Abstract
The effects of pyruvate, the end metabolite of glycolysis, on blood-brain barrier (BBB) impairment and immune reactivity were examined in the quinolinic acid (QA)-injected rat striatum. Extensive disruption of BBB was observed at 7 d post QA-injection as demonstrated by increased immunohistochemical staining using antibody against immunoglobulin G (IgG). Animals receiving pyruvate administration (500 mg/kg) with QA-injection exhibited reduced lgG immunoreactivity (by 45%) relative to QA alone. QA intrastriatal injection also resulted in marked increases in the number of infiltrating T-lymphocytes (by 70-fold) and expression of major histocompatibility complex (MHC-class II) (by 45-fold) relative to unlesioned control. Treatment with pyruvate significantly reduced infiltration of T-cells (by 68%) and MHC class II expression (by 48%) induced by QA. These results indicate that QA injection into rat striatum leads to impairment in BBB function with pyruvate administration reducing immune response and BBB leakiness in excitotoxic injury.
Keywords: Blood-brain barrier (BBB), pyruvate, excitotoxicity, rat striatum, T-cell infiltration, inflammation
Introduction
Excitotoxicity has been implicated in the pathogenesis of a number of neurological disorders including Huntington’s disease (HD) [1], Alzheimer’s disease (AD) [2], stroke [3] and HIV-associated dementia [4]. Several lines of evidence suggest that excitotoxic brain insult is associated with inflammatory responses mediated by activation of resident brain microglial cells [5]. Inflammatory responses from activated microglia involve cellular production of a broad spectrum of proinflammatory mediators including cytokines and oxidative free radicals [6].
The integrity of the blood-brain barrier (BBB) is compromised in diseased brain [7] and is functionally abnormal following excitotoxic insult [8,9]. Roles of neuroinflammation and immune system response in HD have been considered [10,11] with recent work reporting elevated expression of inflammatory mediators in HD tissue [12] and abnormalities in BBB in HD patients and in a mouse model of the disease [13]. Previous work has demonstrated quinolinic acid (QA)-induced oxidative damage in rat striatum which was attenuated using the endogenous energy substrate, pyruvate [14,15]. Although QA-injection is not commonly used as a model for HD, the excitotoxic-induced lesion is similar to that in HD [16] and may have particular utility in emphasizing inflammatory reactivity in the disease [15].
In the present study we have examined BBB permeability to IgG and T-lymphocyte influx as aspects of neuroinflammation in QA-injected rat striatum. The effects of pyruvate treatment on BBB leakiness and T-cell invasion has also been determined following excitotoxic insult.
Material and methods
Animals and QA injection
All experimental procedures were approved by the Committee on Animal Care of the University of British Columbia. Male Sprague-Dawley rats (260-280 g) were anesthetized with intraperitoneal (ip) injection of sodium pentobarbital (50 mg/kg) and then mounted in a stereotaxic apparatus (Kopf Instruments, Tujunga, CA). Animals were subjected to unilateral Iµl QA (60 nmol; Sigma, St. Louis, MO) injection into the striatum (AP: +1.0 mm, ML: -3.0 mm, DV: -5.0 mm, from bregma) as previously described [14,15]. Animals were treated ip with pyruvate (500 mg/kg dissolved in saline; Sigma) at the time of QA injection. This single pyruvate dosage was based on our previous studies showing this dose and treatment regimen to be efficacious against excitotoxic rat brain injury; lower doses were also effective if given daily [15]. At 7 days postinjection, the animals were deeply anesthetized with sodium pentobarbital and then perfused through the heart with cold heparinized saline followed by 4% paraformaldehyde (PFA). The brains were removed, post-fixed overnight in fixative and then left in 30% sucrose. Coronal sections (40 µm) were taken through the striatum on a cryostat microtome.
lmmunohistochemistry
For immunohistochemical analysis, free-floating sections were incubated overnight at 4°C with the antibodies against CD8 (indicative of T-lymphocyte; 1:500, Serotec, Oxford) or OX-6 (indicative of MHC class II; 1:500, Serotec). Biotinylated secondary antibody (1:500; Vector, Burlingame, CA) followed by avidin-biotin-peroxidase complex (ABC, 1:200, Vector) and 3,3’-diaminobenzidine (DAB, Sigma) were used to visualize reaction products. The integrity of BBB was determined by measuring permeability of IgG (immunoglobulin G). The procedures for use of IgG immunohistochemistry to characterize BBB disruption have been previously described [17]. Briefly, free-floating sections were incubated for 1 hr with IgG antibody (1:1000, Vector) and the reaction product was visualized as described above.
