Risk-reducing surgeryd,e |
|---|---|---|---|---|---|
| Average | <1.5 times population risk | All women | Biennial mammogramf | No | No |
| Moderately increased | 1.5–3 times population risk | All women | Annual mammogram | Yes | No |
| High | >3 times population risk | All women | Annual mammogram and breast MRIg | Yes | Yes |
aIncludes regular exercise, not smoking, maintaining a healthy weight, and minimizing alcohol intake
bDoes not reduce risk of breast cancer but may help detect cancer earlier
cIncludes tamoxifen for premenopausal women, and raloxifene, anastrazole, exemestane, or tamoxifen for postmenopausal women
dIncludes risk-reducing mastectomy and premenopausal risk-reducing salpingo-oophorectomy
eThe risk reduction with medication and surgery may not be additive, for example, those who have undergone salpingo-oophorectomy may not benefit further from medication such as tamoxifen
fFrom 50 to 74 years of age
g MRI (magnetic resonance imaging) only in women aged 18–50 years
The risk management options appear as a list, tailored to the woman’s risk category and her input data. The user may choose to click on any or all options in the list to view more details, or she may choose to skip these details altogether.
When details of each risk-reducing option are viewed, estimates are provided of the absolute risk reduction, specific to that woman, for the viewed option (Fig. 3d). Such risk reduction estimates are not available from IBIS and BOADICEA directly. Estimates of the magnitude of relative risk reduction for each option are derived from published data [4, 5, 21–24]. This relative risk reduction is applied to the individual user’s estimated absolute 10-year and residual lifetime breast cancer risk, to give a personalized estimate of the absolute risk reduction for each option, presented in the same range of formats. Information on possible disadvantages/side-effects of each option is also provided. In addition, there are links specific for healthcare providers, e.g., tips for safe prescribing of risk-reducing medication.
Results output: data presentation
An important element in the design of iPrevent® is the presentation of breast cancer risk estimates, risk reduction, and possible side-effects estimates for each management option, in a way that is easily understood by women of varying levels of education and literacy, and for healthcare providers who are not experts in risk presentation. Therefore, all breast cancer risks and risk reductions are presented as words, percentages, a visual scale or pictogram (icon arrays with 1000 women), and graphs. This use of multiple formats to display risk aims to reduce bias in how the numbers may be perceived, while also increasing understanding [25, 26].
With the same aim of maximizing comprehension, the language used in all iPrevent® output pages was aimed at a Flesch–Kincaid reading grade level of eight (the estimated number of years of education required for comprehension) [27]. iPrevent® is designed for use in conjunction with a healthcare provider who can bridge any gaps in understanding.
Future proofing
Updates to IBIS and BOADICEA are expected to occur over time, for example to include mammographic density and single nucleotide polymorphisms (SNPs—variations in the smallest portions of DNA) data into the risk estimation. These updates can readily be integrated into iPrevent® provided that they do not fundamentally alter the way in which iPrevent® interacts with the risk assessment models. The interface with the two models is separated to ensure ease of updating just one interface if required. While many users expressed an interest in the development of iPrevent® as an app for their smart phone or tablet, this would limit the ability of the developers to force updates, preventing users from access to an outdated version in the future.
Verification
Verification relates to ensuring that the computerized model and its implementation are correct, while validation ensures sufficient accuracy of that model in a clinical context [28]. The IBIS and BOADICEA models have already been prospectively validated for calibration and discriminatory accuracy of breast cancer risk estimates [16]. The main objective of verification was to determine the accuracy of the software system, including detecting coding errors and verifying the correct risk estimation model selection according to the iPrevent® algorithm [29]. While iPrevent® is, to some extent, dependant on the validity of the data derived from IBIS and BOADICEA, its operational validity [30], such that clinically appropriate outputs are presented, was also confirmed using a population-based dataset.
