Abstract
Green tea leaf extract (GTLE), used in this experiment has shown great influence on male reproductive system functionally as well as morphologically. The extract was prepared according to the method of Wei. H. et al. The extract was given to two different experimental animal groups with two different doses during 26 consecutive days. After treatment it was found that, the weight of the testis was markedly reduced instead of normal weight gain of all the animals. The sperm count and motility were reduced for the treated groups as compared with control animal group. The enzymes like SGPT and SGOT were as usual and other blood parameters like glucose and protein were also as usual comparing with controlled group. Testosterone level was reduced in the treated groups. FSH and LH levels were also altered accordingly in treated groups. Histological examination showed inhibition of spermatogenesis as evidenced by disintegration of seminiferous tubules of testis. Result of this study showed that GTLE has potent castrative effect on male reproductive system in dose dependent manner.
Keywords: Green tea leaf extract, castrative agent, epigallocatechin, sperm motility, 17β hydroxyl steroid dehydrogenase, catechin
Introduction
Human resource is one of the main weapon of every nation to fight against all the problems which a country generally face but when the number of population increases in uncontrolled way, it becomes a major obstruction against the development of that country. To control this major problem, awareness should be spreaded among the people. It is the compulsory step to be taken to stop the unfavourable condition of explosive growth rate of human population. The main aspect to control the population explosion is family planning. There are various methods for family planning, few are physical methods and another is surgical method. There are some measures taken as drugs also. But these methods are not availed all the times due to uneasiness of uneducated people and sometimes for high cost for those people of below poverty level. Surgical methods are also there to fight against this problem but major people discard this method for its painfulness nature. Besides this there is also a plenty of post operative obligations which are hard to obey due to lack of awareness and knowledge. Due to the above drawbacks of abovementioned contraceptive methods and measures generally used for family planning, it is mandatory to search a new product which can be used parallelly. In this connection, a number of traditional Indian plant products have been used as herbal castrative agents for many years. Several plants are reported to enhance reproductive ability and some are known to hamper such functions. Ethanolic extract of Semecarpus anacardium fruit [1] causes spermatogenic arrest in male albino rats. Oral administration of plumieride [2] causes spermatogenic arrest without any noticeable side effects. Hydro-methanolic extract of leaf of Aegle mermelos [3] has antigonadal effect in male rats. Tulsi (Oscimum sanctum) [4] and neem (Azadirachtaindica) [5,6] are antifertility agents while after ginger (Zingiber officinale) [7] administration causes accumulation of sperms in the lumen of seminiferous tubule. It has been demonstrated that methanolic pod extract of Albizzialebbeck (L) Benth [8] has anti spermatogenic activity. Green tea components theanine and catechin have reproductive effects [9,10]. It has been demonstrated earlier that Aliumsativum [11] bulb extract has its spermicidal activities. Sarcostema acidum [12] stem extract exhibit spermatogenic arrest in male rats without any side effects. It has been reported that there was a reduction in plasma testosterone level by epigallocatechingallate present in green tea [13]. It has been demonstrated earlier on that green tea leaf extract has significant role in decrease in testosterone level as well as changes in morphological character of testis [14]. Green tea catechin has been shown to inhibit tumor cell proliferation and promote the destruction of leukemia cell [15] and breast cancer cells [16,17]. Green tea was also shown to decrease the risk of developing ovarian cancer [18]. It has been suggested that excessive intake of tea should have been avoided by those people who are prone to anaemia [19]. The present study was undertaken to evaluate the morphological and functional changes in testis as well as hormonal level by administration of green tea leaf extract.
Materials and methods
Animal selection, care and grouping
Adult (90±10 days) male albino rats of Wistar strain were taken for this experiment. Animals were maintained as per National guidelines and protocols. Animals were housed in clean polypropylene cages and were maintained in a controlled environmental temperature (22±2°C) in an animal house under a photoperiod of 12 hours of light and 12 hours of darkness with free access to water. Animals were fed on standardized normal diet (20% protein) which consists of 70% wheat, 20% gram, 5% fish meal powder, 4% dry yeast powder and 1% oil and water adlibitum. Rats were equally divided into three groups (n=12). Initial body weights of all the rats were recorded. Animals of group-I were treated as control group and sterile distilled water was given 1 ml/100 gm of body weight. Animals in Group-II were given 2.5% aqueous green tea extract 1 ml/100 gm of body weight to each animal and considered as moderate dose treated group. Animals in Group-III were given 5.0% green tea leaf extract, 1 ml/100 gm of body weight of experimental rats and considered as high dose treated group.
