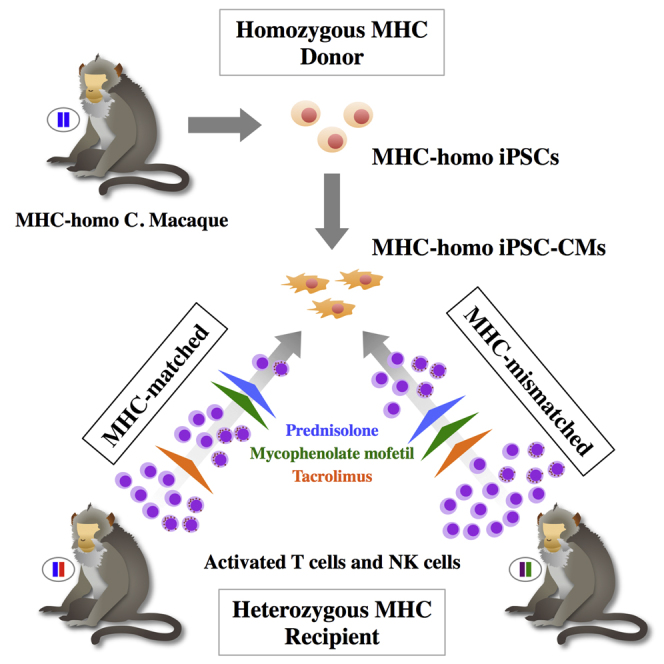
Summary
Induced pluripotent stem cells (iPSCs) can serve as a source of cardiomyocytes (CMs) to treat end-stage heart failure; however, transplantation of genetically dissimilar iPSCs even within species (allogeneic) can induce immune rejection. We hypothesized that this might be limited by matching the major histocompatibility complex (MHC) antigens between the donor and the recipient. We therefore transplanted fluorescence-labeled (GFP) iPSC-CMs donated from a macaque with homozygous MHC haplotypes into the subcutaneous tissue and hearts of macaques having heterozygous MHC haplotypes (MHC-matched; group I) or without identical MHC alleles (group II) in conjunction with immune suppression. Group I displayed a higher GFP intensity and less immune-cell infiltration in the graft than group II. However, MHC-matched transplantation with single or no immune-suppressive drugs still induced a substantial host immune response to the graft. Thus, the immunogenicity of allogeneic iPSC-CMs was reduced by MHC-matched transplantation although a requirement for appropriate immune suppression was retained for successful engraftment.
Graphical Abstract
Highlights
-
•
Cardiomyocytes from iPSCs can treat heart disease
-
•
iPSC-CMs were transplanted into MHC-matched or unmatched cynomolgus macacques
-
•
Matched iPSC-CM grafts had better survival and less host rejection immune response
-
•
Immunosuppression was still required for successful allogeneic iPSC-CM engraftment
In this article, Sawa and colleagues examined whether transplantation of cardiomyocytes generated from induced pluripotent stem cells could alleviate immune-mediated rejection in major histocompatibility antigen-matched non-human primates. Transplantation of matched tissue prevented allogeneic immune rejection and enhanced engraftment with appropriate immunosuppressants. Thus, banked iPSCs with simple MHC profiles might be useful for regenerative therapy for a variety of pathologies.
Introduction
End-stage heart failure is generally characterized by an insufficient number of functional cardiomyocytes (CMs) (Towbin and Bowles, 2002). At this critical stage, cell transplantation is a promising approach for increasing the number of functional CMs. Thus, transplantation with induced pluripotent stem cells (iPSCs) represents a promising treatment for this condition (Yoshida and Yamanaka, 2010, Yoshida and Yamanaka, 2011); accordingly, various studies have examined the potential application of iPSCs for cell transplantation therapy in the heart (Higuchi et al., 2015, Kawamura et al., 2012, Miki et al., 2012).
Cell transplantation therapy using iPSCs theoretically enables autologous transplantation, which could eliminate the need for immunosuppression and avoid related problems such as malignancy and infection. However, the clinical application of this approach is limited by safety concerns and high costs. To overcome the former limitation, banked iPSCs, in which safety has been established in advance, are under development with the aim of transplanting iPSC derivatives in an allogeneic fashion. However, this approach would inevitably induce the host immune response, limiting its therapeutic efficacy in turn.
Several approaches exist to prevent allogeneic cell transplantation-related immune rejection. One is immune suppression therapy using a combination of several different types of immunosuppressants. Others are the use of major histocompatibility complex (MHC)-matched donor cells to reduce immunogenicity, or the suppression of MHC expression via genetic modification. MHC molecules function by binding to pathogen-derived peptide fragments and displaying them on their cell surface for T cell recognition; this process is affected by the high polymorphism of MHC genes. The recognition of non-self MHC molecules causes the rejection of allogeneic organs and tissues (Janeway et al., 2001); therefore, donor/recipient MHC matching can decrease the rate of rejection in organ transplantation (Flomenberg et al., 2004). For these approaches, the establishment of iPSC lines from healthy donors with homozygous MHC alleles is useful for minimizing the number of banked iPSC lines (Nakatsuji et al., 2008, Taylor et al., 2012).
The cynomolgus macaque is a non-human primate that is taxonomically more closely related to humans than other experimental primates. Cynomolgus macaques have a nearly identical genomic organization of the MHC region and drug metabolizing capacity similar to that of humans (Kita et al., 2009, Sano et al., 2006), thus making them a good model for organ transplantation and immunogenicity studies. At least 15 homozygous or semi-homozygous haplotypes (HT1–15) have been identified in a Philippines macaque population (Shiina et al., 2015), with the most frequent haplotype, HT1, detected in 5%–10%.
