Abstract
Progression of cancer is often associated with interactions between cancer cells and extracellular matrix (ECM) surrounding them. Increasing evidence has suggested that accumulation of hyaluronan (HA), a major component of ECM, provides a favorable microenvironment for cancer progression. Pancreatic ductal adenocarcinoma (PDAC) is characterized typically by a dense desmoplastic stroma with a large amount of HA, making this molecule as an attractive target for therapy. Several studies have shown efficacy of inhibitors of HA synthesis or signaling for the treatment of PDAC. Recent studies have also demonstrated substantial improvements in the effects of chemotherapy by a targeted depletion of stromal HA in PDAC using an enzymatic agent. Thus, targeting HA has been recognized as a promising therapeutic strategy to treat this highly aggressive neoplasm. In this review article, we summarize our current understanding of the role of HA in the progression of PDAC and discuss possible therapeutic approaches targeting HA.
Key words: Pancreatic ductal adenocarcinoma, Hyaluronan, Tumor stroma, Desmoplasia, Tumor–stromal interaction, Therapeutic target, 4-Methylumbelliferone, PEGPH20
Graphical abstract
This review article summarizes the current understanding of the role of hyaluronan (HA) in pancreatic ductal adenocarcinoma (PDAC) and discusses possible therapeutic approaches targeting HA, which are inhibition of HA synthesis, blocking HA-receptor signaling, and depletion of stromal HA in combination with chemotherapy.
1. Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of the most aggressive and intractable solid tumors, which often invades surrounding stromal components, including lymphatic, vascular, and perineural systems, ultimately metastasizing to distant organs. Despite recent advances in the clinical management, the survival rate in patients with PDAC remains the lowest among all cancer types, emphasizing the need for a better understanding of its biology. In particular, identification of molecular mechanisms underlying the aggressive behaviors of PDAC can provide the basis for the development of targets for therapeutic intervention1. Although substantial progress has been made in our understanding of the genetic and epigenetic alterations in PDAC, the identification of these molecular defects in cancer cells has led to little progress in developing new treatment strategies2, 3.
The progression of cancer is governed by complex mechanisms and is significantly accelerated by tumor microenvironment composed of extracellular matrix (ECM), such as collagen, fibronectin, laminin, and hyaluronan (HA)4. Among the ECM components, HA has been extensively studied in its relation to cancer initiation and progression. HA, a large polysaccharide composed of repeating disaccharides of glucuronic acid and N-acetyl-glucosamine, plays a critical role in a variety of cellular processes5. HA regulates cell adhesion, migration, and proliferation by interacting with specific cell surface receptors including CD44 and receptor for HA-mediated motility (RHAMM)6. HA is synthesized by hyaluronan synthases (HAS, including HAS1, HAS2, and HAS3) and is degraded by hyaluronidases (such as HYAL1 and HYAL2)5, 7, 8. In normal physiological conditions, the amount of HA is controlled by a balance between synthesis and degradation; however, HA has been shown to be abundantly accumulated in the surrounding stroma of malignant tumor9, 10. The HA-rich microenvironment may promote tumor progression by enhancing cell proliferation, migration, invasion, metastasis, angiogenesis, and resistance to chemotherapeutic agents9, 10.
Because PDAC is characterized typically by a dense desmoplastic stroma containing a large amount of ECM, it is highly probable that HA is involved in the malignant properties of this tumor type. In fact, several studies have shown increased expression of HA and its receptors in PDAC11, 12, 13, 14, 15, 16. In an experimental model of PDAC, accumulation of extracellular HA by HAS overexpression accelerated tumor growth17. These findings strongly suggest that HA could be a therapeutic target in PDAC. Only a few studies, however, have addressed the effects of HA inhibitors for the treatment of PDAC18, 19, 20. More recently, two studies have shown that inhibition of HA by PEGPH20, an HA-targeting enzymatic agent, substantially augments the effect of chemotherapy with gemcitabine in animal models21, 22. These findings suggest a novel therapeutic approach to combat the chemoresistance of PDAC by targeting HA. In this review article, we summarize the current understanding of the role of HA in PDAC and discuss its potential therapeutic applications.
