Abstract
Rationale and objectives
Duration of weaning from mechanical ventilation may be reduced by the use of a systematic approach. We assessed whether a closed-loop knowledge-based algorithm introduced in a ventilator to act as a computer-driven weaning protocol can improve patient outcomes as compared to usual care.
Methods and measurements
We conducted a multicenter randomized controlled study with concealed allocation to compare usual care for weaning to computer-driven weaning. The computerized protocol included an automatic gradual reduction in pressure support, automatic performance of spontaneous breathing trials (SBT), and generation of an incentive message when a SBT was successfully passed. One hundred forty-four patients were enrolled before weaning initiation. They were randomly allocated to computer-driven weaning or to physician-controlled weaning according to local guidelines. Weaning duration until successful extubation and total duration of ventilation were the primary endpoints.
Main results
Weaning duration was reduced in the computer-driven group from a median of 5 to 3 days (P=0.01) and total duration of mechanical ventilation from 12 to 7.5 days (P=0.003). Reintubation rate did not differ (23 vs 16 %, P=0.40). Computer-driven weaning also decreased median intensive-care-unit stay duration from 15.5 to 12 days (P=0.02) and caused no adverse events. The amount of sedation did not differ between groups. In the usual care group compliance to recommended modes and to SBT was estimated respectively at 96% and 51%.
Conclusions
The specific computer-driven system used in this study can reduce mechanical ventilation duration and intensive-care-unit length of stay, as compared to physician-controlled weaning process.
Keywords: Aged; Clinical Protocols; Retrospective Studies; Therapy, Computer-Assisted; Treatment Outcome; Ventilator Weaning; Female; Follow-Up Studies; Humans; Intensive Care Units; Length of Stay; Male; Middle Aged; Respiratory Insufficiency
INTRODUCTION
The weaning process accounts for approximately forty percent of the total duration of mechanical ventilation.(1, 2) Undue prolongation of mechanical ventilation can lead to an increased risk of infectious complications, mainly nosocomial pneumonia,(3, 4) but premature extubation followed by reintubation is associated with increased morbidity and mortality.(5) Thus, a major goal is to recognize readiness for extubation as soon and as reliably as possible. Clinical judgment is far from perfect and often tends to prolong mechanical ventilation.(6–8) Thus, studies have shown that the duration of mechanical ventilation, and most notably of the weaning period, can be shortened by using a systematic approach for reducing the level of assistance and testing the possibility to resume spontaneous breathing.(6, 9)
A closed-loop knowledge-based system has been developed and tested over the last few years as a method for driving pressure-support ventilation.(10) This system interprets clinical data in real-time and provides continuous adjustment of the level of assistance delivered to intubated or tracheotomized patients. The system has been described elsewhere (10–14). In brief, it is embedded in a standard ventilator and adapts the level of pressure support to continuously recorded data on patient’s ventilatory needs, with the goal of keeping the patient within a “comfort” zone. Comfort is defined primarily as a respiratory rate that can vary freely in the 15–30 breaths-per-minute range (up to 34 in patients with neurological disease), a tidal volume above a minimum threshold, and an end-tidal CO2 level below a maximum threshold. The level of pressure support is periodically adapted by the system (10, 13) in steps of 2 to 4 cm of water. The system automatically tries to reduce the pressure level down to a minimal value. At this value, a trial of “spontaneous breathing” with the minimal low pressure support is performed. When successful, a message on the screen recommends separation from the ventilator.
It therefore adapts and reduces the level of assistance at a pace tailored to the individual patient’s needs and evaluates the patient’s ability to be separated from the ventilator. Such a system has previously been shown to reduce the duration of ventilation spent with excessive levels of respiratory work,(13) and to improve extubation readiness prediction.(11) Such a system can be used safely over prolonged periods of mechanical ventilation (15).
Applying guidelines to real-life clinical practice has been found difficult.(16, 17) The closed-loop system constitutes an automated, continuous, protocol-driven ventilation and weaning process that may help to improve compliance with guidelines, including a prompt to physiscians when readiness testing is successful. Although it may not outperform a strictly followed and aggressive weaning protocol, it may be better than usual care. We tested this hypothesis in a multicenter randomized controlled trial versus usual weaning processes. This work has been presented in abstract form (18).
METHODS
A detailed methods section is available in the on-line supplement.
Patients
This study was conducted in five teaching-hospital medical-surgical intensive care units in Barcelona (Spain), Brussels (Belgium), Créteil (France), Geneva (Switzerland), and Paris (France). Each center obtained approval of the study from the ethics committee. Signed informed consent was obtained from each patient or next of kin.
Patients under mechanical ventilation for at least 24 hours and ventilated using an assisted mode were screened for eligibility at an early stage, before usual criteria for weaning readiness were present (Figure 1). Enrolment criteria required absence of the following: do-not-resuscitate order, expected poor short-term prognosis, tracheostomy, and cardiac arrest with a poor neurological prognosis. Inclusion criteria were pulse oximetry > 90 percent with a fraction of inspired oxygen ≤50 percent, positive end-expiratory pressure level ≤ 8 cm of water, no need for epinephrine or norepinephrine at a rate >1 mg per hour, body temperature between 36 °C and 39 °C, and a stable neurological status with little or no sedation.
Figure 1.
