Summary
Heterozygous mutations in the glucocerebrosidase gene (GBA) represent the strongest common genetic risk factor for Parkinson's disease (PD), the second most common neurodegenerative disorder. However, the molecular mechanisms underlying this association are still poorly understood. Here, we have analyzed ten independent induced pluripotent stem cell (iPSC) lines from three controls and three unrelated PD patients heterozygous for the GBA-N370S mutation, and identified relevant disease mechanisms. After differentiation into dopaminergic neurons, we observed misprocessing of mutant glucocerebrosidase protein in the ER, associated with activation of ER stress and abnormal cellular lipid profiles. Furthermore, we observed autophagic perturbations and an enlargement of the lysosomal compartment specifically in dopamine neurons. Finally, we found increased extracellular α-synuclein in patient-derived neuronal culture medium, which was not associated with exosomes. Overall, ER stress, autophagic/lysosomal perturbations, and elevated extracellular α-synuclein likely represent critical early cellular phenotypes of PD, which might offer multiple therapeutic targets.
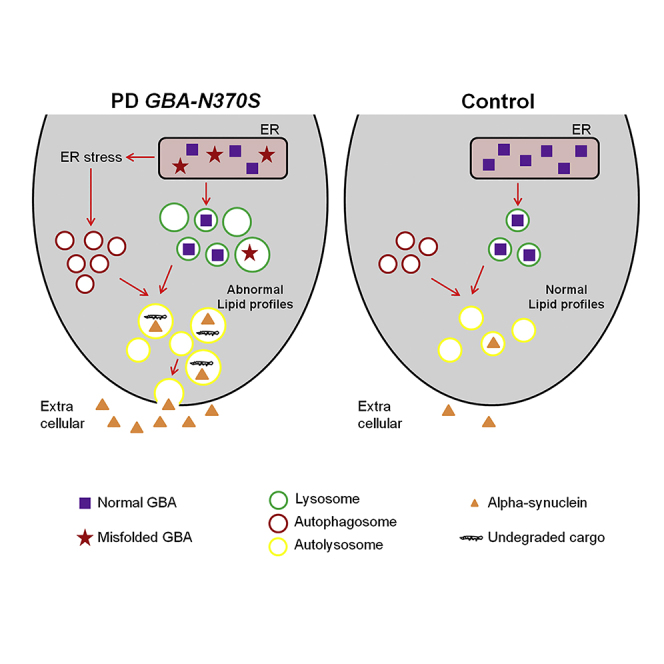
Graphical Abstract
Highlights
-
•
Functional analysis of dopamine neurons from 5 PD GBA-N370S and 5 control iPSC lines
-
•
Perturbed lipid profiles, ER stress, and autophagy in iPSC PD-N370S dopamine neurons
-
•
Enlarged and impaired lysosomal compartment in iPSC PD-N370S dopamine neurons
-
•
Increased extracellular α-synuclein in iPSC PD-N370S dopamine neuronal cultures
In this article, Wade-Martins and colleagues show that in dopamine neurons from Parkinson's disease patients, the GBA-N370S mutation leads to the misprocessing of GCase, increased ER stress, and abnormal lipid profiles. Further, the GBA-N370S mutation impairs autophagic and lysosomal function, ultimately leading to increased α-synuclein release in dopaminergic neuronal cultures, which may be central in the early pathogenesis of Parkinson's disease.
Introduction
Parkinson's disease (PD) is the second most common neurodegenerative disorder, characterized by the preferential degeneration of dopamine neurons in the substantia nigra pars compacta (SNpc). Heterozygous mutations in the glucocerebrosidase gene (GBA) encoding the lysosomal enzyme GCase represent the strongest common genetic risk factor for PD (Sidransky et al., 2009) and have also been associated with other related Lewy body disorders (Goker-Alpan et al., 2006); however, the underlying molecular mechanisms are still poorly understood.