iPrevent® was tested using risk factor data on 127 cases derived from women with no personal history of breast cancer, enrolled in the Australian Breast Cancer Family Study (ABCFS) [31], a population-based case–control breast cancer family study. The ABCFS was approved by the Human Research Ethics Committees of the University of Melbourne, the Cancer Council Victoria, and Cancer Council NSW, and all participants provided written informed consent. The cases were selected for inclusion because they had sufficient risk factor data for the models across the range of breast cancer risks that would be expected to be seen in a variety of clinical settings. The data were manually entered into each of IBIS (v7.02) and BOADICEA (v3.0) models, and iPrevent® independently, and the resulting 10-year and residual lifetime risk estimates recorded and manually categorized as average, moderate, or high risk. Whether the correct (IBIS or BOADICEA) model, according to the iPrevent® algorithm (Fig. 2) was chosen by iPrevent® was also recorded. The numbers of cases at each branch of the algorithm was also noted (Fig. 2) to ensure that a broad variety of clinical scenarios were tested. Estimated 10-year and residual lifetime breast cancer risks from iPrevent® were compared with those attained directly from the chosen model. Variations of greater than 1 % were considered discrepant. The breast cancer risk management options provided by iPrevent® for these 127 cases were assessed for consistency with national guideline recommendations [3], and the absolute risk reductions for each presented risk management option were manually calculated and compared with those calculated by iPrevent®. The output pages presented by iPrevent® for each case were compared to the data entered, as these data were used to present tailored recommendations for lifestyle modifications such as reducing weight (if overweight) and reducing alcohol intake (if consuming greater than national recommendations).
Results
Testing and verification
In the ABCFS derived dataset, iPrevent® used BOADICEA for 75 (59 %) of the 127 cases, including 36, 31, and 8 cases at high, moderate, and average risk, respectively. For the remaining cases where IBIS was used, 21, 12, and 19 were at high, moderate, and average risk, respectively. The correct risk assessment model, according to the iPrevent® algorithm (Fig. 2), was chosen in all 127 cases (100 %).
Discrepant 10-year and residual lifetime risk estimates of >1 % were found for 1 (1 %) and 4 (3 %) cases, respectively, when iPrevent® results were compared with the background risk model (IBIS or BOADICEA) used (Table 2).
Table 2.
Discrepancies in breast cancer risk estimation between iPrevent® and the chosen risk estimation model
| Model selected | iPrevent® risk estimates (%) | IBIS or BOADICEA model risk estimates (%) | Difference between iPrevent® and model used (%) | |||
|---|---|---|---|---|---|---|
| 10-year | Residual lifetime | 10-year | Residual lifetime | 10-year | Residual lifetime | |
| IBIS | 3.1 | 14.3 | 2.8 | 13.2 | 0.3 | 1.1 |
| IBIS | 4.7 | 8.2 | 3.9 | 6.8 | 0.8 | 1.4 |
| IBIS | 4.2 | 14.3 | 2.9 | 10.3 | 1.3 | 4 |
| IBIS | 2.7 | 9.5 | 2.3 | 8.1 | 0.4 | 1.4 |
Discrepancies were seen in 4 of 127 cases tested
All 4 of these cases (for 1 case both 10-year and lifetime risks were discrepant), involved women at population risk of breast cancer, with no personal or family history of breast cancer or cancer predisposition genes (BRCA1 and BRCA2). In order to minimize the data entry required, iPrevent® does not ask users for data on unaffected relatives in these low risk women as the results are not expected to change recommendations. This can lead to very minor, and clinically insignificant variations in the presented estimate for 10-year or residual lifetime breast cancer risk, but greatly enhances ease of use of iPrevent®. All differences noted were considered to be clinically insignificant and none led to a change in the woman’s breast cancer risk category nor to the risk management options presented by iPrevent®.
iPrevent® provided the appropriate risk management options including lifestyle changes, according to Australian guidelines [3] in all 127 (100 %) cases. The correct absolute risk reduction was also shown in 100 % of cases.
