Main Text
The dynamics and interactions between the molecular constituents of life underpin the functioning of biological systems. At the eukaryotic cell level, the spatial partitioning of biochemical and cell biological processes takes place at the cell membrane, and in various membrane-bound organelles embedded in the cytosol. Approximately 50% of the proteins encoded in the genome spend at least some of their time in these membrane-rich areas (1). Consequently, the study of trans-membrane protein motional dynamics has fascinated biophysicists for nearly half a century (2).
The study of protein dynamics on eukaryotic membranes is simplified experimentally because of the reduction in spatial dimensionality from three for free space to two for a plane (3). These studies have revealed the rich behavior of membrane protein motions from random (Brownian), to directed and confined. These experiments have been influential in transforming our understanding of eukaryotic cell membranes from inert two-dimensional fluids to structured, dynamic entities (4). Moreover, the cortical actin cytoskeleton has emerged as one of the key players in driving membrane organization and influencing membrane protein dynamics (5, 6).
A question that naturally emerges from these studies is whether membrane organization and dynamics are evolutionarily conserved. If one were sufficiently naive, one could address this question by measuring the dynamics of proteins in a prokaryotic cell such as a bacterium and compare these dynamics to those observed in eukaryotic cells. However, to measure the positions and movements of individual labeled lipids or protein molecules on bacterial cell membranes is extremely challenging owing to the reduced physical dimensions (size) and non-flat geometry of bacterial cells relative to eukaryotic cells. These reduced dimensions could cause the appearance of confined motion in the dynamics of membrane components, if not properly taken into account. Moreover, the interpretation of the data is complicated by the geometry of the cell membrane envelope, which is sphero-cylindrical in bacteria compared to essentially planar on adherent eukaryotic cells.
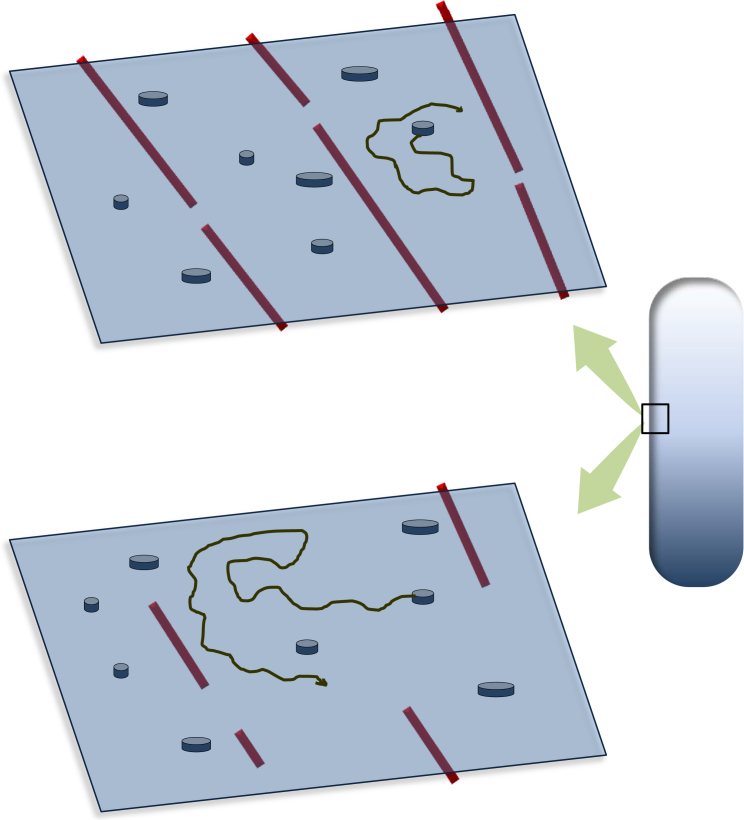
In this issue of Biophysical Journal, Oswald et al. (7) overcome these difficulties and provide robust measurements of lipid and membrane protein positions and dynamics in single E. coli cells. By means of fast single-molecule fluorescence microscopy, combined with high-end quantitative image analysis, the authors provide single-molecule trajectories of lipid or protein dynamics over four dimensions (x, y, z, and t). Time-integrated images of lipid markers directed toward “regions of increased fluidity” allowed visualization of stable lipid domains at steady state, while analysis of time-lapse images revealed hopping dynamics of lipids between domains of varying fluidity. Treatment with drugs that destabilized the bacterial MreB cytoskeleton altered both the stability and dynamics of the lipid domains. The authors then examined transmembrane protein diffusion. Diffusion was found to be homogenous at short times, but examination of longer time trajectories indicated the presence of sub-diffusive motion consistent with confinement. Again, treatment with the MreB depolymerizing drug increased the rate of motion of the transmembrane protein and revealed a reduction in confinement (see Fig. 1). These conclusions were substantiated by computer simulations of three-dimensional motion over the bacterial surface showing that the confinement effects were genuine and not a result of simple Brownian motion. Finally, the authors examined motions of eight different transmembrane proteins of different sizes. These proteins also revealed similar dynamic behavior, homogenous at short times and confined at longer times. However, the diffusion coefficients were weakly dependent on protein radius, in agreement with the predictions of Saffman and Delbrück (8).