In this study, we aimed to investigate the possibility of MHC-matched transplantation using this unique colony of primates, available through Ina Research Inc.. We hypothesized that iPSC-derived CMs (iPSC-CMs) with homozygous MHC haplotypes might prevent allogeneic immune rejection during MHC-matched transplantation.
Results
MHC Genotyping
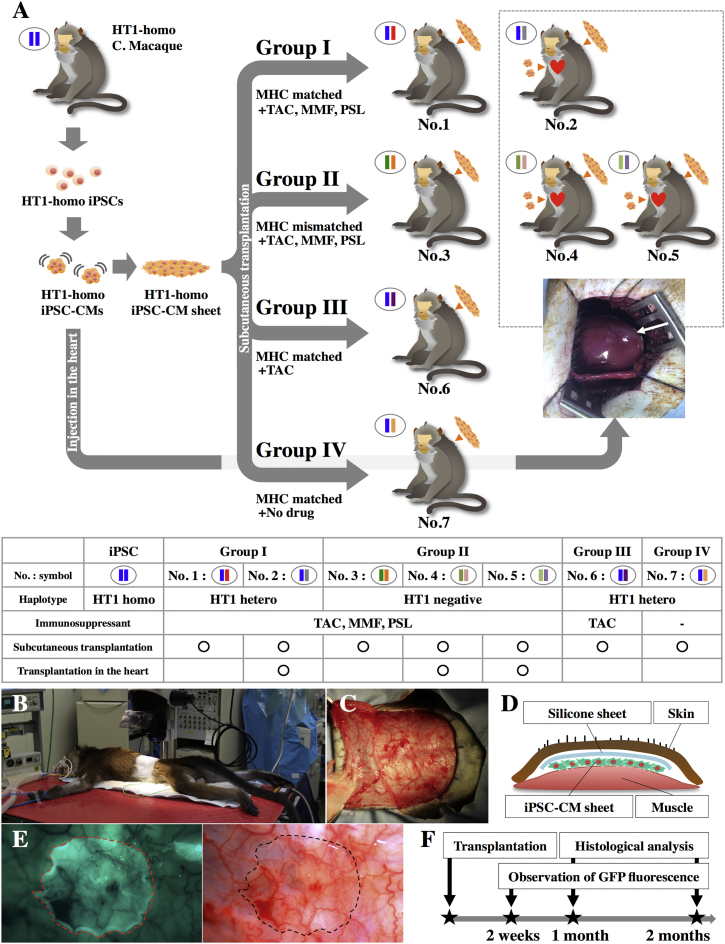
The results of MHC genotyping of iPSCs and seven macaque recipients are described in Table S1. The original macaque supplying the iPSCs expressed only one allele at all MHC gene loci except for the minor allele of A8∗01:01, indicating that it carried a semi-homozygous MHC haplotype (termed HT1). Four macaques (nos. 1, 2, 6, and 7) carried all alleles constituting the HT1 haplotype and were used as MHC-matched recipients. In contrast, animals 3, 4, and 5 had no major HT1 haplotype alleles; these were used as MHC-mismatched recipients (Figure 1A).
Figure 1.
Subcutaneous Transplantation of an iPSC-CM Sheet into Cynomolgus Macaques
(A) Transplantation schema of HT1 homozygous (homo) iPSC-CMs.
(B–D) Schema of subcutaneous transplantation of iPSC-CM sheets into the backs of recipient macaques. Hetero, heterozygous.
(E) Observation of transplanted iPSC-CM sheets expressing GFP.
(F) Follow-up examinations after iPSC-CM sheet transplantation.
Generation of iPSC-CMs
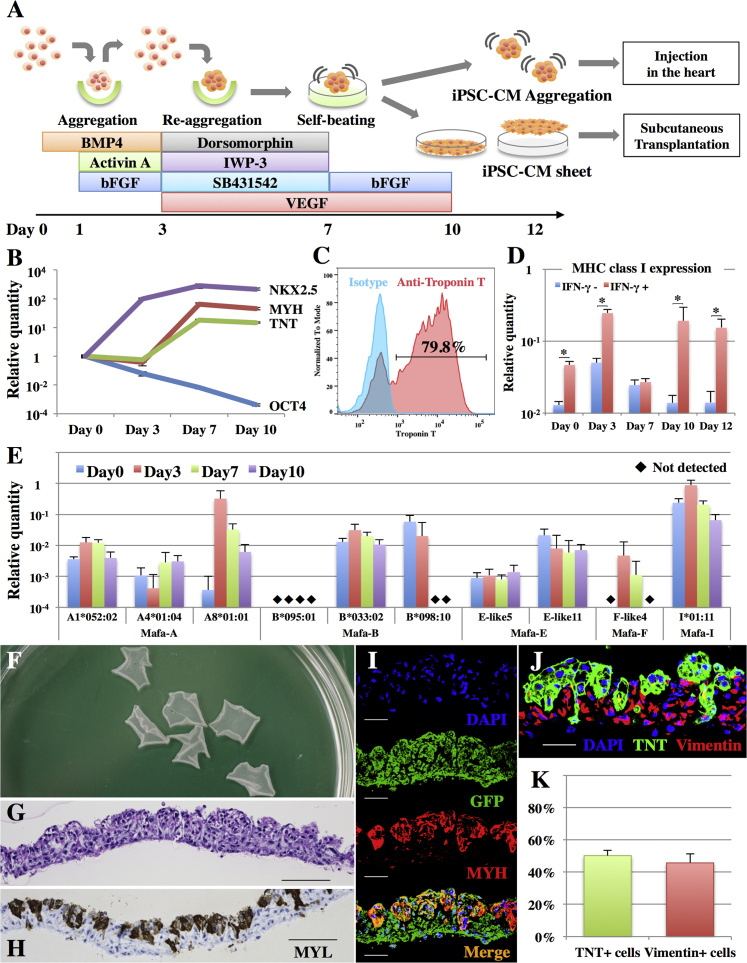
Undifferentiated macaque iPSCs expressed OCT4, TRA-1-60, and SSEA-4 (Figure S1A) and were differentiated to CMs under a protocol using human cytokines and chemicals (Figure 2A), which expressed CM marker genes with decreased OCT4 expression (Figure 2B). Nearly all of the aggregates showed self-beating (Movie S1) at day 10 and exhibited ∼80% purity of troponin T, α actinin, and GFP-positive iPSC-CMs (Figures 2C and S1B).