Discussion
We developed iPrevent®, a web-based decision support tool that integrates two validated risk assessment models to estimate a woman’s personal breast cancer risk and then facilitates discussions between women and their health care providers about evidence-based measures to manage that risk, by providing information tailored to each woman. We took a user-centered approach with the aim of meeting the end user’s needs as identified in our previous research [17, 18]. We verified the coding of iPrevent® using a population-based dataset to ensure the breast cancer risk estimates and risk management information presented were derived correctly according to our algorithm (Fig. 2; Table 1) and thus clinically appropriate. We defined an arbitrary cut-off for the verification of risk estimates of <1 % from the expected breast cancer risk (derived directly from the validated IBIS or BOADICEA model) as an acceptable variation. This definition is strict and much wider variation is likely to be acceptable in a clinical context. A variation of 1 % will only rarely change the risk category (Table 1) that a woman is assigned to and will not substantially alter the risk reduction estimates for any given risk management option. For example, for risk-reducing medication with tamoxifen a variation of 1 % in risk estimate results in a variation of only 0.67 % in the absolute risk reduction estimate, which is unlikely to influence clinical decision making.
Future features
iPrevent® is intended to be a dynamic tool, designed to allow for the incorporation of updated data on breast cancer risk assessment and risk management without major coding changes. Anticipated future changes to breast cancer risk assessment include the incorporation of elements known to affect breast cancer risk but not currently well defined in terms of their interaction with family history and other factors modeled by IBIS and BOADICEA. For example, mammographic density is an important risk factor for breast cancer [32], and the IBIS and BOADICEA developers are currently working on its inclusion in these models. Similarly, SNPs in multiple genes affect breast cancer risk [33], and it is expected these will be included in these models in the future.
Integration of the iPrevent® breast cancer risk with personally controlled health record (PCHR) platforms [34], is also an ideal future use, allowing the risk calculation to be updated over time, with respect to changing circumstances.
While the risk reduction estimates programmed into iPrevent® are based on the best current data, refinements are likely to occur over time. For example, iPrevent® currently applies a 50 % relative risk reduction for breast cancer with risk-reducing salpingo-oophorectomy before 45 years of age [23, 35]. Modeling studies [11] have investigated this research question, but greater data are required for individualization of the risk reduction estimates. It is likely that when more prospective data are available [36], a more accurate age-adapted risk reduction will be known and hence able to be incorporated into iPrevent®.
Ultimately, it is envisaged that iPrevent® will enable healthcare providers to assess and manage a woman’s breast cancer risk easily and routinely as part of a prevention consultation. The current uptake of risk-reducing interventions, even among women at highest risk, is low [37]. iPrevent® will empower women to know their breast cancer risk and understand the pros and cons of various interventions. It will provide users with accurate and personalized risk assessment and risk management information with the intention of improving decision making regarding risk management options.
iPrevent® may be applied to women across the spectrum of breast cancer risk, in a variety of specialist and primary care clinical settings, to provide an evidence-based approach to breast cancer risk assessment and management and to optimize shared decision making between patient and healthcare provider.
IBIS and BOADICEA are excellent breast cancer risk assessment models that have been well validated. iPrevent® provides potential advantages over either model alone, as it automatically uses the most appropriate of these models depending on the data inputted. In addition, the interface has been designed to be easier for women and inexperienced clinicians to use compared with the data input interfaces for IBIS and BOADICEA. Perhaps, the most important distinction though is that IBIS and BOADICEA provide only breast cancer risk information, whereas iPrevent® also provides evidence-based risk management options tailored to the woman’s estimated risk level. Furthermore, iPrevent® displays the absolute risk reduction that can be achieved with each risk management option for each individual woman, providing an excellent platform for informed decision making.
The aim of this project was to develop a personalized, evidence-based, risk assessment, and risk management decision support tool for breast cancer. The results of our verification study show that this goal has been achieved. We are currently undertaking a large pilot study of iPrevent® with 70 women and 20 clinicians across three different clinical settings (primary care, breast surgical clinics, and genetics clinics). The aims of this piloting work is to (i) assess the acceptability of the content, layout, and presentation of iPrevent®, and (ii) identify any issues with usability and potential barriers to implementation which can then be addressed in future iterations of the tool. We believe the user satisfaction with iPrevent® will be a key driver to its widespread use and ultimately better personalized breast cancer risk awareness for all women. It is hoped to make iPrevent® widely and freely available on the web to all healthcare providers in the near future, once piloting is complete.