Quantification and statistical analysis
For quantitative analysis [15], the IgG-stained section (AP: +1.2, +1.0, and +0. 8, according to the atlas of Paxinos and Watson [18]) were captured and digitized using a DVC camera (Diagnostic Instruments, Sterling Heights, Ml) connected to the Zeiss Axioplan 2 microscope. The lgG-immunoreactive area was sharply delineated and measured by calculating the ratio of the IgG-stained area to the unlesioned striatum using Northern Eclipse software (Empix Imaging, Mississauga, ON). For the density of T-lymphocytes, CD8-positive cells in the striatum were counted and presented as a mean per striatum. For MHC class II expressing cells, OX-6-positive cells in the striatum were counted (at x400 magnification) and presented as a mean per 0.06 mm2.
All data are shown as means ± SEM. Statistical significance was assessed using one-tailed nonparametric student’s t-test with significance for p < 0.05.
Results
Initial studies examined the effects of intrastriatal injection of QA on the integrity of the BBB. The levels of IgG immunostaining (measured 7 days after QA injection) were used to assess leakiness and disruption of BBB. The results are presented in Figure 1A, where QA-injected striatum (middle panel, Figure 1A) showed extensive lgG staining relative to unlesioned control (left panel, Figure 1A). Overall, the IgG immunoreactivity (ir) in excitotoxin-injected brain appeared uniformly spread throughout striatum. In the presence of pyruvate (injected ip at a single dose of 500 mg/kg) a different pattern of IgG ir was evident for the QA-injected striatum. As shown (right panel, Figure 1A), pyruvate was highly effective in reducing expressions of IgG relative to that observed with the excitotoxin applied alone. Overall, the IgG ir was reduced by 45% with pyruvate treatment compared with QA injection in the absence of pyruvate (Figure 1B).
Figure 1.
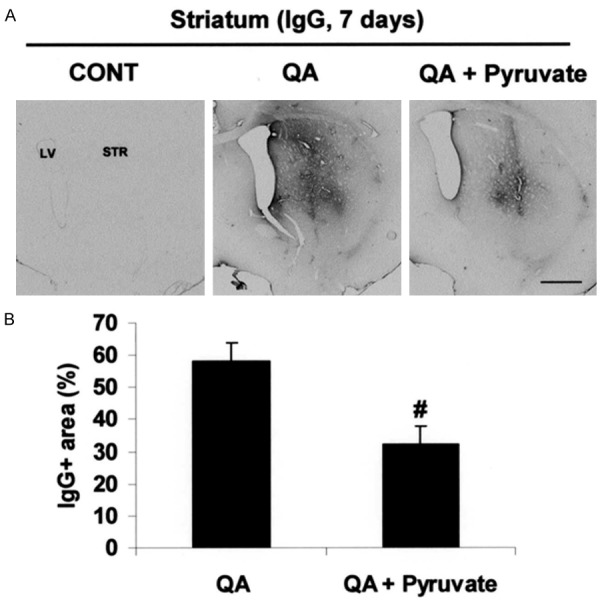
Effect of pyruvate on blood-brain barrier disruption and neuronal damage. A. Photomicrographs of IgG-immunoreactivity in the striatum taken from unlesioned control rats and QA-injected rats, in the absence or presence of pyruvate administration, at 7 days post-QA. B. Quantification of lgG-immunoreactive area. Data are mean ± SEM (n=4/group). #p < 0.05 vs QA. Scale bars = 800 µm. CONT, control; LV, lateral ventricle; STR, striatum.