Preparation of green tea leaf exrtract
Aqueous extract of Green tea leaf was prepared following the method of Wei. H. et al. [20]. To study the effect of Green tea leaf extract on male reproduction, the doses were selected based on the study conducted earlier (Chandra A.K et al. 2010 and Sakamoto Y et al. 2001) [21,22]. At first 5.0 gm green tea was added to 100 ml of boiling water and was steeped for 15 min. The fusion was cooled to room temperature and was filtered. Tea leaves was extracted a second time with 100 ml of boiling water and filtered. Two filtrates were then combined to obtain a 2.5% tea aqueous extract (2.5 gm tea leaves/100 ml of water). Similar procedure was performed with 10 gms green tea to prepare 5.0% aqueous green tea extract. The extract was then ready for oral administration.
Animal treatment, sacrifice and measurement of parameters
After completion of 26 days of treatment, final body weights of all the rats were taken and the rats were anaesthetized one after another with anaesthetic ether and blood was collected directly from hepatic portal vein and allowed to coagulate. Clear serum was collected and stored in 20°C for enzyme assay. Testis of each rat was dissected out and treamed off adipose tissues and weights were taken. One testis from each rat was processed for histology and 5μ thick sections were taken and stained with haematoxylene and eosin for further observation. After sacrifice, the cauda portion was cut and it was kept in 1 ml diluents at 37°C. After scattering it, sperms were dispersed into the fluid and it was taken for the count of sperm and its motility through the process of Majumder and Biswas [23]. Serum glucose was measured using the standard kits. The serum protein was estimated by Biuret method with a standard curve of BSA. Hormonal level like testosterone, FSH and LH in serum of all the animals were estimated with the help of ELISA method. Serum Glutamate Pyruvate Transaminase (SGPT) and Serum Glutamate Oxaloacetate Transaminase (SGOT) were measured of all the control and experimental animals through the process of Kind and King [24]. Finally results were compared with the respective controls with the help of student’s ‘t’ test (Das 2005) [25] to generalize the effect of green tea leaf extract on reproductive system of male albino rat model.
Results
Body weight
Body weight is a common parameter of an individual. In each and every experiment with the application of certain drugs, it is important to measure the general growth of those particular animals. This general growth is mainly reflected through the normal gain of body weight. It has been observed that the present study indicates the net gain of body weight is decreased in moderate dose and high dose group accordingly in comparison with control group. In this present study the net gain of body weight is significantly higher in control group **(p<0.001) in comparison with moderately and highly treated groups (Table 1).
Table 1.
Comparison of net gain of body weight of rats treated with GTLE of different doses and respective controls. Values are mean ± SEM (in %), n=12 rats in each group
| Control | Moderate | High dose | |
|---|---|---|---|
| Net gain of body weight | 30.61±0.102 | 12.63±0.111 | 10.00±0.102 |
Weight of testis
Weight of testis is measured in this present study to show if there is any kind of effect of green tea leaf extract on testis because testis is major organ of male reproductive system. Testicular weight has been reduced significantly **(p<0.01) in moderate group and the level of significance is much higher in high dose treated group **(p<0.001) in this experiment (Table 2).
Table 2.
Comparison of testicular weight (gm%) between controlled and GTLE treated rats, values are mean ± SEM, n=12 rats in each group
| Control | Moderate | High dose | |
|---|---|---|---|
| Testis | 0.93±0.068 | 0.85±0.085 | 0.77±0.060 |
Sperm count
Sperm count is one of the major parameters which is basically taken into account for the assessment of proper functioning of male reproductive system. This is an important criteria on which fertility of a male animal mainly depends. Below mentioned result clearly depicts that sperm count in moderate and high dose groups are influenced in dose dependent manner (Table 3). In comparison with control group, sperm count of the treated groups have been reduced drastically **(p<0.001).