Figure 1.
A schematic representation of the bacterial cell membrane showing confinement of transmembrane proteins by MreB cytoskeleton (top). Upon destabilization of the MreB cytoskeleton, diffusion of the transmembrane proteins is increased due to release of confinement (bottom). The MreB cytoskeleton organization is short-ranged relative to the actin cytoskeleton in eukaryotic cells. To see this figure in color, go online.
Taken together, these results suggest that some of the principles of membrane organization and dynamics, observed in eukaryotic cells, could be applicable to bacterial membranes as well. In this overall context, membrane domains appear to have been conserved during natural evolution, perhaps as an essential strategy for efficient cellular metabolism.
Author Contributions
A.H.A.C. and A.C. wrote the article.
Acknowledgments
We thank G. Aditya Kumar for help with the figure.
Editor: Claudia Steinem.
Contributor Information
Andrew H.A. Clayton, Email: aclayton@swin.edu.au.
Amitabha Chattopadhyay, Email: amit@ccmb.res.in.
References
- 1.Liu Y., Engelman D.M., Gerstein M. Genomic analysis of membrane protein families: abundance and conserved motifs. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-10-research0054. research0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edidin M. Light and life in Baltimore—and beyond. Biophys. J. 2015;108:466–470. doi: 10.1016/j.bpj.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adam G., Delbrück M. Reduction of dimensionality in biological diffusion processes. In: Rich A., Davidson N., editors. Structural Chemistry and Molecular Biology. W. H. Freeman & Company; San Francisco, CA: 1968. pp. 198–215. [Google Scholar]
- 4.Engelman D.M. Membranes are more mosaic than fluid. Nature. 2005;438:578–580. doi: 10.1038/nature04394. [DOI] [PubMed] [Google Scholar]
- 5.Kusumi A., Nakada C., Fujiwara T. Paradigm shift of the plasma membrane concept from the two-dimensional continuum fluid to the partitioned fluid: high-speed single-molecule tracking of membrane molecules. Annu. Rev. Biophys. Biomol. Struct. 2005;34:351–378. doi: 10.1146/annurev.biophys.34.040204.144637. [DOI] [PubMed] [Google Scholar]
- 6.Ganguly S., Pucadyil T.J., Chattopadhyay A. Actin cytoskeleton-dependent dynamics of the human serotonin1A receptor correlates with receptor signaling. Biophys. J. 2008;95:451–463. doi: 10.1529/biophysj.107.125732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oswald F., Varadarajan A., Bollen Y.J.M. MreB-dependent organization of the E. coli cytoplasmic membrane controls transmembrane protein diffusion. Biophys. J. 2016;110:1139–1149. doi: 10.1016/j.bpj.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saffman P.G., Delbrück M. Brownian motion in biological membranes. Proc. Natl. Acad. Sci. USA. 1975;72:3111–3113. doi: 10.1073/pnas.72.8.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]