Graphical abstract
Keywords: DNA methylation, Oral pre-cancer, Bio-marker, Epigenetics, Leukoplakia, Oral sub-mucous fibrosis, Promoter regions, CpG sites, Tumour suppressor genes, Diagnostic marker
Highlights
-
•
We critically reviewed DNA methylation patterns for oral pre-cancer progression.
-
•
Suggestive signals included hyper-methylated loci in p16, p14, MGMT and DAPK.
-
•
Large epigenome-wide explorations are needed to validate these markers and to identify new risk loci and biological pathways of disease progression.
Summary
Although oral cancers are generally preceded by a well-established pre-cancerous stage, there is a lack of well-defined clinical and morphological criteria to detect and signal progression from pre-cancer to malignant tumours. We conducted a critical review to summarize the evidence regarding aberrant DNA methylation patterns as a potential diagnostic biomarker predicting progression. We identified all relevant human studies published in English prior to 30th April 2015 that examined DNA methylation (%) in oral pre-cancer by searching PubMed, Web-of-Science and Embase databases using combined key-searches. Twenty-one studies (18-cross-sectional; 3-longitudinal) were eligible for inclusion in the review, with sample sizes ranging from 4 to 156 affected cases. Eligible studies examined promoter region hyper-methylation of tumour suppressor genes in pathways including cell-cycle-control (n = 15), DNA-repair (n = 7), cell-cycle-signalling (n = 4) and apoptosis (n = 3). Hyper-methylated loci reported in three or more studies included p16, p14, MGMT and DAPK. Two longitudinal studies reported greater p16 hyper-methylation in pre-cancerous lesions transformed to malignancy compared to lesions that regressed (57–63.6% versus 8–32.1%; p < 0.01). The one study that explored epigenome-wide methylation patterns reported three novel hyper-methylated loci (TRHDE; ZNF454; KCNAB3). The majority of reviewed studies were small, cross-sectional studies with poorly defined control groups and lacking validation. Whilst limitations in sample size and study design preclude definitive conclusions, current evidence suggests a potential utility of DNA methylation patterns as a diagnostic biomarker for oral pre-cancer progression. Robust studies such as large epigenome-wide methylation explorations of oral pre-cancer with longitudinal tracking are needed to validate the currently reported signals and identify new risk-loci and the biological pathways of disease progression.
Introduction
Oral cancer is a major public health problem in much of Asia, as well as certain regions of Eastern and Western Europe, Latin America, Caribbean countries and Melanesia [1], [2], [3]. Although high incidence zones in Asia (India, Sri Lanka, Pakistan, Bangladesh and China–Taiwan) contribute to over one third (37.5%) of the total global burden [1], recent trends suggest increasing incidence in the US and in parts of Europe including the United Kingdom [4], [5]. Over a million new cases are reported every year from more developed regions of the World [1], more so among young adults [4], [5].
With a well-defined pre-cancerous stage [6], [7], [8], [9], oral cancer develops through a series of sequential histo-pathological changes (normal, hyperplastic, dysplastic, and carcinoma in-situ) before transforming to invasive disease [7], [9], [10]. Oral pre-cancer can be readily detected in the oral cavity from a visual oral exam and the oral cavity is easily accessible for cytology and biopsy confirmation [11]. Although detection at this early stage significantly reduces the cancer-specific morbidity and mortality [12], oral cancers are mainly detected at a later stage which affects 5-year survival despite improvements in treatment aspects [4]. This is particularly relevant for countries in high incidence zones [3], [4], [13].
Oral pre-cancer is clinically diverse and includes various lesions (leukoplakia, erythroplakia and palatal lesions in reverse smokers) and conditions (submucous fibrosis, lichen planus, actinic keratosis and discoid lupus erythematosus) that are grouped as potentially malignant disorders (PMDs) [14]. Pre-cancerous lesions with dysplasia have shown a 12.3% rate of malignant transformation over a period of 0.5–16 years [10]. The clinico-morphological dilemma pertaining to identification, detection and early treatment of oral pre-cancer, dictates the current ‘wait and watch’ approach for monitoring cancer progression [6], [10]. Both over- and under treatment contribute to considerable patient morbidity [7], [9], [10]. In this scenario where clinical and pathological investigations are very variable in delineating pre-cancer at risk for progression, and a series of epigenetic and genetic alterations signal disease progression, the identification of molecular biomarkers of disease progression could be immensely useful in the early detection of readily reversible lesions, leading to more effective diagnosis and better treatment outcomes [7], [10].
DNA methylation is a physiologic epigenetic modification which occurs primarily on the addition of a methyl group to a CpG dinucleotide in the DNA sequence [15] that regulates gene transcription [16], [17], [18]. Aberrant (more or less) methylation affects the physiological stability of cell division [19], and is considered a mechanism by which environmental risk factors, such as tobacco, alcohol use and diet may influence disease risk [20], [21], [22]. Hyper-methylation of promoter regions (CpG islands) causes silencing of genes primarily involved in tumour suppression such as genes in cell cycle control, DNA repair, and apoptosis pathways [18], [23]. Hypo-methylation of a CpG dinucleotide in the global DNA sequence causes activation of oncogenes such as genes in cell cycle signalling [7], [23]. DNA methylation patterns are reversible and dynamic to adapt with changes in the environment or treatment [18]. Dynamicity if associated with development and progression of cancer might be particularly useful when sensitive detection is required as in the case with early identification of oral pre-cancer that could either progress or regress in stage of disease [17], [18], [23]. Time trends of an increase or a decrease of aberrant methylation could help predict the rate and probability of malignant transformation and also a reversal of disease state respectively. For these reasons, aberrant DNA methylation is thought to be a particularly relevant candidate for evaluation as a biomarker for its potential early diagnostic utility in oral pre-cancer progression [23].
We conducted a review of existing studies on DNA methylation patterns in oral pre-cancer in order to understand the scope of aberrant DNA methylation as a potential diagnostic biomarker for disease progression, and to ascertain knowledge gaps in the literature to guide future research.
Methods
We conducted a literature search in PubMed, Embase and Web of Science to identify all relevant studies of DNA methylation on oral pre-cancer published in the English language prior to April 30th 2015 using the following key words and their combinations in titles and abstracts: “methylation” OR “epigenetics” AND “pre-cancer” OR “premalignant” OR “potentially malignant” OR “leukoplakia” OR “erythroplakia” OR “OSMF” OR “submucous fibrosis” OR “lichen planus” OR “dysplasia” AND “oral” OR “head” OR “neck” AND “humans”. All searches returned studies published after 2001 and the last retrieval was done on 30th of April 2015. A preliminary review of abstracts was conducted to determine study relevance based on the following set of eligibility criteria: (1) DNA methylation in oral pre-cancer from any bio-specimen source (such as tissue or saliva); (2) published in English, and (3) conducted in human subjects (not in vitro or in animals). Studies that met these eligibility criteria were included for further review of the full-text article. Final inclusion was made on availability of quantitative frequency methylation data reported as percent methylation of samples either in cases and controls, or in cases only. Additionally, reference lists of eligible studies were searched for identification of relevant studies.