Figure 2.
Generation of Cynomolgus Macaque iPSC-CM Sheets
(A) Cardiomyogenic differentiation protocol and the generation of iPSC-CM sheets.
(B) Expression of NKX2.5, MYH, TNT, and OCT4 transcripts in iPSCs on days 0, 3, 7, and 10 as analyzed by real-time PCR. Results are relative to those at day 0 and are expressed as the means ±SD (n = 3 independent experiments).
(C) Representative flow cytometry data of iPSC-CMs at day 10, stained with anti-troponin T antibodies or the isotype control.
(D) Expression of MHC class I genes in iPSCs on days 0, 3, 7, 10, and 12 with or without IFN-γ stimulation as analyzed by real-time PCR. Results are relative to those of the peripheral blood and are expressed as the means ±SD (n = 3 independent experiments). ∗p < 0.05.
(E) Expression of MHC class I genes in iPSCs on days 0, 3, 7, and 10 as analyzed by real-time PCR. Mafa-A, B, F, E, and I are the MHC-A, B, F, E, and I genes, respectively, in cynomolgus macaques. The relative quantities of each allele are compared with those of the peripheral blood and are expressed as the means ±SD (n = 3 independent experiments).
(F) Macaque iPSC-CM sheets in a 10-cm dish.
(G) H&E staining of the iPSC-CM sheet. Scale bar, 100 μm.
(H) Immunohistochemistry of MYL in the iPSC-CM sheet. Scale bar, 100 μm.
(I) Immunohistochemistry of GFP (Alexa Fluor 488), MYH (Alexa Fluor 546), and DAPI in the iPSC-CM sheet. Scale bar, 30 μm.
(J) Immunohistochemistry of TNT (Alexa Fluor 488), vimentin (Alexa Fluor 546), and DAPI in the iPSC-CM sheet. Scale bar, 30 μm.
(K) Percentage of TNT- and vimentin-positive cells in the iPSC-CM sheet as analyzed by flow cytometry (representative data are shown in Figure S1C). Results are expressed as the means ±SD (n = 5 independent experiments).
The iPSC-CMs were seeded on temperature-responsive six-well dishes, and iPSC-CM sheets were collected at room temperature just before transplantation. The iPSC-CM sheet showed self-beating (Movie S2) and all cells therein expressed GFP (Figure 2I); approximately half of these cells expressed myosin light chain (MYL; Figure 2H) or myosin heavy chain (MYH; Figure 2I) as a CM marker. Flow cytometry indicated that 50% ± 3% of the cell sheet population was positive for cardiac troponin T and 46% ± 6% was positive for vimentin (n = 5) (Figures 2K and S1C), whereas CD31- or CD144-positive cells were rarely identified (Figure S1C). In addition, the vimentin-positive cells were located on the lower side of the cell sheet, which had attached to the dish (Figure 2J).
Reduced Expression of MHC Genes in iPSC-CMs
The expression of MHC class I genes in the iPSCs and after cardiomyogenic differentiation were relatively lower compared with peripheral blood, although they were significantly increased following in vitro interferon-γ (IFN-γ) stimulation (Figure 2D). Mafa-E (MHC-E) expression was lowest among MHC class I genes (Figure 2E) and MHC class II genes were not expressed at any day of differentiation even following IFN-γ stimulation (data not shown).