Electronic supplementary material
Acknowledgments
We thank community representatives, Ms Debbie Sandler and Ms Leslie Gilham, for their advice on consumer issues during the development of iPrevent®. And Professor Rod Jackson for his advice during the early phases of this project. IBIS computations are provided by the Risk Web Service developed jointly by the Hughes RiskApps Group at the Massachusetts General Hospital and the BayesMendel Lab at the Dana Farber Cancer Institute http://bayesmendel.dfci.harvard.edu/risk/.
Funding
This research was funded by the Australian National Health and Medical Research Council (NHMRC) (#1064244) and by the Australia and New Zealand Breast Cancer Trials Group from public donations made to the Breast Cancer Institute of Australia. KAP is an Australian National Breast Cancer Foundation Practitioner Fellow. JLH is a NHMRC Senior Principal Research Fellow. ACA is a Cancer Research - UK Senior Cancer Research Fellow.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- 1.Trainer AH, Thompson E, James PA. BRCA and beyond: a genome-first approach to familial breast cancer risk assessment. Discov Med. 2011;12(66):433–443. [PubMed] [Google Scholar]
- 2.National Institute of Health (NIH) Precision Medicine Initiative (2015) http://www.nih.gov/precisionmedicine/. Accessed Sept 2015
- 3.Cancer Australia Advice about familial aspects of breast cancer and epithelial ovarian cancer (2010) https://canceraustralia.gov.au/publications-and-resources/cancer-australia-publications/advice-about-familial-aspects-breast-cancer-and-epithelial-ovarian-cancer. Accessed Sept 2015
- 4.Rebbeck TR, Friebel T, Lynch HT, Neuhausen SL, van’t Veer L, Garber JE, Evans GR, Narod SA, Isaacs C, Matloff E, Daly MB, Olopade OI, Weber BL. Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol. 2004;22(6):1055–1062. doi: 10.1200/JCO.2004.04.188. [DOI] [PubMed] [Google Scholar]
- 5.Rebbeck TR, Kauff ND, Domchek SM. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst. 2009;101(2):80–87. doi: 10.1093/jnci/djn442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Visvanathan K, Hurley P, Bantug E, Brown P, Col NF, Cuzick J, Davidson NE, DeCensi A, Fabian C, Ford L. Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2013;2013(2049):3122. doi: 10.1200/JCO.2013.49.3122. [DOI] [PubMed] [Google Scholar]
- 7.National Institute of Health and Clinical Excellence (NICE) (2013) Familial breast cancer: classification, care and managing breast cancer and related risks in people with a family history of breast cancer. Full guideline June 2013. http://www.nice.org.uk/guidance/cg164/evidence/full-guideline-190130941. Accessed Sept 2015 [PubMed]
- 8.Saslow D, Boetes C, Burke W, Harms S, Leach MO, Lehman CD, Morris E, Pisano E, Schnall M, Sener S, Smith RA, Warner E, Yaffe M, Andrews KS, Russell CA, American Cancer Society Breast Cancer Advisory G (2007) American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin 57(2):75-89 [DOI] [PubMed]
- 9.Parkin DM, Boyd L, Walker LC. The fraction of cancer attributable to lifestyle and environmental factors in the UK in 2010. Br J Cancer. 2011;105(Suppl 2):S77–S81. doi: 10.1038/bjc.2011.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amir E, Freedman OC, Seruga B, Evans DG. Assessing women at high risk of breast cancer: a review of risk assessment models. J Natl Cancer Inst. 2010;102(10):680–691. doi: 10.1093/jnci/djq088. [DOI] [PubMed] [Google Scholar]
- 11.Kurian AW, Munoz DF, Rust P, Schackmann EA, Smith M, Clarke L, Mills MA, Plevritis SK. Online tool to guide decisions for BRCA1/2 mutation carriers. J Clin Oncol. 2012;30(5):497–506. doi: 10.1200/JCO.2011.38.6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23(7):1111–1130. doi: 10.1002/sim.1668. [DOI] [PubMed] [Google Scholar]
