Abstract
Discovered by Youyou Tu, one of the 2015 Nobel Prize winners in Physiology or Medicine, together with many other Chinese scientists, artemisinin, artemether and artesunate, as well as other artemisinins, have brought the global anti-malarial treatment to a new era, saving millions of lives all around the world for the past 40 years. The discoveries of artemisinins were carried out beginning from the 1970s, a special period in China, by hundreds of scientists all together under the “whole nation” system. This article focusing on medicinal chemistry research, briefly introduced the discovery and invention course of the scientists according to the published papers, and highlighted their academic contribution and achievements.
KEY WORDS: Antimalarials, Artemisinin, Artemether, Artesunate, Dihydroartemisinin
Graphical abstract
Discovered by Youyou Tu, one of the 2015 Nobel Prize winners in Physiology or Medicine, together with many other Chinese scientists, artemisinin, artemether and artesunate, as well as other artemisinins, have brought the global anti-malarial treatment to a new era, saving millions of lives all around the world for the past 40 years. The discoveries of artemisinins was carried out beginning from the 1970s, a special period in China, by hundreds of scientists all together under the “whole nation” system. This article focusing on medicinal chemistry research, briefly describes the discovery and invention course of the scientists according to the published papers, and highlightes their academic contribution and achievements.
1. Background of artemisinin research
1.1. Discovery of new anti-malarials in a whole nation system
During the Vietnam–US War in the early 1960s, Vietnamese soldiers suffered from serious malaria because a mutated form of Plasmodium falciparum wreaked havoc in Vietnam. Under the request from North Vietnamese government to provide effective drugs against multidrug-resistant malaria, China started, on May 23, 1967, a project to search for new antimalaria drugs; the project, named “Project 523”, was managed under the “whole nation” system, and it involved sixty research organizations and more than 500 scientists1, 2. All activities of the project were directed by the entity known as “Project 523”.
1.2. The program for new drugs or lead compounds from traditional Chinese medicine (TCM) and folk medicine
One of the various programs in the “Project 523” was a team for the “study and survey on effective folk medicine and therapy against malaria”. Making every endeavor, the team obtained several hits, including a sesquiterpene, yingzhaosu A, from Artabotrys hexapetalus, a metal-containing principle in the plant Polyalthiane moralis, analogs of β-dichroine and components from Artemisia3.
1.3. The discovery of artemisia and artemisinin
In 1969, the Institute of Chinese Materia Medica (ICMM), China Academy of Traditional Chinese Medicine, joined the “Group of TCM” of Project 523. Youyou Tu of ICMM was the group leader. She and her ICMM colleague Yagang Yu and Guoming Gu of Academy of Military Medical Sciences (AMMS), collected and screened more than 100 simple and compound recipes from well-recorded folk medicines and TCM, where they found that Artemisia appeared in high frequency for recorded efficacy against malaria (anti-malarial action of Artemisia was recorded in many ancient Chinese herb books in Dynasties Tang, Song, Yuan and Ming). Through systematic bioassay-guided screening by Yu and Gu, an alcoholic extract of Artemisia was discovered to exert inhibitory activity against Plasmodium falciparum by up to 60%–80%1. The extent of the inhibition was unfortunately highly variable; however, the results from Yu and Gu provided valuable references for further investigations.
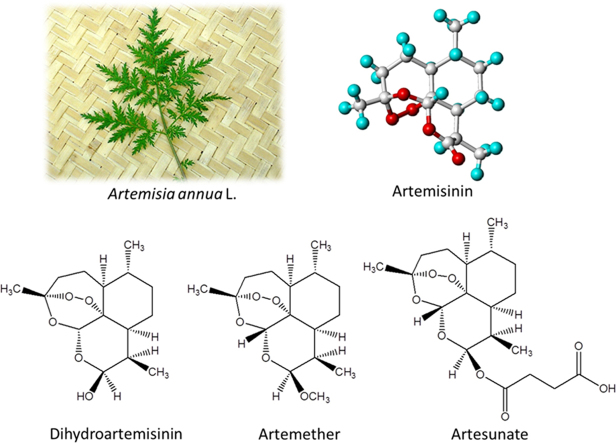
Critical progress was subsequently made by Youyou Tu, who was inspired by the detailed methods of Artemisia usage described in ancient Ge Hong’s book “Zhou Hou Bei Ji Fang”. The book reads: for the treatment of malaria “a handful of Artemisia soaked in two liter of water. Take the pressed juice.” She deduced that the disuse of decoction (by boiling) may imply thermo-instability of active principles, which nonetheless may be lipophilic. Therefore, she switched from ethanol to ether as the extraction solvent. After removing the acidic principles, white solids were isolated from the neutral ethereal extraction, which exerted 100% inhibition against mouse P. falciparum. The white solids were later identified to be artemisinin (1) (Fig. 1). The discovery of artemisinin from the ethereal extraction certainly played a critical role in opening new therapeutic means and saving millions of lives from malaria illness. It also justifies Youyou Tu’s sharing of the 2015 Nobel Prize for Physiology or Medicine.
Figure 1.
Chemical structures of sesquiterpenes identified from the ethereal extraction of Artemisia.
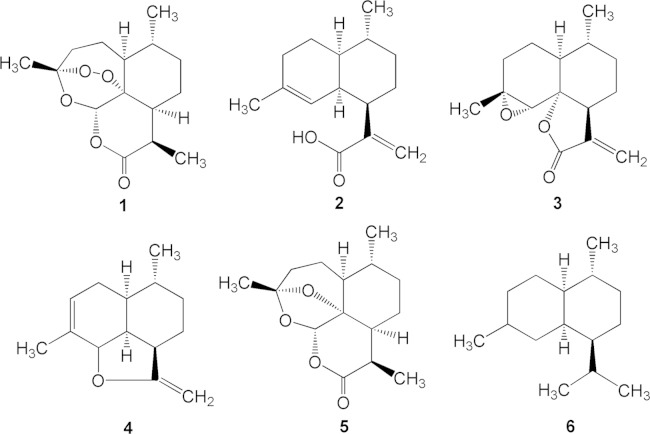
In addition to artemisinin, several other sesquiterpenes were identified from the ethereal extraction, including arteannuic acid (2), arteannuin A (3), arteannuin B (4), arteannuin C (5), and amorphane (6) (Fig. 1). However, these compounds all exerted weak or no anti-malaria activity4.