Engraftment of iPSC-CMs in MHC-Matched Recipients
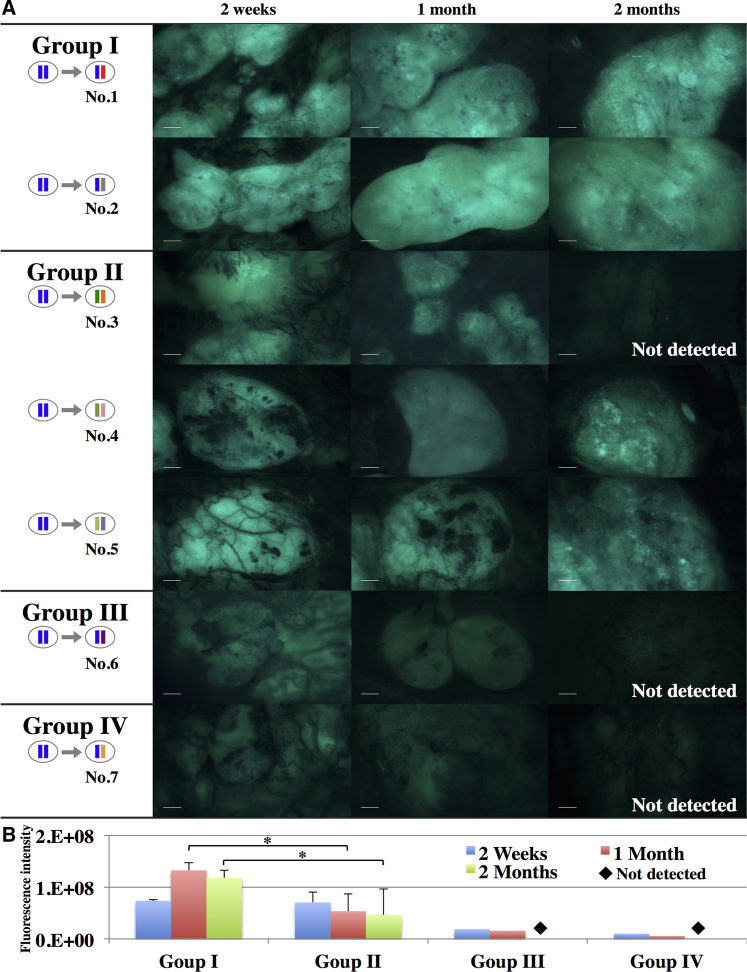
We first subcutaneously transplanted iPSC-CMs sheets into animals 1, 3, 6, and 7 to evaluate iPSC-CM sheet engraftment by quantitative GFP fluorescence evaluation at 2 weeks, 1 month, and 2 months after the transplantation (Figure 3A). GFP fluorescence could be detected in all groups at 2 weeks and 1 month. However, the fluorescence intensity was higher in HT1 heterozygous macaques treated with tacrolimus (TAC), mycophenolate mofetil (MMF), and prednisolone (PSL) (group I) than in the other groups at 1 month (Figure 3B). GFP fluorescence could not be detected at 2 months in recipient 3, which contained no HT1 alleles and was treated with TAC, MMF, and PSL (group II), or in HT1 heterozygous macaques treated with TAC only (group III) and untreated HT1 heterozygous macaques (group IV).
Figure 3.
Engraftment of Subcutaneously Transplanted iPSC-CMs
(A) Images of subcutaneously transplanted iPSC-CMs expressing GFP obtained with a fluorescence stereomicroscope at 2 weeks, 1 month, and 2 months after transplantation. Scale bar, 2 mm.
(B) Quantification of the fluorescence intensity of GFP. Results are expressed as the means ±SD for group I (nos. 1 and 2) and group II (nos. 3–5). ∗p < 0.05.
We next transplanted iPSC-CMs into the heart on the same day but prior to the subcutaneous transplantation in animals 2, 4, and 5 to investigate the engraftment of iPSC-CMs in the heart. The GFP fluorescence intensity in the subcutaneously transplanted graft of animal 2 at 1 or 2 months was higher than that of animal 4 or 5 (Figure 3A). The iPSC-CMs injected into the heart could be detected as GFP-positive cells in recipient 2 (group I) 2 months after the transplantation, but not in recipients 4 and 5 (group II) (Figures S2 and S3). The cells double-positive for GFP and MYH could be detected in recipient 2, although the number was limited (Figure S3).
Immunological Rejection of iPSC-CMs
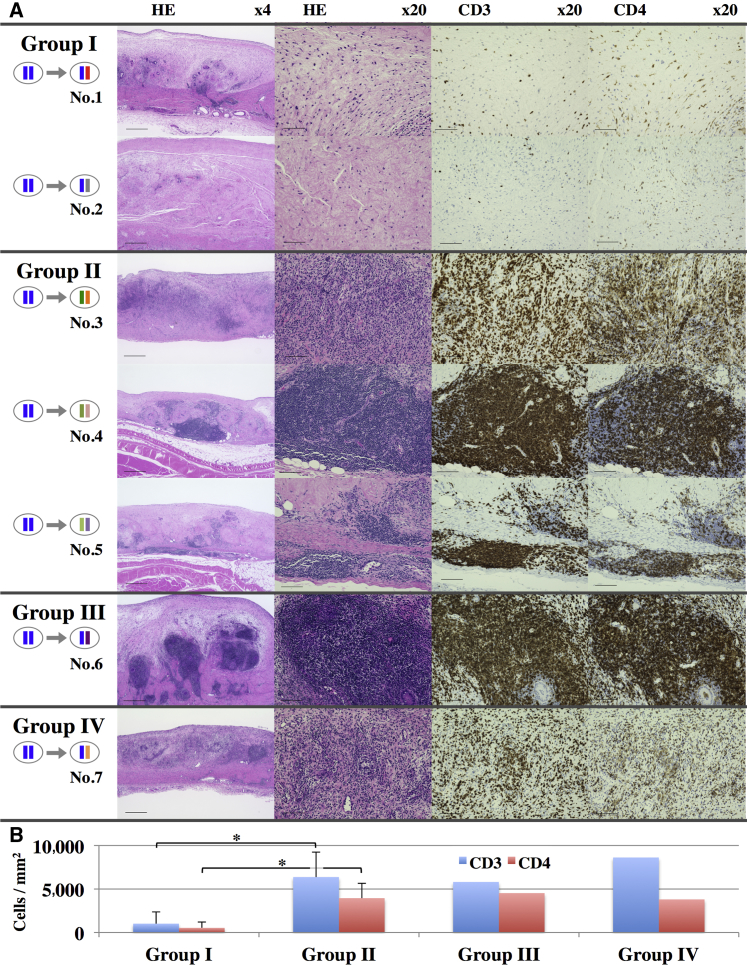
To investigate the immunological rejection of transplanted iPSC-CMs, we histologically compared the subcutaneously transplanted iPSC-CMs (Figure 4) and quantified antibodies against iPSC-CMs in the serum at 1 month after transplantation (Figures S1F–S1I). Although serum levels of immunoglobulin M (IgM) or immunoglobulin G (IgG) against iPSC-CMs were not significantly increased compared with those at day 0, relatively severe infiltration of lymphocytes in the graft was histologically observed for all groups except group I. In detail, diffuse severe lymphocyte infiltration in nearly all parts of the grafts was observed in animals 3 and 7, and relatively localized severe infiltration around the vessels in the graft was observed in animals 4–6. On the other hand, in group I, localized and relatively mild lymphocyte infiltration around the graft vessels was observed in animal 1, and almost none in animal 2. Semi-quantitative scoring for the number of CD3- or CD4-positive cells in the graft showed significantly lower numbers in the grafts of group I (CD3, 1,014/mm2; CD4, 531/mm2) compared with those of group II (CD3, 6,375/mm2; CD4, 3,950/mm2), whereas the values for group III (CD3, 5,810/mm2; CD4, 4,526/mm2) or group IV (CD3, 8,622/mm2; CD4, 3,805/mm2) were markedly higher (Figures 4B and S1D). Furthermore, the expression of interleukin-2 receptor (IL-2R) was decreased in group I grafts compared with the other groups (Figure S1E).
Figure 4.
T Cell-Related Rejection of Transplanted iPSC-CMs
(A) H&E, CD3, or CD4 staining of the harvested iPSC-CM grafts 1 month after transplantation. Scale bars, 500 μm (4×), 100 μm (20×).