The association of GBA with PD first emerged from clinical studies that demonstrated that relatives of patients with Gaucher's disease (GD), a lysosomal storage disease caused by homozygous GBA mutations, had an increased incidence of PD (Goker-Alpan et al., 2004). More recent studies exploring the pathogenic role of homozygous GBA mutations in GD have highlighted a role of GCase in mitochondria function, α-synuclein aggregation, and autophagic machinery (Mazzulli et al., 2011, Sardi et al., 2011). GCase pathology in the context of heterozygous GBA mutations in PD have been addressed in recent postmortem (Gegg et al., 2012) and patient fibroblast (McNeill et al., 2014) studies, and recently in a PD patient human neuronal model (Schöndorf et al., 2014). Overall, mutations in GBA and in LRRK2, also known to play a role in regulating autophagy (Alegre-Abarrategui et al., 2009), have emphasized the role for the autophagic/lysosomal pathway as central to the pathogenesis of PD (Tofaris, 2012).
Human induced pluripotent stem cells (iPSCs) derived from patients carrying disease-associated alleles retain the genetic background likely to include disease-permissive genetic modifiers and can be differentiated into highly physiological models of specific cell types to study cellular mechanisms of disease (Kiskinis and Eggan, 2010). Within this paradigm, iPSCs can be differentiated into functional midbrain dopamine neurons to provide a powerful tool to study the genetic contribution to PD (Hartfield et al., 2012). We have previously developed a highly physiological cellular model of differentiated midbrain dopamine neurons that express key dopaminergic markers, exhibit dopamine synthesis, release, and re-uptake, and show autonomous pace-making and spontaneous synaptic activity (Hartfield et al., 2014).
Here we study the impact of the common GBA-N370S mutation on the phenotype of patient-specific dopaminergic neuronal cultures differentiated from iPSC lines derived from patients with PD and identify deficits in protein homeostasis. Our results suggest that the heterozygous GBA-N370S mutation leads to a cellular gain of function, shown by misprocessing of misfolded GCase protein in the ER resulting in ER stress upregulation and autophagic/lysosomal dysfunction leading to an enlargement of the lysosomal compartment in individually identified vulnerable dopamine neurons. Together, these deficits lead to increased release of extracellular α-synuclein from GBA-N370S PD human iPSC-derived dopamine neuron cultures, which we show is not associated with exosomes. We propose that the combination of these disturbances impairs protein homeostasis in dopamine neurons contributing to their preferential vulnerability in PD. These data highlight the early pathogenic relevance of GCase function in the autophagic/lysosomal pathway in PD, and may explain the higher risk for heterozygous GBA-N370S mutation carriers to develop PD.
Results
Generation of Human iPSCs
PD patients and controls from the Discovery clinical cohort established by the Oxford Parkinson's Disease Centre (OPDC) were screened for the presence of GBA-N370S heterozygous mutation (Figure S1A). We then generated and characterized 22 human iPSC clonal lines from fibroblasts obtained from three unrelated PD patients carrying a GBA-N370S heterozygous mutation and three healthy control individuals (Table S1). Detailed characterization of all PD lines used in this study is shown in Figures S1 and S2. Characterization of lines from two of the control individuals has been published previously (Control-2, van Wilgenburg et al., 2013; Control-1, Hartfield et al., 2014). All iPSC lines displayed embryonic stem cell-like morphology and expressed pluripotency-associated proteins (TRA-1-60, SSEA-4, and Nanog; Figure S1B). Silencing of retroviral transgenes upon establishment of pluripotency was confirmed by qRT-PCR (Figure S1C), and Sendai virus-reprogrammed lines were checked for clearance of the exogenous genes by RT-PCR (Figure S1D). Pluripotency was also assessed using the PluriTest, which is based upon analysis of transcriptome data from iPSCs and human embryonic stem cells and compares gene expression with a large reference set of genome-wide profiles from multiple cell and tissue types (Müller et al., 2011). Accordingly, all lines used were classified as fully reprogrammed and pluripotent (Figure S1E). Genome integrity was confirmed by Illumina SNP arrays (Figure S2) providing detailed resolution of genome integrity compared with traditional karyotyping or M-FISH. The SNP datasets also enabled confirmation that the iPSC lines derived from the expected fibroblast line. The SNP datasets and the Illumina HT12v4 transcriptome array results have been deposited in GEO: GSE53426.