Data extraction
The following information was extracted from each study when possible and applicable, using a standard data collection form with the following elements: first author, year of publication, study population/location, study design (classified based on whether cross-sectional or longitudinal methylation data were presented), sample size, subject description including age, gender, tobacco/alcohol habits, clinical and pathological description of pre-cancer (such as leukoplakia, oral submucous fibrosis, erythroplakia, lichen planus and histopathological features such as hyperkeratosis, hyperplasia and dysplasia), follow-up time for longitudinal studies, source/type of biospecimen used for methylation analysis, loci examined, function of the loci and method of methylation assay (Table 1). Percent of aberrant methylation in cases and in controls was tabulated for loci consistently reported in biopsy confirmed samples in 3 or more studies (the cut-off of 3 studies as baseline criteria was based on previous systematic review [24] and meta-analysis [25]) (Table 2). Controls were of the following types: (1) Paired samples – biopsy confirmed normal mucosa adjacent to pre-cancer/oral squamous cell carcinoma (OSCC); (2) samples from healthy individuals – healthy mucosa from individuals with no evidence of pre-cancer/OSCC, or (3) pre-cancer regressed on longitudinal follow-up. Cases were either: (1) Biopsy-confirmed dysplastic/non-dysplastic pre-cancer, or (2) pre-cancer transformed into cancer on longitudinal follow-up (Table 2).
Table 1.
Characteristics of all reviewed studies (n = 21).
| N | Author/year/study population | Study design | Cases | Controls |
Socio-demographic risk factor data | Sampleanalyzed | Technique | Loci examined | Pathway/function | |
|---|---|---|---|---|---|---|---|---|---|---|
| Type | Report of results | |||||||||
| 1 | Kresty et al. [49] USA |
Longitudinal (1997–2000) | N = 26 dysplastic lesions (including leukoplakia and erythroplakia) | None | NA | Age: 26–87 yrs Sex: 15M/11F |
Tissue | MS-PCR | p16INK4a p14ARF |
Cell cycle control Cell cycle control |
| 2 | Lopez et al. [44] Spanish |
Cross-sectional | (1) N = 19 homogenous leukoplakia (2) N = 15 homogenous leukoplakia with previous OSCC |
None None |
NA NA |
Age: 25-84 yrs Sex: 20M/14F |
Saliva | MS-PCR | p16INK4a p14ARF MGMT |
Cell cycle control Cell cycle control DNA repair |
| 3 | Youssef et al. [51] Caucasians 89.5% Hispanics 4% Asians 4% Blacks 2.5% |
Cross-sectional |
N = 42 dysplastic leukoplakia N = 82 hyperplastic leukoplakia N = 18 HNSCC |
None None N = 22 normal mucosa adjacent to HNSCC (paired sample⁎) |
NA NA |
Age: 23–91 yrs Sex: 63M/61F Tobacco – 75.6% Alcohol – 70.7% |
Tissue | MS-PCR | RAR-β2 | Cell cycle control |
| 4 | Gao et al. [52] Taiwan/Denmark |
Cross-sectional |
N = 4 dysplastic leukoplakia N = 34 OSCC |
None N = 7 normal oral mucosa adjacent to OSCC |
NA Yes |
Age: 35–89 yrs Sex: 32M/6F |
Tissue | MS-PCR | DBCCR1 | Cell cycle control |
| 5 | Sengupta et al. [53] Indians |
Cross-sectional |
N = 27 dysplastic leukoplakia N = 123 HNSCC |
N = 27 normal oral mucosa adjacent to lesion (Paired sample) N = 123 normal mucosa adjacent to HNSCC |
No NA |
Age: 8–80 yrs Sex: 103M/37F Tobacco – 73.9% |
Tissue | MSRA | hMLH1/2 | DNA repair |
| 6 | Hall et al. [31] UK |
Longitudinal (2000–2006) | N = 24 dysplastic leukoplakia/erythroplakia transformed into OSCC | N = 14 regressed dysplastic lesions (different sample†) | Yes | Long term smokers | Tissue | MEP | p16 MGMT CCNA1 CYGB |
Cell cycle control DNA repair Circadian rhythm Oxidative stress |
| 7 | Takeshima et al. [43] Sri Lankans |
Cross-sectional |
N = 64 dysplastic leukoplakia N = 10 OSMF |
N = 10 healthy oral mucosa from non-chewers (different sample) | Yes | Cases: Betel quid chewers Controls: non chewers |
Tissue | MS-PCR | p14 p15 p16 |
Cell cycle control Cell cycle control Cell cycle control |
| 8£ | Ghosh et al. [54] Indians |
Cross-sectional |
N = 52 dysplastic leukoplakia N = 111 HNSCC |
N = 52 normal oral mucosa adjacent to lesion (paired sample) N = 111 normal mucosa adjacent to HNSCC |
No NA |
Age: 22–76 yrs Sex: 155M 33F Tobacco – 69.6% |
Tissue Tissue |
MSRA | LIMD1 LTF RASSF1A CACNA2D2 CDC25 ASCOTIN |
Cell cycle control Immune response Apoptosis signalling Cell cycle control Apoptosis signalling |
| 9 | Cao et al.⁎⁎[30] Chinese |
Longitudinal (1995–2008) | N = 22 dysplastic lesions transformed into OSCC | N = 56 regressed dysplastic lesions (different sample) | Yes | Age: 32–77 yrs Sex: 31M/47F smoking – 36.8% |
Tissue | MS-PCR | p16 | Cell cycle control |
| 10£ | Ghosh et al. [48] Indians |
Cross-sectional |
N = 40 oral dysplastic leukoplakia N = 63 HNSCC |
N = 40 normal oral mucosa adjacent to lesion (paired sample) N = 63 normal mucosa adjacent to HNSCC (paired sample) |
No NA |
Age: 22–76 yrs Sex: 116M/39F Tobacco – 61% |