2. Structural identification of artemisinin
2.1. Physicochemical properties
Artemisinin is white needle-like crystals with mp 151–153 °C. Elementary analysis and mass spectra showed the molecular formula of C15H22O5. It is insoluble in water, but dissolves in acetone, ethanol, ether, petroleum ether and alkali solution. NaOH titration of artemisinin consumes one equivalent. Qualitative analyses give positive color reactions in the oxidation of FeCl2 or NaI. It quantitatively reacts with triphenylphosphine to give one equivalent of triphenylphosphine oxide. These reactions indicated the existence of an oxidative group in its molecule.
2.2. Spectral behavior
The ultraviolet spectra (UV) of artemisinin showed the absence of an aromatic conjugate system. The infrared spectra (IR) indicated carbonyl peak of δ-lactone. 13C NMR revealed the presence of fifteen carbon signals, the numbers of primary, secondary, tertiary and quaternary carbon being 3, 4, 5 and 3, respectively. For the quaternary carbons, one yielded a carbonyl signal, whereas the other two were at low field (79.5 and 105 ppm),indicating linkage to an oxygen atom. Five doublet signals of tertiary carbon atoms appeared at the high field. 1H NMR showed a singlet at 5.68 ppm, indicating the presence of an –O–CH–O– fragment. Based on the biogenesis of sesquiterpene, the 4 oxygen atoms were deduced as ketal, acetal and lactone moieties in the artemisinin scaffold. Yet the 5th oxygen was not defined.
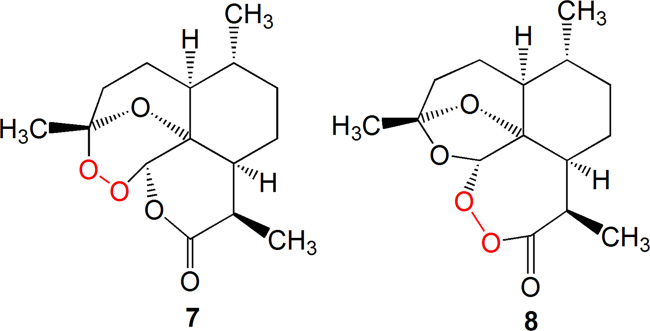
At that time (1975), Dequan Yu, a member of the TCM group of Project 523 at the Institute of Materia Medica, CAMS, promulgated the chemical structure of another anti-malarial natural product, yingzhaosu A,which contains a peroxy linkage5. This information conferred an enormous enlightenment for solving the artemisinin structure. Based on the oxidation of artemisinin in qualitative and quantitative analyses, a peroxy moiety was designated in the structure, at three possible locations, as shown in 1, 7 and 8 (Figure 1, Figure 2).
Figure 2.
Chemical structures of compound 7 and 8 with a peroxy moiety.
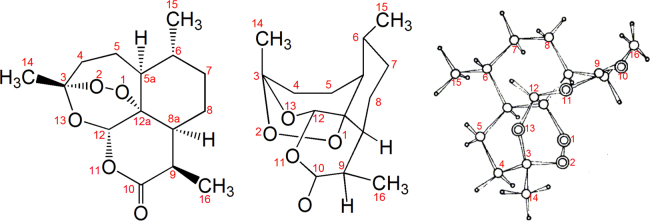
The location of the peroxy linkage and the structure of artemisinin were authentically defined by the method of X-ray crystallography in the Institute of Biophysics, Chinese Academy of Scince (CAS). The absolute configuration was determined by optical rotatory dispersion (ORD)6, 7, 8.
2.3. Chemical reactions of artemisinin—evidence in support of structure
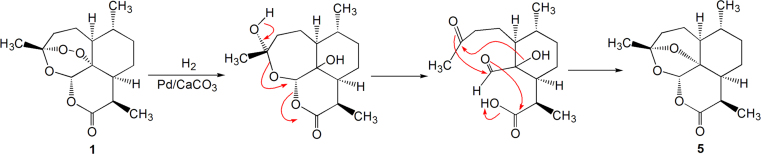
Hydrogenation of artemisinin, under the catalyst of Pd/CaCO3, gave rise to a product, coined reduced artemisinin, with the peroxy moiety being reduced into ether linkage. The structure of reduced artemisinin is identical to that of arteannuin C (5). The mechanism of the reduction is illustrated in Scheme 1.
Scheme 1.
The generation mechanism of reduced artemisinin.
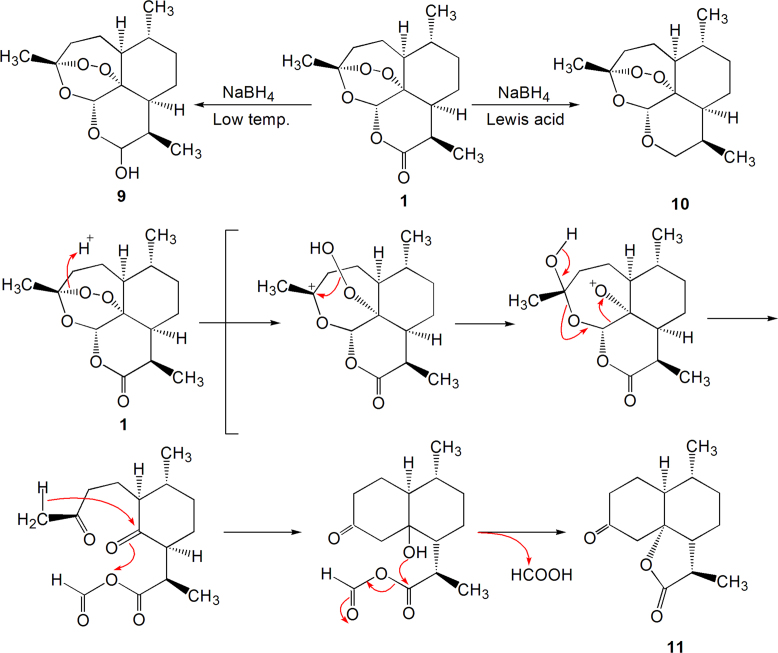
Artemisinin reacts with NaBH4 under low temperature to produce dihydroartemisinin (9, Scheme 2), in which the C10-carbonyl group is reduced into hydroxyl in the form of hemi-acetal. In the presence of Lewis acid with the treatment with NaBH4 the C10-carbonyl group of artemisinin converts into methylene group (10, Scheme 2); artemisinin reacts with acetic-sulfuric acid to generate (11), where the mechanism of reaction involves rearrangement and loss of formic acid, as shown in Scheme 2.
Scheme 2.
Acid-catalytic decarbonylation and rearrangement.