Characterization of Dopaminergic Neuronal Cultures
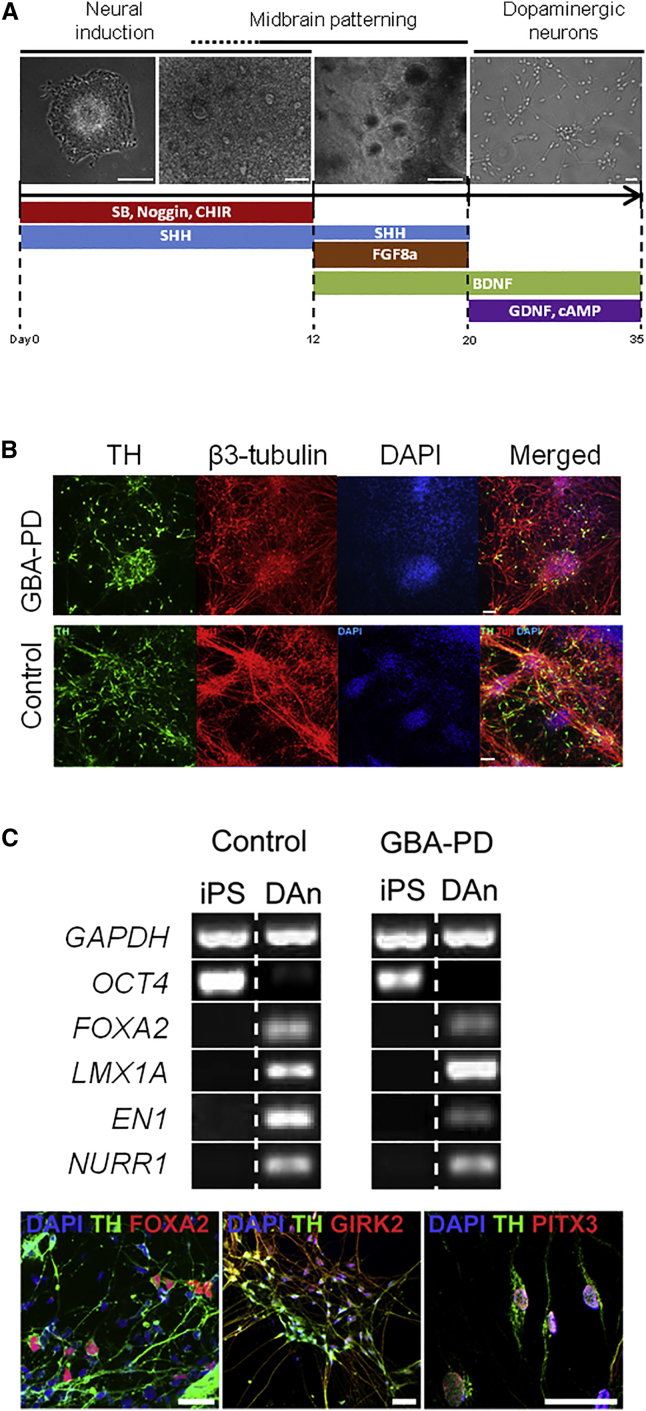
In order to study the effect of the heterozygous GBA-N370S mutation in the context of PD, iPSCs were differentiated into dopaminergic neuronal cultures (Figure 1A). A total of ten different iPSC clonal lines from three control individuals and three PD patients carrying a GBA-N370S heterozygous mutation were differentiated into dopaminergic neuronal cultures, as described previously (Hartfield et al., 2014), with minor modifications. Briefly, embryoid bodies prepared from iPSCs were plated in the presence of SMAD signaling inhibitors (Noggin and SB431542) to initiate neuronal induction, together with CHIR99021 (a potent canonical WNT signaling activator), sonic hedgehog (SHH), and FGF8a for midbrain floor plate induction. By day 20, visible neural rosette structures were manually dissected and re-plated for differentiation into dopaminergic neurons with ascorbic acid, cAMP, brain-derived neurotrophic factor, and glial cell line-derived neurotrophic factor for 15 days. All lines successfully differentiated into dopaminergic neuronal cultures (Figure 1B). The differentiation efficiency, assessed by β-3 tubulin and TH expression using immunofluorescence, was similar across the genotypes used in the study (Figure S3). RT-PCR analysis demonstrated that differentiated dopaminergic cultures expressed the floor plate markers FOXA2 and LMX1A, in addition to the mature dopaminergic neuron markers EN1 and NURR1, while the level of the pluripotency marker OCT4 was much reduced on differentiation (Figure 1C). We confirmed the co-expression of TH with the floorplate marker FOXA2, the marker of the A9 dopaminergic neurons susceptible in PD GIRK2, and the post-mitotic midbrain neuronal marker PITX3, further confirming the mature midbrain identity of the differentiated dopaminergic neurons (Figure 1C). We have previously confirmed the highly mature, functional dopaminergic phenotype in neurons derived from iPSCs using this protocol (Hartfield et al., 2014). Neurons exhibit the correct pharmacological and physiological characteristics expected of mature dopamine neurons, including expression of a wide range of midbrain markers, dopamine synthesis and uptake, spontaneous slow autonomous Ca2+ pace-making activity, and spontaneous synaptic activity at 5–10 Hz, typical of dopamine neurons of the substantia nigra pars compacta (Hartfield et al., 2014).