Tissue | MSRA | SH3GL2 p14 p15 p16 |
Cell cycle signalling Cell cycle control Cell cycle control Cell cycle control |
| 11 | Pattani et al. [45] Caucasians 69.6% Afro-Americans 23% others 7.3% |
Cross-sectional |
N = 43 dysplastic leukoplakia/erythroplakia N = 113 pre-cancer lesions(with/without hyperplasia) N = 35 OSCC |
None None |
NA NA |
Age: 18–90 yrs Sex: 132M/59F Tobacco – 69.1% Alcohol – 72.8% |
Saliva | MS-PCR | KIF1A EDNRB |
Unknown Cell cycle signalling |
| 12£ | Ghosh et al. [50] Indians |
Cross-sectional |
N = 54 dysplastic lesions N = 84 HNSCC samples |
N = 54 normal oral mucosa adjacent to lesion (paired sample) N = 84 normal mucosa adjacent to HNSCC (paired sample) |
No NA |
Age: 22–76 yrs Sex: 113M/36F Tobacco – 62% |
Tissue | MSRA | hMLH1I TGA9 RBSP |
DNA repair Cell cycle control Cell cycle signalling |
| 13 | Liu et al. [33] USA |
Cross-sectional |
N = 34 dysplastic leukoplakia N = 77 hyperkeratotic/hyperplastic leukoplakia N = 10OSCC |
None None |
NA NA |
Age: 24-90 yrs Sex: 59M/52F Smoking – 80.2% Alcohol – 70.2% |
Tissue | MS-PCR | p16 DAPK MGMT GSTP1 |
Cell cycle control Apoptosis signalling DNA repair Carcinogen metabolism |
| 14 | Silva et al. [47] Brazilians |
Cross-sectional | N = 48 dysplastic lesions | N = 24 healthy oral mucosa (mucoceles) (different sample) | Yes | Age: 15–74 yrs Sex: M39/F33 Tobacco – 81.8% Alcohol – 72.7% |
Tissue | MS-PCR | p16 CDKN2A | Cell cycle control |
| 15 | Liu et al. [46] Chinese |
Cross-sectional |
N = 64 dysplastic leukoplakia N = 13 non-dysplastic leukoplakia N = 32 OSCC |
None None |
NA NA |
Age: 26–86 yrs Sex: 42M/35F Smoking – 48.6% Alcohol – 52% Family history – 19.5% Spicy hot food – 18.2% |
Tissue, blood, saliva | MS-PCR | DAPK | Apoptosis signalling |
| 16£ | Ghosh et al. [55] Indians |
Cross-sectional |
N = 58 dysplastic lesions N = 62 HNSCC samples |
N = 58 normal oral mucosa adjacent to lesion (paired sample) N = 62 normal mucosa adjacent to HNSCC (paired sample) |
No NA |
Age: 22–76 yrs Sex: 113M/36F Tobacco – 62% |
Tissue | MSRA | FANCC PTCH1 PHF2 |
Cell cycle signalling Transcription activator |
| 17 | Xu et al. [36] Chinese |
Cross-sectional |
N = 50 dysplastic OSMF N = 60 OSCC samples |
N = 50 healthy oral mucosa from non-chewers (different sample) N = 50 healthy oral mucosa from non-chewers (different sample) |
Yes Yes |
Age: 19–53 yrs Sex: 48M/2F Cases: Areca nut chewers Controls: non chewers |
Tissue | MS-PCR | E-cadherin COX-2 |
Intercellular adhesion Inflammatory pathway |
| 18 | Dang et al. [35] Chinese |
Cross-sectional |
N = 20 non-dysplastic OLP N = 12 OSCC samples |
N = 10 healthy oral mucosa (different sample) N = 10 healthy oral mucosa (different sample) |
Yes Yes |
Cases: Mean age 49.6 yrs Sex: 8M/12F Tobacco: 30% Alcohol: 20% Controls: Mean age 27.8 yrs Sex: 5M/5F Tobacco: 30% Alcohol: none |
Tissue | MS-PCR | p16 miR-137 |
Cell cycle control Micro-RNA |
| 19 | Towle et al. [39] Caucasians |
Cross-sectional |
N = 10 dysplastic lesions N = 10 CIS/OSCC |
N = 10 normal adjacent oral mucosa (paired sample) N = 10 normal adjacent oral mucosa (paired sample) |
Yes | Age: 31–68 yrs Sex: 5M/5F Smoking – 30% |
Tissue | Agilant Microarray 4X 44 K | Whole genome | Mainly Wnt and map kinase pathways of cell cycle signalling |
| Yes | ||||||||||
| 20 | Bhatia et al. [32] Indians |
Cross-sectional |
N = 11 dysplastic leukoplakia N = 22 non-dysplastic leukoplakia N = 13 OSMF N = 8 OLP |
N = 16 healthy oral mucosa (different sample) | Yes | Cases: Mean age 34–40 ± 8–13 yrs Sex: 47M/7F Control: Mean age 29 ± 8.2 yrs Sex: 12M/4F |
Tissue | MS-PCR | MGMT p16 |
DNA Repair Cell cycle control |
| N = 76 OSCC | N = 16 healthy oral mucosa (different sample) | Yes | ||||||||
| 21 | Asokan et al. [34] Indians |
Cross-sectional |
N = 10 leukoplakia N = 10 OSCC samples |
N = 5 healthy oral mucosa (different sample) N = 5 healthy oral mucosa (different sample) |
Yes Yes |
NR | Tissue | MS-PCR | P 15/16 MGMT E-cadherin hMLH |
Cell cycle control DNA repair Intercellular adhesion DNA repair |
OSCC – oral squamous cell carcinoma; HNSCC – head and neck squamous cell carcinoma; MSP – methylation specific PCR; MSRA – methylation sensitive restriction analysis PCR; MEP – methylation enrichment pyrosequencing; M – male; F – female; OSMF – oral sub-mucous fibrosis; CIS – carcinoma-in-situ; OLP – oral lichen planus; NA – not applicable; NR – not reported.
Paired control samples refer to samples obtained from healthy sites of the same set of individuals (i.e., cases).
Different control samples refer to samples obtained from a different set of individuals either from healthy sites free of any oral disease/from muco-celes or from regressing pre-cancer sites as in cohort studies.
Oral dysplastic lesions included leukoplakia, oral lichen planus and discoid lupus erythmatosus.
Studies in rows 8, 10, 12 and 16 used same samples.
Table 2.