Table 3.
Effect of GTLE on sperm count in control and treated groups, Values are mean ± SEM (million/ml), n=12 rats in each group
| Control | Moderate | High dose | |
|---|---|---|---|
| Sperm count | 72.40±0.511 | 61.10±0.298 | 47.40±0.469 |
Sperm motility
Not only the number of sperm but the motility of the sperm is also an important aspect regarding the proper functioning of male reproductive system. In this present study Sperm motility has also been severely reduced after 26 days consecutive treatment of GTLE in dose dependent manner **(p<0.001) in comparison with control group (Table 4).
Table 4.
Effect of GTLE on sperm motility in control and treated groups, Values are mean ± SEM (%), n=12 rats in each group
| Control | Moderate | High dose | |
|---|---|---|---|
| Sperm motility | 69.80±0.426 | 58.40±0.512 | 52.60±0.512 |
SGPT and SGOT
These two enzymes are taken as metabolic indicator. In present study, these enzymes were measured to ensure whether there is any side effect of presently used drug on gastrointestinal system. In present study, the enzymes system, SGPT and SGOT have not been changed significantly in the present study in both the moderate and high dose treated groups. These two enzymes system are considered as metabolic marker. So it means that GTLE has no influence on the metabolism of albino rat (Table 5).
Table 5.
Effect of GTLE on SGPT and SGOT activity in male albino rats, Values are mean ± SEM (IU/L), n=12 rats in each group
| Control | Moderate | High dose | |
|---|---|---|---|
| SGPT | 39.35±0.170 | 39.40±0.145 | 39.30±0.128 |
| SGOT | 137.40±0.045 | 137.38±0.162 | 137.50±0.264 |
Glucose and total protein
Glucose and total protein both are general parameters of blood serum. Both these molecules play important role in biochemical events mainly for energy production. Protein has its role in back bone formation. GTLE has no significant influence on serum glucose and total protein in moderate and high dose treated groups when compared with control (Table 6).
Table 6.
Comparison of glucose and serum protein between controlled and treated groups, values are mean ± SEM (gm/100 ml), n=12 rats in each group
| Control | Moderate | High dose | |
|---|---|---|---|
| Glucose | 99.60±0.205 | 99.70±0.239 | 99.72±0.026 |
| Total protein | 7.70±0.127 | 7.78±0.172 | 7.80±0.052 |
Testosterone
Testosterone is main reproductive hormone for male. It has important role in spermatogenesis. In present study this hormone is being measured to show the efficacy of the drug used in this experiment. Oral administration of GTLE causes reduction in serum testosterone level after 26 days treatment. The change is significant **(p<0.001) in both moderate and high dose of treatment groups (Table 7).
Table 7.
Effect of GTLE on testosterone level, values are mean ± SEM (ng/ml), n=12 rats in each group
| Control | Moderate | High dose | |
|---|---|---|---|
| Testosterone | 2.64±0.119 | 2.01±0.179 | 1.61±0.094 |
FSH and LH
These two hormones are released from pituitary gland and play important roles in spermatogenic activity. FSH and LH act for spermatogenesis after influencing on sertoli cell and leydig cell respectively. FSH has been altered in two treated groups significantly **(p<0.001) but in case of LH, significant **(p<0.001) change has taken place only in high dose treated group (Table 8).
Table 8.
Effect of GTLE on FSH & LH, values are mean ± SEM (mlU/ml), n=12 rats in each group
| Control | Moderate | High dose | |
|---|---|---|---|
| FSH | 3.36±0.171 | 4.19±0.068 | 4.52±0.136 |
| LH | 4.29±0.307 | 4.46±0.136 | 5.38±0.153 |
Histological study
Histological structure has been changed in both the doses in treated groups. Disintegration of seminiferous tubules of testis alongwith decrease in somatic indices occurs. Reduction in the accumulation of spermatozoa is also observed when compared with control (Figure 1).
Figure 1.