2. Role of HA in the progression of PDAC
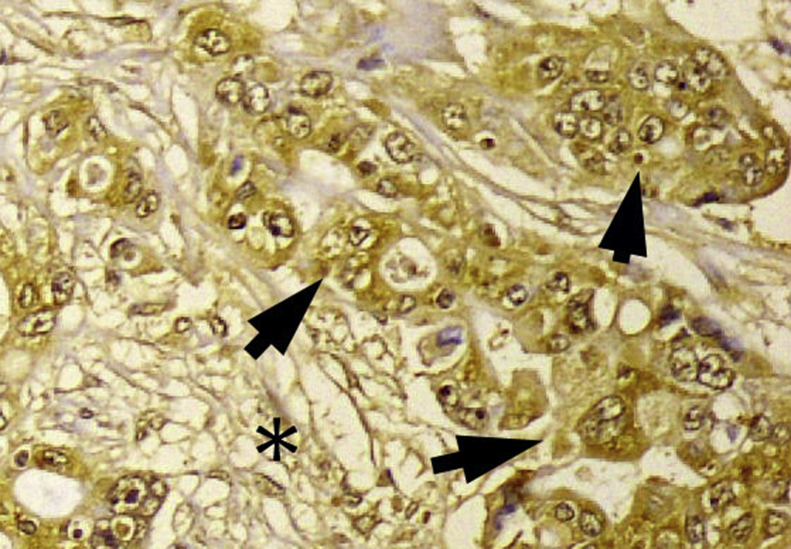
HA is a large, linear glycosaminoglycan that consists of repeating disaccharide subunits of glucuronic acid and N-acetylglucosamine produced by HA synthases (HAS1, HAS2, and HAS3) and degraded by hyaluronidases (mainly HYAL1 and HYAL2)5. In normal physiological conditions, the amounts of HA in tissues are tightly regulated by a balance between synthesis and degradation. In certain cancers, HA is often increased or highly concentrated in tumor cells and, particularly, in their surrounding ECM. There have been several studies investigating the degree of HA concentration and/or pattern of HA expression in PDAC. For example, a previous study showed that HA is secreted from cultured human pancreatic cancer cell lines11. In addition, the amount of HA is increased in human PDAC tissues (12-fold increase) as compared to the normal pancreas14. Using a biotinylated HA-binding protein isolated from bovine cartilage, Fries et al.12 demonstrated that in primary PDAC tissues, HA was found predominantly in the connective tissue immediately around tumor cells or at the border between the tumor and normal pancreatic tissue. A comprehensive analysis of the HA content in a variety of human malignant tumors revealed that PDAC had the highest incidence of detectable HA content, which was predominantly associated with the desmoplastic stroma rather than with tumor cells21. We also used immunohistochemistry to analyze the expression of HA and its regulators (including HAS2 and HYAL1) in primary PDAC16, and demonstrated that HA is strongly expressed in 80% of primary PDAC tissues with a staining being detected both in tumor and stromal components (Fig. 1). Importantly, strong HA expression was an independent prognostic factor in patients with PDAC undergoing resection, suggesting a prognostic significance of HA in PDAC16.
Figure 1.
Overexpression of hyaluronan in human pancreatic ductal adenocarcinoma tissue by immunohistochemical staining. Strong staining is observed mainly in tumor cells (arrows) but is also present in stroma (⁎).
