Abstract
We used neutron-scattering experiments to probe the conformational dynamics of the light, oxygen, voltage (LOV) photoreceptor PpSB1-LOV from Pseudomonas putida in both the dark and light states. Global protein diffusion and internal macromolecular dynamics were measured using incoherent neutron time-of-flight and backscattering spectroscopy on the picosecond to nanosecond timescales. Global protein diffusion of PpSB1-LOV is not influenced by photoactivation. Observation-time-dependent global diffusion coefficients were found, which converge on the nanosecond timescale toward diffusion coefficients determined by dynamic light scattering. Mean-square displacements of localized internal motions and effective force constants, <k′>, describing the resilience of the proteins were determined on the respective timescales. Photoactivation significantly modifies the flexibility and the resilience of PpSB1-LOV. On the fast, picosecond timescale, small changes in the mean-square displacement and <k′> are observed, which are enhanced on the slower, nanosecond timescale. Photoactivation results in a slightly larger resilience of the photoreceptor on the fast, picosecond timescale, whereas in the nanosecond range, a significantly less resilient structure of the light-state protein is observed. For a residue-resolved interpretation of the experimental neutron-scattering data, we analyzed molecular dynamics simulations of the PpSB1-LOV X-ray structure. Based on these data, it is tempting to speculate that light-induced changes in the protein result in altered side-chain mobility mostly for residues on the protruding Jα helix and on the LOV-LOV dimer interface. Our results provide strong experimental evidence that side-chain dynamics play a crucial role in photoactivation and signaling of PpSB1-LOV via modulation of conformational entropy.
Introduction
Flavin-binding light, oxygen, voltage (LOV) photoreceptors are ubiquitously distributed throughout all kingdoms of life (1). Most LOV photoreceptors are modularly built, multidomain sensory receptors with the light-sensing LOV domain coupled to a diverse set of different effector domains, such as kinases, anti-sigma factors, phosphodiesterases, cyclases, or DNA-binding domains (2, 3). By exploiting this modularity, a number of artificial LOV “photoreceptor” proteins have been constructed in recent years. In those so-called LOV-based optogenetic tools, the light-induced structural changes in the LOV domain have been harnessed to allow the control of the biological activity of fused protein domains (reviewed in (4)). Thus, both for understanding of LOV photoactivation and signaling and for the rational design and mutational optimization of recently constructed LOV-based optogenetic tools, a detailed understanding of the photoactivation mechanism is essential. The light-sensing function of all LOV proteins is intricately linked to the photochemistry of a flavin chromophore, which in the dark is noncovalently bound within the LOV sensory domain (5). In the dark-adapted state, the bound flavin chromophore absorbs maximally at 450 nm, enabling blue light absorption by the protein. Upon photon capture, a photocycle is initiated, which results in the formation of a covalent bond between a totally conserved cysteine residue and the C4a atom of the flavin chromophore (6). As the longest-living intermediate of the LOV photocycle, the adduct state represents the signaling state of the photoreceptor. In the ultraviolet-visible (UV/Vis) spectrum, adduct formation manifests as a loss of absorbance at 450 nm and the formation of a new maximum at ∼380 nm (7). In the dark, this covalent bond is broken within seconds to days, depending on the specific LOV domain (7, 8, 9). Several LOV photoreceptors and isolated LOV domains have been crystallized and dark-adapted and light-state structures are available (3, 10, 11, 12, 13, 51). In most cases, the latter show only small-scale structural changes compared to the corresponding dark-state structures, since larger-scale structural changes and motions are impeded by the crystal lattice (51).
Dynamics and motions in proteins play an important role for biological function. Dynamics in biological macromolecules typically extend over a very broad range of relaxation times from the subpicosecond range up to several seconds (14). Fast motions of amino acid side chains and methyl groups occur on the picosecond timescale (15), whereas slower fluctuations of mostly amino acid side chains and of the protein backbone extend into the nanosecond time range (16, 17). Quasielastic incoherent neutron spectroscopy (QENS) is a technique well suited to measuring localized dynamics of biological macromolecules on the picosecond to nanosecond timescale and on the Ångstrom length range (18). The technique is predominantly sensitive to the motions of protons due to the large incoherent scattering cross section of 1H compared to all other chemical elements occurring in biological macromolecules, including 2H. Average dynamics are probed by QENS as hydrogen atoms are distributed uniformly in proteins. Concerning the properties of LOV photoreceptors, detailed knowledge about the change of conformational side-chain dynamics during photoactivation is still lacking.
In recent years, several stand-alone so-called “short” LOV proteins have been described in bacteria and fungi, which lack a fused effector domain (8, 9, 19, 51). Bacterial short LOV proteins, such as the PpSB1-LOV protein of Pseudomonas putida strain KT2240, represent at ∼13% the third-largest group of bacterial LOV photoreceptors (20). Several recent studies have shown that bacterial short LOV proteins, although lacking a fused effector domain, can regulate cellular functions such as photosynthetic gene expression and photopigment synthesis in phototrophic bacteria (21, 51). PpSB1-LOV crystallized under constant illumination is present as a dimer in the asymmetric unit (10). Due to the lack of a PpSB1-LOV dark-state crystal structure, the structural changes associated with the dark-to-light-state transition are currently still unclear. Importantly, for our case, the PpSB1-LOV protein is characterized by a unique and exceptionally long average dark recovery time constant of 2467 min at 20°C (9), which allows QENS measurements of the dark and light states under quasistationary conditions.
In this article, we report on a comparative experimental study on the picosecond to nanosecond dynamics of the photoreceptor PpSB1-LOV in the dark and light states using neutron time-of-flight (TOF) spectroscopy and neutron backscattering (BS) spectroscopy. The extracted dynamic parameters showed clear differences between the two states, indicative of the relevance of conformational dynamics for the signaling mechanism of the LOV photoreceptor.
Materials and Methods
Sample preparation
PpSB1-LOV was expressed as a hexahistidin-tagged fusion protein, as described previously (9), employing a riboflavin-auxotrophic strain, Escherichia coli Cmpx131 (22). All media were supplemented with 50 μM riboflavin. The use of E. coli Cmpx131 hereby improves the flavin mononucleotide (FMN) load of the heterologously produced PpSB1-LOV protein. PpSB1-LOV was purified using immobilized-metal affinity chromatography (IMAC) by employing an ÄKTA FPLC purifier system (GE Healthcare, Pittsburgh, PA), as described previously (9). The sample was concentrated to ∼70 mg/mL (10 kDa molecular mass cutoff concentrator, Pall Corporation, Port Washington, NY). All further sample handling was carried out under dim-red safety light to avoid population of the light state. The protein sample was lyophilized and resuspended in the initial-volume D2O. This procedure was repeated once to achieve complete substitution of all exchangeable protons for deuterium. As a last step, the sample was dialyzed against the heavy water buffer (10 mM NaH2PO4/Na2HPO4, 10 mM NaCl, 1 mM dithiothreitol, pD 8.0, 99.9 atom % D D2O). The dialysate was used as a buffer reference for neutron-scattering experiments. The final protein concentration was 62 mg/mL. Protein solutions and buffers were filled in flat aluminum sample holders (1 mm internal thickness and 30 mm width). A volume of 1.5 mL of each sample was used, which was sufficient to illuminate the samples with the full neutron beam height.
UV/Vis spectrophotometry
Light-dependent absorption changes in the visible region were measured using a Cary 60 UV/vis spectrophotometer (Agilent, Santa Clara, CA) equipped with a Peltier-thermostatted single-cell holder. All measurements were carried out at 303.2 K. Protein samples were diluted to a final absorbance of ∼0.3 at 450 nm in the heavy-water buffer. The same buffer was used as a reference. Chromophore loading was estimated from dark-state spectra of PpSB1-LOV by employing experimentally determined molar extinction coefficients of FMN at 450 nm (εFMN450nm = 11.765 M−1 cm−1) and 280 nm (εFMN280nm = 18.107 M−1 cm−1) and the molar extinction coefficient of the protein (εPpSB1-LOV280nm = 16.424 M−1 cm−1) derived from the amino acid sequence using the Protparam webservice (http://www.web.expasy.org/protparam). After recording of the protein dark-state spectra, the samples were illuminated for 30 s using a blue-light-emitting high-power light-emitting diode (LED) (Luxeon Lumileds, Phillips, Aachen, Germany) mounted on top of the cuvette. LED illumination was controlled using an Arduino UNO (Smart Projects, Ivrea, Italy) microcontroller. After illumination, the light-state spectrum was recorded. Dark-state recovery was measured from illuminated samples in the dark by recording consecutive spectra for 96 h, with one spectrum recorded every 30 min. For determination of the light-state lifetime, a time trace for the absorption recovery at 485 nm was extracted from the data set. The corresponding dark recovery time traces were fitted using a single-exponential decay function. All measurements were carried out in triplicate.