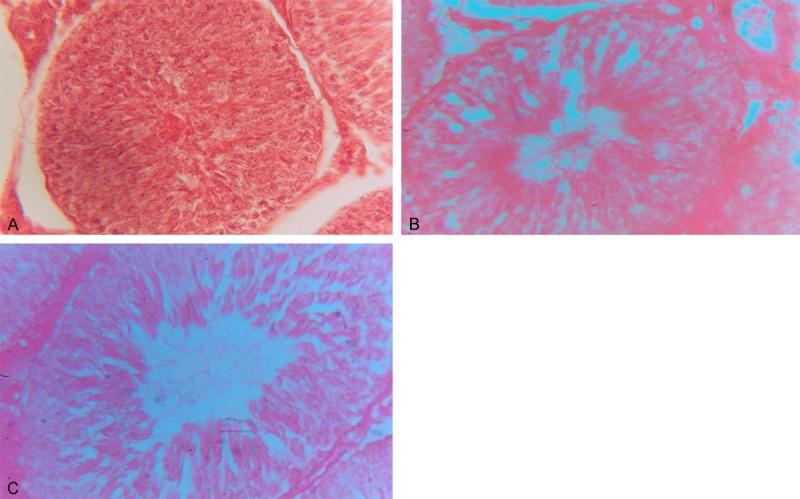
A: H/E stained section of control group (group-I) testis (10 X 40x). B: H/E stained section of moderate dose (group-II) of GTLE treated testis (10 X 40x). C: H/E staines section of high dose (group-III) of GTLE treated testis (10 X 40x) showing an increase in luminal area, reduced spermatozoal mass and disorganized cellular orientation.
Discussion
It has been found from these observations, that GTLE is a potent herbal castrative agent when applied in a specific dose. When GTLE was administered orally in different groups of animals, it produced reduction in net gain of body weight in moderate group and high dose treated group of animals in comparison to that of their respective control. Through various experiments earlier on, it has been shown that the body weight was reduced after the treatment of green tea and green tea powder [13,26]. It has been discovered that, the cardinal antioxidative ingredient in the green tea extract is green tea catechins (GTC), which comprise four major epicatechin derivatives; namely, epicatechin (EC), epigallocatechin (EGC), epicatechin gallate (ECG), and epigallocatechin gallate (EGCG). Of which, EGCG (Structure given in Figure 2) accounts for more than 40% of the total content (taken from Wikipedia). It has also been shown that the reduction of body weight after application of green tea extract may be due to inhibition of catechol-O-methyl transferase (COMT) enzyme by epigallocatechingallate (EGCG) of the green tea [27,28].
Figure 2.
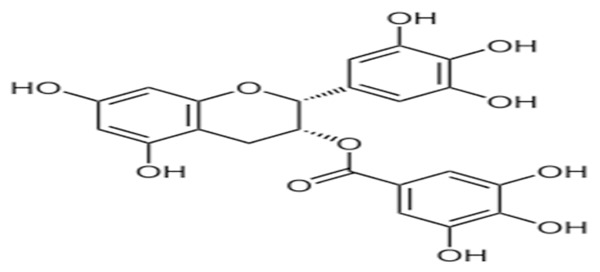
Structure of EGCG.
This enzyme (COMT) is responsible to degrade the effect of nor epinephrine which can stimulate thermogenesis and responsible for oxidation of fat [29].
Besides body weight reduction, high dose of tea extract can cause significant reduction in testicular weight in dose dependent manner. Weight of testis generally depends on the mass of spermatogenic cells. So it may be said that, testicular weight loss is due to the decreased number of spermatogenic cells [30].
After application of GTLE in various doses, the sperm count and sperm motility were decreased in both treated groups in comparison to their control. It may be due to the reduction in testosterone level [14]. Because testosterone and other gonadotrophins like FSH and LH maintain the spermatogenesis and act as a useful marker for male infertility [31].
Present observation suggests that no change in the level of SGPT and SGOT occurs after treatment of animals with GTLE when compared with controlled animals. Analysis also depicts that generally these two enzymes used as metabolic marker are inhibited due to failure in gastro-protective and repair mechanism leading to disrupted mucosal barrier [32]. So it can be concluded that the oral administration of GTLE does not hamper the metabolic activity.