Figure 1.
Generation and Characterization of iPSC-Derived Dopaminergic Neuronal Cultures
(A) Schematic overview of conditions used for differentiation of iPSCs into dopaminergic neuronal cultures, with bright field representations of cells at several stages of differentiation (scale bar, 50 μm).
(B) Immunohistochemistry representation of differentiated dopaminergic neuronal cultures at day 35 showing high expression levels for the neuronal marker β-3 tubulin (red), the dopaminergic neuron marker TH (green), and DAPI (blue).
(C) RT-PCR analysis showed reduced expression of the pluripotency marker OCT4 and increased expression of floor plate markers (FOXA2 and LMX1A) and mature dopaminergic markers (EN1 and NURR1) in differentiated dopaminergic neuronal cultures (DAn) versus undifferentiated (iPS). Immunofluorescence analyses also confirmed the co-expression of TH with FOXA1, GIRK2, and PITX3 (scale bar, 50 μm). Results are representative of at least three independent differentiation experiments performed in triplicate per cell line.
See also Figures S1–S3.
GBA-N370S Leads to Aberrant GCase Post-translation Modification and Abnormal Lipid Profiles
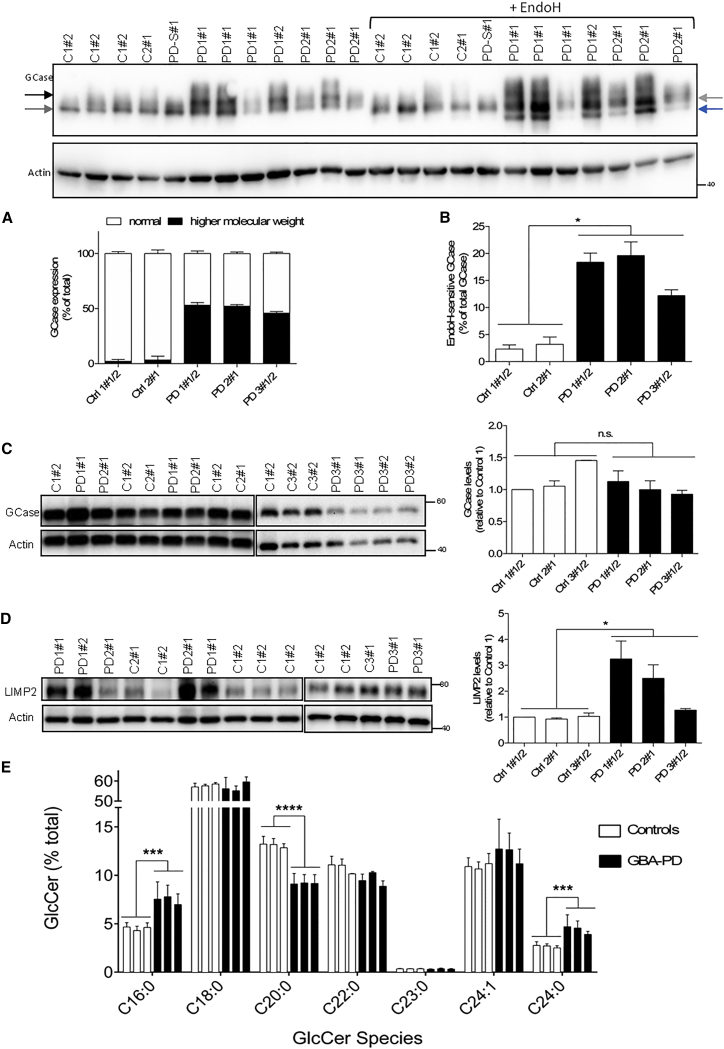
Once we had established a human dopaminergic neuronal culture model consistent between different lines from multiple individuals, we next determined the effect of the heterozygous GBA-N370S mutation on GCase protein level in the GBA-N370S PD lines. In addition to the expected 60 kDa isoform corresponding to mature GCase, we observed an extra isoform of apparently higher molecular weight only in dopaminergic neuronal cultures derived from GBA-N370S PD patients, which represented up to 50% of total GCase in these cultures (Figure 2A). This N370S-specific isoform was sensitive to treatment with EndoH, which yielded a third, lower molecular weight isoform (Figure 2B). This demonstrates that a proportion of GCase in the N370S GBA cell lines had retained high-mannose oligosaccharides, indicating failure to be processed in the Golgi, likely because of retention in the ER. In GBA-N370S-derived dopaminergic neuronal cultures, this EndoH-sensitive GCase isoform was significantly