We next examined for effects of pyruvate on immune response by staining for CD8, a specific marker for infiltrating T-lymphocytes. As shown in Figure 2A, QA-injected striatum (middle panel, Figure 2A) showed a considerable increase (by 70-fold) in the infiltration of CD8-positive cells compared with unlesioned control (left panel, Figure 2A). Administration of pyruvate (at 500 mg/kg) with QA injection markedly diminished CD8-positive T-cells in the lesioned area (right panel, Figure 2A). Overall, pyruvate was found to reduce infiltration of CD8-positive T-cells by 68% compared with levels in excitotoxin injected striatum (Figure 2B).
Figure 2.
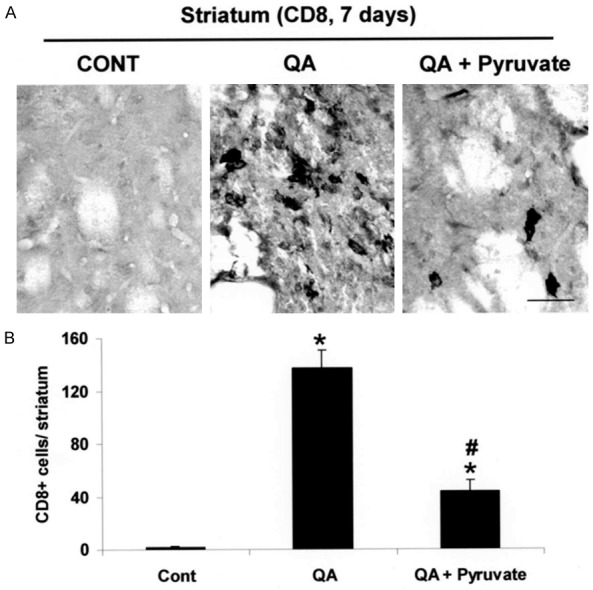
Effect of pyruvate on CD8-positive T lymphocytes infiltration. A. Photomicrographs of CD8-positive cells in the striatum taken from unlesioned control rats and QA injected rats in the absence or presence of pyruvate administration, at 7 days post-QA. B. Quantification of CD8-positive cells in the QA-injected striatum. Data are mean ± SEM (n=4/group). *p < 0.05 vs control; #p < 0.05 vs QA. Scale bar = 30 µm. CONT, control.
MHC class II serves as a cellular marker for inflammatory responses to T-cell activation. An increased MHC class II ir (by 45-fold) was observed in the striatum following QA injection (Figure 3A, middle panel) relative to unlesioned control (Figure 3A, left panel). Indeed, unlesioned striatum exhibited minimal staining for this marker (Figure 3A). Pyruvate treatment was highly effective in diminishing MHC class II expression in QA-lesioned striatum (Figure 3A, right panel). Quantification of MHC class II-positive cells revealed that the number of MHC class II-positive cells was significantly reduced (by 48% relative to QA-injected striatum) with pyruvate treatment (Figure 3B).
Figure 3.
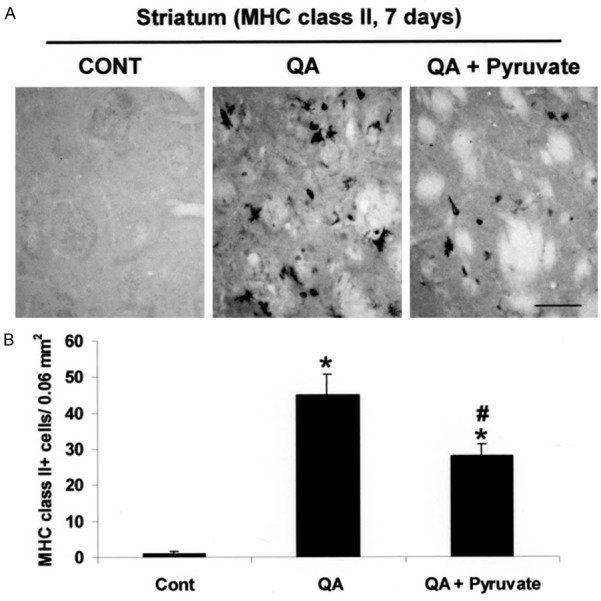
Effect of pyruvate on MHC class II expression. A. Photomicrographs of MHC class II-positive cells in the striatum taken from unlesioned control rats and QA injected rats in the absence or presence of pyruvate administration, at 7 days post-QA. B. Quantification of MHC class II-positive cells in the QA-injected striatum. Data are mean ± SEM (n=4/group). *p < 0.05 vs control; #p < 0.05 vs QA. Scale bar = 60 µm. CONT, control.