Present experiment shows that glucose and total protein remain unchanged after treatment of animals with GTLE. These two components generally change their level in serum depending on oral ingestion of food stuffs but no alteration in their level in serum comparing the control suggest that there is no metabolic disruption after oral administration of GTLE.
This experiment clearly shows the decrease in serum testosterone level in GTLE treated groups in a dose dependent manner in comparison with their respective control. The decreased concentration of serum testosterone has also been reported earlier by green tea epigallocatechingallate [33]. This reduced concentration of testosterone may be due to decreased activity of steroidogenic enzymes, Δ5 3β HSD and 17β HSD [34]. Kao et al. [35] also reported the decrease in serum testosterone level after exposure of catechin in green tea. It has also been explained earlier that green tea extract polyphenols mainly EGCG has inhibitory effect on leydig cell testosterone production probably through cell signaling pathway, P-450 side chain cleavage and the function of 17β HSD [36].
In present observation, GTLE treated groups of animals showed decreased synthesis of testosterone and also decreased serum level of this androgen which reflects in elevated level of serum LH through feedback mechanism [37]. Diminished level of testosterone also increases the FSH level but not significantly. Because all these hormones are treated as useful marker in management of male infertility [38].
Observation also suggests that, there are changes in histo architectures confirmed from the histological slide prepared from the moderate group and high treated group of GTLE. Comparing with control group, vacuolization in treated groups are also observed. The sperm heads are not properly distributed at right places. Heads of the sperms are scattered in case of high dose treated group.
Conclusion
So, from the above study it is revealed that, there are some changes in histo architecture and functional status of testis and also some impairment in sex organ in dose dependent manner after oral administration of GTLE. So, it can be concluded that GTLE may be used as potent herbal castrative agent in near future.
Acknowledgements
Authors are grateful to all respected teachers and other support stuffs of K.N College, Berhampore, Murshidabad, W.B.
References
- 1.Sharma A, Verma PK, Dixit VP. Effect of Semecarpus anacardium fruits extract on reproductive function of male albino rat. Asian J Androl. 2003;5:121–4. [PubMed] [Google Scholar]
- 2.Gupta RS, Bhatnaqer AK, Joshi YC, Sharma R, Sharma A. Effect of plumieride, an iridoid on spermatogenesis in male albino rats. Phytomedicine. 2004;11:169–74. doi: 10.1078/0944-7113-00346. [DOI] [PubMed] [Google Scholar]
- 3.Das UK, De D, Chatterjee K, Mallick C, Bera TK, Ghosh D. Journal Of Medicinal Plants Research Vol 3(10) 2009. Oct, Antigonadal effect induced by hydromethanolic extract of leaf of Aegle mermelos in male rat: Effect of Hcg co-administration; pp. 728–735. [Google Scholar]
- 4.Kashinathan S, Basu S, Ramakrishnan SL. Antifertility effect of Ocimum sanctum L. Indian J Exp Biol. 1972;10:23–5. [PubMed] [Google Scholar]
- 5.Joshi AR, Ahamed RN, Pathan KM, Manivannan B. Effect of Azadirachtaindica leaves on testis and its recovery in albino rats. Indian J Exp Biol. 1996;34:1091–4. [PubMed] [Google Scholar]
- 6.Choudhary DN, Singh JN, Verma SK, Singh BP. Antifertility effects of leaf extracts of some plants in male rats. Indian J Exp Biol. 1990;28:714–6. [PubMed] [Google Scholar]