2.4. Total synthesis of artemisinin—validation of structure
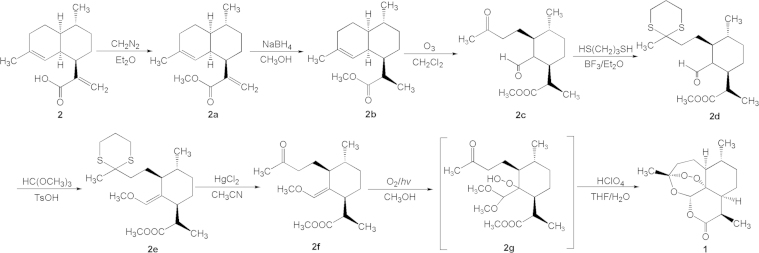
The success in total synthesis of artemisinin in China was accomplished by Xingxiang Xu and co-workers in Shanghai Institute of Organic Chemistry, CAS. Based upon biogenic principle of artemisinin, it was believed that arteannuic acid (2), in abundant content in the plant, may be the precursor for the biosynthesis of artemisinin. So a synthetic scheme was designed and performed as concisely illustrated in Scheme 3. Esterification of arteannuic acid (2) gives 2a, which is treated with NaBH4 to saturate the exo-double bond to give 2b. Opening the octahydronaphthalene through ozonization creates the monocyclic keto-aldehyde 2c. Selective protection of the ketone group by 1,3-dimercaptopropane gives 2d, which is treated with orthoformate to modify the aldehyde group, giving rise to 2e. Removal of the protecting sulfoether group produces 2f, which is converted to the key intermediate 2g by the oxygen–photooxidation reaction. Acidolysis of 2g is automatically followed by cascade reactions to form the peroxy, ether and lactone-containing compound artemisinin (1)9.
Scheme 3.
Synthetic route of artemisinin starting from arteannuic acid (2).
Simultaneously, Schmid and Hofheinz10 using a different route completed the total synthesis of 1. Several synthetic strategies and improvements were subsequently reported. The success in total synthesis of artemisinin provided authentic evidence for the artemisinin structure (Fig. 3), and also paved the way for its industrial production.
Figure 3.
The location of the peroxy linkage and the structure of artemisinin defined by the method of X-ray crystallography and the absolute configuration determined by optical rotatory dispersion (ORD).
2.5. Preliminary clinic trails
After conducting the safety experiments in animals and healthy volunteers with neutral components of ethereal extraction, Tu’s group proceeded to clinic therapy in Beijing and Hainan Province, China. A definite therapeutic efficacy was demonstrated in the treatment of 30 patients with various types of malaria. The neutral component mainly contains artemisinin (at that time called Artemisinin II). Tu’s work both on the discovery of artemisinin and the proof of its action unequivocally created a new area of malaria treatment.
2.6. The large-scale production of artemisinin and validation of clinic efficacy
Inspired by the success of the ethereal extraction method and the preliminary clinical effect, Zeyuan Luo (member of Project 523 in Yunnan Institute of Material Medica) and Zhenxing Wei (member of Project 523 in Shandong Institute of Chinese Traditional Medicine) obtained artemisinin in pure quality from the local plant Artemisia annua L. Especially, Luo’s invention of “Petro-solvent extraction” laid the material foundation for the large scale production of artemisinin and subsequent clinical trials.
Cooperating with Luo, who provided pure artemisinin, Guoqiao Li (member of Project 523 at Guangzhou Institute of Chinese Traditional Medicine) conducted clinical trails for malaria patients in Yunnan Province. Artemisinin (originally called Huang Hao Su) was orally administered to patients. Li showed that the fine ring forms of P. falciparum in human body ceased development and their abundance rapidly decreased, indicating that the efficacy of artemisinin was far superior to those of quinine and chloroquine. Following oral administration of artemisinin, 18 patients suffering different forms of malaria were totally cured, including cerebral falciparum (1 case), P. falciparum jaundice type (2 cases), uncomplicated falciparum (11 cases) and vivax malaria (4 cases). Li’s clinical research was the first to show artemisinin to be an effective, fast-acting, and low-adverse-effect antimalarial medicine. His work conveyed an important contribution to the clinical application of artemisinins11.
3. Lead optimization—modification of artemisinin
As a special sesquiterpene, artemisinin contains five interweaving oxygen atoms to form cyclic ether and peroxy ether, cyclic acetal and ketal, as well as lactone. Given the absence of a solubilizing moiety in its structure, artemisinin is insoluble in water, and poorly soluble in lipids. The poor biopharmaceutic properties and low bioavailability of artemisinin restricts its clinical application, even though artemisinin was approved and launched by China Food and Drugs Administration (CFDA) in 1985. In order to develop artemisinin-related drugs, the “Project 523” took on further optimization of the lead compound. Because of lack of knowledge on the mechanism of action by artemisinin, phenotype evaluation and analysis of structure–activity relationships were also performed in the optimization process.
3.1. Critical structural features—peroxy linkage and special scaffold
The change in peroxy moiety of artemisinin into ether linkage results in reduced artemisinin (5), which is devoid of anti-malarial activity. Considering the existence of peroxy group in antimalarial yingzhaosu A, it was inferred that the peroxy moiety is one of the pharmacophoric features. However, other peroxy-containing compounds with different skeletons did not exert the anti-malarial activity, suggesting that the specific scaffold of artemisinin is also one of the critical factors. Therefore, these two features were kept constant in the further optimization and the design of derivatives.
3.2. Artemisinin-related drug in China—artemether
The first important derivative dihydroartemisinin (9) was obtained for identifying artemisinin structure. Compound 9 exerted stronger activities than did the lead compound 1, a result suggesting that alterations in the lactone moiety retains or raises the activity. In order to further increase the anti-malarial activity and chemical stability, Ying Li and co-workers synthesized additional derivatives with two types of functional groups: ethers and esters.