Little is known about the mechanism by which HA is aberrantly accumulated in PDAC. One possible mechanism is increased production of HA from cancer cells themselves through its accelerated synthesis. In fact, our previous study demonstrated overexpression of one of the HA synthases, HAS2, in PDAC tissues16. We and other researches also demonstrated that cultured PDAC cells secrete a certain amount of HA into conditioned media23, 24. Furthermore, we recently discovered that epigenetic mechanism (namely DNA methylation) is involved in the regulation of HA synthesis in PDAC cells24. Another mechanism may be related to the enhanced secretion of HA from stromal cells, including fibroblasts. In support of this, it has been shown that HA staining was predominantly associated with the desmoplastic stroma rather than with tumor cells in human PDAC tissues15, 21. Interestingly, Knudson et al.25 demonstrated that direct coculture between tumor cells (including PDAC cells) and normal fibroblasts promotes the production of HA into the culture medium. Thus, tumor-stromal interactions may play a pivotal role in the increased HA accumulation in PDAC.
Recently, a study used a genetic model to investigate the role of HA in the progression of PDAC. Kultti et al.17 demonstrated that forced production and accumulation of HA by HAS3 overexpression promoted the growth of PDAC tumor in mice through initiation of epithelial-mesenchymal transition (EMT) as evidenced by loss of plasma membrane E-cadherin and accumulation of cytoplasmic β-catenin. Further studies are needed to elucidate the exact mechanism by which HA promotes the progression of PDAC.
3. Therapeutic strategies targeting HA in PDAC
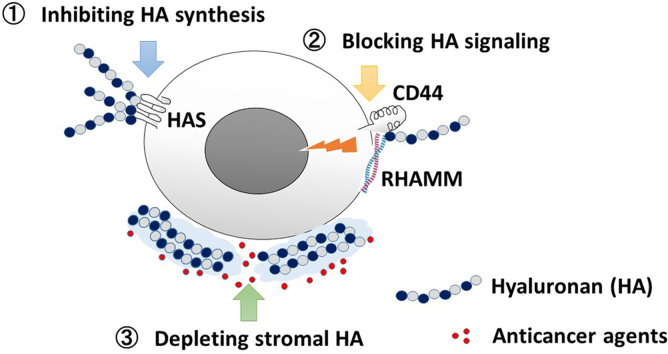
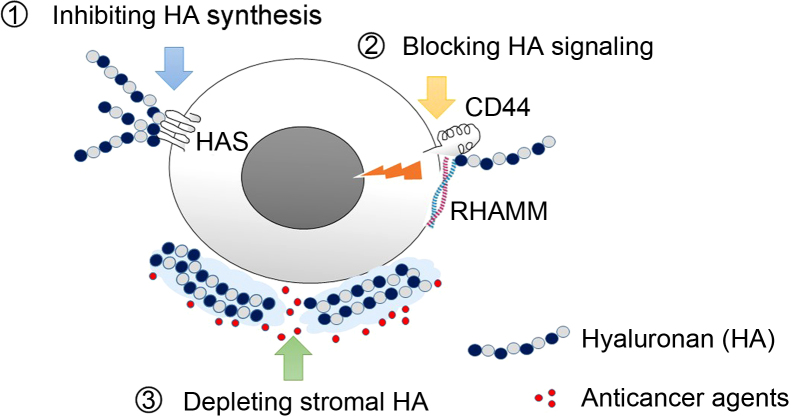
In light of these critical contributions of HA to tumor progression, there have been great interest in developing therapeutic strategies targeting HA. Three different therapeutic approaches may be identified: (1) inhibiting HA synthesis, (2) blocking HA signaling, and (3) depleting stromal HA in PDAC to improve chemosensitivity (Fig. 2).
Figure 2.
Strategies of targeting hyaluronan for the treatment of pancreatic cancer. Currently, three different therapeutic approaches may are considered: (1) inhibiting HA synthesis, (2) blocking HA signaling, and (3) depleting stromal HA barrier in PDAC to improve chemosensitivity.