Discussion
This work reports impairment of BBB in QA-lesioned striatum as demonstrated by increased membrane permeability to IgG. Furthermore, increased leakiness of BBB subsequent to QA insult was associated with infiltration of T-cells and upregulation in expression of the immune cell marker, MHC class II.
Pyruvate was found to significantly reduce BBB disruption, T-cell invasion and immune cell expression in excitotoxic brain. Previous work has reported pyruvate efficacy in reducing glial-mediated oxidative damage in QA-injected animals [14,15]. One possibility is that BBB impairment, demonstrated by increased IgG influx, is secondary to oxidative stress induced by QA striatal injection. In a similar manner the increased T-cell invasion following excitotoxic insult could be associated with oxidative-induced abnormalities in BBB. In the latter case, activation of MHC-II positive microglia could act as a source of reactive oxygen species following excitotoxic insult. Another possibility is that the enhanced T-cell presence in QA-injected rat striatum promotes BBB dysfunction as reported in an immune murine model of multiple sclerosis [19,20]. To our knowledge a measure of T-lymphocytes in HD has not been published but one study has noted no difference in CD247, a T-cell marker, expression between control and HD tissue [21].
Clearly an acute excitotoxic insult has limited applicability as an animal model of HD, however, the procedure may be useful to help characterize roles of inflammatory reactivity in the disease [15]. HD tissue shows evidence of neuroinflammation [12,21] which has been considered in some detail in relation to pathology in the disease [11]. In this regard recent work has reported cerebrovasculature abnormalities and BBB impairment in HD patients and also in a mouse model of HD [13]. These findings suggest the utility in studies designed to examine inflammatory processes and mechanisms underlying BBB dysfunction in HD animal models.
Our results demonstrate efficacy for pyruvate in reducing inflammatory reactivity and stabilizing the BBB following excitotoxic insult. It is noteworthy that pyruvate was administered as a single dose at the time of QA-striatal injection; previous work has reported a daily dosage regimen shows enhanced efficacy compared with a single treatment [14]. Since pyruvate is endogenous in brain, we suggest elevated levels of the compound could serve as a non-toxic effector and plausible adjunctive pharmacological strategy to inhibit neuroinflammation in pathological conditions of oxidative stress including HD.
In summary, we provide evidence that excitotoxic insult in rat striatum leads to disruption and leakiness of BBB. Furthermore, excitotoxicity in rat striatum was associated with infiltration of T-cells and enhanced expression of MHC class II immune marker. Our findings suggest that the endogenous compound, pyruvate, may have utility in attenuating neuroinflammation under conditions which compromise the intactness of BBB.
Acknowledgements
This work was supported by a grant from Huntington Foundation of Canada.
Disclosure of conflict of interest
None.