Dynamic light scattering
Dynamic light scattering (DLS) was measured on a Zetasizer Nano ZS instrument (Malvern Instruments, Malvern, UK). The concentrated PpSB1-LOV protein solutions were diluted with the heavy-water buffer to a concentration of 3 mg/mL. The diluted protein solution was illuminated with the blue-light LED before the DLS measurements. The autocorrelation functions were analyzed using the CONTIN algorithm (23).
Neutron-scattering experiments
Neutron scattering was measured using the multichopper TOF spectrometer TOFTOF (24, 25), operated by Technische Universität München, and the high-resolution BS instrument SPHERES, operated by the Jülich Centre for Neutron Science (26, 27). Both instruments are located at the Heinz Meier-Leibnitz Zentrum (MLZ), Garching, Germany. On TOFTOF the incident neutron wavelength was 9 Å, the chopper speed 12,000 rpm, and the chopper ratio 6. Data were analyzed between 0.40 and 1.00 Å−1. The settings resulted in an energy resolution of ∼20 μeV full width at half-maximum. On SPHERES, the incident neutron wavelength was 6.27 Å. SPHERES has an energy resolution of ∼0.65 μeV full width at half-maximum. SPHERES data were analyzed between 0.60 and 1.55 Å−1. Spectra of the TOFTOF detectors were grouped. Energy channels were binned for better statistics. Measured intensities were corrected for energy-dependent neutron detector efficiency, normalized to vanadium, and transformed into energy transfer and scattering vector space. Multiple scattering corrections were not performed, as the transmissions of all samples were above or close to 0.9. All samples were mounted in Al-flat cells and were oriented at 135° with respect to the incident beam. Resolution functions were measured with a 1-mm-thick vanadium slab. The transmissions, T, of the protein solution and of the D2O buffer with 1 mm thickness were measured on the small-angle neutron camera KWS2 located at the MLZ (Tprotein = 0.892, TD2O = 0.927 at 6.2 Å; Tprotein = 0.878, TD2O = 0.940 at 9.1 Å).
Scattered intensities of the buffer were subtracted from the measured data of the protein solution for TOF data according to
| (1) |
where is the volume fraction of the protein with average partial specific volume = 0.73 mL/g (28) and measured protein concentration c = 0.062 g/mL, and f = Tprotein/TD2O = 0.934 is the correction factor for neutron beam attenuation. For BS data, the buffer contributed to the spectra only as a flat background in the scattering vector range >0.6 Å−1, and the empty Al sample holder was subtracted from the protein solution. The D2O contribution was fitted as a flat background.
On TOFTOF, all samples received 5–6 h neutron beam time per temperature point. On SPHERES, the protein samples were measured between 18 and 27 h and the empty cell between 11 and 19 h at each temperature. The light-state sample was reexcited after each temperature by illumination with a blue-light LED during the experiment on TOFTOF. During the measurement on SPHERES, the light-state sample was illuminated regularly after 12 h (after 6 h at 298 K). For each illumination, the sample holder was removed from the cryostat and opened. The protein solution was taken out of the sample holder with a transparent syringe with a needle long enough to reach the bottom of the sample holder. The protein solution was then illuminated in the syringe and put back into the sample holder. The sample holder was sealed with indium wire and put back into the cryostat.
QENS data analysis
A simplified model for QENS spectra of internal protein dynamics is given by (18, 29)
| (2) |
where A0(q) is the elastic incoherent structure factor (EISF). The quasielastic broadening is approximated with one effective Lorentzian.
In solution, proteins additionally show translational and rotational diffusion. The total scattering function, Stotal(q,ω), for the observed motions of the proteins is then the convolution between the scattering functions for global diffusion, SG(q,ω), and for internal protein dynamics, SI(q,ω). It was shown that in the q-range covered by neutron TOF and BS spectrometers, rotational and translational diffusion of the protein can be described by one effective Lorentzian (30, 31, 32). Using the simplified model for internal protein dynamics given in Eq. 2, the total theoretical model function then reads
| (3) |
with the two Lorentzians
| (4) |
and
| (5) |
where and are the half-widths at half-maximum of the Lorentzians. The and account for global and internal diffusive motions, respectively.
The total theoretical scattering function plus linear background B(q,ω) was convoluted with the instrumental resolution function and fitted to the measured spectra according to
| (6) |
The <xvib2> are mean-square displacements (MSDs) of fast vibrational motions. The fits were performed over the energy-transfer range from −0.5 meV to +1.0 meV for data measured on TOFTOF and from −30 μeV to +30 μeV for spectra obtained with SPHERES.
Results and Discussion
Characterization by UV/Vis absorption
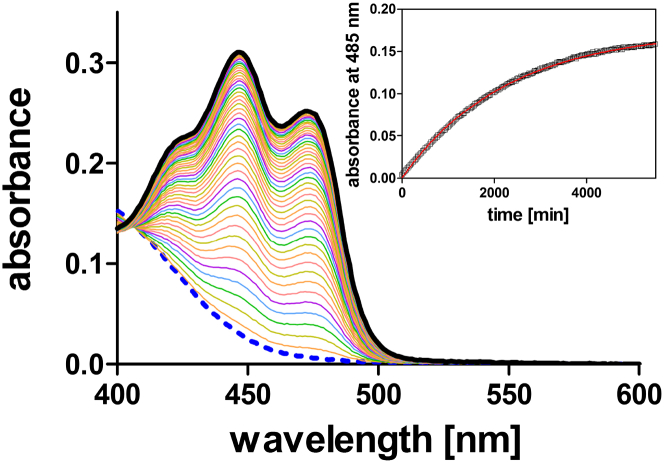
As a prerequisite for the neutron-scattering experiments, and to ensure optimal experimental conditions, the protein solution was first characterized by UV/Vis spectrophotometry (see Fig. 1). The final protein preparation showed a chromophore load of ∼92%. The light-state lifetime of PpSB1-LOV dissolved in heavy water was determined at 303.2 K. A very slow dark recovery with a light-state lifetime, τREC, of 2347 ± 67 min was determined (Fig. 1, inset). Hence, after 6 h at 303 K, which corresponds to the longest measuring time during neutron TOF experiments at the highest temperature, ∼86% of the light state is still present in the sample. Extrapolating the measured relaxation time in D2O to the temperature range below 290 K assuming Arrhenius behavior, we find that after the long measurement times of 12 h using neutron BS, >80% of the sample is in the light state. Therefore, to a large extent, the measured dynamics of the illuminated samples of PpSB1-LOV by neutron scattering reflect the dynamic properties of the light state. The presence of the dark state in the illuminated sample has not been accounted for during QENS data analysis due to the exceptionally long lifetime of the light state.
Figure 1.
Absorption changes and dark recovery of PpSB1-LOV in heavy-water buffer. The dark-state spectrum shows a maximum at ∼450 nm (black solid line). After illumination with blue light, the absorbance at 450 nm decreases, indicative of light-state formation (blue dashed line). The absorbance band is fully recovered in the dark (rainbow-colored spectra). For clarity, only every fourth spectrum (time increment, 120 min) is shown. The inset shows the time trace for the absorption recovery at 485 nm. Experimental data (open squares) were fitted to a single-exponential function (red solid line). To see this figure in color, go online.