3.1. Targeting HA synthesis
First, inhibition of HA synthesis may be an ideal and straightforward treatment strategy. One agent that has received increasing attention is 4-methylumbelliferone (4-MU). 4-MU inhibits HA synthesis by acting as a competitive substrate for UDP-glucuronosyltransferase (UGT) and by downregulating HAS2 and HAS326, 27. Notably, 4-MU, also known as hymecromone, is already used in several countries as a drug to improve liver function or to treat biliary spasm without any serious side effects reported28. Previous studies have shown that 4-MU and its derivatives inhibit the growth and metastasis of PDAC in vitro and in vivo18, 20. In addition, Nakazawa et al.19 showed that 4-MU enhanced the anticancer activity of a commonly used chemotherapeutic drug, gemcitabine, in PDAC cell line and animal model. This finding is consistent with a recent study showing that chemotherapy with carboplatin induces HA production which can contribute to chemoresistance by regulating ABC transporter expression in ovarian cancer29. Recently, in addition to its anticancer efficacy, chemopreventive efficacy of 4-MU has been shown in prostate cancer animal models30. However, these studies used cancer cell lines and/or xenograft models which basically lack human tumor stroma, raising a concern about the potential therapeutic benefits of 4-MU in patients with PDAC. Further preclinical and clinical studies are required to determine the efficacy of 4-MU for the treatment of PDAC.
3.2. Targeting HA signaling
In addition to the synthesis process of HA, signal transduction pathways induced by HA can be a target for anticancer therapy. HA interacts with several cell-surface receptors, including CD44 and RHAMM, to activate intracellular signaling pathways that regulate a variety of cellular processes. In many cancers including PDAC, HA-CD44 interactions may play an important role in tumor cell growth, survival, migration, invasion, multidrug resistance, and cancer stem cell self-renewal6, 31, 32, 33. Previous studies34, 35 have shown that CD44 regulates invasion of PDAC. Therefore, blocking this interaction may prevent progression of PDAC. For example, Sugahara et al.36 showed that tumor-derived HA fragments enhance CD44 cleavage and cell migration in a CD44-dependent manner and that inhibition of CD44-HA interaction by digesting the HA oligosaccharides using hyaluronidase results in complete abrogation of these cellular events. As downstream of CD44-HA interactions, Ras-mitogen-activated protein kinase pathway and phosphoinositide 3-kinase (PI3K)-Akt signaling pathway are known to be involved in the cancer-promoting effects of HA37. These signaling pathways are thus a target for therapy. Teranishi et al.38 demonstrated that enhanced cell motility and peritoneal metastasis of PDAC induced by HA were blocked by the PI3K inhibitor wortmannin in mice.
RHAMM is another receptor for HA known to be implicated in various cancers6. RHAMM mRNA is overexpressed in pancreatic cancer cell lines exhibiting a poorly differentiated phenotype and a high metastatic potential when injected into nude mice13. Recently, we also show that RHAMM is overexpressed in primary PDAC tissues and its expression correlates with poor survival in patients who underwent surgical resection39. Therefore, RHAMM may also be a promising target but has not yet been investigated in terms of its therapeutic efficacy in PDAC.
3.3. Depleting stromal HA in PDAC
PDAC is characterized typically by its extensive fibrosis in a stromal region as a result of desmoplastic reaction. It has been suggested that accumulation of HA in tumor stroma may increase tumor interstitial pressure, thereby blocking delivery of drugs to the tumor cells. Consequently, targeting the components of ECM, particularly HA, has been considered an attractive therapeutic strategy to overcome chemoresistance40, 41, 42. Although this idea of depleting stromal HA has been previously proposed and tested in other tumor types43, it had not been tested in a model of PDAC until recently. Provenzano et al.22 investigated intravenous administration of PEGPH20, an HA-targeting enzymatic agent, in mice bearing PDAC. Systemic administration of PEGPH20 depleted stromal HA, normalized interstitial pressure, re-expanded microvasculature, and consequently improved the effects of gemcitabine22. Similarly, Jacobetz et al.21 also used a genetically engineered mouse model, the LSL-KrasG12D/+; LSL-Trp53R172H/+; Pdx-1-Cre (KPC) mice, to demonstrate that PEGPH20 depletes HA, induces the re-expansion of collapsed blood vessels in PDA, and increases the intratumoral delivery of two chemotherapeutic agents, doxorubicin and gemcitabine. Furthermore, combination therapy with PEGPH20 and gemcitabine inhibits tumor growth and prolongs survival in the KPC mice21. Importantly, treatment with PEGPH20 alone had no significant effects on the tumor growth and survival in mice21, 22, suggesting that the potential therapeutic benefit of HA inhibition is obtained primarily by overcoming the stromal barrier and sensitizing chemotherapy rather than by its own anticancer effect.