3.2.1. C10-ethereal compounds
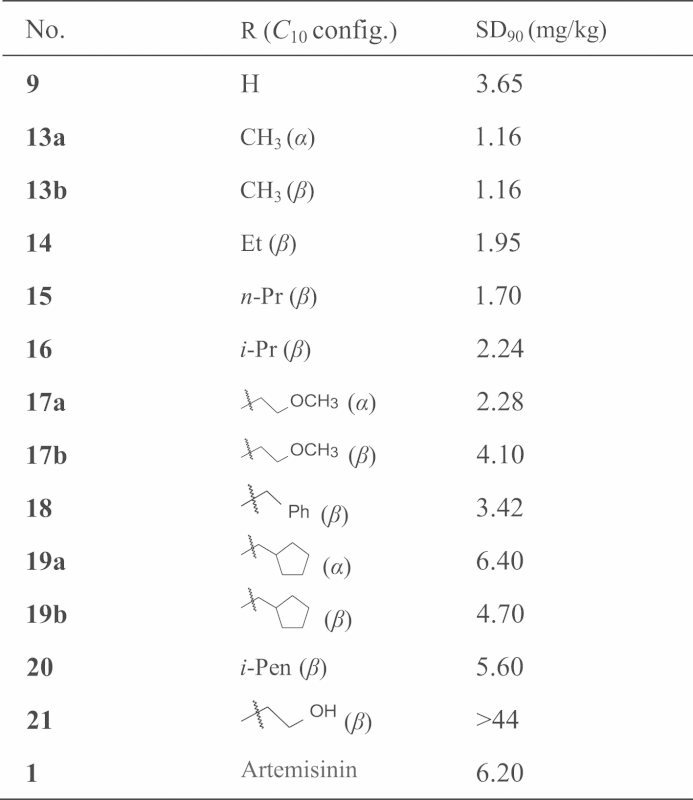
Under the catalysis of BF3-ether, dihydroartemisinin (9) reacts with various alcohols to yield acetal-ethers (general structure 12, Fig. 4). The products consist of two diastereomers (α- and β-epimers; β-epimer predominates), which are both stable compounds. The separated ethereal α and β-monomers were evaluated against chloroquine-resistant mouse P. berghei (compared to artemisinin as the control), for a determination of the dose for inhibition of 90% Plasmodium (SD90). The structure and activity of typical ethereal compounds are listed in Table 1. The data show that the activity of dihydroartemisinin (9) is one time stronger than that of artemisinin (1). The methyl ether (13) increases the activity two times compared to 9. However, with increase in the size of alkyl group the activities decrease. Generally β-epimers show higher activity than corresponding α-epimers. β-C10-O-methyl dihydroartemisinin (β-13, also named as artemether) was the strongest congener in the ethereal series.
Figure 4.
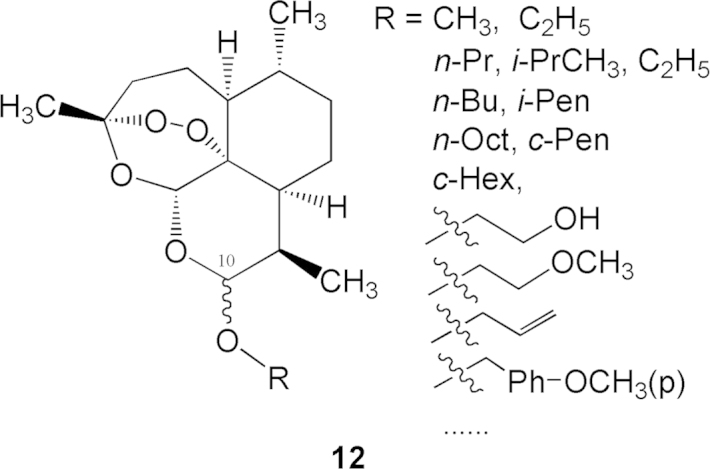
General chemical structures of acetal-ethers.
Table 1.
The anti-malarial activity against P. berghei of ethereal compounds of dihydroartemisinin.
 |
3.2.2. C10-ester compounds
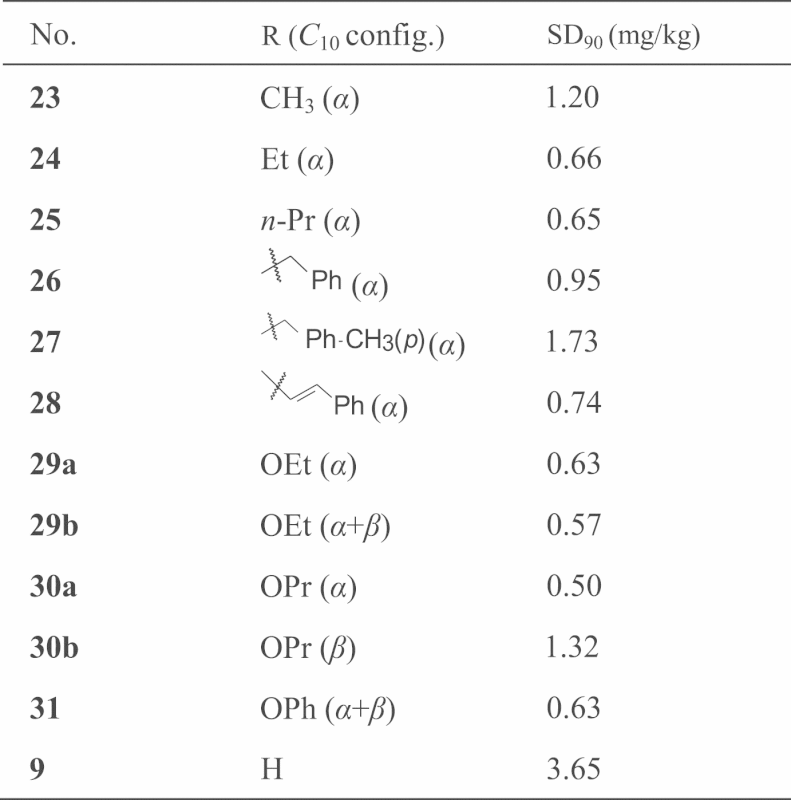
Dihydroartemisinin (9) reacts with anhydrides, acyl chlorides, or chloroformates in pyridine medium to yield C10-carboxy esters or carbonate esters (general structure 22, Fig. 5) 12, 13.The structures and activities of these esters against P. berghei are listed in Table 2. The activity of ester compounds in general are stronger than that of the ethereal series. The order of activity strength is as follows: carbonates>carboxy esters>ethers>dihydroartemisinin>artemisinin.
Figure 5.
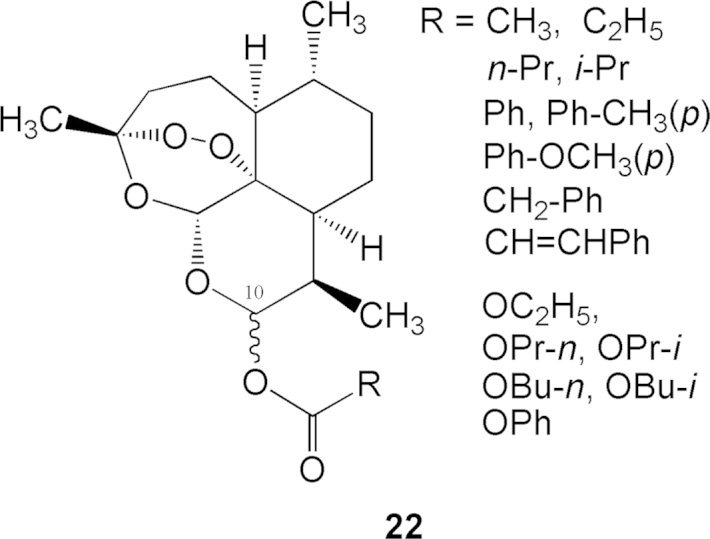
General chemical structures of C10-esters compounds.