- 7.Khaki A, Fathiazad F, Nouri M, Khaki AA, Khamenehi HJ, Hamadeh M. The effect of ginger on spermatogenesis and sperm parameters of rat. Folia Morphol (Warsz) 2009;68:45–51. [PubMed] [Google Scholar]
- 8.Gupta RS, Kachhawa JB, Chaudhary R. Antifertility effects of methanolic pod extract of Albizzialebbeck (L. ) Benth in male rats. Asian J Andrology. 2004;6:155–9. [PubMed] [Google Scholar]
- 9.Yokogoshi H, Kobayashi M. Hypotensive effect of gamma-glutamylmethylamide in spontaneously hypertensive rats. Life Sci. 1998;62:1065–8. doi: 10.1016/s0024-3205(98)00029-0. [DOI] [PubMed] [Google Scholar]
- 10.Kakuda T. Neuroprotective effect of the green tea components theanine and catechins. Biol Pharm Bull. 2007;25:1513–8. doi: 10.1248/bpb.25.1513. [DOI] [PubMed] [Google Scholar]
- 11.Chakraborty K, Pal S, Bhattacharya AK. Sperm immobilization activity of Allium sativumL. and other plants extracts. Asian J Androl. 2003;5:131–5. [PubMed] [Google Scholar]
- 12.Venma PK, Sharma A, Mathur A, Sharma P, Gupta RS, Joshi SC, Dixit VP. Effect of Sarcostemmaacidum stem extract on spermatogenesis in male albino rats. Asian J Androl. 2002;4:43–7. [PubMed] [Google Scholar]
- 13.Kao YH, Hiipakka RA, Liao S. Modulation of endocrine systems and food intake by green tea epigallocatechin gallate. Endocrinology. 2000;141:980–7. doi: 10.1210/endo.141.3.7368. [DOI] [PubMed] [Google Scholar]
- 14.Chandra AK, Choudhury SR, De N, Sarkar M. Effect of green tea Cameliasinensis L extract on morphological and functional changes in adult male gonads of albino rats. Indian J Exp Biol. 2011;49:689–97. [PubMed] [Google Scholar]
- 15.Smith DM, Dou QP. Green tea polyphenols epigallocatechin inhibits DNA replication and consequently induces leukemia cell apoptosis. Int J Mol Med. 2001;7:645–52. doi: 10.3892/ijmm.7.6.645. [DOI] [PubMed] [Google Scholar]
- 16.Vergote D, Cren-Olivé C, Chopin V, Toillon RA, Rolando C, Hondermarck H, Le Bourhis X. (-)-Epigallocatechin (EGC) of green tea induces apoptosis of human breast cancer cells but not of their normal counterparts. Breast Cancer Res Treat. 2002;76:195–201. doi: 10.1023/a:1020833410523. [DOI] [PubMed] [Google Scholar]
- 17.Masuda M, Suzui M, Lim JT, Deguchi A, Soh JW, Weinstein IB. Epigallocatechin-3-gallate decreases VEGF production in head and neck and breast carcinoma cells by inhibiting EGFRrelated pathways of signal transduction. J Exp Ther Oncol. 2002;2:350–9. doi: 10.1046/j.1359-4117.2002.01062.x. [DOI] [PubMed] [Google Scholar]
- 18.Zhang M, Binns CW, Lee AH. Tea consumption and ovarian cancer risk: A case control study in China. Cancer Epidemiol Biomarkers Prev. 2002;11:713–8. [PubMed] [Google Scholar]
- 19.Samman S, Sandström B, Toft MB, Bukhave K, Jensen M, Sørensen SS, Hansen M. Green tea or rosemary extract added to foods reduces nonheme-iron absorption. Am J Clin Nutr. 2001;73:607–12. doi: 10.1093/ajcn/73.3.607. [DOI] [PubMed] [Google Scholar]
- 20.Wei H, Zhang X, Zhao JF, Wang ZY, Bickers D, Lebwohl M. Scavenging of hydrogen peroxide and inhibition of ultraviolet light-induced oxidative DNA damage by aqueous extracts from green and black teas. Free Radic Biol Med. 1999;26:1427–35. doi: 10.1016/s0891-5849(99)00005-2. [DOI] [PubMed] [Google Scholar]
- 21.Chandra AK, De N. Goitrogenic/antithyroidal potential of green tea extract in relation to catechin in rats. Food Chem Toxicol. 2010;48:2304–11. doi: 10.1016/j.fct.2010.05.064. [DOI] [PubMed] [Google Scholar]