Summary of quantitative findings of the reviewed studies.
| N | Author/year/ ocation | Cases | Controls | Loci identified | Biological pathway | Methylated cases (%) | Unmethylated cases (%) | Methylated controls (%) | Unmethylated controls (%) |
|---|---|---|---|---|---|---|---|---|---|
| Longitudinal studies | |||||||||
| 1 | Kresty et al. [49] Ohio, USA |
Oral dysplastic lesions | None | p16 | Cell cycle control | 57.7 | 42.3 | NR | NR |
| p14 | Cell cycle control | 3.8 | 96.2 | ||||||
| 2 | Hall et al. 2008[31] UK |
Oral dysplastic lesions transformed into OSCC | Regressed oral dysplastic lesions | p16 | Cell cycle control | 57.0 | 43.0 | 8.0 | 92.0 |
| MGMT | DNA repair | 4.0 | 96.0 | 3.0 | 97.0 | ||||
| 3 | Cao⁎ et al. [30] China |
Oral dysplastic lesions transformed into OSCC | Regressed oral dysplastic lesions | p16 | Cell cycle control | 63.6 | 46.4 | 32.1 | 67.9 |
| Cross sectional studies | |||||||||
| 4 | Takeshima, et al. [43] Sri Lanka |
Oral dysplastic leukoplakia | Healthy oral mucosa | p16 | Cell cycle control | 29.6 | 70.4 | 0.0 | 100.0 |
| p14 | Cell cycle control | 73.4 | 26.6 | 0.0 | 100.0 | ||||
| Non dysplastic OSMF | Healthy oral mucosa | p16 | Cell cycle control | 70.0 | 30.0 | 0.0 | 100.0 | ||
| p14 | Cell cycle control | 80.0 | 20.0 | 0.0 | 100.0 | ||||
| 5 | Liu et al. [33] USA |
Oral dysplastic leukoplakia | None | p16 | Cell cycle control | 41.1 | 58.9 | NR | NR |
| DAPK | Apoptosis | 35.2 | 64.8 | NR | NR | ||||
| MGMT | DNA repair | 38.2 | 61.8 | NR | NR | ||||
| Oral hyperplastic leukoplakia | None | p16 | Cell cycle control | 19.4 | 80.6 | NR | NR | ||
| DAPK | Apoptosis | 18.2 | 81.8 | NR | NR | ||||
| MGMT | DNA repair | 24.6 | 75.4 | NR | NR | ||||
| 6 | Silva et al. [47] Brasil |
Oral dysplastic leukoplakia | Healthy oral mucosa | p16 | Cell cycle control | 87.5 | 12.5 | 8.3 | 91.7 |
| 7 | Liu et al. [46] China |
Oral dysplastic leukoplakia | None | DAPK | Apoptosis | 19.5 | 80.5 | NR | NR |
| 8 | Dang et al. [35] China |
Non dysplastic oral lichen planus | Healthy oral mucosa | p16 | Cell cycle control | 25.0 | 75.0 | 0.0 | 100.0 |
| 9 | Ghosh et al. [48] India |
Oral dysplastic lesions | Normal mucosa adjacent to lesions | p16 | Cell cycle control | 17.5 | 82.5 | NR | NR |
| p14 | Cell cycle control | 20.0 | 80.0 | NR | NR | ||||
| 10 | Bhatia et al. [32] India |
Oral dysplastic leukoplakia | Healthy oral mucosa | p16 | cell cycle control | 36.4 | 63.6 | 14.3 | 85.7 |
| OSMF | Healthy oral mucosa | MGMT | DNA repair | 72.7 | 27.3 | 14.3 | 85.7 | ||
| p16 | Cell cycle control | 61.5 | 38.5 | 14.3 | 85.7 | ||||
| MGMT | DNA repair | 43.1 | 53.9 | 14.3 | 85.7 | ||||
| OLP | Healthy oral mucosa | p16 | cell cycle control | 50.0 | 50.0 | 14.3 | 85.7 | ||
| MGMT | DNA repair | 25.0 | 75.0 | 14.3 | 85.7 | ||||
| 11 | Towle et al [39] Caucasians |
Oral dysplastic lesions | Normal mucosa adjacent to lesions | p16 | Cell cycle control | 50.0 | 50.0 | NR | NR |
| MGMT | DNA repair | 60.0 | 40.0 | NR | NR | ||||
| DAPK | Apoptosis | 70.0 | 30.0 | NR | NR | ||||
| 12 | Asokan et al. [34] India |
Oral leukoplakia | Healthy oral mucosa | p16 | Cell cycle control | 60.0 | 40.0 | 0.0 | 100.0 |
| MGMT | DNA repair | 30.0 | 70.0 | 0.0 | 100.0 | ||||
Epigenome wide methylation study [39] reported a total of 605 hyper-methylated genes and 90 hypo-methylated genes including Wnt and MAP kinase pathway genes and 3 novel sites in TRHDE, ZNF454, KCNAB3.
NR – not reported; OSMF – oral submucous fibrosis; OLP – oral lichen planus.
Oral dysplastic lesions included leukoplakia, oral lichen planus and discoid lupus erythmatosus.
Results
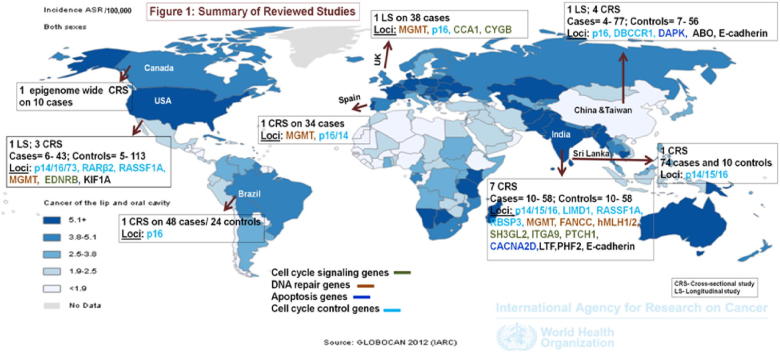
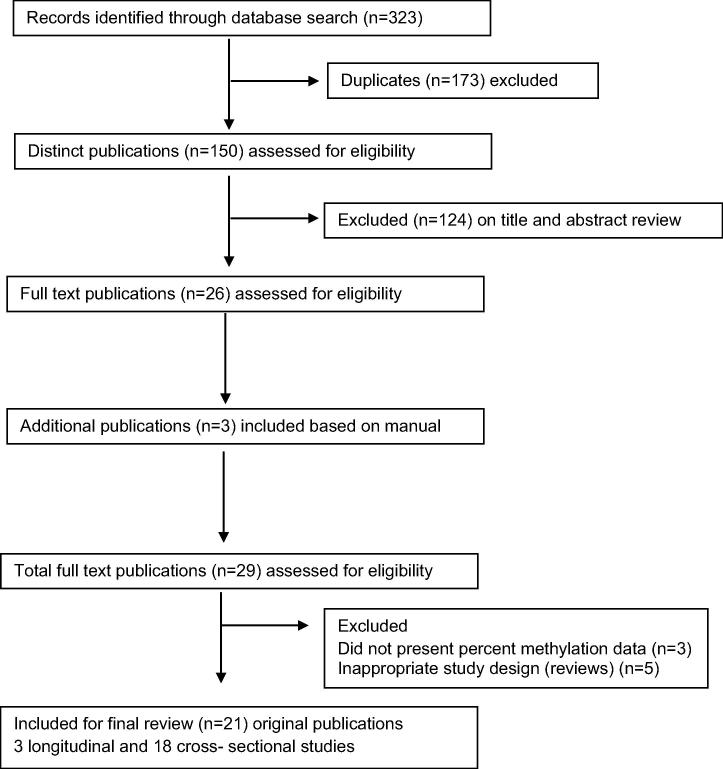
A total of 323 articles were retrieved from the combined key term search on Pubmed (N = 72), Embase (N = 96) and Web-of-Science (N = 155). After removal of duplicates, 150 distinct articles were identified. Based on a review of titles and abstracts, 22 original articles and 4 review articles were eligible for further review, and full-text articles were retrieved for these. A manual search of reference lists of the 26 studies yielded two more original research articles and 1 review article meeting the inclusion criteria. A total of 21 eligible original article studies were included for final review. The geographical distribution of study locations across the globe is shown in Fig. 1. Fig. 2 summarizes the evidence search and eligible studies included for final review.