Table 2.
The anti-malarial activity against P. berghei of carboxylic ester and carbonate compounds of dihydroartemisinin.
 |
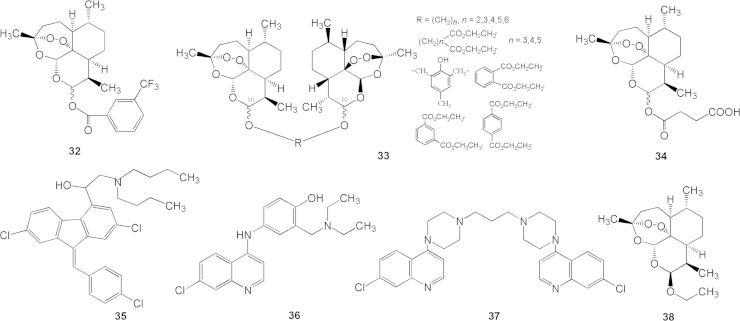
Additionally, Yu and co-workers synthesized C10-substituted benzoates of dihydroartemisinin. The biological results indicated that the halogen- and nitro-containing molecules were similar to artemisinin in activity14, 15, except compound 32 (Fig. 6), whose activity is 10 times stronger than artemisinin.
Figure 6.
Chemical structures of compound 32–38.
3.2.3. Bis-ethereal dihydroartemisinin
Using polymethylene as a linkage to connect two dihydroartemisinin, Chen and co-workers16 synthesized a series of bis-ethereal dihydroartemisinins (general structure 33, Fig. 6) that had C10,10′-α, β or β, β configurations. However, the biological evaluation showed that none of them was more active than compound 13 (artemether).
3.2.4. The QSAR of dihydroartemisinin derivatives
Multiple regression analysis was performed for the ester and ether derivatives of dihydroartemisinin using Hansch analysis method, correlating antimalarial activities to the linear free-energy related descriptors. The significant equations are as follows17:
for ester and ether derivatives:
| (1) |
for ester derivatives:
| (2) |
for ether derivatives:
| (3) |
In the equations, C is the molar concentration of a congener for inhibiting 90% growth in P. berhgei; P is the partition coefficient determined in the system of n-octanol and water; indicator variable I is assigned the value of 1 for congeners of C10 β-epimers and zero for α-epimers; and σ* means Taft electronic substituent constant.
As Eqs. (1), (2) indicate, the variance in antimalarial activities of dihydroartemisinin ester and ether series is well explained by the parabolic logP, and the optimal partition coefficients for maximum activities (2.6–2.9) are consistent with those in the other antimalarial compounds. More potent activity in α-epimers than in the corresponding β-epimers is indicated by the minus coefficient of indicator variable I [α : β = antilog (0.612 : 0) = 4.2 : 1]. For the ester series the correlation Eq. (2) is the same as Eq. (1), although the coefficients are different. In the series of ethers, however, an additional term (electronic parameter σ*) had to be added to make the correlation significant. This suggests the mode of action and/or transport process of the ether series be different from those of the ester derivatives.
3.2.5. Artemether—one of the candidates selected for drug development
Three compounds with high antimalarial activity were selected from the above-described dihydroartemisinin ethers, carboxylic esters, and carbonates for further evaluation of efficacy in experimental therapy against P. berhgei in mice. The results for compounds 13 (β-epimer, artemether), 24 (α-epimer of propionate), and 29 (α-epimer of ethoxycarbonate) are listed in Table 3. Data show that the 3 compounds possess favorable therapeutic effects for infected mice. Furthermore, the therapeutic efficacy of these compounds for P. berhgei-infected cynomolgus monkeys was demonstrated (data not shown)18. After comparing the three compounds for the toxicities in mice, rats, rabbits, dogs, and monkeys, as well as the physic–chemical properties, artemether (13, β-epimer) was chosen as the candidate to start with for further pre-clinical investigations18. In 1978, the clinical trials commenced. In 1987, artemether was approved by CFDA as a new molecule entity and launched in various formulations.
Table 3.
The comparison of antimalarial activity of compounds 13, 24, and 29 against P.berhgei in mice.
| Compd. | SD50a (mg/kg) | SD90b (mg/kg) | CD50c (mg/kg) | CD100d (mg/kg) |
|---|---|---|---|---|
| 13 (Artemether) | 0.60 | 1.00 | 1.22 | 1.80 |
| 24 (Propionate) | – | 0.50 | 0.47 | 0.82 |
| 29 (Ethoxycarbonate) | 0.32 | 0.66 | 0.76 | 0.91 |
| 1 (Artemisinin) | – | 6.2 | – | 25 |
| 9 (Dihydroartemisinin) | – | 3.7 | – | – |
The dose required for 50% suppression of the parasitemia.
The dose required for 90% suppression of the parasitemia.
Mouse, p.o., once daily, after 5 days the minimum dose required for 50% negative conversion of the parasitemia.
Mouse, p.o., once daily, after 5 days the minimum dose required for 100% negative conversion of the parasitemia.
3.2.6. Another candidate selected for drug development —artesunate
To develop water-soluble artemisinin-related drugs for injection formulations, Xu Liu of Guilin Pharmaceutical Company designed and synthesized dihydroartemisinin C10-monoesters of diacids. One of the compounds is succinic acid monoester (34, Code No. 804, Fig. 6), the structure of which was defined by IR, NMR, MS, and X-ray crystallography. The sodium salt of 34 is soluble in water and suitable for injection dosage19, 20.
Preclinical investigations indicate that compound 34 (coined as artesunate) exerts strong antimalarial effects. In mouse malaria, the activity against chloroquine-resistant and -sensitive strains was respectively 5.2 and 5.3 times greater than that of artemisinin (1) when sodium artesunate was given intravenously. In monkey malaria, the time required for disappearance of plasmodia from the blood stream was 16–20 h when artesunate was administered intravenously at a dosage of 6–12 mg/kg q.d. for three days.
Sub-acute toxicity experiments on rabbits and dogs given i.v. sodium artesunate showed no harmful effects on cardiac, hepatic and renal functions at test dosage, nor were any apparent structural changes found upon pathological examination21.