Based on these promising results of preclinical studies, PEGPH20 is now being tested in a clinical trial (NCT01839487) to determine its efficacy when used in combination with nab-paclitaxel plus gemcitabine in patients with metastatic PDAC (https://clinicaltrials.gov/show/NCT01839487). The results of this and future trials will reveal the clinical efficacy of HA inhibitors and offer a novel treatment option for otherwise untreatable patients with PDAC.
4. Future prospective
In summary, there are currently three major strategies targeting HA (inhibition of HA synthesis, blocking HA-receptor signaling, and depletion of stromal HA in combination with chemotherapy) in the treatment of PDAC. In addition to these strategies, there may be other potential strategies to target HA for the treatment of PDAC. For example, inhibition of HA degradation, as well as HA synthesis, could be an ideal strategy, because accumulating evidence suggests that low-molecular-weight or fragmented HA, produced through degradation by hyaluronidase, plays a critical role in cancer progression44, 45. In fact, previous studies have shown antitumor effects of hyaluronidase inhibitors in some types of cancers46, 47. Although further preclinical and clinical studies are required, controlling the amount and/or size of HA by modulating the production and degradation process may be a promising therapeutic strategy to improve the prognosis of this deadly disease in the future.
Acknowledgments
We thank Ms. Yuko Ueda for her technical assistance. This study was supported in part by a grant-in-aid from the Ministry of Education, Culture, Sports, Science and Technologies of Japan (26462076).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 2.Sato N., Goggins M. The role of epigenetic alterations in pancreatic cancer. J Hepatobiliary Pancreat Surg. 2006;13:286–295. doi: 10.1007/s00534-005-1057-1. [DOI] [PubMed] [Google Scholar]
- 3.Vincent A., Herman J., Schulick R., Hruban R.H., Goggins M. Pancreatic cancer. Lancet. 2011;378:607–620. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rowe R.G., Weiss S.J. Navigating ECM barriers at the invasive front: the cancer cell–stroma interface. Annu Rev Cell Dev Biol. 2009;25:567–595. doi: 10.1146/annurev.cellbio.24.110707.175315. [DOI] [PubMed] [Google Scholar]
- 5.Toole B.P. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4:528–539. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- 6.Misra S., Hascall V.C., Markwald R.R., Ghatak S. Interactions between hyaluronan and its receptors (CD44, RHAMM) regulate the activities of inflammation and cancer. Front Immunol. 2015;6:201. doi: 10.3389/fimmu.2015.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stern R. Hyaluronan catabolism: a new metabolic pathway. Eur J Cell Biol. 2004;83:317–325. doi: 10.1078/0171-9335-00392. [DOI] [PubMed] [Google Scholar]
- 8.Itano N., Kimata K. Mammalian hyaluronan synthases. IUBMB Life. 2002;54:195–199. doi: 10.1080/15216540214929. [DOI] [PubMed] [Google Scholar]
- 9.Itano N., Zhuo L., Kimata K. Impact of the hyaluronan-rich tumor microenvironment on cancer initiation and progression. Cancer Sci. 2008;99:1720–1725. doi: 10.1111/j.1349-7006.2008.00885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sironen R.K., Tammi M., Tammi R., Auvinen P.K., Anttila M., Kosma V.M. Hyaluronan in human malignancies. Exp Cell Res. 2011;317:383–391. doi: 10.1016/j.yexcr.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 11.Mahlbacher V., Sewing A., Elsasser H.P., Kern H.F. Hyaluronan is a secretory product of human pancreatic adenocarcinoma cells. Eur J Cell Biol. 1992;58:28–34. [PubMed] [Google Scholar]