- 13.Lee AJ, Cunningham AP, Kuchenbaecker KB, Mavaddat N, Easton DF, Antoniou AC, Consortium of Investigators of Modifiers of BRCA1/2, Breast Cancer Association Consortium (2014) BOADICEA breast cancer risk prediction model: updates to cancer incidences, tumour pathology and web interface. Br J Cancer 110(2):535–545. doi:10.1038/bjc.2013.730 [DOI] [PMC free article] [PubMed]
- 14.Antoniou AC, Cunningham AP, Peto J, Evans DG, Lalloo F, Narod SA, Risch HA, Eyfjord JE, Hopper JL, Southey MC, Olsson H, Johannsson O, Borg A, Pasini B, Radice P, Manoukian S, Eccles DM, Tang N, Olah E, Anton-Culver H, Warner E, Lubinski J, Gronwald J, Gorski B, Tryggvadottir L, Syrjakoski K, Kallioniemi OP, Eerola H, Nevanlinna H, Pharoah PD, Easton DF. The BOADICEA model of genetic susceptibility to breast and ovarian cancers: updates and extensions. Br J Cancer. 2008;98(8):1457–1466. doi: 10.1038/sj.bjc.6604305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacInnis RJ, Bickerstaffe A, Apicella C, Dite GS, Dowty JG, Aujard K, Phillips KA, Weideman P, Lee A, Terry MB, Giles GG, Southey MC, Antoniou AC, Hopper JL. Prospective validation of the breast cancer risk prediction model BOADICEA and a batch-mode version BOADICEACentre. Br J Cancer. 2013;109(5):1296–1301. doi: 10.1038/bjc.2013.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quante AS, Whittemore AS, Shriver T, Hopper JL, Strauch K, Terry MB. Practical problems with clinical guidelines for breast cancer prevention based on remaining lifetime risk. J Natl Cancer Inst. 2015 doi: 10.1093/jnci/djv124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collins IM, Steel E, Mann GB, Emery JD, Bickerstaffe A, Trainer A, Butow P, Pirotta M, Antoniou AC, Cuzick J, Hopper J, Phillips KA, Keogh LA. Assessing and managing breast cancer risk: clinicians’ current practice and future needs. Breast. 2014;23(5):644–650. doi: 10.1016/j.breast.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 18.Phillips KA, Steel EJ, Collins I, Emery J, Pirotta M, Mann GB, Butow P, Hopper JL, Trainer A, Moreton J, Antoniou AC, Cuzick J, Keogh L. Transitioning to routine breast cancer risk assessment and management in primary care: what can we learn from cardiovascular disease? Aust J Prim Health. 2015 doi: 10.1071/PY14156. [DOI] [PubMed] [Google Scholar]
- 19.University of Cambridge Centre for Cancer Genetic Epidemiology BOADICEA risk estimation tool. https://pluto.srl.cam.ac.uk/cgi-bin/bd3/v3/bd.cgi. Accessed Sept 2015
- 20.Australian Institute of Health and Welfare (AIHW) ACIM (Australian Cancer Incidence and Mortality) Books (2012) http://aihw.gov.au/acim-books/. Accessed Sept 2015
- 21.Lostumbo L, Carbine NE, Wallace J (2010) Prophylactic mastectomy for the prevention of breast cancer. Cochrane Database Syst Rev (11):CD002748. doi:10.1002/14651858.CD002748.pub3 [DOI] [PubMed]
- 22.Nelson HD, Fu R, Griffin JC, Nygren P, Smith ME, Humphrey L (2009) Systematic review: comparative effectiveness of medications to reduce risk for primary breast cancer. Ann Intern Med 151(10):703–715, W-226-735. doi:10.7326/0003-4819-151-10-200911170-00147 [DOI] [PubMed]
- 23.Eisen A, Lubinski J, Klijn J, Moller P, Lynch HT, Offit K, Weber B, Rebbeck T, Neuhausen SL, Ghadirian P, Foulkes WD, Gershoni-Baruch R, Friedman E, Rennert G, Wagner T, Isaacs C, Kim-Sing C, Ainsworth P, Sun P, Narod SA. Breast cancer risk following bilateral oophorectomy in BRCA1 and BRCA2 mutation carriers: an international case–control study. J Clin Oncol. 2005;23(30):7491–7496. doi: 10.1200/JCO.2004.00.7138. [DOI] [PubMed] [Google Scholar]