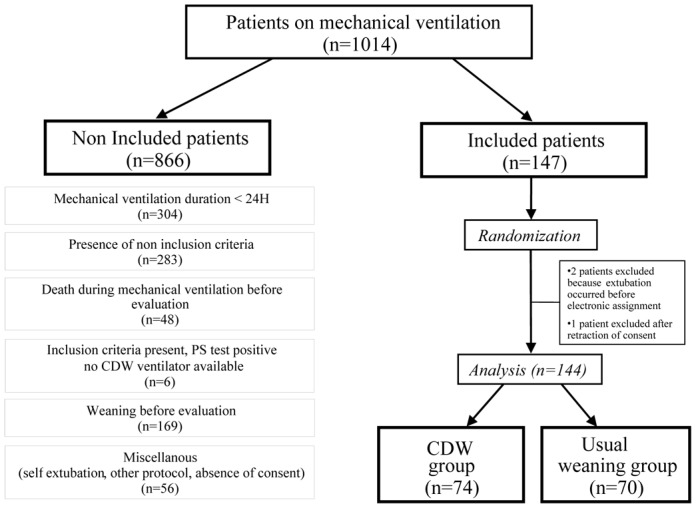
Flow chart of the study. This chart shows the results of daily screening for study inclusion in the five participating centers during the study period. Mean duration of center participation was 171 days (range, 79 to 284 days).
PS denotes pressure support and CDW computer-driven weaning.
Study protocol
As soon as patients met the inclusion criteria, a pre-inclusion test with pressure support at ≥ 15 cm of water was performed to assess the patient’s ability to tolerate this mode. The test was positive at 30 minutes if the patient remained clinically stable, with no hemodynamic or respiratory distress.
Patients were then allocated at random to ventilation with an Evita 4 ventilator (Dräger, Lübeck, Germany) equipped with the system or to the usual care (control) group. In the usual care arm, weaning was conducted according to usual local practice (guidelines were available in four units). In all centers, weaning was conducted based on written guidelines, as follows (i) Once daily or more, screening for criteria to decide for a SBT (T-piece or PSV±PEEP) had to be performed, (ii) SBT might be performed as soon as criteria were present (iii) after succeeding a SBT, standardized extubation criteria were used. These principles and the local guidelines are detailed in the online supplement. We did not assess compliance to guidelines not to influence practice. In this group, ventilatory settings were chosen by the physician in charge of the patient.
Randomization was concealed and generated by an electronic-mail system. The randomization was stratified by center and on the presence of an underlying disease (chronic obstructive pulmonary disease, central neurological disease, or none).
Identical criteria were used in both groups to switch back to assist-control ventilation in case of worsening. The patient was then retested, and returned to the same arm when the test was positive.
End-points
The primary end-points were the time to successful extubation, defined as the time from inclusion until successful extubation (followed by 72 hours without ventilator support) and the total duration of mechanical ventilation.
Secondary end-points were the duration of ventilatory support until first extubation, length of intensive care unit and hospital stay, number of complications in the intensive care unit, number of nosocomial pneumonia, and mortality rates in the intensive care unit and hospital.
Statistical analysis
The sample size of 75 in each group was chosen to give power of 0.80 to detect a reduction in weaning time of 2 days (from 7 to 5 days, 30%), assuming a standard deviation of 5 days and a two-sided test at the 0.05 level. The analysis was performed in the two groups as treated. Results are given as medians (25th–75th interquartile ranges). Proportions were compared using the chi-square test or the Fisher exact test when required. The Mann-Whitney U test was used to analyze mechanical ventilation durations or length of stay. The cumulative probability of remaining on mechanical ventilation was analysed by the Kaplan-Meier method and a log-rank test was used to assess differences. P values smaller than 0.05 were considered significant. All the P values were two-sided.
RESULTS
Patients
Patients were enrolled from September 1st, 2002, to July 12th, 2003. Mean duration of participation per center was 171 days; 40 patients were enrolled in Brussels, 39 in Barcelona, 34 in Créteil, 18 in Geneva, and 16 in Paris. Figure 1 indicates the number of patients receiving invasive mechanical ventilation in the study centers, and the 147 patients included. Two patients were extubated before being randomized to the computer-driven weaning group, due to a delay in the electronic randomization procedure, and one control group patient was excluded because the family withdrew their consent. This left 144 patients for the data analysis, 74 in the intervention group and 70 in the control group.
Patient characteristics at baseline are shown in Table 1. Patients were similar for most characteristics, including the number of patients with chronic obstructive pulmonary disease or central neurological disorders. Duration of mechanical ventilation before inclusion was similar in the two groups. The values used for the pressure-support test, including the positive end-expiratory pressure and fraction of inspired oxygen, were also similar.
TABLE 1.