Protein dynamics measured by QENS
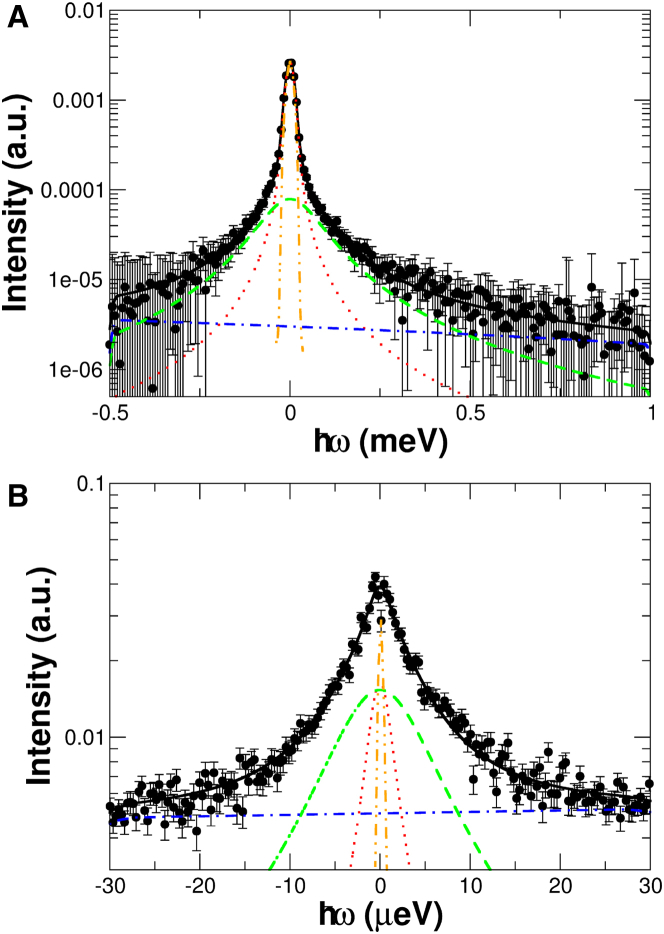
The settings of the neutron TOF spectrometer TOFTOF (24, 25) enable the investigation of protein dynamics with relaxation times of up to several tens of picoseconds. The high resolution of the BS spectrometer SPHERES (26, 27) allows resolution of slower molecular motions with relaxation times up to several nanoseconds. As protein solutions were investigated in the experiments, the measured QENS spectra contain information about both global protein diffusion and localized internal macromolecular dynamics. Typical QENS spectra of the measured PpSB1-LOV solutions after background subtraction are shown in Fig. 2 A for data recorded with TOFTOF and in Fig. 2 B for intensities measured with SPHERES.
Figure 2.
QENS spectra of PpSB1-LOV in the light state. (A) Data measured with TOFTOF (q = 1.0 Å−1, T = 293 K). Only every second experimental data point is shown for clarity. (B) Spectrum measured with SPHERES (q = 1.42 Å−1, T = 283 K). The black solid lines are fits to the data using the model described by Eq. 6. The components of the model are plotted as red dotted, green dashed, and blue dash-dotted lines corresponding to the narrow and broad Lorentzians and the linear background, respectively. All curves are convoluted with the instrumental resolution function. The orange dot-dot-dashed lines show the measured resolution function. To see this figure in color, go online.
The measured QENS spectra can be described well using the theoretical model described by Eq. 6, consisting of a narrow and a broad Lorentzian that describe the global diffusion dynamics of the protein and the internal diffusion, respectively (see Materials and Methods, QENS data analysis, for details). In the following, the global and internal diffusion dynamics are presented and discussed.
Global protein diffusion
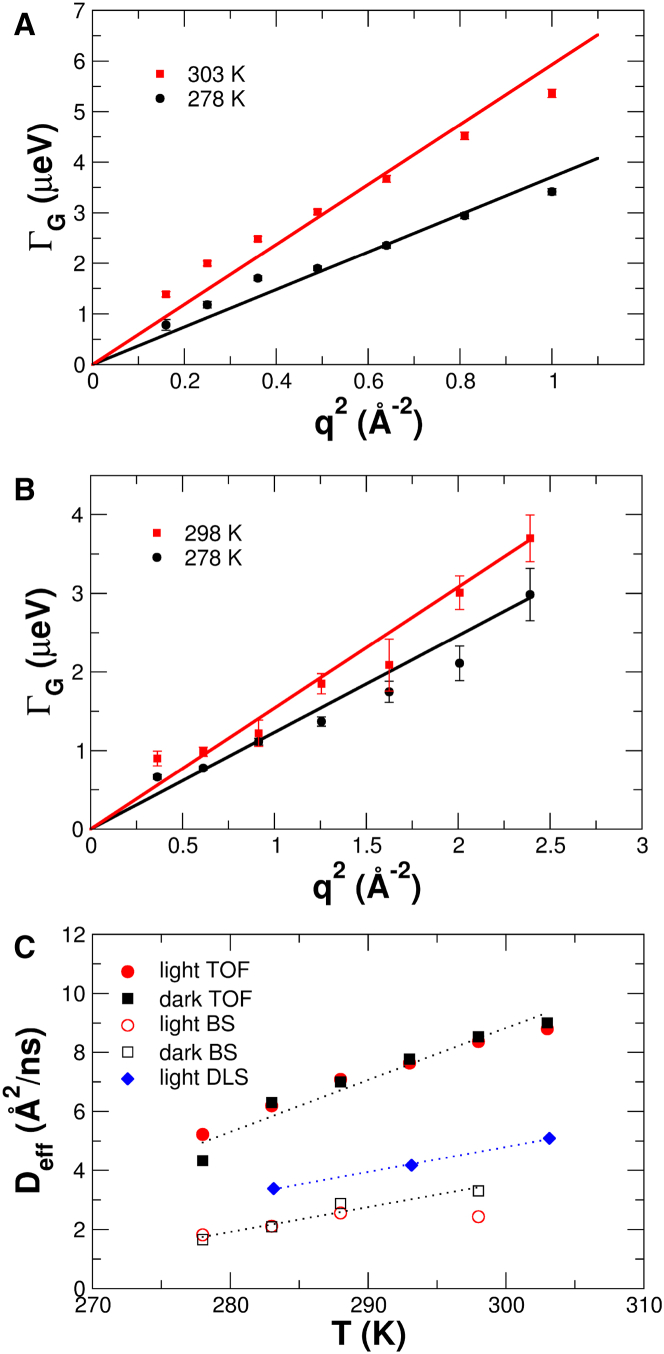
Information about global protein diffusion can be extracted from the measured QENS spectra. To determine the narrow line-widths, ΓG(q), accurately, the internal dynamics were described with a q-independent internal relaxation rate ΓI (see also the next section, Relaxation times of internal dynamics). Representative ΓG(q) of the narrow Lorentzian measured with TOF and BS are given in Fig. 3, A and B.
Figure 3.
(A and B) Line widths of the narrow Lorentzian accounting for global protein diffusion of PpSB1-LOV at different temperatures. The solid lines are linear fits to the measured data points. (A) Dark state measured by TOF. (B) Light state measured by BS. (C) Diffusion coefficients of global protein diffusion of PpSB1-LOV in the dark and light states measured by TOF, BS, and DLS. To see this figure in color, go online.
The line widths follow the expected ΓG(q) = Deffq2 behavior indicative of free Brownian diffusion, where Deff is an effective self-diffusion coefficient of the protein that contains the contributions of rotational and translational diffusion. The Deff determined from the TOF and BS measurements of the concentrated 62 mg/mL protein solution are shown in Fig. 3 C. The center-of-mass diffusion in the limit of infinite dilution, Dt (protein concentration 3 mg/mL), was measured using DLS, and the obtained values are also given in Fig. 3 C.
For spherical globular proteins, the contribution of rotational diffusion results in Deff values that are ∼27% larger than the value of pure translational self-diffusion, Dself, alone (30, 31, 32). The self-diffusion coefficients of proteins at high concentrations are reduced by hydrodynamic interactions, as compared to the center-of-mass diffusion, Dt, at infinite dilution. The hydrodynamic function, H, is related to the intrinsic viscosity, [η], and the protein concentration, c, according to H = 1 − [η]c. The intrinsic viscosity can be calculated from the protein structure using the computer program HYDROPRO (33). Using the structure of the light-state PpSB1-LOV dimer (PDB: 3SW1) and the known protein concentration 62 mg/mL, we obtain H = 0.76. That value, however, is an upper limit, as PpSB1-LOV contains flexible end regions, which are not resolved in the structure (10). Including these regions in the HYDROPRO calculation, we obtain a more realistic estimated value of H = 0.63. Both effects bring the Deff measured by BS closer to the center-of-mass diffusion, Dt, at infinite dilution (estimated Deff = 1.27 × 0.63 × Dt = 0.8 × Dt).