Fig. 1.
Reference: Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Perklin DM. GLOBOCAN 2012 v2.0 cancer incidence and mortality worldwide: IARC CancerBase No. 10 [Internet]. Lyon, France: International Agency for Research on Cancer; 2012. Available from: http://globocan.iarc.fr [accessed on 30th April 2015].
Fig. 2.
Summary of evidence search and selection for DNA methylation and oral pre-cancer (up to 30th April 2015).
Table 1 summarizes key characteristics of the reviewed studies. Eighteen cross-sectional and 3 longitudinal studies reported hyper-methylation patterns of promoter regions of tumour suppressor genes involved in cell cycle control (n = 15 studies), DNA repair (n = 7 studies), cell cycle signalling (n = 4 studies) and apoptotic pathways (n = 3 studies). Sample sizes ranged from 4 to 156 affected cases/regions. Only one study to date has explored epigenome-wide methylation patterns on 10 dysplastic pre-cancer samples. Socio-demographic and lifestyle risk factors were inconsistently reported. A majority of studies (n = 19) used tissue samples (paraffin fixed/fresh frozen) for methylation analysis and 2 studies used buccal samples (saliva) for the analysis. Fourteen studies analyzed methylation patterns using a methylation-specific PCR technique, one study used pyrosequencing, and the remaining studies (n = 5) used methylation sensitive restriction analysis. Epigenome-wide methylation analysis was conducted using the Agilant Whole Human Genome Microarray 4X 44 K platform. Only nine studies (43% of studies) reported methylation frequency data for control samples. The remaining studies (n = 12) either did not report control data or did not have any controls.
Most reviewed studies used biopsy-confirmed tissue samples and standard (validated and reproducible) methods such as methylation specific or sensitive restriction analysis PCR for methylation analysis. However, heterogeneity existed among the studies with respect to sample size, control sampling (paired vs. different healthy samples) and adequate reporting of percent methylation, socio-economic and lifestyle (e.g., tobacco, alcohol) characteristics, with less emphasis on reporting of data from controls.
Table 2 summarizes percent hyper-methylation data for cases and controls for CpG sites of promoter regions of tumour suppressor genes consistently reported in 3 or more studies. The most commonly reported hyper-methylated loci were p16 (17.5–87.5% in cases vs. 0–14.3% in controls); p14 (20.0–73.4% in cases vs. null in controls); MGMT (24.6–72.7% in cases vs. 0–14.3% in controls) and DAPK (19.5–35.2% in cases). Epigenome-wide methylation study confirmed these loci (p16 in 50% of dysplastic cases; MGMT in 60% of dysplastic cases and DAPK in 70% of dysplastic cases) and identified 3 novel hyper-methylated loci as well (TRHDE, ZNF454, KCNAB3). Two longitudinal studies observed higher p16 hyper-methylation in pre-cancerous lesions transformed to malignancy compared to ones that regressed (57–63.6% vs. 8–32.1%; p < 0.01). A number of hyper-methylated loci (n = 23) were reported only once or twice in the literature. One previous epigenome-wide methylation study reported 90 hypomethylated and 605 hypermethylated loci.
Discussion
Epigenetic alterations such as aberrant DNA CpG methylation patterns, which silence tumour suppressor genes and/or activate oncogenes, are some of the earliest molecular changes in oral carcinogenesis [7], [26], [27]. These methylation patterns correlate with a person’s genetic profile as well as environmental risk exposure (e.g., tobacco, diet, alcohol, etc.) [26], and occur in all stages of carcinogenesis, including initial stages before any morphological changes [28], [29]. Thus, DNA methylation patterns stand out in their potential as a good early diagnostic marker. These methylation changes appear gradually and may be reversible with environmental influences, removal of risk factors or with therapeutic interventions at the early stages, which also make them ideal targets for intervention in the disease pathway (pharmacogenomics) [27].
We conducted a comprehensive critical review of studies on DNA methylation and oral pre-cancer (n = 21 studies after exclusions) to understand the current status of evidence, and to assess the potential diagnostic utility of DNA methylation as a marker for oral cancer progression. With the exception of one epigenome-wide methylation profile exploration, all other studies examined CpG sites of promoter regions of tumour suppressor genes. Only three studies explored longitudinal methylation patterns; the rest reported cross-sectional methylation profiles.
Based on the review of current evidence, a few loci involved in cell cycle control (p16, p14), DNA repair (MGMT) and apoptosis (DAPK) have been consistently reported in 3 or more studies and confirmed by an epigenome-wide methylation analysis and appear to be promising markers of choice for further evaluation. Longitudinal studies have reported higher hyper-methylation (p16) for dysplastic lesions that transformed to malignancy compared to lesions that regressed [30], [31], indicating possible dynamic alterations of methylation patterns through disease progression. p16 hyper-methylation was more frequently observed during dysplastic stages of pre-cancer than in non-dysplastic stages (hyperkeratotic/hyperplastic or non-dysplastic oral pre-cancer) [32], [33]. Interestingly, hyper-methylation of p16 has also been found to be a promising prognostic bio-marker for recurrence-free survival of oral and oro-pharyngeal cancers [19]. Other loci such as E-cadherin (adhesion molecule), mi-RNA genes and various other DNA repair genes which have been studied in oral cancers [7] are also being evaluated in oral pre-cancer [34], [35], [36].
Whilst locus-specific methylation analytical techniques primarily measure promoter hyper-methylation, high-density arrays allow aberrant (hyper- and hypo-) methylation at single sites to be measured throughout the genome [37] in a standardized manner replicable across populations [38]. The epigenome-wide methylation analysis of oral pre-cancer identified three novel loci (TRHDE, ZNF454, KCNAB3) previously unreported in any cancer site [39] in addition to confirming the loci in p16, MGMT and DAPK. The functional significance of the novel sites are yet to be characterized [39].