Pharmacokinetic experiments in animals showed that artesunate was rapidly distributed and hydrolyzed to dihydroartemisinin. The plasma half-life (t1/2) was 15.6 min in rats and 10–45 min in dogs. After oral administration in volunteers, the bioavailability of artesunate was 40% and t1/2 was 41.35 min22. Clinical trials indicated that artesunate exerts significant therapeutic effect for the treatment of patients with tertian falciparum and cerebral malaria. Artesunate was approved by CFDA in 1987 as a new molecule entity and launched in various formulations.
3.2.7. Compound formulations—the combination artemisinin-related drugs with other anti-malarials
In spite of the high efficiency and fast-action advantages against all kinds of malaria, artemether and artesunate still suffer from a common drawback. With very short half-lives, artemrther and artesunate are so quickly cleared from plasma that the plasmodia of patients cannot be completely removed. Furthermore, as drug resistance develops to existing drugs, new ones need to be introduced. For P. falciparum, the use of two or more drugs with different modes of action in combination is now recommended, to provide adequate cure rate and delay development of resistance. Therefore, artemisinin-based combination therapy (ACT) is recommended for the treatment of P. falciparum malaria. Fast acting artemisinin-based compounds are combined with a drug from a different class. Compound formulations have been developed for artemether, artesunate and dihydroartemisinin, as described below.
-
(1)
Compound artemether tablet is the first fixed-dose artemisinin-based combination formulation, which contains artemether (13) and lumefantrine (35, Fig. 6). Lumefantrine was synthesized and developed as a new anti-malarial drug by Rongxian Deng and coworkers23 in 1981. The compound artemether tablet was designed by Rongxian Deng and Dingxi Ning and then recommended and pre-qualified by World Health Organization (WHO) for the treatment of uncomplicated malaria caused by P. falciparum. It has been shown to be effective for multi-drug resistant P. falciparum and recommended as first-line treatment for uncomplicated malaria in several countries. In 1992, the tablet formulation was approved in China under the trade name Coartem®; each tablet contains artemether 20 mg, lumefantrine 120 mg. In 2002 Coartem® was included in the 12th edition of the “Essential drug list” by WHO. In 2009, the FDA approved Coartem® in the USA.
-
(2)
Artesunate (34) /amodiaquine (36, Fig. 6), another dose-fixed tablet formulation, was launched in 2007, under the trade name Coarsucum. Each tablet contains artesunate 100 mg and amodiaquine 270 mg. In 2007 it was pre-qualified in the therapeutic list by WHO.
-
(3)
Dihydroartemisinin (9) /piperaquine (37, Fig. 6) is a clinically investigated ACT tablet under the trade name Artekin®. Each tablet contains dihydroartemisinin 40 mg and piperaquine 320 mg24.
3.3. The mechanism of action of artemisinin-related drugs
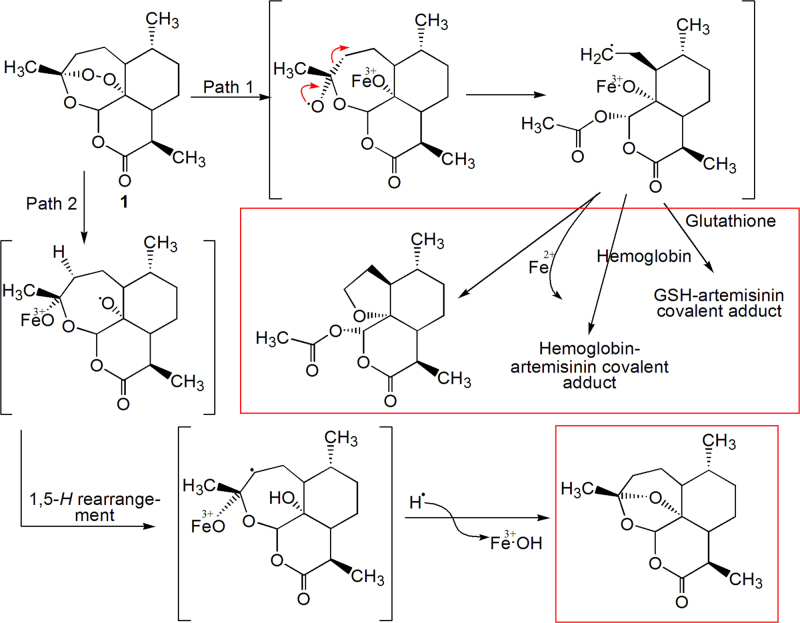
Most artemisinin-related drugs in use today are prodrugs, which are activated through hydrolysis to the metabolite dihydroartemisinin. Several lines of evidence indicate that artemisinin drugs exert their anti-malarial action by radical formation via their peroxide linkage. During the stage when the parasite is located inside red blood cells, it consumes hemoglobin within its digestive vacuole by the hemoglobin degradation enzyme. The process releases ferrous ions, which catalyze the cleavage of the peroxy moiety to form oxygen or carbon free radicals. These free radical intermediates disrupt the biomembrane and inhibit the cysteine protease of the vacuoles. Scheme 4 illustrates the reaction process catalyzed by ferrous ions via two proposed pathways. The products are marked by the boxes25.
Scheme 4.
The products and biochemical mechanism of artemisinins' action.
Another plausible mechanism of action for artemisinin drugs consists of the inhibition of the target “P. ferghei calcium ATP protein 6” (PfATP6). PfATP6 is a SERCA-type protein enzyme, which modulates the calcium concentration within P. plasma through consumption of ATP to maintain calcium homeostasis. Inhibition of PfATP6 by artemisinins causes the increase of calcium levels within P. plasma and kills P. Heritability studies suggest that the observed artemisinin resistance phenotype of the parasites has a genetic basis—the mutation of PfATP626, 27.
4. The subsequent antimalarial artemisinins
4.1. Arteether
Arteether (38, artemotil, Fig. 6),or dihydroartemisinin-C10-β-ethylether,was developed by Brocacef Co.28 in Netherlands and launched in 2000. Arteether is used as a sesame oil formulation for intramuscular injection to treat patients with falciparum malaria. At 3–12 h after injection, it reaches peak plasma concentration; plasma half-life (t1/2) was 1–2 days. Oxidative deethylation of arteether by liver CYP3A4 occurs to form dihydroartemisinin, which is glycosylated by glucuronic acid, leading to be clearance from bile. As a follow-on medicine, arteether does not show any advantage over artemether.