We find diffusion coefficients depending on the observation time of the neutron spectrometer. The Deff values measured on TOFTOF are on average 2.8 times larger than the values determined with SPHERES. On the other hand, the Deff values determined with SPHERES are in better agreement with the Dt in the long-time limit and at long length scales, as probed by DLS. Observation-time-dependent self-diffusion coefficients of hemoglobin in red blood cells (32, 34), and also of liquid medium-chain n-alkanes measured by QENS (35), have been reported before. In the article by Unruh et al. (35), a distribution of relaxation times in the n-alkanes results in observation-time-dependent diffusion coefficients. For our case, the simplest explanation would be that global protein diffusion couldn’t be fully resolved by the lower resolution of TOFTOF. A slow internal process (see the next section) probably contributes to the measured narrow line widths, and the medium resolution does not allow separation of both components accurately. However, using three Lorentzians for the description of the spectra measured with TOFTOF does not give stable results.
The diffusion coefficients, Deff, measured with the same neutron spectrometer do not differ between the dark and light states. This result demonstrates that the protein in the dark state at the investigated concentration and buffer condition has a shape similar to that of the PpSB1-LOV dimer in the light state (10).
Relaxation times of internal dynamics
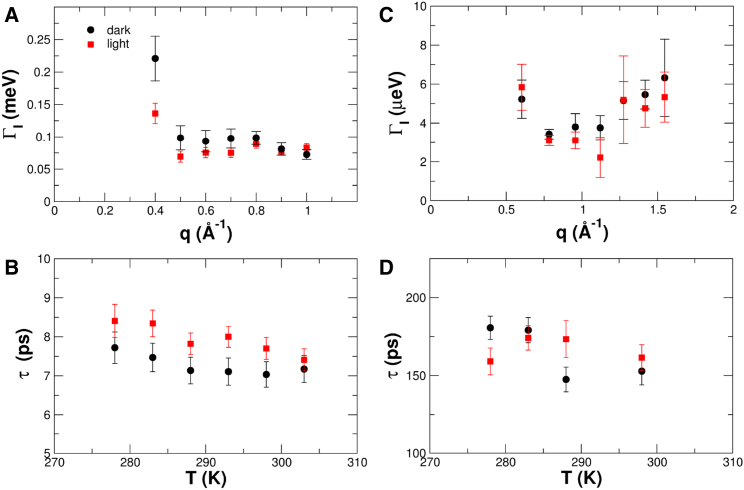
Internal diffusive motions are visible in the QENS spectra recorded on both neutron spectrometers. The quasielastic broadening due to the combined effect of global protein diffusion and internal fluctuations can be described effectively with one broad Lorentzian with half-widths ΓG+I(q) (see Fig. 2, A and B). The contribution of global protein diffusion to the line widths ΓG+I(q) was subtracted and the ΓI(q) values, describing internal protein dynamics in the light- and dark-adapted states at selected temperatures, are shown in Fig. 4, A and C, for spectra obtained with TOFTOF and SPHERES, respectively.
Figure 4.
(A and C) Half-widths of the broad Lorentzian describing internal protein dynamics of PpSB1-LOV in the dark and light states as a function of the scattering vector. Data were measured by TOF at the sample temperature, T = 293 K (A), and by BS at T = 288 K (C). (B and D) Relaxation times of internal dynamics as a function of the sample temperature, determined using TOF (B) and BS (D). To see this figure in color, go online.
The fast process seen with TOF is outside of the detectable energy-transfer range of the BS instrument and appears only as a flat background. Within the error bars, the line widths recorded with both instruments are constant as a function of the scattering vector, which is the signature of a localized dynamic process. The discrepancy at q = 0.4 Å−1 on TOFTOF is caused by the weak contribution of the internal dynamics to the overall scattered intensity, which leads to a large uncertainty of the line width. To determine the relaxation rate of the internal processes with higher accuracy, all measured spectra at one temperature were fitted in a global fit approach with a q-independent relaxation rate, ΓI. The obtained relaxation times, τ = 1/ΓI, are given in Fig. 4, B and D, for TOFTOF and SPHERES, respectively. Concerning the fast process observed with TOF we find relaxation times for the dark-state PpSB1-LOV that are similar to those of different folded conformations of apomyoglobin determined by neutron TOF spectroscopy (τ = 6.9 ps at 289.2 K) (36). The light state of PpSB1-LOV, however, has slightly larger relaxation times at all measured temperatures. Therefore, this seems to be a systematic effect, which demonstrates that the formation of the covalent bond between the photoactive cysteine residue (C53) and the C4a atom of the FMN chromophore in the light state strengthens the interaction network in PpSB1-LOV, leading to slower relaxation times on the picosecond scale. Activation energies, Ea, were extracted from the Arrhenius behavior of the relaxation rates, and the obtained values are given in Table 1. Although the activation energy of fast motions in the light state appears to be larger than in the dark state, the uncertainty is rather large and does not allow us to draw final conclusions. Further measurements with high statistics would be required to verify that aspect more precisely and reliably.
Table 1.
Activation Energies, Ea, and Effective Force Constants, <k′>
| PpSB1-LOV | TOFTOF |
SPHERES |
|
|---|---|---|---|
| Ea (kJ/mol) | <k′> (N/m) | <k′> (N/m) | |
| Light state | 3.4 ± 1.4 | 0.28 ± 0.07 | 0.018 ± 0.003 |
| Dark state | 2.2 ± 1.7 | 0.16 ± 0.03 | 0.10 ± 0.02a (0.14 ± 0.07b) |
The activation energies were derived from the relaxation times, and the effective force constants were calculated from the MSDs.
aValue calculated excluding the MSD at 283 K.
bValue calculated over all MSDs.
The slow relaxation times measured with the high-resolution BS instrument are rather similar between the light- and dark-adapted states of PpSB1-LOV and do not show clear systematic differences between the two states. The results, therefore, show that light excitation does not significantly modify the relaxation times of slow, nanosecond motions in PpSB1-LOV. The absolute values are close to the slow dynamical process found in alcohol dehydrogenase, as determined with high-resolution TOF and BS spectroscopy (τ = 160 ps at 278.2 K) (37). Due to the large error bars and the narrower temperature range, the activation energies could not be determined.
MSDs of internal dynamics
MSDs, <u2>, describing the extent of localized internal dynamics of PpSB1-LOV can be extracted from the measured EISFs (see Eq. 3 and Materials and Methods, QENS data analysis). Within the Gaussian approximation, the <u2> values were calculated according to (38, 39)
| (7) |
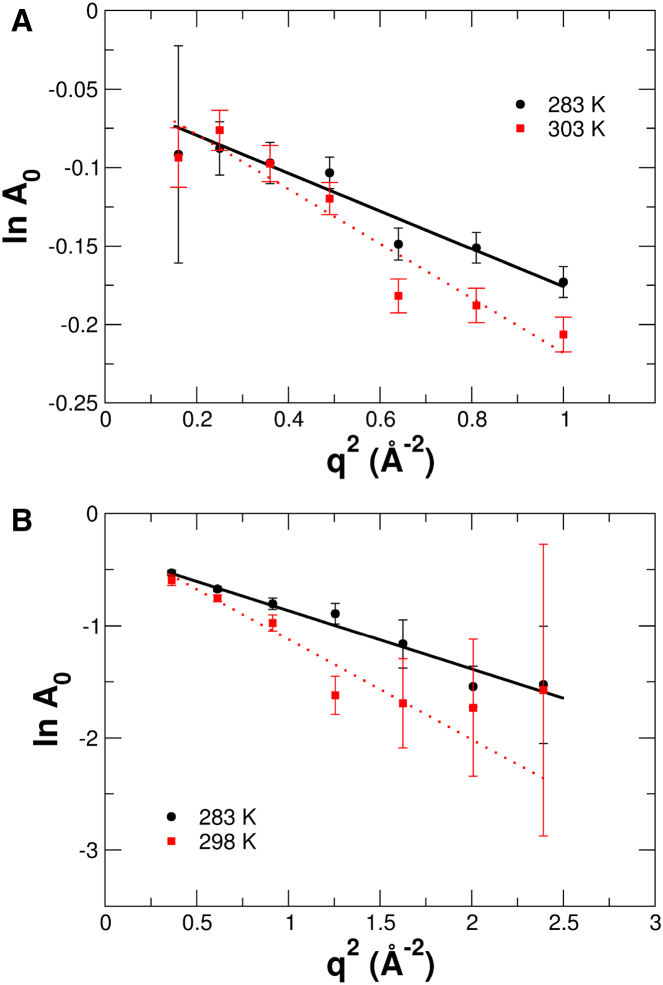
over the measured q2-range, where A0(q) is the measured EISF. Examples of EISFs determined with Gaussian fits are shown in Fig. 5 for the dark and light states of the protein measured with both instruments.