- 12.Fries H., Elsasser H.P., Mahlbacher V., Neumann K., Kern H.F. Localisation of hyaluronate (HA) in primary tumors and nude mouse xenografts of human pancreatic carcinomas using a biotinylated HA-binding protein. Virchows Arch. 1994;424:7–12. doi: 10.1007/BF00197386. [DOI] [PubMed] [Google Scholar]
- 13.Abetamann V., Kern H.F., Elsasser H.P. Differential expression of the hyaluronan receptors CD44 and RHAMM in human pancreatic cancer cells. Clin Cancer Res. 1996;2:1607–1618. [PubMed] [Google Scholar]
- 14.Theocharis A.D., Tsara M.E., Papageorgacopoulou N., Karavias D.D., Theocharis D.A. Pancreatic carcinoma is characterized by elevated content of hyaluronan and chondroitin sulfate with altered disaccharide composition. Biochim Biophys Acta. 2000;1502:201–206. doi: 10.1016/s0925-4439(00)00051-x. [DOI] [PubMed] [Google Scholar]
- 15.Whatcott C.J., Diep C.H., Jiang P., Watanabe A., LoBello J., Sima C. Desmoplasia in primary tumors and metastatic lesions of pancreatic cancer. Clin Cancer Res. 2015;21:3561–3568. doi: 10.1158/1078-0432.CCR-14-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng X.B., Sato N., Kohi S., Yamaguchi K. Prognostic impact of hyaluronan and its regulators in pancreatic ductal adenocarcinoma. PLoS One. 2013;8:e80765. doi: 10.1371/journal.pone.0080765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kultti A., Zhao C., Singha N.C., Zimmerman S., Osgood R.J., Symons R. Accumulation of extracellular hyaluronan by hyaluronan synthase 3 promotes tumor growth and modulates the pancreatic cancer microenvironment. Biomed Res Int. 2014;2014:817613. doi: 10.1155/2014/817613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hajime M., Shuichi Y., Makoto N., Masanori Y., Ikuko K., Atsushi K. Inhibitory effect of 4-methylesculetin on hyaluronan synthesis slows the development of human pancreatic cancer in vitro and in nude mice. Int J Cancer. 2007;120:2704–2709. doi: 10.1002/ijc.22349. [DOI] [PubMed] [Google Scholar]
- 19.Nakazawa H., Yoshihara S., Kudo D., Morohashi H., Kakizaki I., Kon A. 4-Methylumbelliferone, a hyaluronan synthase suppressor, enhances the anticancer activity of gemcitabine in human pancreatic cancer cells. Cancer Chemother Pharmacol. 2006;57:165–170. doi: 10.1007/s00280-005-0016-5. [DOI] [PubMed] [Google Scholar]
- 20.Morohashi H., Kon A., Nakai M., Yamaguchi M., Kakizaki I., Yoshihara S. Study of hyaluronan synthase inhibitor, 4-methylumbelliferone derivatives on human pancreatic cancer cell (KP1-NL) Biochem Biophys Res Commun. 2006;345:1454–1459. doi: 10.1016/j.bbrc.2006.05.037. [DOI] [PubMed] [Google Scholar]
- 21.Jacobetz M.A., Chan D.S., Neesse A., Bapiro T.E., Cook N., Frese K.K. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut. 2012;62:112–120. doi: 10.1136/gutjnl-2012-302529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Provenzano P.P., Cuevas C., Chang A.E., Goel V.K., Von Hoff D.D., Hingorani S.R. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:418–429. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang P., Li X., Thompson C.B., Huang Z., Araiza F., Osgood R. Effective targeting of the tumor microenvironment for cancer therapy. Anticancer Res. 2012;32:1203–1212. [PubMed] [Google Scholar]