(B) Semi-quantitative scoring of the number of CD3- or CD4-positive cells in the graft 1 month after transplantation. The results are shown as the means ±SD for group I (nos. 1 and 2) and group II (nos. 3–5). ∗p < 0.05.
Natural Killer Cell-Related Rejection of iPSC-CMs
The low expression of MHC class I genes, including MHC-E in iPSC-CMs (Figure 2E) might cause natural killer (NK) cell-related immune reactions because HLA-E inhibits the cytotoxic activity of NK cells (Janeway et al., 2001). Therefore, we examined the number of NK cells in the subcutaneously transplanted iPSC-CMs by quantifying the expression of NK cell markers, i.e., immunoglobulin gamma Fc region receptor III (FcγR III) and natural killer group 2, member D (NKG2D). Their expression levels were increased in macaques not treated with immunosuppressants (group IV) or treated with TAC only (group III) compared with HT1 heterozygous macaques treated with TAC, MMF, and PSL (group I) (Figure S1E).
Discussion
In this study, we generated an iPSC line from the cynomolgus macaque with a homozygous MHC HT1 haplotype and obtained iPSC-CM cell sheets in which approximately one half of the cells were troponin T-positive CMs and the remainder were vimentin-positive cells; CD31- or CD144-positive cells were rarely identified. Because vimentin is an intermediate filament expressed in mesenchymal cells such as fibroblasts or endothelial cells, of which CD31 or CD144 is a marker, the vimentin-positive cells in the iPSC-CMs might possibly represent fibroblasts. Using the iPSC-CMs, we provided evidence of the efficacy of MHC-matched allogeneic transplantation in a non-human primate model. Under the same TAC, MMF, and PSL immunosuppressive regimen, grafts in MHC-matched recipients showed significantly increased numbers of engrafted iPSC-CMs compared with those of MHC-mismatched recipients. Consistent with this, less severe infiltration of CD3+ and CD4+ T cells, suggestive of less severe rejection, were observed in MHC-matched recipients. However, all recipients regardless of MHC status exhibited severe rejection without appropriate immunosuppressants. These findings indicated that immunosuppressants in addition to TAC might be required to prevent rejection in MHC-matched iPSC-CM transplantation.
The efficacy and limitations of MHC-matched transplantation are related to the role of MHC in allogeneic cell transplantation. Theoretically, the main mechanism of MHC-matched transplantation is prevention of the host immune reaction through MHC-restricted direct recognition for the donor cells as self by the recipient immunocompetent cells, e.g., T cells. In general, approximately 1%–10% of naive T cells are activated by non-self MHC molecules, but not by self MHC molecules via such a “direct pathway” (Janeway et al., 2001). Therefore, MHC-matched transplantation might decrease the initial population of T cells activated by transplanted cell MHCs by recognizing them as self. In this study, the less severe rejection effected by MHC-matched versus mismatched iPSC-CM transplantation suggested that immune rejection via the direct pathway might have been minimal in the former. In addition, allogeneic iPSC-CMs caused severe rejection in MHC-mismatched transplantation with combined immune suppression within 1 month, even though organ transplantation, including heart, lung, liver, and kidney transplantations, is usually performed in an MHC-mismatched manner without severe rejection under the appropriate immunosuppression therapy. Therefore, MHC-matched transplantation of iPSC-CMs represents a promising approach for clinical applications to avoid immunosuppression-related problems.