BASELINE CHARACTERISTICS OF THE STUDY PATIENTS
| VARIABLE | CDW group (N = 74) | Usual weaning group (N = 70) | P Value |
|---|---|---|---|
| Age - yr | 60 (51–74) | 62 (52 – 72) | 0.76 |
| Sex Male/Female (no.) | 47/27 | 45/25 | 0.99 |
| SAPS II at amission | 49 (39 – 57) | 47.50 (38 – 50) | 0.89 |
| LODS at admission | 7 (5–9) | 7 (5–10) | 0.65 |
| LODS at inclusion | 5 (3–7) | 5 (3–7) | 0.65 |
| Mc Cabe no. (%) | |||
| 1 | 38 (51) | 37 (53) | |
| 2 | 31 (42) | 28 (40) | 0.97 |
| 3 | 5 (7) | 5 (7) | |
| Admission type no. (%) | |||
| Medical | 51 (68) | 47 (67) | |
| Elective surgery | 11 (15) | 10 (14) | 0.93 |
| Emergent surgery | 12 (16) | 13 (19) | |
| Comorbidities no. (%) | |||
| COPD | 16 (22) | 13 (19) | 0.68 |
| Restrictive respiratory insufficiency | 3 (4) | 4 (6) | 0.71 |
| Asthma | 2 (3) | 1 (1) | 0.99 |
| Ischemic heart disease | 12 (16) | 6 (9) | 0.21 |
| Hypertensive heart disease | 5 (7) | 6 (9) | 0.76 |
| Valvular heart disease | 5 (7) | 7 (10) | 0.56 |
| Peripheral neurological disorder | 1 (1) | 4 (6) | 0.20 |
| Central neurological disorder | 8 (11) | 5 (7) | 0.56 |
| Psychiatric disorder | 9 (12) | 5 (7) | 0.40 |
| Immunosuppression | 8 (11) | 9 (13) | 0.79 |
| At least one comorbidities | 51 (69) | 43 (63) | 0.48 |
| Pressure support test at inclusion | |||
| Level of PS (cmH 2O) | 18 (15–20) | 16 (15–20) | 0.14 |
| Level of PEEP (cmH 2O) | 5 (5–6) | 5 (5–6) | 0.52 |
| Level of FiO 2 (%) | 35 (30–40) | 35 (30–40) | 0.95 |
| Duration of invasive mechanical ventilation before inclusion (Days) † | 3.50 (2–6) | 4 (3–7) | 0.08 |
CDW denotes computer-driven weaning, SAPS II simplified acute physiologic score II, LODS logistic organ dysfunction score, COPD chronic obstructive pulmonary disease, PS pressure support, and PEEP positive end expiratory pressure. Values are expressed as medians (interquartile range), or numbers (percentage).
The duration of invasive mechanical ventilation before inclusion is the time on endotracheal mechanical ventilation prior to study inclusion.
Outcome
The main results are shown in Table 2. The weaning time was greatly reduced with the computer-driven weaning as compared to usual weaning, whether or not the time on post-extubation noninvasive ventilation was counted. The total duration of mechanical ventilation and the duration of the intensive-care-unit stay were also significantly reduced with the computer-driven weaning, when considering the total population as well as patients alive at ICU discharge (Table E1). No difference was found for hospital length of stay.
TABLE 2.
COMPARISON OF THE OUTCOMES IN THE TWO GROUPS
| OUTCOME median no. of days (interquartile range) | CDW group (N = 74) | Usual weaning group (N = 70) | P Value |
|---|---|---|---|
| Time to first extubation† | 2.00 (1.75–6.25) | 4.00 (2.00–8.25) | 0.02 |
| Duration of mechanical ventilation until first extubation† | 6.50 (3.00–12.25) | 9.00 (5.75–16.00) | 0.03 |
| Time to successful extubation * | 3.00 (2.00–8.00) | 5.00 (2.00–12.00) | 0.01 |
| Total duration of mechanical ventilation * | 7.50 (4.00–16.00) | 12.00 (7.00–26.00) | 0.003 |
| Intensive care length of stay | 12.00 (6.00–22.00) | 15.50 (9.00–33.00) | 0.02 |
| Hospital length of stay | 30.00 (17.00–54.75) | 35.00 (21.00–60.25) | 0.22 |
CDW denotes computer-driven weaning.
The time to first extubation is the time from study inclusion (first positive pressure-support test) to first extubation.
The time to successful extubation is the time from study inclusion (first positive pressure-support test) to last successful extubation. Total duration of mechanical ventilation is the time from intubation to first or last successful extubation. Data are expressed as medians (25th–75th interquartile range).
Mortality in the intensive care unit was similar in the computer-driven weaning group and the usual group (21.6 vs. 22.9 percent, P=1.0), as was hospital mortality (37.8 vs. 28.6 percent, P=0.29). Mortality while connected to the ventilator during the weaning phase was also similar in the computer-driven weaning and control groups (6 and 5 patients, respectively, P=0.70).
The probability of remaining on mechanical ventilation is shown in Figure 2, and was significantly reduced with the computer-driven weaning (log-rank test P=0.015). Data concerning survivors only are shown in Table E1 in the on-line supplement.
Figure 2.
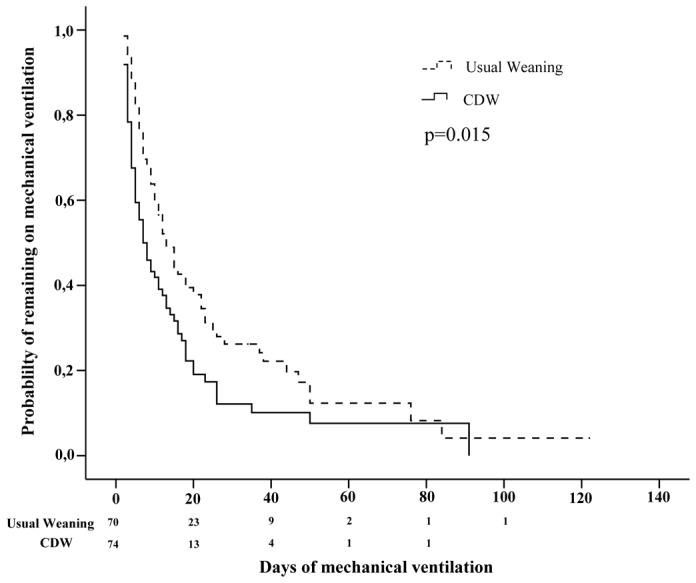
Kaplan-Meier analysis of weaning time until successful extubation or death after inclusion for all included patients, in each study group.
CDW denotes computer-driven weaning.