- 24.Kohi S, Sato N, Cheng XB, Koga A, Higure A, Hirata K. A novel epigenetic mechanism regulating hyaluronan production in pancreatic cancer cells. Clin Exp Metastasis 2015 Nov 20. Available from: 10.1007/s10585-015-9771-9 [DOI] [PubMed]
- 25.Knudson W., Biswas C., Toole B.P. Interactions between human tumor cells and fibroblasts stimulate hyaluronate synthesis. Proc Natl Acad Sci U S A. 1984;81:6767–6771. doi: 10.1073/pnas.81.21.6767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kultti A., Pasonen-Seppänen S., Jauhiainen M., Rilla K.J., Kärnä R., Pyöriä E. 4-Methylumbelliferone inhibits hyaluronan synthesis by depletion of cellular UDP-glucuronic acid and downregulation of hyaluronan synthase 2 and 3. Exp Cell Res. 2009;315:1914–1923. doi: 10.1016/j.yexcr.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Kakizaki I., Kojima K., Takagaki K., Endo M., Kannagi R., Ito M. A novel mechanism for the inhibition of hyaluronan biosynthesis by 4-methylumbelliferone. J Biol Chem. 2004;279:33281–33289. doi: 10.1074/jbc.M405918200. [DOI] [PubMed] [Google Scholar]
- 28.Nagy N., Kuipers H.F., Frymoyer A.R., Ishak H.D., Bollyky J.B., Wight T.N. 4-Methylumbelliferone treatment and hyaluronan inhibition as a therapeutic strategy in inflammation, autoimmunity, and cancer. Front Immunol. 2015;6:123. doi: 10.3389/fimmu.2015.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ricciardelli C., Ween M.P., Lokman N.A., Tan I.A., Pyragius C.E., Oehler M.K. Chemotherapy-induced hyaluronan production: a novel chemoresistance mechanism in ovarian cancer. BMC Cancer. 2013;13:476. doi: 10.1186/1471-2407-13-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yates T.J., Lopez L.E., Lokeshwar S.D., Ortiz N., Kallifatidis G., Jordan A. Dietary supplement 4-methylumbelliferone: an effective chemopreventive and therapeutic agent for prostate cancer. J Natl Cancer Inst. 2015;107:djv085. doi: 10.1093/jnci/djv085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Misra S., Heldin P., Hascall V.C., Karamanos N.K., Skandalis S.S., Markwald R.R. Hyaluronan–CD44 interactions as potential targets for cancer therapy. FEBS J. 2011;278:1429–1443. doi: 10.1111/j.1742-4658.2011.08071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toole B.P. Hyaluronan–CD44 interactions in cancer: paradoxes and possibilities. Clin Cancer Res. 2009;15:7462–7468. doi: 10.1158/1078-0432.CCR-09-0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chanmee T., Ontong P., Kimata K., Itano N. Key roles of hyaluronan and its CD44 receptor in the stemness and survival of cancer stem cells. Front Oncol. 2015;5:180. doi: 10.3389/fonc.2015.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takada M., Yamamoto M., Saitoh Y. The significance of CD44 in human pancreatic cancer: II. The role of CD44 in human pancreatic adenocarcinoma invasion. Pancreas. 1994;9:753–757. doi: 10.1097/00006676-199411000-00014. [DOI] [PubMed] [Google Scholar]