more abundant, representing around 20% of total GCase (Figure 2B). Samples were further treated with PNGase F to remove both high-mannose and complex N-linked glycans, and under these conditions no significant differences were observed in GCase protein levels between N370S mutant and control dopaminergic neuronal cultures (Figure 2C). Overall, these results indicate that the GBA-N370S mutation likely results in retention in the ER as a result of disrupting the structure and/or folding of the protein, without affecting the total amount of GCase.
Figure 2.
Aberrant GCase Post-translation Modification in Heterozygous GBA-N370S PD Dopaminergic Neuronal Cultures
Representative western blot for differentiated dopaminergic neuronal cultures demonstrates the presence of two isoforms (gray and black arrows) for GCase protein specific for GBA-N370S cultures.
(A) Quantification of GCase isoforms by western blot (as percentage of total GCase) confirms the higher molecular weight isoform (black bars) to be specific to GBA-N370S dopaminergic neuronal cultures, representing up to 50% of total GCase levels.
(B) The GCase top isoform was sensitive to EndoH treatment shifting to a lower size band (blue arrow). Quantification of the EndoH-sensitive band represented as a percentage of total GCase for the respective line. Student's t test; ∗p < 0.0001. PD-S#1 corresponds to a sporadic PD sample run but not analyzed further in this study.
(C) No change of total GCase expression levels after PNGase F treatment. Representative western blot and quantification of GCase is shown. Student's t test; not significant.
(D) Increased LIMP2 protein levels in heterozygous GBA-N370S PD dopaminergic cultures. Student's t test; ∗p < 0.005. In each case, data represent the mean ± SEM from performing at least three independent differentiation experiments per line each analyzed in triplicate.
(E) Analysis of different GlcCer species (C16:0, C18:0, C20:0, C22:0, C23:0, C24:0, C24:1, C24:0) as a percentage of total GlcCer shows a difference in the distribution of GlcCer species in GBA-N370S PD dopaminergic cultures compared with controls. Each bar represents the mean ± SEM of independent differentiated lines: three control and three GBA-N370S PD done in triplicate (n = 3). Student's t test; ∗∗∗p < 0.005; ∗∗∗∗p < 0.0001.