Aberrant methylation is a potential candidate for evaluation as a biomarker for guiding patient-related clinical decisions [18], especially for cancer sites such as the oral cavity [11], cervix [11] and colon [40], [41] where a well-established pre-cancer stage is detected and treated. The heterogeneity in anatomical and pathological aspects of disease progression associated with colon cancer [40], [41] and variations in pathological types and multiple virulent strains of Human Papilloma Virus (HPV) causing cervical cancer [11], [42] make identification of methylation markers more complicated for these sites compared to oral cancer [7].
Although our review revealed some promising leads for follow up, many of the studies were subject to limitations in the sample size, study design and/or the reporting of quantitative results. Most prior studies lack data on socio-demographic and life-style risk factors. The sampling scheme was largely non-uniform, especially in terms of control sample selection. One third of the studies either did not report control data, or did not include any controls in their study design. Those studies with controls varied greatly regarding control selection (Table 1). Although paired control samples obtained from the same individual can be helpful for controlling potential confounding factors [39] associated with using unpaired samples such as tobacco/betel quid use, [43] this approach does not take into account the ‘field cancerization’ normally found in patients of oral pre-cancer [14]. With the exception of one longitudinal study wherein repeated samples on 38 affected cases were collected (total n = 284 samples), all other studies had small sample sizes, and thus limited power for meaningful interpretation. A large number of hyper-methylated loci were reported only once and lacked any validation effort. Finally, the majority of published studies use a cross-sectional design, which cannot assess temporality thus making inference regarding causality difficult. Given these limitations, it is currently not possible to indicate strong inference for any of the markers identified to date.
On the other hand, most published studies used standard validated bisulfite conversion and MS-PCR method to measure DNA methylation status with adequate quality control procedures. Additionally, the majority of studies used biopsy-confirmed tissue samples for methylation analysis. Notably, two studies [44], [45] suggest that saliva could be a potential non-invasive medium for investigation of methylation markers, although Liu et al. [46] reported a lower yield of DAPK methylation in saliva (2.8%) compared to tissue (19.5%) or blood (20.9%). Methylation patterns are tissue specific [18] and the methylation profile of tissue may differ from that for blood or saliva [17]. Since methylation is the cause of differential gene expression, tissue-specific samples could reveal accurate epigenetic methylation patterns that contribute to the disease pathway [17]. Whole blood and saliva can also be used for methylation analysis. Whole blood is a non-target agent with many different cells that can have different methylation patterns [20]. Saliva, on the other hand, has the problem of potential contamination with food debris, residual cells and microorganisms [29]. Nonetheless, some studies have shown good results with whole blood [20] and saliva samples for highly specific salivary bio-markers such as EDNRB and KIF1A [29]. Reasonably good correlations have also been observed between tissue and blood sample results (R = 0.49, p < 0.001) [46].
Although the current evidence is inconclusive, we observed some level of consistency in terms of loci [30], [31], [32], [33], [34], [35], [43], [46], [47], [48], [49] and evidence for dynamic changes during disease progression [30], [31]. A few studies also reported concomitant dysregulated protein/mRNA expression in aberrantly methylated dysplastic oral pre-cancer [32], [39], [47], [48], [50]. Studies which analyzed methylation patterns secondary to genetic alterations such as deletions [48], [50] indicated that aberrant methylation could be the earliest molecular change signalling disease development and progression. These data suggest that methylation patterns may serve as a potential diagnostic biomarker for oral pre-cancer progression. Future large-scale epigenome wide methylation studies of oral pre-cancer with adequate replication and sequential follow-up data to capture the dynamic variations of methylation profile can help identify robust loci marking disease progression to guide early diagnosis during critical windows. It is important to emphasize the need for adequate study design, appropriate definition of controls, adherence to quality control and reporting recommendations, and collection of associated socio-demographic, life-style risk factors, and relevant clinical and histo-pathological data in order to facilitate the development of clinically relevant markers.
Conflict of interest statement
None declared.
Ethical approval
Not required as we utilized already published reports.
Acknowledgements
Dr. Krithiga Shridhar is supported by PHFI–UKC Wellcome Trust Capacity Building Programme-Extension Phase and by Wellcome Trust Strategic award – South Asia Network for Chronic Disease (SANCD) (WT 084674).
References
- 1.Ferlay J., Shin H.R., Bray F., Forman D., Mathers C., Perklin D.M. International Agency for Research on Cancer; Lyon (France): 2012. GLOBOCAN 2012 v2.0 cancer incidence and mortality worldwide: IARC CancerBase No. 1.0. Available from: http://globocan.iarc.fr [accessed on 30th April 2015] [Google Scholar]
- 2.Gupta B., Ariyawardana A., Johnson N.W. Oral cancer in India continues in epidemic proportions: evidence base and policy initiatives. Int Dent J. 2013;63(1):12–25. doi: 10.1111/j.1875-595x.2012.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45(4–5):309–316. doi: 10.1016/j.oraloncology.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Bessell A., Glenny A.M., Furness S., Clarkson J.E., Oliver R., Conway D.I. Interventions for the treatment of oral and oropharyngeal cancers: surgical treatment. Cochrane Database Syst Rev. 2011;9:CD006205. doi: 10.1002/14651858.CD006205.pub3. [DOI] [PubMed] [Google Scholar]