4.2. Artemisone
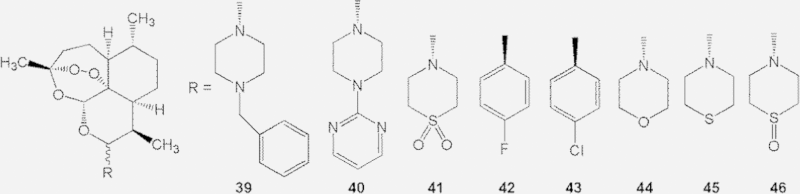
Bayer and Hong Kong University of Science & Technology set up a project with the aim to improve artemisinin pharmacokinetics. Artemether, arteether, and artesunate are rapidly hydrolyzed in plasma to dihydroartemisinin, which is conjugated to glucuronic acid and excreted from bile, events that cause these drugs’ short duration of action. Moreover, dihydroartemisinin exerts some neurotoxicity. To prolong time period of action and avoid the production of dihydroartemisinin, a series of compounds were synthesized. Some notable compounds (39–46) and their physico-chemical properties, as well as anti-malarial activity, are listed in Table 4 29. The data show that compounds 39, 40, 42, 44 and 45 exert strong activities. However, in rat primary neuronal brain stem cell culture tests, these compounds showed toxicities. Compounds 42 and 43 at the dose of 50 mg/kg/day, caused substantial body-weight loss, coupled with reduced motility, uncoordinated gait, and piloerection. Compound 41, which has excellent physic–chemical and pharmacological properties, was validated to be a safe and effective antimalarial in aotus monkeys; the compound, named artemisone, is now in phase II clinical trial.
Table 4.
The structure, physico-chemical properties, and antimalarial activitya of compounds 39–46.
| Compd. | Solubility (mg/L) | logP n-octanol/buffer |
P. berghei ED90 (mg/kg) |
P. berghei artesunate indexb |
P. yoelii ED90 (mg/kg) |
P. yoelii artesunate index | |||
|---|---|---|---|---|---|---|---|---|---|
| sc | po | sc | po | sc | po | sc | |||
| Artesunate | 565 | 2.77 | 7.2 | 7.1 | 1.0 | 1.0 | 22.0 | -- | 1.0 |
| 39 | 8 | 5.62 | 0.8 | 3.5 | 9.0 | 2.0 | 0.85 | 3.0 | 25.9 |
| 40 | < 2 | 4.78 | 0.6 | 2.8 | 12 | 2.5 | 0.52 | 2.0 | 42.3 |
| 41 | 89 | 2.49 | 1.5 | 3.1 | 4.8 | 2.3 | 3.9 | 5.0 | 5.6 |
| 42 | < 1 | 5.59 | 1.16 | 5.0 | 6.2 | 1.4 | 1.08 | -- | 20.4 |
| 43 | < 1 | 6.15 | 3.8 | 4.6 | 1.9 | 1.7 | 3.0 | -- | 7.3 |
| Artesunatec | 565 | 2.77 | 4.6 | 9.3 | 1.0 | 1.0 | 42.0 | -- | 1.0 |
| 44 | 28.4 | 3.05 | 0.18 | 1.3 | 25.6 | 7.15 | 1.25 | 1.84 | 33.6 |
| 45 | < 2 | 4.97 | 0.51 | 1.9 | 9.0 | 4.9 | 0.61 | 2.0 | 81.0 |
| Artesunatec | 565 | 2.77 | 12.0 | -- | 1.0 | -- | 50.0 | -- | 1.0 |
| 46 | 1251 | 2.63 | 9.0 | -- | 1.3 | -- | 10.0 | -- | 5.0 |
| Artemisinin | 63 | 2.94 | -- | -- | -- | -- | -- | -- | -- |
| Artemether | 117 | 3.98 | -- | -- | -- | -- | -- | -- | -- |
In vivo screens against CQ-sensitive P. berghei N strain and CQ-resistant P. yoelii NS strain. Peters׳ four-day test with mice treated daily subcutaneously (sc) or orally (po) from the day of infection (Day 0) through Day 3; results (ED90 values) were based on parasite counts in peripheral blood on day 4.
ED90 (artesunate) / ED90 (tested compound).
The variation in artesunate activity in different experiments.
5. Concluding remarks
The discovery of artemisinin and the development of a series of related drugs in China have pioneered a new era for the treatment of malaria. These drugs have served as the standard regimen for treating P. falciparum and saved millions of malarial patients worldwide. The tremendous successes of the artemisinin anti-malarial therapy were due to the serendipitous discovery by Youyou Tu and the efforts of the organizers and other participants of “the 523 Project”, including many scientists/technologists, covering the fields of chemistry, pharmacognosy, medicinal chemistry, pharmacology, toxicology, pharmaceutics and processing, as well as clinical and industrial partners. Today this type of “whole-nation” R&D program, which was a product of the unique historical period that encompassed the “Cultural Revolution” and a period that suffered significant economic hardship and paucity of modern infrastructure for drug development, will unlikely be mimicked or reproduced. Furthermore, this particular case is also flawed by the lack of intellectual property protection and the low share of market profit by the inventors. However, as a significant contribution to human health, the R&D program of artemisinins deserves the ultimate recognition by the society, as symbolized by the Noble Prize.
Scientifically, with the unique structure of the artemisinins, there remains an enormous space for further investigations. Extensive efforts have to be devoted to the elucidation of drug targets and mechanisms of action, the improvement of pharmacokinetic properties, and the identification of a novel generation of artemisinins against resistant strains of plasmodia. In addition, the promiscuity of artemisinin has also provided clues for the search for drugs in other therapeutic fields30, 31, 32.
Biography

Prof. Zongru Guo is a senior scientist in Medicinal Chemistry. He has devoted his whole life to the endeavor of drug development. Imrecoxib®, one of his many accomplishments, has been launched on the market since 2011
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.The history of discovery of artemisinin and development of artemisinin-based antimalarials. In: Li GQ, et. al., editors. Artemisinin-based antimalarials. Beijing: Science Publishing House; 2015. p. 1.