In contrast, MHC-matched transplantation might not have substantial effects on the prevention of allogeneic cell transplantation rejection in the indirect pathway, in which the transplanted cell antigens are presented after being phagocytosed and digested by recipient antigen-presenting cells to activate recipient T cells, as observed in animal 6 or 7. This might arise because the peptide antigens would be derived from MHC proteins and other non-self peptides, acting as minor antigens. In this study, GFP might represent one of the minor antigens, as has previously been reported (Stripecke et al., 1999), or non-peptide antigens such as cell surface glycans, which have been reported to be highly expressed in iPSC-CMs (Kawamura et al., 2014, Kawamura et al., 2015), might serve as well. The antigen causing rejection in MHC-matched transplantation will need to be identified in further studies to overcome this limitation of allogeneic iPSC-CM transplantation.
Rejection might also have occurred as iPSC-CMs might fail to suppress recipient NK cells in MHC-matched transplantation. MHC class I molecules such as MHC-E, which are expressed on almost all cells in the body, bind to receptors on NK cells to inhibit cytotoxic activity and, following the immune reaction of NK cells (Janeway et al., 2001), are recognized as self. Since the expression levels of MHC class I genes in iPSC-CMs were low in this study, the grafts might have been recognized as non-self and rejected by recipient NK cells. Consistent with this, MHC-matched transplantation using TAC only or without immune suppressants induced elevated expression of the NK cell markers FcγR III and NKG2D in the grafts (Figure S1E), which might suggest that NK cell-related immune rejection might occur during MHC-matched iPSC-CM transplantation if the recipient did not receive appropriate immunosuppressants.
There were a few limitations to this study. The group sizes in this study were very small, which limits statistical robustness. In addition, we mainly performed subcutaneous transplantation of iPSC-CMs using silicone sheets to evaluate engraftment and rejection by direct observation of the transplanted iPSC-CMs and histological analysis of the grafts. This might not be the optimal location or the appropriate method as clinical applications would be expected to include transplantation to the heart without foreign material, which could provoke the host inflammatory response (Tang and Eaton, 1995) and might have worsened rejection. Notably, the iPSC-CMs transplanted into the heart in this study exhibited poorer survival compared with a previous report (Chong et al., 2014) in which human embryonic stem cell-derived cardiomyocytes were shown to survive for 3 months in the immune-suppressed macaque. This discrepancy might be due to the difference between the healthy heart in this study and the myocardial infarction model in the study of Chong et al., or might result from the different immunosuppressive regimens, both of which are expected to have a significant influence on the survival of transplanted cardiomyocytes. Further study would be needed for the detailed evaluation of immunological rejection and survival of the transplanted iPSC-CMs in disease model hearts according to the clinical applications.
In conclusion, the transplantation of MHC-matched iPSC-CMs was effective for preventing allogeneic immune rejection and allowing engraftment of transplanted cells with appropriate immunosuppressants. The use of homozygous MHC banked iPSC lines might be useful for future regenerative therapy for a variety of pathologies.
Experimental Procedures
Non-human Primates
Seven male cynomolgus macaques (Ina Research) were used in this study. Animal care procedures were consistent with the Guide for the Care and Use of Laboratory Animals (NIH). Experimental protocols were approved by the Ethics Review Committee for Animal Experimentation of Osaka University Graduate School of Medicine (reference no. 25-077-003).
Generation of iPSCs from Cynomolgus Macaques with Homozygous MHC
We used the cynomolgus macaque iPSC line, 1123C1-G (a gift from Professor Yamanaka), generated from an animal with a homozygous MHC haplotype as previously described (Okita et al., 2013). iPSCs were cultured using AK02 medium (Ajinomoto) on an LN511E8-coated (Nippi) dish as previously described (Miyazaki et al., 2012, Nakagawa et al., 2014). The iPSCs were then transfected with GFP as a donor cell marker as previously described (Morizane et al., 2013).
Cardiomyogenic Differentiation of Macaque iPSCs In Vitro
Cardiomyogenic differentiation of macaque iPSCs was performed using a previously described protocol (Miki et al., 2015) with modifications (Figure 2A, Supplemental Experimental Procedures). On day 12, the dish was incubated at room temperature, which induced the cells to detach from the dish forming scaffold-free iPSC-CM sheets.
Transplantation of iPSC-CMs
Transplantation was performed under general anesthesia, with intubation and ventilation using sevoflurane. Macaque iPSC-CM sheets, made from 3.3 × 106 cells per sheet, were subcutaneously transplanted into the backs of recipient animals (three sheets per animal). A 0.5-mm silicone sheet was placed on the iPSC-CM sheet to prevent its adhesion to the skin for repeat observations (Figures 1B–1E). In addition, injection of the iPSC-CMs into the heart was performed for animal 2, 4, and 5. The heart was exposed through left thoracotomy and iPSC-CM aggregations in PBS (approximately 3.3 × 106 cells) were delivered intramyocardially into the anterior wall of the left ventricle using an 18G needle. Needle tips were placed within a preformed mattress suture, which was closed to facilitate cell retention before needle withdrawal.