Complications
Complications are reported in Table 3. The need for noninvasive ventilation was almost halved in the group. The total number of ventilation-related complications (reintubation, self-extubation, need for noninvasive ventilation, mechanical ventilation longer than 21 days, and tracheotomy) was reduced by 30 percent with the computer-driven weaning compared to the usual group. In the computer-driven weaning and control groups, ventilator-associated pneumonia occurred in 13 and 11 patients, and pneumothorax in 0 and 2 patients respectively.
TABLE 3.
COMPLICATIONS OF MECHANICAL VENTILATION
| COMPLICATION | CDW group (N = 74) | Usual weaning group (N = 70) | P Value |
|---|---|---|---|
| no. of patients (%) | |||
| Reintubation within 72 h | 12 (16) | 16 (23) | 0.40 |
| Any reintubation | 17 (23) | 23 (33) | 0.20 |
| Need for non invasive ventilation | 14 (19) | 26 (37) | 0.02 |
| Self-extubation | 8 (11) | 7 (10) | 0.99 |
| Tracheostomy | 12 (16) | 13 (19) | 0.83 |
| Mechanical ventilation duration > 14 d | 12 (16) | 20 (29) | 0.11 |
| Mechanical ventilation duration > 21 d | 5 (7) | 11 (16) | 0.11 |
CDW denotes computer-driven weaning. Figures denote number of patients (percentage).
Mechanical ventilation
Patients were ventilated with pressure support for 392 days in the usual weaning group and 293 days in the computer-driven weaning group. The modes of ventilation recommended in the guidelines (PS for the weaning phase and ACV in case of worsening) were used 92 and 96% of the time after inclusion in the computer driven weaning group and in the usual weaning group respectively. Alternatively, SIMV was used in 8% and 4% of the time (Table E2). A T-piece trial was performed 124 times in the usual weaning group and 12 times (in 8 patients) in the computer-driven weaning group. In the usual care group, we estimated compliance to recommendations for using spontaneous breathing trials: T-piece trials were performed 51% of the days of ventilation with PSV or SIMV at FiO2 below 50% in the usual weaning group. In the computer-driven weaning group, the mean time from display of the message recommending separation from the ventilator to extubation was 0.6 ± 2.65 days (median 1, 25th – 75th 0–2), minimum of 0 days, and maximum of 15 days. Only 42% of the patients were extubated the day of the message.
Technical problems recorded with the computer-driven weaning were as follows. In five patients, a total of eleven episodes of transient system interruption occurred over a total of 293 days of ventilation using this system. During the interruptions, ventilatory assistance was delivered in standard pressure-support mode. In 10 patients, the system was voluntarily stopped because worsening of the clinical condition required assist-control ventilation. In five patients, a manual increase in pressure support was deemed necessary by the physician, and a manual decrease in three patients. Two instances of CO2 sensor dysfunction requiring removal of the computer-driven weaning system occurred in one center.
The amount of sedatives used did not differ between the groups during the intubation-to-inclusion period and the inclusion-to-extubation period (see Table 4 and Table E3 in the on-line supplement). Use of steroids and neuromuscular blocking agents before and after inclusion was also similar in the two groups.
TABLE 4.
USE OF OPIOIDS, SEDATIVES, NEUROMUSCULAR BLOCKERS, AND CORTICOSTEROIDS*
| CDW group (N = 74) | Usual weaning group (N = 70) | P value | |
|---|---|---|---|
| Sedative agents | |||
| Cumulative daily dosage (midazolam-equivalent, mg) | |||
| Before inclusion | 49 (25–81) | 46 (28–81) | 0.74 |
| After inclusion | 0 (0–8) | 0.7 (0–16) | 0.14 |
| Opioids | |||
| Cumulative daily dosage (fentanyl-equivalent, μg) | |||
| Before inclusion | 100 (0–795) | 170 (0–1312) | 0.51 |
| After inclusion | 0 (0–50) | 0 (0–100) | 0.08 |
| Neuromuscular blockers | |||
| % of days with NMBs before inclusion | 0 (0–0) | 0 (0–0) | 0.10 |
| % of days with NMBs after inclusion | 0 (0–0) | 0 (0–0) | 0.25 |
| Corticosteroids | |||
| % of days with corticosteroids before inclusion | 0 (0–33) | 0 (0–62) | 0.49 |
| % of days with corticosteroids after inclusion | 0 (0–0) | 0 (0–34) | 0.36 |
Opioids, sedatives, neuromuscular blocking agents (NMB), and corticosteroids used in each group. This table shows the cumulative daily dosages of sedatives per patient (in mg midazolam equivalent) (34) and opioids (in μg fentanyl equivalent) (35) and the percentage of days under neuromuscular blockers and corticosteroids. These data are given for the periods of ventilation before and after study inclusion. Data are expressed as medians (25th–75th interquartile range).
CDW denotes computer-driven weaning, NMB neuromuscular blockers
DISCUSSION
In this study, a computer-driven weaning protocol performed better than usual care based on written weaning guidelines. Weaning time was nearly halved with the computer-driven weaning as compared to usual weaning. The system used in this study was developed several years ago and has been repeatedly evaluated since then. (10–14) It ensures that the desired ventilation protocol is applied. In the usual weaning group, weaning was performed according to local guidelines, representing the usual care in these university centers involved in respiratory and weaning research. The reduction in weaning duration was associated with decreases in both the total duration of mechanical ventilation, and the intensive care unit length of stay.