- 2.The discovery of artemisinin . In: Artemisia and the artemisin drugs. Yu Y.Y., editor. Chemical Industry Press; Beijing: 2009. p. 1. [Google Scholar]
- 3.Zhang W.H. Social relations in innovations: several arguments about the artemisinin. J Dialectics Nat. 2009;31:32–39. [Google Scholar]
- 4.Tu Y.Y., Ni M.Y., Zhong Y.R., Li L.N., Cui S.L., Zhang M.Q. Studies on the constituents of Artemisia annua L. Acta Pharm Sin. 1981;16:366–370. [PubMed] [Google Scholar]
- 5.Liang X.T., Yu D.Q., Wu W.L., Deng H.C. The structure of yingzhaosu A. Acta Chim Sinica. 1979;37:215–230. [Google Scholar]
- 6.Qinghaosu Research Group Institute of Biophysics, Academia Sinica. Crystal structure and absolute cofiguration of qinghaosu. Sci Sin. 1980;23:380–396. [Google Scholar]
- 7.Liu J.M., Ni M.Y., Fan J.F., Tu Y.Y., Wu Z.H., Wu Y.L. Structure and reaction of arteannuin. Acta Chim Sin. 1979;37:129–141. [Google Scholar]
- 8.Coordinating Research Group for the structure of Artemisinin A new type of sesquiterpene lactone-artemisinin. Chin Sci Bull. 1977;22:142. [Google Scholar]
- 9.Xu X.X., Zhu J., Huang D.Z., Zhou W.S. Studies on structure and syntheses of artennuin and related compound. X. The stereocotrolled synthesis of artennuin and deoxy artennuin from arteneuic acid. Acta Chim Sin. 1983;41:574–576. [Google Scholar]
- 10.Schmid G., Hofheinz W. Total synthesis of quinghaosu. J Am Chem Soc. 1983;105:624–625. [Google Scholar]
- 11.Jianfang Zhang. Yangcheng Evening News Press; Guangzhou: 2006. A detailed chronological record of Project 523 and the discovery and development of qinghaosu (artemisinin) [Google Scholar]
- 12.Li Y., Yu P.L., Chen Y.X., Li L.Q., Ge Y.Z. Studies on analogs of artemisinine. I. The synthesis of ethers, carboxylic esters and carbonates of dihydroartemisinine. Acta Pharm Sin. 1981;16:429–439. [PubMed] [Google Scholar]
- 13.Gu H.M., Lü B.F., Qu Z.X. Antimalarial activities of 25 derivatives of artemisinine against chloroquine-resistent Plasmodium berghei. Acta Pharmacol Sin. 1980;1:48–50. [PubMed] [Google Scholar]
- 14.Yu P.L., Chen Y.X., Li Y., Ji R.Y. Studies on analogs of qinghaosu (artemisinine, artennuin). IV. Synthesis of derivatives of qinghaosu containing halogen, nitrogen and sulfer heteroatoms. Acta Pharm Sin. 1985;20:357–365. [PubMed] [Google Scholar]
- 15.China Cooperative Research Group on Qinghaosu and its derivatives as antimalarials The chemistry and synthesis of Qinghaosu derivatives. J Tradit Chin Med (Eng edition) 1982;2:9–16. [PubMed] [Google Scholar]
- 16.Chen Y.X., Yu P.L., Li Y., Ji R.Y. Studies on analogs of qinghaosu. VII. The synthesis of ethers of bis(dihydroqinghaosu) and bis(dihydrodeoxyqinghaosu) Acta Pharm Sin. 1985;20:470–473. [PubMed] [Google Scholar]
- 17.Wu J.A., Ji R.Y. A quantitative structure–activity study on artemisinin analogues. Acta Pharmacol Sin. 1982;3:55–60. [PubMed] [Google Scholar]
- 18.Gu H.M., Liu M.Z., Lü B.F., Xu J.Y., Chen L.J., Wang M.Y. Antimalarial effect and toxicity of methyl-dihydroartemisinine in animals. Acta Pharmacol Sin. 1981;2:138–144. [PubMed] [Google Scholar]
- 19.Liu X. Study on artemisinin derivatives. Chin Pharm Bull. 1980;15:39. [Google Scholar]
- 20.Pu JL, Chen RG, Cai JL. The structure determination of artemisinin derivatives. In: Proceedings of 2nd Annual Meeting. Guangxi Pharmaceutical Society. 1979. p. 82
- 21.Yang Q.C., Gan J., Li P.S., Zhang S.F., Wei Z.Q., Shi W.Z. Artemisinin derivatives—anti-malarial activity and toxicity of artesunate. J Guangxi Med Univ. 1981;1981:1–6. [Google Scholar]
- 22.Yang Q.C., Shi W.Z., Li R., Gan J. The antimalarial and toxic effect of artesunate on animal models. J Tradit Chin Med. 1982;2:99–103. [PubMed] [Google Scholar]
- 23.Deng R.X., Yu L.B., Zhang H.B., Geng R.L., Ye K.L., Zhang D.F. Studied on antimalarial agents—α-(alkylaminomethyl)-halogenated-4-fluorinemethanol. Acta Pharm Sin. 1981;16:920–924. [PubMed] [Google Scholar]
- 24.The Clinical trials of dihydroartemisinin/piperaquine phosphate tsblets. In: Li GQ, Li Y, et al., editors. Artemisinin-based antimalarials. Beijing: Science Publishing House; 2015. p. 416–9.
- 25.Robert A., Dechy-Cabaret O., Cazelles J., Meunier B. From mechanistic studies on artemisinin derivatives to new modular antimalarial drugs. Acc Chem Res. 2002;35:167–174. doi: 10.1021/ar990164o. [DOI] [PubMed] [Google Scholar]
- 26.Dondorp A.M., Yeung S., White L., Nguon C., Day N.P., Socheat D. Artemisinin resistance: current ststus and scenarios for containment. Nat Rev Microbiol. 2010;8:272–280. doi: 10.1038/nrmicro2331. [DOI] [PubMed] [Google Scholar]
- 27.Shandilya A., Chacko S., Jayaram B., Ghosh I. A plausible mechanism for the antimalarial activity of artemisinin: a computational approach. Sci Rep. 2013;3:2513. doi: 10.1038/srep02513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brossi A., Venugopalan B., Dominguez Gerpe L., Yeh H.J., Flippen-Anderson J.L., Buchs P. Arteether, a new antimalarial drug: synthesis and antimalarial properties. J Med Chem. 1988;31:645–650. doi: 10.1021/jm00398a026. [DOI] [PubMed] [Google Scholar]
- 29.Haynes R.K., Fugmann B., Stetter J., Rieckmann K., Heilmann H.D., Chan H.W. Artemisone—a high active antimalarial drug of the artemisinin class. Angew Chem Int Ed Engl. 2006;45:2082–2088. doi: 10.1002/anie.200503071. [DOI] [PubMed] [Google Scholar]
- 30.Crespo-Ortiz M.P., Wei M.Q. Antitumor activity of artemisinin and its derivatives: from a well-known antimalarial agent to a potential anticancer drug. J Biomed Biotechnol. 2012;2012:247597. doi: 10.1155/2012/247597. . Available from: 10.1155/2012/247597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang J.X., Tang W., Zuo J.P. The anti-inflammatory and imunosuppressive activity of artemisinin derivatives. Int J Pharm Res. 2007;4:336–340. [Google Scholar]
- 32.Li H.J., Wang W., Liang Y.S. Advances in research of dihydroartemisinin against parasitic diseases. Chin J Schisto Control. 2011;23:460–464. [PubMed] [Google Scholar]