Immune Suppression
Macaques were divided into four groups as follows (Figure 1A): treated with TAC, MMF, and PSL; treated with TAC only; given no immunosuppressants; and those with no HT1 alleles (MHC-mismatched allogeneic transplantation model) treated with TAC, MMF, and PSL. Oral administration of 2 mg kg−1 day−1 TAC or continuous infusion of 0.5 mg kg−1 day−1 TAC to the femoral vein using an ALZET osmotic pump (DURECT Corp.) was performed from 2 days before transplantation to maintain serum trough levels at >10 ng ml−1 until the end of the study. In addition, 40 mg kg−1 day−1 MMF and 1 mg kg−1 day−1 PSL were orally administered to the appropriate groups from 2 days before the transplantation until the end of the study. Drug dosages were based on clinical uses, as previously reported (Kinugasa et al., 2008, Song et al., 2014).
Quantitatively Observation of Transplanted iPSC-CMs
Transplanted iPSC-CM sheets were observed using a fluorescence stereomicroscope (MVX-10, Olympus) with fixed excitation laser intensity and identical exposure times. The GFP fluorescence intensities in the iPSC-CM sheets were quantified using ImageJ software as the green signal intensities standardized by subtracting the vessel intensities as the background and multiplied by the area. The GFP intensity correlation with cell number was confirmed by observing the two-layer and one-layer sections of the cell sheet on day 0 (Figure S4A).
Author Contributions
T.K. provided study materials, collected and/or assembled the data, and wrote the manuscript; Shigeru M. provided financial support, and analyzed and interpreted the data; S.F., A.M., N.K., A.K., K.M., K. Okita, Y.Y., K.T., and H.O. analyzed and interpreted the data; T.S., K. Ogasawara, and Shuji M. conceived and designed the study, collected and/or assembled the data, and wrote the manuscript; Y.S. conceived and designed the study and provided financial support.
Acknowledgments
We thank Professor Shinya Yamanaka of the Center for iPS Cell Research and Application, Kyoto University, who kindly provided the cynomolgus macaque iPSCs. We also thank Seiko Eiraku, Akima Harada, and Hiromi Nishinaka for their technical support. This study was supported by the Japan Science and Technology Agency as a part of the project, Center for the Development of Myocardial Regenerative Treatments Using iPS Cells.
Published: February 18, 2016
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Supplemental Information includes Supplemental Experimental Procedures, four figures, two tables, and two movies and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2016.01.012.
Supplemental Information
References
- Chong J.J., Yang X., Don C.W., Minami E., Liu Y.W., Weyers J.J., Mahoney W.M., Van Biber B., Palpant N.J., Gantz J.A. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510:273–277. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flomenberg N., Baxter-Lowe L.A., Confer D., Fernandez-Vina M., Filipovich A., Horowitz M., Hurley C., Kollman C., Anasetti C., Noreen H. Impact of HLA class I and class II high-resolution matching on outcomes of unrelated donor bone marrow transplantation: HLA-C mismatching is associated with a strong adverse effect on transplantation outcome. Blood. 2004;104:1923–1930. doi: 10.1182/blood-2004-03-0803. [DOI] [PubMed] [Google Scholar]
- Higuchi T., Miyagawa S., Pearson J.T., Fukushima S., Saito A., Tsuchimochi H., Sonobe T., Fujii Y., Yagi N., Astolfo A. Functional and electrical integration of induced pluripotent stem cell-derived cardiomyocytes in a myocardial infarction rat heart. Cell Transplant. 2015;24:2479–2489. doi: 10.3727/096368914X685799. [DOI] [PubMed] [Google Scholar]
- Janeway C.A., Jr., Travers P., Walport M., Shlomchik M.J. Fifth edition. Garland Science; 2001. Immunobiology: The Immune System in Health and Disease. [Google Scholar]
- Kawamura M., Miyagawa S., Miki K., Saito A., Fukushima S., Higuchi T., Kawamura T., Kuratani T., Daimon T., Shimizu T. Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation. 2012;126:S29–S37. doi: 10.1161/CIRCULATIONAHA.111.084343. [DOI] [PubMed] [Google Scholar]
- Kawamura T., Miyagawa S., Fukushima S., Yoshida A., Kashiyama N., Kawamura A., Ito E., Saito A., Maeda A., Eguchi H. N-Glycans: phenotypic homology and structural differences between myocardial cells and induced pluripotent stem cell-derived cardiomyocytes. PLoS One. 2014;9:e111064. doi: 10.1371/journal.pone.0111064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura T., Miyagawa S., Fukushima S., Kashiyama N., Kawamura A., Ito E., Saito A., Maeda A., Eguchi H., Toda K. Structural changes in N-Glycans on induced pluripotent stem cells differentiating toward cardiomyocytes. Stem Cells Transl. Med. 2015;4:1258–1264. doi: 10.5966/sctm.2015-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinugasa F., Nagatomi I., Ishikawa H., Nakanishi T., Maeda M., Hirose J., Fukahori H., Ooshima S., Noto T., Higashi Y. Efficacy of oral treatment with tacrolimus in the renal transplant model in cynomolgus monkeys. J. Pharmacol. Sci. 2008;108:529–534. doi: 10.1254/jphs.08142fp. [DOI] [PubMed] [Google Scholar]