- 35.Jiang W., Zhang Y., Kane K.T., Collins M.A., Simeone D.M., di Magliano M.P. CD44 regulates pancreatic cancer invasion through MT1-MMP. Mol Cancer Res. 2015;13:9–15. doi: 10.1158/1541-7786.MCR-14-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sugahara K.N., Hirata T., Hayasaka H., Stern R., Murai T., Miyasaka M. Tumor cells enhance their own CD44 cleavage and motility by generating hyaluronan fragments. J Biol Chem. 2006;281:5861–5868. doi: 10.1074/jbc.M506740200. [DOI] [PubMed] [Google Scholar]
- 37.Sohara Y., Ishiguro N., Machida K., Kurata H., Thant A.A., Senga T. Hyaluronan activates cell motility of v-Src-transformed cells via Ras-mitogen-activated protein kinase and phosphoinositide 3-kinase-Akt in a tumor-specific manner. Mol Biol Cell. 2001;12:1859–1868. doi: 10.1091/mbc.12.6.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teranishi F., Takahashi N., Gao N., Akamo Y., Takeyama H., Manabe T. Phosphoinositide 3-kinase inhibitor (wortmannin) inhibits pancreatic cancer cell motility and migration induced by hyaluronan in vitro and peritoneal metastasis in vivo. Cancer Sci. 2009;100:770–777. doi: 10.1111/j.1349-7006.2009.01084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng X.B., Sato N., Kohi S., Koga A., Hirata K. Receptor for hyaluronic acid-mediated motility is associated with poor survival in pancreatic ductal adenocarcinoma. J Cancer. 2015;6:1093–1098. doi: 10.7150/jca.12990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whatcott C.J., Han H., Posner R.G., Hostetter G., von Hoff D.D. Targeting the tumor microenvironment in cancer: why hyaluronidase deserves a second look. Cancer Discov. 2011;1:291–296. doi: 10.1158/2159-8290.CD-11-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu M., Tannock I.F. Targeting tumor architecture to favor drug penetration: a new weapon to combat chemoresistance in pancreatic cancer? Cancer Cell. 2012;21:327–329. doi: 10.1016/j.ccr.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 42.Provenzano P.P., Hingorani S.R. Hyaluronan, fluid pressure, and stromal resistance in pancreas cancer. Br J Cancer. 2013;108:1–8. doi: 10.1038/bjc.2012.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baumgartner G., Gomar-Hoss C., Sakr L., Ulsperger E., Wogritsch C. The impact of extracellular matrix on the chemoresistance of solid tumors—experimental and clinical results of hyaluronidase as additive to cytostatic chemotherapy. Cancer Lett. 1998;131:85–99. [PubMed] [Google Scholar]
- 44.Wu M., Cao M., He Y., Liu Y., Yang C., Du Y. A novel role of low molecular weight hyaluronan in breast cancer metastasis. FASEB J. 2015;29:1290–1298. doi: 10.1096/fj.14-259978. [DOI] [PubMed] [Google Scholar]
- 45.Schmaus A., Klusmeier S., Rothley M., Dimmler A., Sipos B., Faller G. Accumulation of small hyaluronan oligosaccharides in tumour interstitial fluid correlates with lymphatic invasion and lymph node metastasis. Br J Cancer. 2014;111:559–567. doi: 10.1038/bjc.2014.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang R.Y., Chu Y.L., Jiang Z.B., Chen X.M., Zhang X., Zeng X. Glycyrrhizin suppresses lung adenocarcinoma cell growth through inhibition of thromboxane synthase. Cell Physiol Biochem. 2014;33:375–388. doi: 10.1159/000356677. [DOI] [PubMed] [Google Scholar]
- 47.Benitez A., Yates T.J., Lopez L.E., Cerwinka W.H., Bakkar A., Lokeshwar V.B. Targeting hyaluronidase for cancer therapy: antitumor activity of sulfated hyaluronic acid in prostate cancer cells. Cancer Res. 2011;71:4085–4095. doi: 10.1158/0008-5472.CAN-10-4610. [DOI] [PMC free article] [PubMed] [Google Scholar]