- 22.Sakamoto Y, Mikuriya H, Tayama K, Takahashi H, Nagasawa A, Yano N, Yuzawa K, Ogata A, Aoki N. Goitrogenic effects of green tea extract catechins by dietary administration in rats. Arch Toxicol. 2001;75:591–6. doi: 10.1007/s00204-001-0286-6. [DOI] [PubMed] [Google Scholar]
- 23.Majumder GC, Biswas R. Evidence for the occurrence of an ecto-(adenosine triphosphatase) in rat epididymal spermatozoa. Biochem J. 1979;183:737–43. doi: 10.1042/bj1830737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kind PR, King EJ, Inverley H, Gowenlock AH. Method of practical clinical biochemistry. pp. 899–900p. [Google Scholar]
- 25.Das D, Das A. Statistics in biology and psychology. 4th ed. Kolkata: Academic publishers; 2005. pp. 117–26p. [Google Scholar]
- 26.Sayana K, Lin S, Oguni I, Zheng G. Effects of green tea on growth, food utilization and lipid metabolism in mice. In Vivo. 2000;14:481–4. [PubMed] [Google Scholar]
- 27.Chantre P, Lairon D. Recent findings of green tea extract AR25 (Exolise) and its activity for the treatment of obesity. Phytomedicine. 2002;9:3–8. doi: 10.1078/0944-7113-00078. [DOI] [PubMed] [Google Scholar]
- 28.Dulloo AG, Duret C, Rohrer D, Girardier L, Mensi N, Fathi M, Chantre P, Vandermander J. Efficacy of a green tea extract rich in catechin polyphenols and caffeine in increasing 24-h energy expenditure and fat oxidation in humans. Am J Clin Nutr. 1999;70:1040–5. doi: 10.1093/ajcn/70.6.1040. [DOI] [PubMed] [Google Scholar]
- 29.Dulloo AG, Seydoux J, Girardier L, Chantre P, Vandermander J. Green tea and thermogenesis: interactions between catechin-polyphenols, caffeine and sympathetic activity. Int J Obes Relat Metab Disord. 2000;24:252–8. doi: 10.1038/sj.ijo.0801101. [DOI] [PubMed] [Google Scholar]
- 30.Chapin RE, Harris MW, Davis BJ, Ward SM, Wilson RE, Mauney MA, Lockhart AC, Smialowicz RJ, Moser VC, Burka LT, Collins BJ. The effects of perinatal/juvenile methoxychlor exposure on adult rat nervous, immune, and reproductive system function. Fundam Appl Toxicol. 1997;40:138–57. doi: 10.1006/faat.1997.2381. [DOI] [PubMed] [Google Scholar]
- 31.Zabul J, Mierzejewski W, Rogoza A. [Usefulness of examining gonadotropin hormones and testosterone in men with abnormal semen] . Ginekol Pol. 1994;65:71–4. [PubMed] [Google Scholar]
- 32.Dhikav V, Singh S, Pande S, Chawla A, Singh A, JK Non-steroidal drug-induced gastrointestinal toxicity:Mechanisms and management. J Acad Clin Med. 2003;4:315–22. [Google Scholar]
- 33.Adani VM, Ahmad N, Mukhtar H. Molecular targets for green tea in prostate cancer prevention. J Nutr. 2013;133:2417S–2424S. doi: 10.1093/jn/133.7.2417S. [DOI] [PubMed] [Google Scholar]
- 34.Chandra AK, Choudhury SR, De N, Sarkar M. Effect of green tea (Camellia sinensis L. ) extract on morphological andfunctional changes in adult male gonads of albino rats. IIndian J Exp Biol. 2011;49:689–97. [PubMed] [Google Scholar]
- 35.Kao YH, Hiipaka RA, Liao S. Modulation of endocrine systems and food intake by green tea epigallocatechin gallate. Endocrinology. 2000;141:980–7. doi: 10.1210/endo.141.3.7368. [DOI] [PubMed] [Google Scholar]
- 36.Figueiroa MS, César Vieira JS, Leite DS, Filho RC, Ferreira F, Gouveia PS, Udrisar DP, Wanderley MI. Green tea polyphenols inhibit testosterone production in rat leydig cells. Asian J Androl. 2009;11:362–370. doi: 10.1038/aja.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Das D. Biochemistry. Tenth edition 2000. [Google Scholar]
- 38.Zabul J, Mierzejewski W, Rogoza A. Usefulness of examining gonadotropin hormones and testosterone in men with abnormal semen. Ginekol Pol. 1994;65:71–4. [PubMed] [Google Scholar]