- 24.Hartmann LC, Schaid DJ, Woods JE, Crotty TP, Myers JL, Arnold PG, Petty PM, Sellers TA, Johnson JL, McDonnell SK, Frost MH, Jenkins RB. Efficacy of bilateral prophylactic mastectomy in women with a family history of breast cancer. N Engl J Med. 1999;340(2):77–84. doi: 10.1056/NEJM199901143400201. [DOI] [PubMed] [Google Scholar]
- 25.Trevena L, Zikmund-Fisher FB, Edwards A et al (2012) Presenting probabilities: update of the International Patient Decision Aids Standards (IPDAS) Collaboration’s background document, chap C. http://ipdas.ohri.ca/IPDAS-Chapter-C.pdf. Accessed Sept 2015
- 26.Dorval M, Bouchard K, Chiquette J, Glendon G, Maugard CM, Dubuisson W, Panchal S, Simard J. A focus group study on breast cancer risk presentation: one format does not fit all. Eur J Hum Genet. 2013;21(7):719–724. doi: 10.1038/ejhg.2012.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kincaid JP, Fishburne RP Jr, Rogers RL, Chissom BS (1975) Derivation of new readability formulas (automated readability index, fog count and flesch reading ease formula) for navy enlisted personnel. DTIC Document
- 28.Sargent RG. Verification and validation of simulation models. J Simul. 2013;7(1):12–24. doi: 10.1057/jos.2012.20. [DOI] [Google Scholar]
- 29.East EW, Kirby JG, Liu LY. Verification and validation of a project collaboration tool. Autom Constr. 2008;17(2):201–214. doi: 10.1016/j.autcon.2007.04.003. [DOI] [Google Scholar]
- 30.Sargent RG (2005) Verification and validation of simulation models. In: Proceedings of the 37th conference on winter simulation, pp 130–143
- 31.Dite GS, Jenkins MA, Southey MC, Hocking JS, Giles GG, McCredie MR, Venter DJ, Hopper JL. Familial risks, early-onset breast cancer, and BRCA1 and BRCA2 germline mutations. J Natl Cancer Inst. 2003;95(6):448–457. doi: 10.1093/jnci/95.6.448. [DOI] [PubMed] [Google Scholar]
- 32.Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, Jong RA, Hislop G, Chiarelli A, Minkin S, Yaffe MJ. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356(3):227–236. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- 33.Mavaddat N, Pharoah PD, Michailidou K, et al. Prediction of breast cancer risk based on profiling with common genetic variants. J Natl Cancer Inst. 2015 doi: 10.1093/jnci/djv036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mandl KD, Kohane IS. Tectonic shifts in the health information economy. N Engl J Med. 2008;358(16):1732–1737. doi: 10.1056/NEJMsb0800220. [DOI] [PubMed] [Google Scholar]
- 35.Kotsopoulos J, Lubinski J, Lynch HT, Kim-Sing C, Neuhausen S, Demsky R, Foulkes WD, Ghadirian P, Tung N, Ainsworth P, Senter L, Karlan B, Eisen A, Eng C, Weitzel J, Gilchrist DM, Blum JL, Zakalik D, Singer C, Fallen T, Ginsburg O, Huzarski T, Sun P, Narod SA. Oophorectomy after menopause and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. Cancer Epidemiol Biomark Prev. 2012;21(7):1089–1096. doi: 10.1158/1055-9965.EPI-12-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Terry MB, Phillips KA, Daly MB, John EM, Andrulis IL, Buys SS, Goldgar DE, Knight JA, Whittemore AS, Chung WK, Apicella C, Hopper JL. Cohort profile: the Breast Cancer Prospective Family Study Cohort (ProF-SC) Int J Epidemiol. 2015 doi: 10.1093/ije/dyv118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Collins IM, Milne RL, Weideman PC, McLachlan SA, Friedlander ML, Kathleen Cuningham Foundation Consortium For Research Into Familial Breast Cancer, Hopper JL, Phillips KA (2013) Preventing breast and ovarian cancers in high-risk BRCA1 and BRCA2 mutation carriers. Med J Aust 199(10):680–683 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.