- Kita Y.F., Hosomichi K., Kohara S., Itoh Y., Ogasawara K., Tsuchiya H., Torii R., Inoko H., Blancher A., Kulski J.K. MHC class I A loci polymorphism and diversity in three Southeast Asian populations of cynomolgus macaque. Immunogenetics. 2009;61:635–648. doi: 10.1007/s00251-009-0390-y. [DOI] [PubMed] [Google Scholar]
- Miki K., Uenaka H., Saito A., Miyagawa S., Sakaguchi T., Higuchi T., Shimizu T., Okano T., Yamanaka S., Sawa Y. Bioengineered myocardium derived from induced pluripotent stem cells improves cardiac function and attenuates cardiac remodeling following chronic myocardial infarction in rats. Stem Cells Transl. Med. 2012;1:430–437. doi: 10.5966/sctm.2011-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki K., Endo K., Takahashi S., Funakoshi S., Takei I., Katayama S., Toyoda T., Kotaka M., Takaki T., Umeda M. Efficient detection and purification of cell populations using synthetic MicroRNA switches. Cell Stem Cell. 2015;16:699–711. doi: 10.1016/j.stem.2015.04.005. [DOI] [PubMed] [Google Scholar]
- Miyazaki T., Futaki S., Suemori H., Taniguchi Y., Yamada M., Kawasaki M., Hayashi M., Kumagai H., Nakatsuji N., Sekiguchi K. Laminin E8 fragments support efficient adhesion and expansion of dissociated human pluripotent stem cells. Nat. Commun. 2012;3:1236. doi: 10.1038/ncomms2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morizane A., Doi D., Kikuchi T., Okita K., Hotta A., Kawasaki T., Hayashi T., Onoe H., Shiina T., Yamanaka S. Direct comparison of autologous and allogeneic transplantation of iPSC-derived neural cells in the brain of a nonhuman primate. Stem Cell Rep. 2013;1:283–292. doi: 10.1016/j.stemcr.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa M., Taniguchi Y., Senda S., Takizawa N., Ichisaka T., Asano K., Morizane A., Doi D., Takahashi J., Nishizawa M. A novel efficient feeder-free culture system for the derivation of human induced pluripotent stem cells. Sci. Rep. 2014;4:3594. doi: 10.1038/srep03594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuji N., Nakajima F., Tokunaga K. HLA-haplotype banking and iPS cells. Nat. Biotechnol. 2008;26:739–740. doi: 10.1038/nbt0708-739. [DOI] [PubMed] [Google Scholar]
- Okita K., Yamakawa T., Matsumura Y., Sato Y., Amano N., Watanabe A., Goshima N., Yamanaka S. An efficient nonviral method to generate integration-free human-induced pluripotent stem cells from cord blood and peripheral blood cells. Stem Cells. 2013;31:458–466. doi: 10.1002/stem.1293. [DOI] [PubMed] [Google Scholar]
- Sano K., Shiina T., Kohara S., Yanagiya K., Hosomichi K., Shimizu S., Anzai T., Watanabe A., Ogasawara K., Torii R. Novel cynomolgus macaque MHC-DPB1 polymorphisms in three South-East Asian populations. Tissue Antigens. 2006;67:297–306. doi: 10.1111/j.1399-0039.2006.00577.x. [DOI] [PubMed] [Google Scholar]
- Shiina T., Yamada Y., Aarnink A., Suzuki S., Masuya A., Ito S., Ido D., Yamanaka H., Iwatani C., Tsuchiya H. Discovery of novel MHC-class I alleles and haplotypes in Filipino cynomolgus macaques (Macaca fascicularis) by pyrosequencing and Sanger sequencing: Mafa-class I polymorphism. Immunogenetics. 2015;67:563–578. doi: 10.1007/s00251-015-0867-9. [DOI] [PubMed] [Google Scholar]
- Song L., Ma A., Dun H., Hu Y., Zeng L., Bai J., Zhang G., Kinugasa F., Sudo Y., Miyao Y. Effects of ASKP1240 combined with tacrolimus or mycophenolate mofetil on renal allograft survival in Cynomolgus monkeys. Transplantation. 2014;98:267–276. doi: 10.1097/TP.0000000000000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stripecke R., Carmen Villacres M., Skelton D., Satake N., Halene S., Kohn D. Immune response to green fluorescent protein: implications for gene therapy. Gene Ther. 1999;6:1305–1312. doi: 10.1038/sj.gt.3300951. [DOI] [PubMed] [Google Scholar]
- Tang L., Eaton J.W. Inflammatory responses to biomaterials. Am. J. Clin. Pathol. 1995;103:466–471. doi: 10.1093/ajcp/103.4.466. [DOI] [PubMed] [Google Scholar]
- Taylor C.J., Peacock S., Chaudhry A.N., Bradley J.A., Bolton E.M. Generating an iPSC bank for HLA-matched tissue transplantation based on known donor and recipient HLA types. Cell Stem Cell. 2012;11:147–152. doi: 10.1016/j.stem.2012.07.014. [DOI] [PubMed] [Google Scholar]
- Towbin J.A., Bowles N.E. The failing heart. Nature. 2002;415:227–233. doi: 10.1038/415227a. [DOI] [PubMed] [Google Scholar]
- Yoshida Y., Yamanaka S. Recent stem cell advances: induced pluripotent stem cells for disease modeling and stem cell-based regeneration. Circulation. 2010;122:80–87. doi: 10.1161/CIRCULATIONAHA.109.881433. [DOI] [PubMed] [Google Scholar]
- Yoshida Y., Yamanaka S. iPS cells: a source of cardiac regeneration. J. Mol. Cell Cardiol. 2011;50:327–332. doi: 10.1016/j.yjmcc.2010.10.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.