Figure 5.
EISFs determined from the QENS experiments at different temperatures. (A) EISFs of the light state of PpSB1-LOV, measured with TOF. (B) EISFs of the dark state of PpSB1-LOV, determined with BS. To see this figure in color, go online.
The EISFs were calculated from the QENS data sets using a global fit with a q-independent relaxation rate. The determined MSDs of PpSB1-LOV in the light and dark states are shown in Fig. 6, A and B, respectively. Effective force constants, <k′>, were determined from the temperature dependence of the <u2> according to (38, 39)
| (8) |
The obtained values of the effective force constants of the light and dark states measured with TOFTOF and SPHERES are reported in Table 1. The values of the uncertainties result from formal error propagation. The large error of the force constant of the dark state measured by BS is due to the small deviation of the MSD at 283 K from linear behavior. When we exclude that data point, we obtain an effective force constant of 0.10 ± 0.02 N/m for the dark-state protein measured by BS, which might be a more realistic estimate.
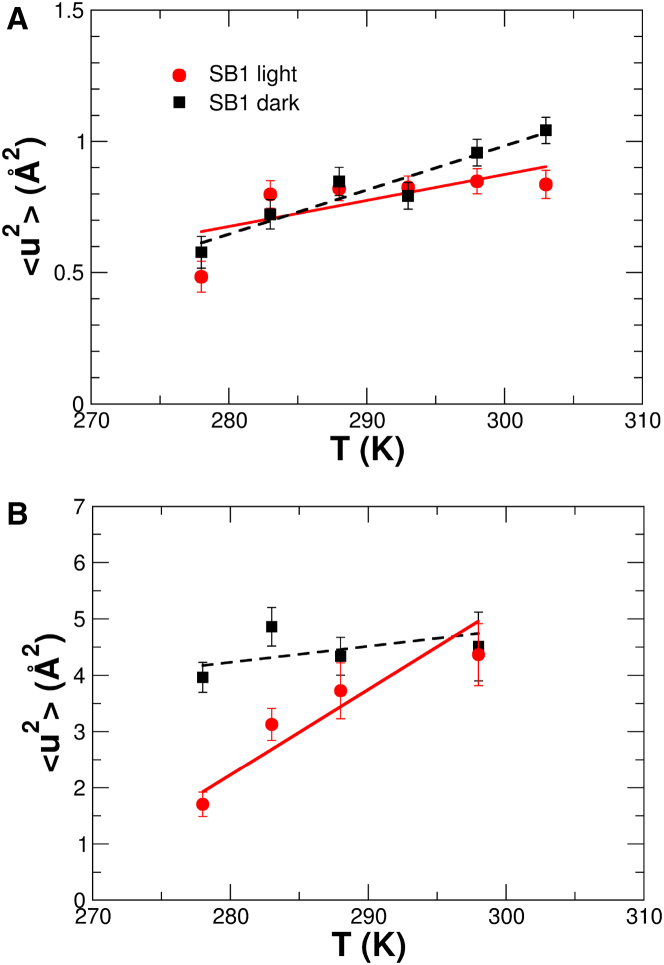
Figure 6.
MSDs of the internal dynamics of PpSB1-LOV measured with (A) TOFTOF on the picosecond timescale,and (B) SPHERES on the nanosecond timescale. To see this figure in color, go online.
The <u2> reveal a distinct dynamical behavior of the light and dark states of PpSB1-LOV on the picosecond and nanosecond timescales. The values of the <u2> determined by TOF are smaller than in the long-time limit observed by BS, which is an expected result. Observation-time-dependent <u2> values determined by neutron scattering have been reported before (40, 41). On the fast, picosecond time scale the observed amplitudes of motion are similar below 295 K for both light and dark states of PpSB1-LOV and diverge at the highest temperatures, where the light-excited state is less flexible than the dark-adapted protein. Measured differences in the <u2> values observed with SPHERES are more pronounced. At low temperatures, the flexibility of the light-state protein in the nanosecond timescale is significantly reduced compared to the dark state.
More information can be obtained from the determined effective force constants, <k′> (38, 39). The underlying assumption is that molecular forces in the protein can be approximated by a quasiharmonic potential (38). Effective force constants inform about the “stiffness”, or resilience, of the protein. A protein with strong intermolecular forces is characterized by a large resilience, whereas biomacromolecules with weak internal forces are “soft” objects with low resilience. The extracted <k′> values depend on the observation time of the neutron spectrometer, which has also been observed before (40, 41). This demonstrates that internal forces acting on motions in the observed long-time limit are smaller than on the faster, picosecond timescale, in particular for the light state of PpSB1-LOV. Concerning changes in the dynamics between the light and dark states of PpSB1-LOV, we find that on the short timescale observed by TOF, the light state appears more resilient within the errors than the dark-adapted state. The situation changes on the nanosecond timescale, where the light state is significantly less resilient than the dark-adapted state.
On the picosecond timescale, the observed motions were attributed to fast rotational and librational motions of side chains along their chemical bonds, including methyl-group rotations (15), whereas on the slow, nanosecond timescale, the detectable motions are identified predominantly as motions of whole amino acid side chains (37). Photoactivation leads to the formation of a covalent bond between the cysteine residue (C53) and the C4a atom of the FMN chromophore. On the fast, picosecond timescale, the induced changes due to bond formation after photoactivation lead to a stabilization of the light state, which is visible by reduced MSDs at higher temperatures, stronger internal forces in PpSB1-LOV, and longer relaxation times, with a larger activation energy, as derived from the QENS broadening.
We can determine the difference in conformational entropy between the light and dark states from the measured <u2> according to (42)
| (9) |
where R = 8.3144 J K−1 mol−1 is the gas constant. The derived values of ΔSconf and the difference, TΔSconf, per monomer between the light and dark states at 303 K on the picosecond timescale are given in Table 2. The value of ΔSconf needs to be interpreted as an average value on a per residue basis. The derived value of TΔSconf is in the range of TΔSconf between the free and DNA-bound catabolite activator protein determined by NMR measurements of side-chain methyl-group dynamics on the pico- to nanosecond timescale (43), but it is significantly smaller than the TΔSconf between the folded and unfolded states of globular proteins investigated by QENS on the picosecond timescale (36, 44). Within the error, ΔSconf of PpSB1-LOV is close to zero at the other temperatures.
Table 2.
Difference of Conformational Entropy, ΔSconf and TΔSconf, between the Light and Dark States of PpSB1-LOV Derived from the Measured MSDs
| T (K) | ΔSconf (J K−1 mol−1) | TΔSconf (kJ mol−1) |
|---|---|---|
| TOFTOF | ||
| 303 | 2.7 ± 1.0 | 133 ± 49 |
| SPHERES | ||
| 278 | 10.5 ± 1.8 | 473 ± 81 |
| 283 | 5.5 ± 1.4 | 252 ± 64 |
The light-sensing mechanism, however, has a larger impact on slower motions occurring on the nanosecond timescale. The light-activated signaling state of PpSB1-LOV is significantly softer and with reduced flexibility compared to the dark-state protein. The derived values of ΔSconf and TΔSconf per monomer between the light and dark states at 278 and 283 K on the nanosecond timescale are given in Table 2. At the other two temperatures, ΔSconf is close to zero within the errors.