References
- 1.Beal MF. Energetics in the pathogenesis of neurodegenerative diseases. Trends Neurosci. 2000;23:298–304. doi: 10.1016/s0166-2236(00)01584-8. [DOI] [PubMed] [Google Scholar]
- 2.Hynd MR, Scott HL, Dodd PR. Glutamatemediated excitotoxicity and neurodegeneration in Alzheimer’s disease. Neurochem Int. 2004;45:583–595. doi: 10.1016/j.neuint.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Prog Brain Res. 1994;100:47–51. doi: 10.1016/s0079-6123(08)60767-0. [DOI] [PubMed] [Google Scholar]
- 4.Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- 5.Tikka T, Fiebich BL, Goldsteins G, Keinaren R, Koistinaho J. Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activated and proliferating microglia. J Neurosci. 2001;21:2580–2588. doi: 10.1523/JNEUROSCI.21-08-02580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzalez-Scarano F, Baltuch G. Microglia as mediators of inflammatory and degenerative diseases. Ann Rev Neurosci. 1999;22:219–240. doi: 10.1146/annurev.neuro.22.1.219. [DOI] [PubMed] [Google Scholar]
- 7.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Bolton SJ, Perry VH. Differential bloodbrain barrier breakdown and leukocyte recruitment following excitotoxic lesions in juvenile and adult rats. Exp Neurol. 1998;154:231–240. doi: 10.1006/exnr.1998.6927. [DOI] [PubMed] [Google Scholar]
- 9.Parathath SR, Parathath S, Tsirka SE. Nitric oxide mediated neurodegeneration and breakdown of the blood-brain barrier in tPA-dependent excitotoxic injury in mice. J Cell Sci. 2006;119:339–349. doi: 10.1242/jcs.02734. [DOI] [PubMed] [Google Scholar]
- 10.Ellrichmann G, Reick C, Saft C, Linker RA. The role of the immune system in Huntington’s disease. Clin Dev Immunol. 2013;2013:541259. doi: 10.1155/2013/541259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moller T. Neuroinflammation in Huntington’s disease. J Neural Transm. 2013;117:1001–1008. doi: 10.1007/s00702-010-0430-7. [DOI] [PubMed] [Google Scholar]
- 12.Crotti A, Glass CK. The choreography of neuroinflammation in Huntington’s disease. Trends Immunol. 2015;36:364–373. doi: 10.1016/j.it.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drouin-Ouellet J, Sawiak SJ, Cisbani G, Lagace M, Kuan WL, Saint-Pierre M, Dury RJ, Alata W, St-Amour I, Mason SL, Calon F, Lacroix S, Gowland PA, Francis ST, Barker R, Cicchetti F. Cerebrovascular and blood-brain barrier impairments in Huntington’s Disease: Potential implications for its pathophysiology. Ann Neurol. 2015;78:160–177. doi: 10.1002/ana.24406. [DOI] [PubMed] [Google Scholar]
- 14.Ryu JK, Kim SU, McLarnon JG. Neuroprotective effects of pyruvate in the quinolinic acid rat model of Huntington’s disease. Exp Neurol. 2003;183:700–704. doi: 10.1016/s0014-4886(03)00214-0. [DOI] [PubMed] [Google Scholar]
- 15.Ryu JK, Kim SU, McLarnon JG. Blockade of quinolinic acid-induced neurotoxicity by pyruvate is associated with inhibition of glial activation in a model of Huntington’s disease. Exp Neurol. 2004;187:150–159. doi: 10.1016/j.expneurol.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Beal MF, Ferrante RJ, Swartz KJ, Kowall NW. Chronic quinolinic acid lesions in rats closely resemble Huntington’s disease. J Neurosci. 1991;11:1649–1659. doi: 10.1523/JNEUROSCI.11-06-01649.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Todd KG, Butterworth RF. Early microglial response in experimental thiamine deficiency: an immunohistochemical analysis. Glia. 1999;25:190–198. doi: 10.1002/(sici)1098-1136(19990115)25:2<190::aid-glia9>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 18.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Ed 2. New York: Academic Press; 1986. [Google Scholar]
- 19.Suidan GL, Mcdole JR, Chen Y, Pirko I, Johnson AJ. Induction of blood brain barrier tight junction protein alterations by CD8 T cells. PLoS One. 2008;3:e3037. doi: 10.1371/journal.pone.0003037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suidan GL, Dickerson JW, Chen Y, Mcdole JR, Tripathi P, Pirko I, Seroogy KB, Johnson AJ. CD8 T cell-initiated vascular endothelial growth factor expression promotes central nervous system vascular permeability under neuroinflammatory conditions. J Immunol. 2010;184:1031–1040. doi: 10.4049/jimmunol.0902773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silvestroni A, Faull RL, Strand AD, Moller T. Distinct neuroinflammatory profile in postmortem human Huntington’s disease. Neuroreport. 2009;20:1098–1103. doi: 10.1097/WNR.0b013e32832e34ee. [DOI] [PubMed] [Google Scholar]