Weaning protocols or guidelines recommending a systematic approach have been shown to reduce the duration of weaning and mechanical ventilation (6, 9) and are often recommended.(19) In a randomized controlled study, Ely et al. showed that routine daily screening and identification of the patients able to breathe spontaneously reduced weaning duration from a median of 3 days to 1 day and the total duration of mechanical ventilation from 6 days to 3.5 days.(6) Kollef et al. also showed a reduction in the duration of mechanical ventilation in patients weaned using protocols, from a median of 1.8 to 1.4 days.(9) The implementation of protocols, however, is time-consuming,(16) requires staff training, is not always followed faithfully,(17) and varies in efficacy according to all these factors.(20–22) Protocols may even not be necessary in well-staffed centres (20). In the present study, written weaning guidelines were compared to a closed-loop knowledge-based ventilation. The duration of weaning was significantly decreased, from a median of 5 days to 3 days in the computer-driven weaning group, and the total duration of mechanical ventilation decreased from 12 to 7.5 days. The duration of weaning was slightly longer in the present study than in the previously mentioned studies. This is in part because the type of patients was different,(9) and also because patients were included at an early stage, as soon as they were able to tolerate moderate to high pressure support levels and before they met criteria for readiness testing and weaning.
In our study, several reasons may explain the reduction of mechanical ventilation duration in the computer-driven weaning group. Automation of the weaning protocol may explain an essential part of the results. The system is designed to perform several tasks comparable to a weaning protocol 24 hours a day and 7 days a week: to automatically and gradually reduce the ventilatory assistance, to automatically perform the equivalent of a spontaneous breathing trial and to display an incentive message when the patient is deemed ready to breathe spontaneously. Although the reduction in pressure support applied by the system is gradual, complete weaning can be obtained in less than 24 hours, thus allowing rapid detection of readiness for extubation. This computer-driven weaning protocol has advantages compared to a human-driven protocol. The computer-driven weaning protocol does not depend on the willingness or availability of the staff, and full compliance with the weaning protocol is therefore ensured. A permanent evaluation and adjustment of ventilatory support can not be continuously performed by care-givers, and the system has the ability to determine more easily and rapidly than usual care the time for a possible separation from the ventilator. It is likely that the message delivered by the system also constitutes a strong incentive for the clinician to consider a possible extubation. This visual prompt constitutes an important aspect of the “computer-driven protocol”.
Other specific features of the computerized protocol used in the study, which may differ from human directed protocols, should be underlined. The computerized protocol used in the study, takes into account the history of breathing pattern and the previous modifications of the assistance level to decide for the setting. One important feature is that the decision process of the system is designed to accept transient instabilities, such as a short increase in respiratory frequency, without changing the ventilation classification. The system is also able to perform the final test at any time and to repeat it whenever possible, increasing the opportunity to find a successful test. This temporal reasoning may differ from an automated or even a human-driven approach where one single measurement or test is performed.
It is possible, however, that the rigor with which weaning assessment was performed in the control group was suboptimal (e.g. with less assessment on week-ends or in case of major variations in overall workload in the units), as often observed in the real life. Such a suboptimal approach could also participate in the difference between the two groups, but our design did not allow addressing this question.
The need for reintubation within 72 hours after extubation tended to be lower with the computer-driven weaning (16.2 vs. 22.9 percent), but not significantly. This failure rate is in the higher end of the reported range. In recent studies, reintubation rates were 11 percent,(23) 15.7 percent,(24) 23.5 percent,(25) and 14.5 percent.(26) A relatively high extubation rate was expected because patients on mechanical ventilation for less than 24 hours were not included in the study. The need for noninvasive ventilation after extubation was reduced to 18.9 percent in the computer-driven weaning group as compared to 37.1 percent in the usual group. The rate of respiratory failure after extubation with a potential need for noninvasive ventilation was 23 percent in the study by Keenan et al.(27) and 22.5 percent in the study by Esteban et al.(28) The difference with our study may be ascribable to differences in patient selection, with higher severity scores in our population, and to the experience of the centers with this technique.
The trend for a reduction in reintubation and in the need for non-invasive ventilation in the computer-driven weaning group may be explained by physiological benefits of the system previously demonstrated, since adjusting the level of assistance to the breathing pattern may avoid periods of excessive work of breathing. In a previous study,(13) patients were ventilated successively with the computer-driven weaning and with standard pressure support. The time spent in the comfort zone of ventilation was 93±8 percent with automatic pressure support and 66±24 percent with standard pressure support (p<0.05). The time spent with a high airway occlusion pressure (suggesting excessive work of breathing) was significantly lower with automatic pressure support. The level of pressure support was modified 56±40 times over a 24-hour period in the computer-driven weaning group versus 1±2 times in the standard pressure support group. Repeated periods of excessive workload during mechanical ventilation may slow recovery from diaphragmatic fatigue and/or aggravate diaphragm weakness, a frequent finding in difficult-to-wean patients.(29)
This study has limitations. The results can not be generalized to all patients since only a small proportion of eligible patients were randomized (14%). The rationale, however, extends at least to patients with a short weaning duration and further studies will be needed including this group. In a few patients, the closed-loop was interrupted, either for technical reasons or because the clinicians disagreed with the settings. More work is needed to determine which patients may be poor candidates for ventilation with the system. Another limitation is that blinding of the investigators was not feasible, which may have favored the computer-driven weaning group. The selection of controls is an important issue in randomized trials of mechanical ventilation and has recently been a focus of debate.(30) It has been suggested that usual care should be applied in the control group when feasible,(31) as in the study by Ely et al.(6) The control group in our study was managed based on written weaning guidelines used routinely in each center. These guidelines had been in use for several years in all study centers and included daily screening and spontaneous breathing tests. Compliance with guidelines, however, was not evaluated in the usual weaning group, as our goal was to keep usual weaning practices unchanged. Compliance with weaning protocols is frequently relatively low.(17, 22, 32) In the study by Ely et al., after the training period, compliance was 81 percent in medical intensive care units and 63 percent in surgical units, and poor compliance was often related to the T-piece trial.(17) With the computer-driven weaning, T-piece trials are not required to test the patient’s readiness for extubation, as the spontaneous breathing trials are automatically carried out with low levels of pressure support. In the present study, 12 T-piece trials were nevertheless performed in the computer-driven weaning arm, as compared to 124 in the usual weaning group. In the usual weaning group, we estimated from the number of performed T-piece trials that compliance with recommendations for testing spontaneous breathing with trials was about 51%. This calculation, however, only takes into account spontaneous breathing trials performed with T-piece, and not those performed in pressure support, which were not recorded. The level of compliance may then have been underestimated by this estimation. In the future, comparison with protocolized weaning rather than usual care may be required.