The modulation of molecular dynamics (MD) of the membrane protein bacteriorhodopsin on the picosecond timescale was investigated by Pieper et al. (45) using kinetic QENS measurements through the photocycle of the protein. A softening of the photoexcited M intermediate, with increased flexibility compared to the bacteriorhodopsin ground state, was observed. On the other hand, slow structural changes of whole thylakoid membranes in green algae upon light illumination and successive dark adaptation have been studied by small-angle neutron scattering (46). Our results demonstrate that photoactivation has a different impact on fast, picosecond side-chain dynamics of a LOV photoreceptor than on the light-controlled integral membrane proton pump bacteriorhodopsin.
An interesting and surprising finding of our experimental study is that the resiliences on the pico- and nanosecond timescales follow opposite evolutions upon photoactivation. The physical reason for that observation is related to the heterogeneity of protein dynamics occurring on different timescales. Intuitively, one would relate the larger resilience on the fast, picosecond timescale to a stronger hydrogen-bonding network induced by the covalent bond between the cysteine residue (C53) and the C4a atom of the FMN chromophore. A general softening of the protein on the fast, picosecond timescale in the active state apparently is not required for its biological function, in contrast to the proton pump bacteriorhodopsin (45). PpSB1-LOV functions as a photoswitch, and not as a proton pump like bacteriorhodopsin, which requires a softer structure for the active working cycle. On the other hand, reduced side-chain dynamics acting mostly on the nanosecond timescale seem to play a fundamental and essential role for signal propagation in PpSB1-LOV. One can speculate about the biological significance of that observation. Slow side-chain dynamics on the nanosecond timescale may reflect motions of whole structural elements or secondary structure elements. The smaller MSDs of the light state at low temperatures might reflect less flexible and more ordered structural elements, which are important for interaction with possible binding partners and thus could be essential for transfer of the signal. In the dark state, these structural elements are more flexible but are confined by the surrounding structure in their maximal amplitudes of motion, which can account for the observed larger resilience of the dark-state protein. In the next section, we used MD simulations to infer structural regions with potentially modified flexibility upon photoactivation.
Potential structural basis of the observed dynamics of PpSB1-LOV
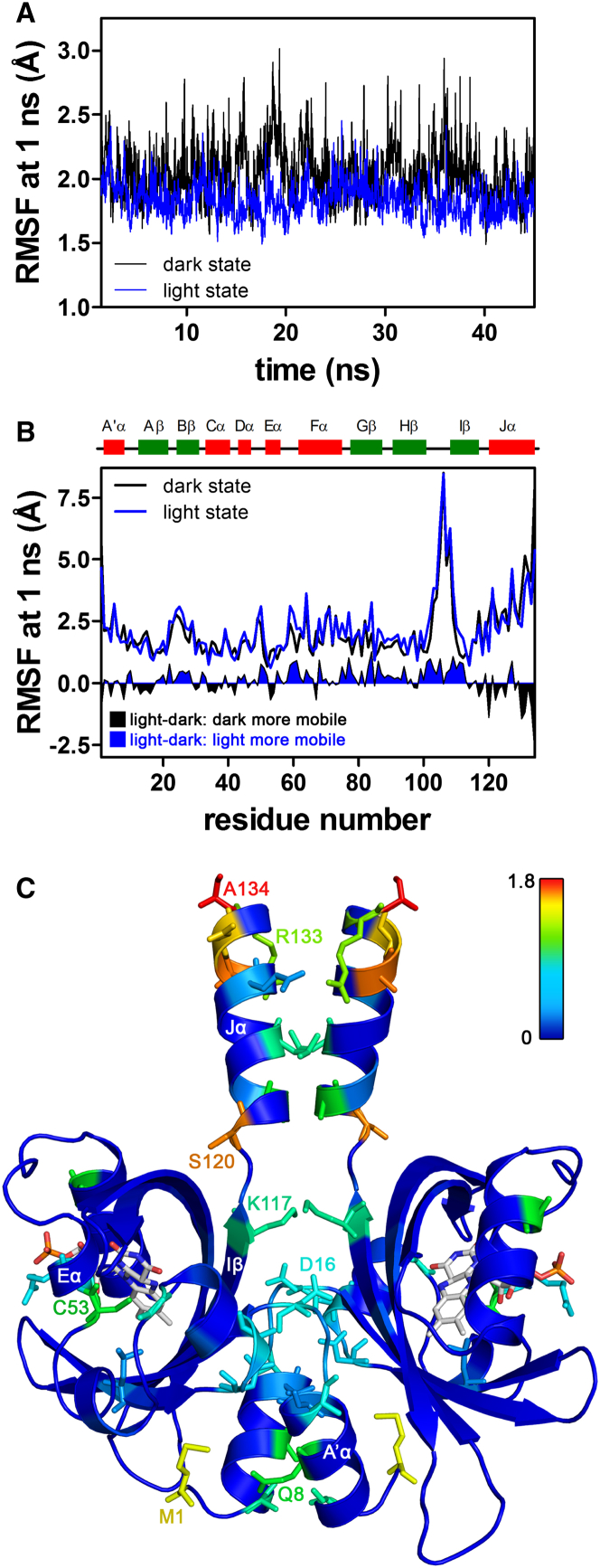
PpSB1-LOV possesses a mixed α/β Per-ARNT-SIM fold in the topological order Aβ-Bβ-Cα-Dα-Eα-Gβ-Hβ-Iβ (Fig. 7). The FMN chromophore is anchored above the central Iβ sheet within a binding pocket constituted by the β-scaffold and the surrounding Cα-Dα-Eα helices. Outside of the LOV-core domains, the N-terminal A′α helix and the C-terminal Jα-helix protrude from the LOV core, constituting part of the LOV-LOV dimer interface (Fig. 7). Due to the lack of a dark-state x-ray structure of PpSB1-LOV, the structural consequences of photoactivation currently remain elusive. To address this issue, we recently performed MD simulations of PpSB1-LOV, which revealed increased A′α- and Jα-helix mobility in the respective dark-state simulations (47). To evaluate differences in side-chain mobility, we here reanalyzed the previously presented MD simulation data (Fig. 7) by calculating side-chain root mean-square fluctuations (RMSFs), as a descriptor for side-chain flexibility, over the respective trajectories. Here, PpSB1-LOV shows globally increased side-chain RMSF values in the dark-state simulation compared to the respective light-state trajectory (Fig. 7 A), which is in line with the experimentally observed increased <u2> values derived for the dark state of PpSB1-LOV by SPHERES (Fig. 6). A residue-resolved RMSF plot identifies residues/structural regions showing increased side-chain fluctuations in the dark-state simulations on the nanosecond timescale (Fig. 7 B, light-dark RMSF plot). To highlight structural regions that show increased side-chain fluctuations, the PpSB1-LOV dimer x-ray structure is color coded according to the calculated light-dark RMSF absolute values, showing an increased flexibility for residues located in the dimer interface region and near the FMN chromophore (Fig. 7 C).
Figure 7.
Time- and residue-resolved reanalysis of the previously presented MD simulation data for PpSB1-LOV (47). To evaluate the possibility of altered side-chain flexibilities accompanying PpSB1-LOV photoactivation, we reanalyzed the respective MD trajectories for altered side-chain RMSFs. (A) By averaging over a time window of 1 ns (time resolution of the SPHERES instrument), globally increased RMSF values are calculated for the dark-state trajectory (black line) compared to the light-state simulation (blue line). (B) Residue-resolved RMSF plot obtained for the dark- (black line) and light-state simulations (blue line). The corresponding light-dark RMSF plot highlights structural regions/residues showing increased fluctuations in the dark state (black filled peaks below the y = 0 line), compared to regions that are more mobile in the light state (blue-filled peaks above the y = 0 line). (C) For visualization, the corresponding light-dark RMSF absolute values were mapped on the light-state x-ray structure of PpSB1-LOV (PDB: 3SW1), colored according to RMSF, with low absolute values in blue and high values in red. For clarity, only residues that show increased side-chain RMSF values in the dark state are shown in stick representation. For orientation, only residues relevant for the discussion are labeled. To see this figure in color, go online.