- 5.Chaturvedi A.K., Anderson W.F., Lortet-Tieulent J., Curado M.P., Ferlay J., Franceschi S. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol. 2013;31(36):4550–4559. doi: 10.1200/JCO.2013.50.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Napier S.S., Speight P.M. Natural history of potentially malignant oral lesions and conditions: an overview of the literature. J Oral Pathol Med. 2008;37(1):1–10. doi: 10.1111/j.1600-0714.2007.00579.x. [DOI] [PubMed] [Google Scholar]
- 7.Towle R., Garnis C. Methylation-mediated molecular dysregulation in clinical oral malignancy. J Oncol. 2012;2012:170–172. doi: 10.1155/2012/170172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mishra R. Biomarkers of oral premalignant epithelial lesions for clinical application. Oral Oncol. 2012;48(7):578–584. doi: 10.1016/j.oraloncology.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 9.Pitiyage G., Tilakaratne W.M., Tavassoli M., Warnakulasuriya S. Molecular markers in oral epithelial dysplasia: review. J Oral Pathol Med. 2009;38(10):737–752. doi: 10.1111/j.1600-0714.2009.00804.x. [DOI] [PubMed] [Google Scholar]
- 10.Mehanna H.M., Rattay T., Smith J., McConkey C.C. Treatment and follow-up of oral dysplasia – a systematic review and meta-analysis. Head Neck. 2009;31(12):1600–1609. doi: 10.1002/hed.21131. [DOI] [PubMed] [Google Scholar]
- 11.Rajaraman P., Anderson B.O., Basu P., Belinson J.L., D’Cruz A., Dhillon P.K. Recommendations for screening and early detection of common cancers in India. Lancet Oncol. 2015;16(7):e352–e361. doi: 10.1016/S1470-2045(15)00078-9. [DOI] [PubMed] [Google Scholar]
- 12.Sankaranarayanan R., Ramadas K., Thomas G., Muwonge R., Thara S., Mathew B. Effect of screening on oral cancer mortality in Kerala, India: a cluster-randomised controlled trial. Lancet. 2005;365(9475):1927–1933. doi: 10.1016/S0140-6736(05)66658-5. [DOI] [PubMed] [Google Scholar]
- 13.Kulkarni V., Saranath D. Concurrent hypermethylation of multiple regulatory genes in chewing tobacco associated oral squamous cell carcinomas and adjacent normal tissues. Oral Oncol. 2004;40(2):145–153. doi: 10.1016/s1368-8375(03)00143-x. [DOI] [PubMed] [Google Scholar]
- 14.Warnakulasuriya S., Johnson N.W., van der Waal I. Nomenclature and classification of potentially malignant disorders of the oral mucosa. J Oral Pathol Med. 2007;36(10):575–580. doi: 10.1111/j.1600-0714.2007.00582.x. [DOI] [PubMed] [Google Scholar]
- 15.Bird A. Perceptions of epigenetics. Nature. 2007;447(7143):396–398. doi: 10.1038/nature05913. [DOI] [PubMed] [Google Scholar]
- 16.Deaton A.M., Bird A. CpG islands and the regulation of transcription. Genes Dev. 2011;25(10):1010–1022. doi: 10.1101/gad.2037511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baumgartel K., Zelazny J., Timcheck T., Snyder C., Bell M., Conley Y.P. Molecular genomic research designs. Annu Rev Nurs Res. 2011;29:1–26. doi: 10.1891/0739-6686.29.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esteller M. Epigenetics in cancer. N Engl J Med. 2008;358(11):1148–1159. doi: 10.1056/NEJMra072067. [DOI] [PubMed] [Google Scholar]
- 19.Al-Kaabi A., van Bockel L.W., Pothen A.J., Willems S.M. p16(INK4A) and p14(ARF) gene promoter hypermethylation as prognostic biomarker in oral and oropharyngeal squamous cell carcinoma: a review. Disease Markers. 2014 doi: 10.1155/2014/260549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeilinger S., Kuhnel B., Klopp N., Baurecht H., Kleinschmidt A., Gieger C. Tobacco smoking leads to extensive genome-wide changes in DNA methylation. PLoS ONE. 2013;8(5):e63812. doi: 10.1371/journal.pone.0063812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerhauser C. Cancer chemoprevention and nutriepigenetics: state of the art and future challenges. Top Curr Chem. 2013;329:73–132. doi: 10.1007/128_2012_360. [DOI] [PubMed] [Google Scholar]
- 22.Jaenisch R., Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33(suppl.):245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- 23.Radhakrishnan R., Kabekkodu S., Satyamoorthy K. DNA hypermethylation as an epigenetic mark for oral cancer diagnosis. J Oral Pathol Med. 2011;40(9):665–676. doi: 10.1111/j.1600-0714.2011.01055.x. [DOI] [PubMed] [Google Scholar]
- 24.Chao C., Chi M., Preciado M., Black M.H. Methylation markers for prostate cancer prognosis: a systematic review. Cancer Causes Control. 2013;24(9):1615–1641. doi: 10.1007/s10552-013-0249-2. [DOI] [PubMed] [Google Scholar]
- 25.Jiang D., Hong Q., Shen Y., Xu Y., Zhu H., Li Y. The diagnostic value of DNA methylation in leukemia: a systematic review and meta-analysis. PLoS ONE. 2014;9(5):e96822. doi: 10.1371/journal.pone.0096822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verma M. Epigenetic biomarkers in cancer epidemiology. Methods Mol Biol. 2012;863:467–480. doi: 10.1007/978-1-61779-612-8_28. [DOI] [PubMed] [Google Scholar]
- 27.Mascolo M., Siano M., Ilardi G., Russo G., Merolla F., Rosa G.D. Epigenetic dysregulation in oral cancer. Int J Mol Sci. 2012;13:2331–2353. doi: 10.3390/ijms13022331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warnakulasuriya S., Reibel J., Bouquot J., Dabelsteen E. Oral epithelial dysplasia classification systems: predictive value, utility, weaknesses and scope for improvement. J Oral Pathol Med. 2008;37(3):127–133. doi: 10.1111/j.1600-0714.2007.00584.x. [DOI] [PubMed] [Google Scholar]
- 29.Schussel J., Zhou X.C., Zhang Z., Pattani K., Bermudez F., Jean-Charles G. EDNRB and DCC salivary rinse hypermethylation has a similar performance as expert clinical examination in discrimination of oral cancer/dysplasia versus benign lesions. Clin Cancer Res. 2013;19(12):3268–3275. doi: 10.1158/1078-0432.CCR-12-3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao J., Zhou J., Gao Y., Gu L., Meng H., Liu H. Methylation of p16 CpG island associated with malignant progression of oral epithelial dysplasia: a prospective cohort study. Clin Cancer Res. 2009;15(16):5178–5183. doi: 10.1158/1078-0432.CCR-09-0580. [DOI] [PubMed] [Google Scholar]
- 31.Hall G.L., Shaw R.J., Field E.A., Rogers S.N., Sutton D.N., Woolgar J.A. P16 promoter methylation is a potential predictor of malignant transformation in oral epithelial dysplasia. Cancer Epidemiol Biomarkers Prev. 2008;17(8):2174–2179. doi: 10.1158/1055-9965.EPI-07-2867. [DOI] [PubMed] [Google Scholar]