In conclusion, we have shown in the present study that weaning duration from mechanical ventilation could be reduced using a system which automatically drives the level of pressure support, automatically performs spontaneous breathing trials and displays an incentive message when the trial is successfully passed. Milic-Emili asked whether weaning was an art or a science.(33) Science is gaining ground as knowledge accumulates from physiological studies and randomized trials. We think that incorporation of this knowledge into a computer-driven weaning system is a step forward in a scientific approach to weaning.
Supplementary Material
Figure E1: This schema shows the phases that preceded and followed study inclusion. In patients who met the inclusion criteria, informed consent was sought from the patient or family. Daily pre-inclusion tests were performed (“pressure-support test”), and randomization occurred when the test was positive. Patients were then weaned from mechanical ventilation either with the computer-driven weaning (CDW) or according to local protocols.
In patients with clinical deterioration, the patients were ventilated in assist-control mode and reassessed daily with a pressure support test. When the pressure support test was again positive, the patient was returned to the ventilation mode assigned by randomization, which was continued throughout the rest of the time on ventilation.
Table E1 Comparisons of outcomes for ICU survivors in the two groups
Table E2. This table shows the modes of ventilation (expressed in days of ventilation and in percentage of the total days of ventilation after inclusion) used after study inclusion in each study group. The number of T-piece trials performed in each group is also displayed.
Table E3 This table shows the average daily doses of sedatives and opioids according to prescription preferences in each centers. Only Brussels used propofol as main sedative, the other centers used midazolam. Creteil, Geneva and Paris used fentanyl as opioid, Barcelona used morphine and Brussels used sufentanyl.
Acknowledgments
Acknowledgment, conflict of interest and source of funding
Stefan Mersmann is employed by Dräger Medical. Laurent Brochard, as head of the clinical research group, has received funding through research contracts with Dräger for the conduct of clinical trials concerning the system. Dräger Medical has provided the centers with the equipment necessary for the study (including the ventilators (EVITA 4) equipped with the Evita Weaning System), and has provided a grant necessary to cover insurance costs, Ethics Committee’s administrative fees, organization of meetings for the investigators and for monitoring purposes.
Footnotes
This article has an online data supplement, which is accessible from this issue’s table of content online at www.atsjournals.org.
References
- 1.Esteban A, Alia I, Ibanez J, Benito S, Tobin MJ. Modes of mechanical ventilation and weaning. A national survey of Spanish hospitals. The Spanish Lung Failure Collaborative Group. Chest. 1994;106:1188–93. doi: 10.1378/chest.106.4.1188. [DOI] [PubMed] [Google Scholar]
- 2.Esteban A, Anzueto A, Frutos F, Alia I, Brochard L, Stewart TE, Benito S, Epstein SK, Apezteguia C, Nightingale P, Arroliga AC, Tobin MJ. Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28-day international study. Jama. 2002;287:345–55. doi: 10.1001/jama.287.3.345. [DOI] [PubMed] [Google Scholar]
- 3.Torres A, Aznar R, Gatell JM, Jimenez P, Gonzalez J, Ferrer A, Celis R, Rodriguez-Roisin R. Incidence, risk, and prognosis factors of nosocomial pneumonia in mechanically ventilated patients. Am Rev Respir Dis. 1990;142:523–8. doi: 10.1164/ajrccm/142.3.523. [DOI] [PubMed] [Google Scholar]
- 4.Fagon JY, Chastre J, Domart Y, Trouillet JL, Pierre J, Darne C, Gibert C. Nosocomial pneumonia in patients receiving continuous mechanical ventilation. Prospective analysis of 52 episodes with use of a protected specimen brush and quantitative culture techniques. Am Rev Respir Dis. 1989;139:877–84. doi: 10.1164/ajrccm/139.4.877. [DOI] [PubMed] [Google Scholar]
- 5.Epstein SK, Ciubotaru RL. Independent effects of etiology of failure and time to reintubation on outcome for patients failing extubation. Am J Respir Crit Care Med. 1998;158:489–93. doi: 10.1164/ajrccm.158.2.9711045. [DOI] [PubMed] [Google Scholar]
- 6.Ely EW, Baker AM, Dunagan DP, Burke HL, Smith AC, Kelly PT, Johnson MM, Browder RW, Bowton DL, Haponik EF. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N Engl J Med. 1996;335:1864–9. doi: 10.1056/NEJM199612193352502. [DOI] [PubMed] [Google Scholar]