Regions/residues with increased mobility in the dark state include 1) N- and C-termini (M1 and A134), as well as the photoactive cysteine (C53), 2) residues on the A′α helix (e.g., Q8), the A′α-Aβ loop (e.g., D16), and 3) the C-terminal end of Iβ (K117), as well as several residues on the Jα helix (e.g., S120 and R133). Interestingly, residues that appear more mobile in the dark state are predominately localized at the LOV-LOV dimer interface, corroborating our previous model, in which photoactivation of PpSB1-LOV causes a rearrangement of the two dimer subunits relative to each other. In addition to our previous model based on MD data, the here-presented neutron-scattering data suggest that those changes are accompanied (or driven) by altered side-chain motions in the dark state compared to the light state. This hypothesis is also supported by an NMR study on the Bacillus subtilis YtvA LOV photoreceptor, where a low order parameter of the Jα-helix was reported, indicating a high degree of mobility of this region in the dark state (48).
Taken together, global conformational changes of the dimer accompanied by altered side-chain dynamics and conformational entropies would represent a feasible mode of signal relay to modify interactions with potential, yet-to-be-identified binding partners, which would enable a physiological response mediated by an effector-domain-less LOV photoreceptor.
Conclusions
We present, to our knowledge, the first neutron-scattering study of a LOV photoreceptor, revealing systematic and significant differences in fast, picosecond to nanosecond side-chain dynamics between the dark and light states of PpSB1-LOV. Photoactivation results in a larger resilience of the photoreceptor on the fast, picosecond timescale accompanied by a slightly reduced flexibility at higher temperatures and significantly longer relaxation times of diffusive motions. That observation is in agreement with the assumption that the formation of the covalent bond between the cysteine residue (C53) and the C4a atom of the FMN chromophore in the light-state protein strengthens the interaction network in PpSB1-LOV. On the slower, nanosecond timescale, however, a significant softening of the light-state protein is observed, accompanied by a reduced flexibility of the photosensor. Our observation is in agreement with the assumption that side chains located mostly on the protruding Jα helix and the LOV-LOV dimer interface become dynamically more stabilized upon photoactivation. The reduction of side-chain dynamics of the Jα helix could then transfer the light signal to other binding partners. Our results stress the importance of side-chain dynamics on the picosecond to nanosecond timescale for photoactivation and signaling in the LOV photosensor family, and they thus contribute to the emerging view of protein flexibility as a major source of conformational entropy (36, 43, 49, 50), enabling dynamically controlled allosteric responses.
Author Contributions
A.S. and U.K. designed the research; A.S., E.K.G., M.B., W.L., M.Z., and U.K. performed the research; A.S. and U.K. analyzed the data; and A.S. and U.K. wrote the article.
Acknowledgments
This work is based on experiments performed on the TOFTOF instrument operated by the Physik Department E13, Technische Universität München, and on SPHERES, operated by the Jülich Centre for Neutron Science at the Heinz Maier-Leibnitz Zentrum (MLZ), Garching, Germany.
U.K. acknowledges funding by the Federal Ministry of Education and Research (BMBF) in the framework of the collaborative research project “OptoSys” (FKZ 031A16).
Editor: Michael Sattler.
References
- 1.Krauss U., Minh B.Q., Jaeger K.E. Distribution and phylogeny of light-oxygen-voltage-blue-light-signaling proteins in the three kingdoms of life. J. Bacteriol. 2009;191:7234–7242. doi: 10.1128/JB.00923-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herrou J., Crosson S. Function, structure and mechanism of bacterial photosensory LOV proteins. Nat. Rev. Microbiol. 2011;9:713–723. doi: 10.1038/nrmicro2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Möglich A., Yang X., Moffat K. Structure and function of plant photoreceptors. Annu. Rev. Plant Biol. 2010;61:21–47. doi: 10.1146/annurev-arplant-042809-112259. [DOI] [PubMed] [Google Scholar]
- 4.Shcherbakova D.M., Shemetov A.A., Verkhusha V.V. Natural photoreceptors as a source of fluorescent proteins, biosensors, and optogenetic tools. Annu. Rev. Biochem. 2015;84:519–550. doi: 10.1146/annurev-biochem-060614-034411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crosson S., Moffat K. Structure of a flavin-binding plant photoreceptor domain: insights into light-mediated signal transduction. Proc. Natl. Acad. Sci. USA. 2001;98:2995–3000. doi: 10.1073/pnas.051520298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swartz T.E., Corchnoy S.B., Bogomolni R.A. The photocycle of a flavin-binding domain of the blue light photoreceptor phototropin. J. Biol. Chem. 2001;276:36493–36500. doi: 10.1074/jbc.M103114200. [DOI] [PubMed] [Google Scholar]
- 7.Salomon M., Christie J.M., Briggs W.R. Photochemical and mutational analysis of the FMN-binding domains of the plant blue light receptor, phototropin. Biochemistry. 2000;39:9401–9410. doi: 10.1021/bi000585+. [DOI] [PubMed] [Google Scholar]
- 8.Rani R., Jentzsch K., Krauss U. Conservation of dark recovery kinetic parameters and structural features in the pseudomonadaceae “short” light, oxygen, voltage (LOV) protein family: implications for the design of LOV-based optogenetic tools. Biochemistry. 2013;52:4460–4473. doi: 10.1021/bi400311r. [DOI] [PubMed] [Google Scholar]
- 9.Jentzsch K., Wirtz A., Krauss U. Mutual exchange of kinetic properties by extended mutagenesis in two short LOV domain proteins from Pseudomonas putida. Biochemistry. 2009;48:10321–10333. doi: 10.1021/bi901115z. [DOI] [PubMed] [Google Scholar]
- 10.Circolone F., Granzin J., Batra-Safferling R. Structural basis for the slow dark recovery of a full-length LOV protein from Pseudomonas putida. J. Mol. Biol. 2012;417:362–374. doi: 10.1016/j.jmb.2012.01.056. [DOI] [PubMed] [Google Scholar]
- 11.Fedorov R., Schlichting I., Hegemann P. Crystal structures and molecular mechanism of a light-induced signaling switch: the Phot-LOV1 domain from Chlamydomonas reinhardtii. Biophys. J. 2003;84:2474–2482. doi: 10.1016/S0006-3495(03)75052-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halavaty A.S., Moffat K. N- and C-terminal flanking regions modulate light-induced signal transduction in the LOV2 domain of the blue light sensor phototropin 1 from Avena sativa. Biochemistry. 2007;46:14001–14009. doi: 10.1021/bi701543e. [DOI] [PubMed] [Google Scholar]
- 13.Zoltowski B.D., Schwerdtfeger C., Crane B.R. Conformational switching in the fungal light sensor Vivid. Science. 2007;316:1054–1057. doi: 10.1126/science.1137128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCammon J.A., Harvey S.C. Cambridge University Press; Cambridge, United Kingdom: 1987. Dynamics of Proteins and Nucleic Acids. [Google Scholar]
- 15.Zanotti J.M., Bellissent-Funel M.C., Parello J. Hydration-coupled dynamics in proteins studied by neutron scattering and NMR: the case of the typical EF-hand calcium-binding parvalbumin. Biophys. J. 1999;76:2390–2411. doi: 10.1016/S0006-3495(99)77395-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henzler-Wildman K.A., Lei M., Kern D. A hierarchy of timescales in protein dynamics is linked to enzyme catalysis. Nature. 2007;450:913–916. doi: 10.1038/nature06407. [DOI] [PubMed] [Google Scholar]