- 32.Bhatia V., Goel M.M., Makker A., Tewari S., Yadu A., Shilpi P. Promoter region hypermethylation and mRNA expression of MGMT and p16 genes in tissue and blood samples of human premalignant oral lesions and oral squamous cell carcinoma. Biomed Res Int. 2014;2014:248419. doi: 10.1155/2014/248419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu M., Feng L., Tang X., Guo S. Gene promotor hypermethylation in leukoplakia of the oral mucosa. Pathol Lab Med Int. 2010;2:71–77. [Google Scholar]
- 34.Asokan G.S., Jeelani S., Gnanasundaram N. Promoter hypermethylation profile of tumour suppressor genes in oral leukoplakia and oral squamous cell carcinoma. J Clin Diagn Res. 2014;8(10):ZC09–ZC12. doi: 10.7860/JCDR/2014/9251.4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dang J., Bian Y.Q., Sun J.Y., Chen F., Dong G.Y., Liu Q. MicroRNA-137 promoter methylation in oral lichen planus and oral squamous cell carcinoma. J Oral Pathol Med. 2013;42(4):315–321. doi: 10.1111/jop.12012. [DOI] [PubMed] [Google Scholar]
- 36.Xu C., Zhao J., Loo W.T., Hao L., Wang M., Cheung M.N. Correlation of epigenetic change and identification of risk factors for oral submucous fibrosis. Int J Biol Markers. 2012;27(4):e314–e321. doi: 10.5301/JBM.2012.9937. [DOI] [PubMed] [Google Scholar]
- 37.Bibikova M., Lin Z., Zhou L., Chudin E., Garcia E.W., Wu B. High-throughput DNA methylation profiling using universal bead arrays. Genome Res. 2006;16(3):383–393. doi: 10.1101/gr.4410706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michels K.B., Binder A.M., Dedeurwaerder S., Epstein C.B., Greally J.M., Gut I. Recommendations for the design and analysis of epigenome-wide association studies. Nat Methods. 2013;10(10):949–955. doi: 10.1038/nmeth.2632. [DOI] [PubMed] [Google Scholar]
- 39.Towle R., Truong D., Hogg K., Robinson W.P., Poh C.F., Garnis C. Global analysis of DNA methylation changes during progression of oral cancer. Oral Oncol. 2013;49(11):1033–1042. doi: 10.1016/j.oraloncology.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 40.Yamane L., Scapulatempo-Neto C., Reis R.M., Guimaraes D.P. Serrated pathway in colorectal carcinogenesis. World J Gastroenterol. 2014;20(10):2634–2640. doi: 10.3748/wjg.v20.i10.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lochhead P., Chan A.T., Giovannucci E., Fuchs C.S., Wu K., Nishihara R. Progress and opportunities in molecular pathological epidemiology of colorectal premalignant lesions. Am J Gastroenterol. 2014;109(8):1205–1214. doi: 10.1038/ajg.2014.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wentzensen N., Sherman M.E., Schiffman M., Wang S.S. Utility of methylation markers in cervical cancer early detection: appraisal of the state-of-the-science. Gynecol Oncol. 2009;112(2):293–299. doi: 10.1016/j.ygyno.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takeshima M., Saitoh M., Kusano K., Nagayasu H., Kurashige Y., Malsantha M. High frequency of hypermethylation of p14, p15 and p16 in oral pre-cancerous lesions associated with betel-quid chewing in Sri Lanka. J Oral Pathol Med. 2008;37(8):475–479. doi: 10.1111/j.1600-0714.2008.00644.x. [DOI] [PubMed] [Google Scholar]
- 44.Lopez M., Aguirre J.M., Cuevas N., Anzola M., Videgain J., Aguirregaviria J. Gene promoter hypermethylation in oral rinses of leukoplakia patients – a diagnostic and/or prognostic tool? Eur J Cancer. 2003;39(16):2306–2309. doi: 10.1016/s0959-8049(03)00550-1. [DOI] [PubMed] [Google Scholar]
- 45.Pattani K.M., Zhang Z., Demokan S., Glazer C., Loyo M., Goodman S. Endothelin receptor type B gene promoter hypermethylation in salivary rinses is independently associated with risk of oral cavity cancer and premalignancy. Cancer Prev Res (Phila) 2010;3(9):1093–1103. doi: 10.1158/1940-6207.CAPR-10-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu Y., Zhou Z.T., He Q.B., Jiang W.W. DAPK promoter hypermethylation in tissues and body fluids of oral precancer patients. Med Oncol. 2012;29(2):729–733. doi: 10.1007/s12032-011-9953-5. [DOI] [PubMed] [Google Scholar]
- 47.Fonseca-Silva T., Farias L.C., Cardoso C.M., Souza L.R., Carvalho Fraga C.A., Oliveira M.V. Analysis of p16(CDKN2A) methylation and HPV-16 infection in oral mucosal dysplasia. Pathobiology. 2012;79(2):94–100. doi: 10.1159/000334926. [DOI] [PubMed] [Google Scholar]
- 48.Ghosh A., Ghosh S., Maiti G.P., Sabbir M.G., Alam N., Sikdar N. SH3GL2 and CDKN2A/2B loci are independently altered in early dysplastic lesions of head and neck: correlation with HPV infection and tobacco habit. J Pathol. 2009;217(3):408–419. doi: 10.1002/path.2464. [DOI] [PubMed] [Google Scholar]
- 49.Kresty L.A., Mallery S.R., Knobloch T.J., Song H., Lloyd M., Casto B.C. Alterations of p16(INK4a) and p14(ARF) in patients with severe oral epithelial dysplasia. Cancer Res. 2002;62(18):5295–5300. [PubMed] [Google Scholar]
- 50.Ghosh A., Ghosh S., Maiti G.P., Sabbir M.G., Zabarovsky E.R., Roy A. Frequent alterations of the candidate genes hMLH1, ITGA9 and RBSP3 in early dysplastic lesions of head and neck: clinical and prognostic significance. Cancer Sci. 2010;101(6):1511–1520. doi: 10.1111/j.1349-7006.2010.01551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Youssef E.M., Estecio M.R., Issa J.P. Methylation and regulation of expression of different retinoic acid receptor beta isoforms in human colon cancer. Cancer Biol Ther. 2004;3(1):82–86. doi: 10.4161/cbt.3.1.591. [DOI] [PubMed] [Google Scholar]
- 52.Gao S., Worm J., Guldberg P., Eiberg H., Krogdahl A., Sorensen J.A. Loss of heterozygosity at 9q33 and hypermethylation of the DBCCR1 gene in oral squamous cell carcinoma. Br J Cancer. 2004;91(4):760–764. doi: 10.1038/sj.bjc.6601980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sengupta S., Chakrabarti S., Roy A., Panda C.K., Roychoudhury S. Inactivation of human mutL homolog 1 and mutS homolog 2 genes in head and neck squamous cell carcinoma tumors and leukoplakia samples by promoter hypermethylation and its relation with microsatellite instability phenotype. Cancer. 2007;109(4):703–712. doi: 10.1002/cncr.22430. [DOI] [PubMed] [Google Scholar]
- 54.Ghosh S., Ghosh A., Maiti G.P., Alam N., Roy A., Roy B. Alterations of 3p21.31 tumor suppressor genes in head and neck squamous cell carcinoma: correlation with progression and prognosis. Int J Cancer. 2008;123(11):2594–2604. doi: 10.1002/ijc.23834. [DOI] [PubMed] [Google Scholar]
- 55.Ghosh A., Ghosh S., Maiti G.P., Mukherjee S., Mukherjee N., Chakraborty J. Association of FANCC and PTCH1 with the development of early dysplastic lesions of the head and neck. Ann Surg Oncol. 2012;19(suppl. 3):S52–S538. doi: 10.1245/s10434-011-1991-x. [DOI] [PubMed] [Google Scholar]