- 7.Coplin WM, Pierson DJ, Cooley KD, Newell DW, Rubenfeld GD. Implications of extubation delay in brain-injured patients meeting standard weaning criteria. Am J Respir Crit Care Med. 2000;161:1530–6. doi: 10.1164/ajrccm.161.5.9905102. [DOI] [PubMed] [Google Scholar]
- 8.Stroetz RW, Hubmayr RD. Tidal volume maintenance during weaning with pressure support. Am J Respir Crit Care Med. 1995;152:1034–40. doi: 10.1164/ajrccm.152.3.7663780. [DOI] [PubMed] [Google Scholar]
- 9.Kollef MH, Shapiro SD, Silver P, St John RE, Prentice D, Sauer S, Ahrens TS, Shannon W, Baker-Clinkscale D. A randomized, controlled trial of protocol-directed versus physician-directed weaning from mechanical ventilation. Crit Care Med. 1997;25:567–74. doi: 10.1097/00003246-199704000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Dojat M, Brochard L, Lemaire F, Harf A. A knowledge-based system for assisted ventilation of patients in intensive care units. Int J Clin Monit Comput. 1992;9:239–50. doi: 10.1007/BF01133619. [DOI] [PubMed] [Google Scholar]
- 11.Dojat M, Harf A, Touchard D, Laforest M, Lemaire F, Brochard L. Evaluation of a knowledge-based system providing ventilatory management and decision for extubation. Am J Respir Crit Care Med. 1996;153:997–1004. doi: 10.1164/ajrccm.153.3.8630586. [DOI] [PubMed] [Google Scholar]
- 12.Dojat M, Pachet F, Guessoum Z, Touchard D, Harf A, Brochard L. NeoGanesh: a working system for the automated control of assisted ventilation in ICUs. Artif Intell Med. 1997;11:97–117. doi: 10.1016/s0933-3657(97)00025-0. [DOI] [PubMed] [Google Scholar]
- 13.Dojat M, Harf A, Touchard D, Lemaire F, Brochard L. Clinical evaluation of a computer-controlled pressure support mode. Am J Respir Crit Care Med. 2000;161:1161–6. doi: 10.1164/ajrccm.161.4.9904064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dojat M, Brochard L. Knowledge-based systems for automatic ventilatory management. Respir Care Clin N Am. 2001;7:379–96. viii. doi: 10.1016/s1078-5337(05)70048-4. [DOI] [PubMed] [Google Scholar]
- 15.Bouadma L, Lellouche F, Cabello B, Taille S, Mancebo J, Dojat M, Brochard L. Computer-driven management of prolonged mechanical ventilation and weaning: a pilot study. Intensive Care Med. 2005;31:1446–50. doi: 10.1007/s00134-005-2766-2. [DOI] [PubMed] [Google Scholar]
- 16.Vitacca M, Clini E, Porta R, Ambrosino N. Preliminary results on nursing workload in a dedicated weaning center. Intensive Care Med. 2000;26:796–9. doi: 10.1007/s001340051249. [DOI] [PubMed] [Google Scholar]
- 17.Ely EW, Bennett PA, Bowton DL, Murphy SM, Florance AM, Haponik EF. Large scale implementation of a respiratory therapist-driven protocol for ventilator weaning. Am J Respir Crit Care Med. 1999;159:439–46. doi: 10.1164/ajrccm.159.2.9805120. [DOI] [PubMed] [Google Scholar]
- 18.Lellouche F, Mancebo J, Roesler J, Jolliet P, Schortgen F, Cabello M, Bouadma L, Rodriguez P, Maggiore S, Qader S, Taille S, Brochard L. Computer-driven ventilation reduces duration of weaning: a multicenter randomized controlled study. Intensive Care Medecine. 2004;30:S1–S234. [Google Scholar]
- 19.MacIntyre NR, Cook DJ, Ely EW, Jr, Epstein SK, Fink JB, Heffner JE, Hess D, Hubmayer RD, Scheinhorn DJ. Evidence-based guidelines for weaning and discontinuing ventilatory support: a collective task force facilitated by the American College of Chest Physicians; the American Association for Respiratory Care; and the American College of Critical Care Medicine. Chest. 2001;120:375S–95S. doi: 10.1378/chest.120.6_suppl.375s. [DOI] [PubMed] [Google Scholar]
- 20.Krishnan JA, Moore D, Robeson C, Rand CS, Fessler HE. A Prospective, Controlled Trial of a Protocol-based Strategy to Discontinue Mechanical Ventilation. Am J Respir Crit Care Med. 2004;169:673–678. doi: 10.1164/rccm.200306-761OC. [DOI] [PubMed] [Google Scholar]
- 21.Namen AM, Ely EW, Tatter SB, Case LD, Lucia MA, Smith A, Landry S, Wilson JA, Glazier SS, Branch CL, Kelly DL, Bowton DL, Haponik EF. Predictors of successful extubation in neurosurgical patients. Am J Respir Crit Care Med. 2001;163:658–64. doi: 10.1164/ajrccm.163.3.2003060. [DOI] [PubMed] [Google Scholar]
- 22.Randolph AG, Wypij D, Venkataraman ST, Hanson JH, Gedeit RG, Meert KL, Luckett PM, Forbes P, Lilley M, Thompson J, Cheifetz IM, Hibberd P, Wetzel R, Cox PN, Arnold JH. Effect of mechanical ventilator weaning protocols on respiratory outcomes in infants and children: a randomized controlled trial. Jama. 2002;288:2561–8. doi: 10.1001/jama.288.20.2561. [DOI] [PubMed] [Google Scholar]
- 23.Brochard L, Rauss A, Benito S, Conti G, Mancebo J, Rekik N, Gasparetto A, Lemaire F. Comparison of three methods of gradual withdrawal from ventilatory support during weaning from mechanical ventilation. Am J Respir Crit Care Med. 1994;150:896–903. doi: 10.1164/ajrccm.150.4.7921460. [DOI] [PubMed] [Google Scholar]