See also Figure S4A.
LIMP2 is a GCase-specific lysosomal receptor (Reczek et al., 2007), which has been associated with PD risk (Do et al., 2011). In dopaminergic neuronal cultures derived from GBA-N370S PD patients, we observed increased LIMP2 protein expression compared with controls, although there was inter-individual variation between the GBA-N370S PD lines (Figure 2D). This result supports a role for LIMP2 in trafficking ER-retained mutant GCase from the ER to the lysosome (Reczek et al., 2007).
We next quantified the GCase substrate lipids in GBA-N370S PD dopaminergic cultures. No GlcCer accumulation was observed in patient cells, as measured by mass spectrometry quantification of individual species normalized to the ceramide precursor (Figure S4A). Notably, the distribution of GlcCer species was markedly different in GBA-N370S PD dopaminergic cultures compared with controls, with a 30% reduction for C20:0 GlcCer and a ∼65% increase of C16:0 and C24:0 species (Figure 2E). Overall, these data indicate that the GBA-N370S heterozygous mutation results in abnormal lipid profiles not previously reported.
ER Stress and Autophagic Disturbances in GBA-N370S Dopaminergic Neuronal Cultures
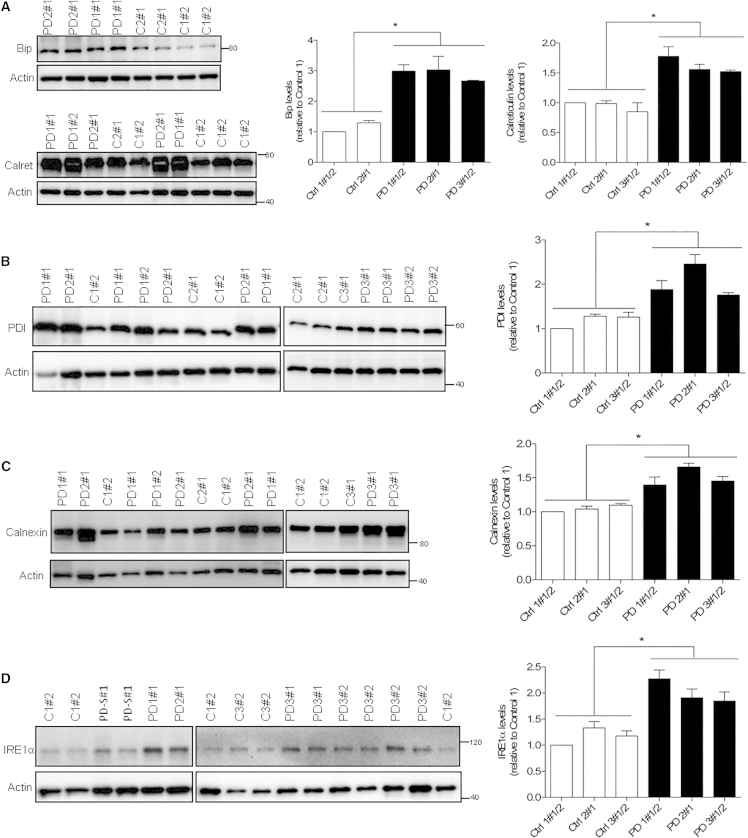
Accumulation of misfolded proteins in the ER can overload the cellular capacity for protein re-folding and induce ER stress, leading to activation of the unfolded protein response (UPR) (Høyer-Hansen and Jäättelä, 2007). Analysis of the two ER-resident chaperones, Bip/GRP78 and calreticulin, revealed significant upregulation in GBA-N370S dopaminergic neuronal cultures when compared with control-derived dopaminergic cultures (Figure 3A). Upregulation of additional UPR mediators, PDI, calnexin, and IRE1alpha, confirmed UPR activation (Figures 3B–3D), although cleavage of XBP1 mRNA was not observed (Figure S4B). XBP1 splicing might not be a crucial transducer in this context as it has been previously detected in the brain in only one-third of GBA mutant PD patients (Gegg et al., 2012).