- 17.Stadler A.M., Stingaciu L., Richter D. Internal nanosecond dynamics in the intrinsically disordered myelin basic protein. J. Am. Chem. Soc. 2014;136:6987–6994. doi: 10.1021/ja502343b. [DOI] [PubMed] [Google Scholar]
- 18.Fitter J., Gutberlet T., Katsaras J., editors. Neutron Scattering in Biology: Techniques and Applications. Springer; New York: 2006. [Google Scholar]
- 19.El-Arab K.K., Pudasaini A., Zoltowski B.D. Short LOV proteins in methylocystis reveal insight into LOV domain photocycle mechanisms. PLoS One. 2015;10:e0124874. doi: 10.1371/journal.pone.0124874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Losi A., Gärtner W. Bacterial bilin- and flavin-binding photoreceptors. Photochem. Photobiol. Sci. 2008;7:1168–1178. doi: 10.1039/b802472c. [DOI] [PubMed] [Google Scholar]
- 21.Metz S., Jäger A., Klug G. Role of a short light, oxygen, voltage (LOV) domain protein in blue light- and singlet oxygen-dependent gene regulation in Rhodobacter sphaeroides. Microbiology. 2012;158:368–379. doi: 10.1099/mic.0.054700-0. [DOI] [PubMed] [Google Scholar]
- 22.Mathes T., Vogl C., Hegemann P. In vivo generation of flavoproteins with modified cofactors. J. Mol. Biol. 2009;385:1511–1518. doi: 10.1016/j.jmb.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Provencher S.W. CONTIN: a general purpose constrained regularization program for inverting noisy linear algebraic and integral equations. Comput. Phys. Commun. 1982;27:229–242. [Google Scholar]
- 24.Unruh T., Neuhaus J., Petry W. The high-resolution time-of-flight spectrometer TOFTOF. Nucl. Instrum. Methods Phys. Res. A. 2007;580:1414–1422. [Google Scholar]
- 25.Lohstroh W., Evenson Z. TOFTOF: cold neutron time-of-flight spectrometer. J. Large-scale Res. Facil. 2015;1:A15. [Google Scholar]
- 26.Wuttke J., Budwig A. SPHERES, Jülich’s high-flux neutron backscattering spectrometer at FRM II. Rev. Sci. Instrum. 2012;83:075109. doi: 10.1063/1.4732806. [DOI] [PubMed] [Google Scholar]
- 27.Zamponi M., Khaneft M. SPHERES: backscattering spectrometer. J. Large-scale Res. Facil. 2015;1:A30. [Google Scholar]
- 28.Murphy L.R., Matubayasi N., Levy R.M. Protein hydration and unfolding--insights from experimental partial specific volumes and unfolded protein models. Fold. Des. 1998;3:105–118. doi: 10.1016/S1359-0278(98)00016-9. [DOI] [PubMed] [Google Scholar]
- 29.Bée M. Adam Hilger; Bristol, United Kingdom: 1988. Quasielastic Neutron Scattering: Principles and Applications in Solid State Chemistry, Biology, and Materials Science. [Google Scholar]
- 30.Pérez J., Zanotti J.M., Durand D. Evolution of the internal dynamics of two globular proteins from dry powder to solution. Biophys. J. 1999;77:454–469. doi: 10.1016/S0006-3495(99)76903-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roosen-Runge F., Hennig M., Schreiber F. Protein self-diffusion in crowded solutions. Proc. Natl. Acad. Sci. USA. 2011;108:11815–11820. doi: 10.1073/pnas.1107287108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stadler A.M., van Eijck L., Artmann G. Macromolecular dynamics in red blood cells investigated using neutron spectroscopy. J. R. Soc. Interface. 2011;8:590–600. doi: 10.1098/rsif.2010.0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.García De La Torre J., Huertas M.L., Carrasco B. Calculation of hydrodynamic properties of globular proteins from their atomic-level structure. Biophys. J. 2000;78:719–730. doi: 10.1016/S0006-3495(00)76630-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stadler A.M., Digel I., Büldt G. Hemoglobin dynamics in red blood cells: correlation to body temperature. Biophys. J. 2008;95:5449–5461. doi: 10.1529/biophysj.108.138040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Unruh T., Smuda C., Petry W. Diffusive motions in liquid medium-chain n-alkanes as seen by quasielastic time-of-flight neutron spectroscopy. J. Chem. Phys. 2008;129:121106. doi: 10.1063/1.2990026. [DOI] [PubMed] [Google Scholar]
- 36.Stadler A.M., Koza M.M., Fitter J. Determination of conformational entropy of fully and partially folded conformations of holo- and apomyoglobin. J. Phys. Chem. B. 2015;119:72–82. doi: 10.1021/jp509732q. [DOI] [PubMed] [Google Scholar]
- 37.Monkenbusch M., Stadler A., Richter D. Fast internal dynamics in alcohol dehydrogenase. J. Chem. Phys. 2015;143:075101. doi: 10.1063/1.4928512. [DOI] [PubMed] [Google Scholar]
- 38.Bicout D.J., Zaccai G. Protein flexibility from the dynamical transition: a force constant analysis. Biophys. J. 2001;80:1115–1123. doi: 10.1016/S0006-3495(01)76089-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zaccai G. How soft is a protein? A protein dynamics force constant measured by neutron scattering. Science. 2000;288:1604–1607. doi: 10.1126/science.288.5471.1604. [DOI] [PubMed] [Google Scholar]
- 40.Trapp M., Trovaslet M., Peters J. Energy landscapes of human acetylcholinesterase and its Huperzine A-inhibited counterpart. J. Phys. Chem. B. 2012;116:14744–14753. doi: 10.1021/jp304704h. [DOI] [PubMed] [Google Scholar]
- 41.Laulumaa S., Nieminen T., Natali F. Dynamics of the peripheral membrane protein P2 from human myelin measured by neutron scattering—a comparison between wild-type protein and a hinge mutant. PLoS One. 2015;10:e0128954. doi: 10.1371/journal.pone.0128954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Receveur V., Calmettes P., Durand D. Picosecond dynamical changes on denaturation of yeast phosphoglycerate kinase revealed by quasielastic neutron scattering. Proteins. 1997;28:380–387. [PubMed] [Google Scholar]
- 43.Tzeng S.-R., Kalodimos C.G. Protein activity regulation by conformational entropy. Nature. 2012;488:236–240. doi: 10.1038/nature11271. [DOI] [PubMed] [Google Scholar]
- 44.Fitter J. A measure of conformational entropy change during thermal protein unfolding using neutron spectroscopy. Biophys. J. 2003;84:3924–3930. doi: 10.1016/S0006-3495(03)75120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pieper J., Buchsteiner A., Hauss T. Transient protein softening during the working cycle of a molecular machine. Phys. Rev. Lett. 2008;100:228103. doi: 10.1103/PhysRevLett.100.228103. [DOI] [PubMed] [Google Scholar]
- 46.Nagy G., Ünnep R., Minagawa J. Chloroplast remodeling during state transitions in Chlamydomonas reinhardtii as revealed by noninvasive techniques in vivo. Proc. Natl. Acad. Sci. USA. 2014;111:5042–5047. doi: 10.1073/pnas.1322494111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bocola M., Schwaneberg U., Krauss U. Light-induced structural changes in a short light, oxygen, voltage (LOV) protein revealed by molecular dynamics simulations—implications for the understanding of LOV photoactivation. Front. Mol. Biosci. 2015;2:55. doi: 10.3389/fmolb.2015.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jurk M., Dorn M., Schmieder P. Blue flickers of hope: secondary structure, dynamics, and putative dimerization interface of the blue-light receptor YtvA from Bacillus subtilis. Biochemistry. 2011;50:8163–8171. doi: 10.1021/bi200782j. [DOI] [PubMed] [Google Scholar]
- 49.Wand A.J. Dynamic activation of protein function: a view emerging from NMR spectroscopy. Nat. Struct. Biol. 2001;8:926–931. doi: 10.1038/nsb1101-926. [DOI] [PubMed] [Google Scholar]
- 50.Henzler-Wildman K., Kern D. Dynamic personalities of proteins. Nature. 2007;450:964–972. doi: 10.1038/nature06522. [DOI] [PubMed] [Google Scholar]
- 51.Endres S., Granzin J., Batra-Safferling R. Structure and function of a short LOV protein from the marine phototrophic bacterium Dinoroseobacter shibae. BMC Microbiology. 2015;15:30. doi: 10.1186/s12866-015-0365-0. [DOI] [PMC free article] [PubMed] [Google Scholar]