- 24.Esteban A, Frutos F, Tobin MJ, Alia I, Solsona JF, Valverdu I, Fernandez R, de la Cal MA, Benito S, Tomas R, et al. A comparison of four methods of weaning patients from mechanical ventilation. Spanish Lung Failure Collaborative Group. N Engl J Med. 1995;332:345–50. doi: 10.1056/NEJM199502093320601. [DOI] [PubMed] [Google Scholar]
- 25.Torres A, Gatell JM, Aznar E, el-Ebiary M, Puig de la Bellacasa J, Gonzalez J, Ferrer M, Rodriguez-Roisin R. Re-intubation increases the risk of nosocomial pneumonia in patients needing mechanical ventilation. Am J Respir Crit Care Med. 1995;152:137–41. doi: 10.1164/ajrccm.152.1.7599812. [DOI] [PubMed] [Google Scholar]
- 26.Epstein SK, Ciubotaru RL, Wong JB. Effect of failed extubation on the outcome of mechanical ventilation. Chest. 1997;112:186–92. doi: 10.1378/chest.112.1.186. [DOI] [PubMed] [Google Scholar]
- 27.Keenan SP, Powers C, McCormack DG, Block G. Noninvasive Positive-Pressure Ventilation for Postextubation Respiratory Distress. JAMA. 2002;287:3238–3244. doi: 10.1001/jama.287.24.3238. [DOI] [PubMed] [Google Scholar]
- 28.Esteban A, Frutos-Vivar F, Ferguson ND, Arabi Y, Apezteguia C, Gonzalez M, Epstein SK, Hill NS, Nava S, Soares MA, D’Empaire G, Alia I, Anzueto A. Noninvasive positive-pressure ventilation for respiratory failure after extubation. N Engl J Med. 2004;350:2452–60. doi: 10.1056/NEJMoa032736. [DOI] [PubMed] [Google Scholar]
- 29.Laghi F, Cattapan SE, Jubran A, Parthasarathy S, Warshawsky P, Choi YS, Tobin MJ. Is weaning failure caused by low-frequency fatigue of the diaphragm? Am J Respir Crit Care Med. 2003;167:120–7. doi: 10.1164/rccm.200210-1246OC. [DOI] [PubMed] [Google Scholar]
- 30.Silverman HJ, Miller FG. Control group selection in critical care randomized controlled trials evaluating interventional strategies: An ethical assessment. Crit Care Med. 2004;32:852–7. doi: 10.1097/01.ccm.0000114814.62759.06. [DOI] [PubMed] [Google Scholar]
- 31.Tobin M. Of Principles of Protocols and Weaning. Am J Respir Crit Care Med. 2004;169:661–662. doi: 10.1164/rccm.2401006. [DOI] [PubMed] [Google Scholar]
- 32.Iregui M, Ward S, Clinikscale D, Clayton D, Kollef MH. Use of a handheld computer by respiratory care practitioners to improve the efficiency of weaning patients from mechanical ventilation. Crit Care Med. 2002;30:2038–43. doi: 10.1097/00003246-200209000-00014. [DOI] [PubMed] [Google Scholar]
- 33.Milic-Emili J. Is weaning an art or a science? Am Rev Respir Dis. 1986;134:1107–1108. doi: 10.1164/arrd.1986.134.6.1107. [DOI] [PubMed] [Google Scholar]
- 34.Higgins TL, Yared JP, Estafanous FG, Coyle JP, Ko HK, Goodale DB. Propofol versus midazolam for intensive care unit sedation after coronary artery bypass grafting. Crit Care Med. 1994;22:1415–23. doi: 10.1097/00003246-199409000-00011. [DOI] [PubMed] [Google Scholar]
- 35.Jacobi J, Fraser GL, Coursin DB, Riker RR, Fontaine D, Wittbrodt ET, Chalfin DB, Masica MF, Bjerke HS, Coplin WM, Crippen DW, Fuchs BD, Kelleher RM, Marik PE, Nasraway SA, Jr, Murray MJ, Peruzzi WT, Lumb PD. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002;30:119–41. doi: 10.1097/00003246-200201000-00020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure E1: This schema shows the phases that preceded and followed study inclusion. In patients who met the inclusion criteria, informed consent was sought from the patient or family. Daily pre-inclusion tests were performed (“pressure-support test”), and randomization occurred when the test was positive. Patients were then weaned from mechanical ventilation either with the computer-driven weaning (CDW) or according to local protocols.
In patients with clinical deterioration, the patients were ventilated in assist-control mode and reassessed daily with a pressure support test. When the pressure support test was again positive, the patient was returned to the ventilation mode assigned by randomization, which was continued throughout the rest of the time on ventilation.
Table E1 Comparisons of outcomes for ICU survivors in the two groups
Table E2. This table shows the modes of ventilation (expressed in days of ventilation and in percentage of the total days of ventilation after inclusion) used after study inclusion in each study group. The number of T-piece trials performed in each group is also displayed.
Table E3 This table shows the average daily doses of sedatives and opioids according to prescription preferences in each centers. Only Brussels used propofol as main sedative, the other centers used midazolam. Creteil, Geneva and Paris used fentanyl as opioid, Barcelona used morphine and Brussels used sufentanyl.