Figure 3.
ER Stress Upregulation in Heterozygous GBA-N370S Dopaminergic Neuronal Cultures
(A) Representative western blot and quantification shows increased protein levels of the ER chaperones Bip and calreticulin.
(B–D) Representative western blot and quantification of expression of further ER stress markers, namely PDI (B), calnexin (C), and IRE1α (D).
In each case, data represent the mean ± SEM from performing at least three independent differentiation experiments per line each analyzed in triplicate. Student's t test; ∗p < 0.0001. PD-S#1 corresponds to a sporadic PD sample run but not analyzed further in this study.
See also Figure S4B.
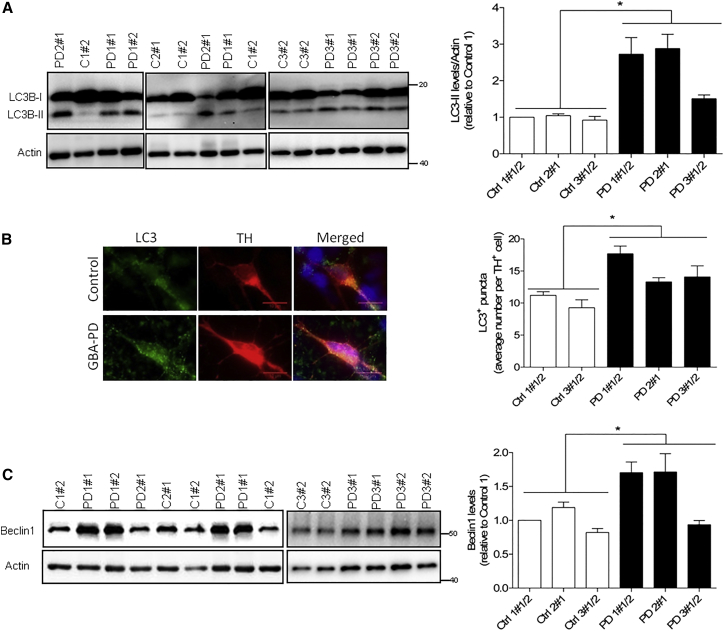
Since ER stress can result in autophagic disturbances (Yorimitsu et al., 2006) through the accumulation of misfolded proteins (Høyer-Hansen and Jäättelä, 2007), we examined the autophagosome content in our cultures by quantifying the levels of LC3B-II and LC3 puncta (Klionsky et al., 2012). LC3B-II, the lipidated form of the autophagosome marker LC3/Atg8, was significantly increased in GBA-N370S-derived dopaminergic neuronal cultures reflecting increased levels of autophagosomes (Figure 4A). This increase in LC3B-II was supported by immunofluorescence analysis that confirmed an increased number of LC3+ puncta in dopamine neurons in GBA-N370S compared with controls (Figure 4B). Furthermore, beclin1, a key regulator of autophagy used to study autophagosome formation, was found increased in GBA-N370S dopaminergic neuronal cultures from two patients, consistent with an autophagic perturbation (Figure 4C).
Figure 4.
Autophagic Disturbances in Heterozygous GBA-N370S PD Dopaminergic Neuronal Cultures
(A) Representative western blot analysis and quantification showing increased LC3B-II protein levels. Student's t test; ∗p < 0.0001.
(B) Increased number of LC3+ puncta in TH+ cells. Immunofluorescence staining for LC3B (green) in dopaminergic TH-positive neurons (red) in control and PD patient dopaminergic neuronal cultures (scale bar, 20 μm). Student's t test; ∗p < 0.0001.
(C) Representative western blot and quantification of Beclin1 protein levels in differentiated dopaminergic cultures. Student's t test; ∗p < 0.05. In each case, data represent the mean ± SEM from performing at least three independent differentiation experiments per line each analyzed in triplicate.
Impaired Lysosomal Degradation in GBA-N370S Dopaminergic Neurons
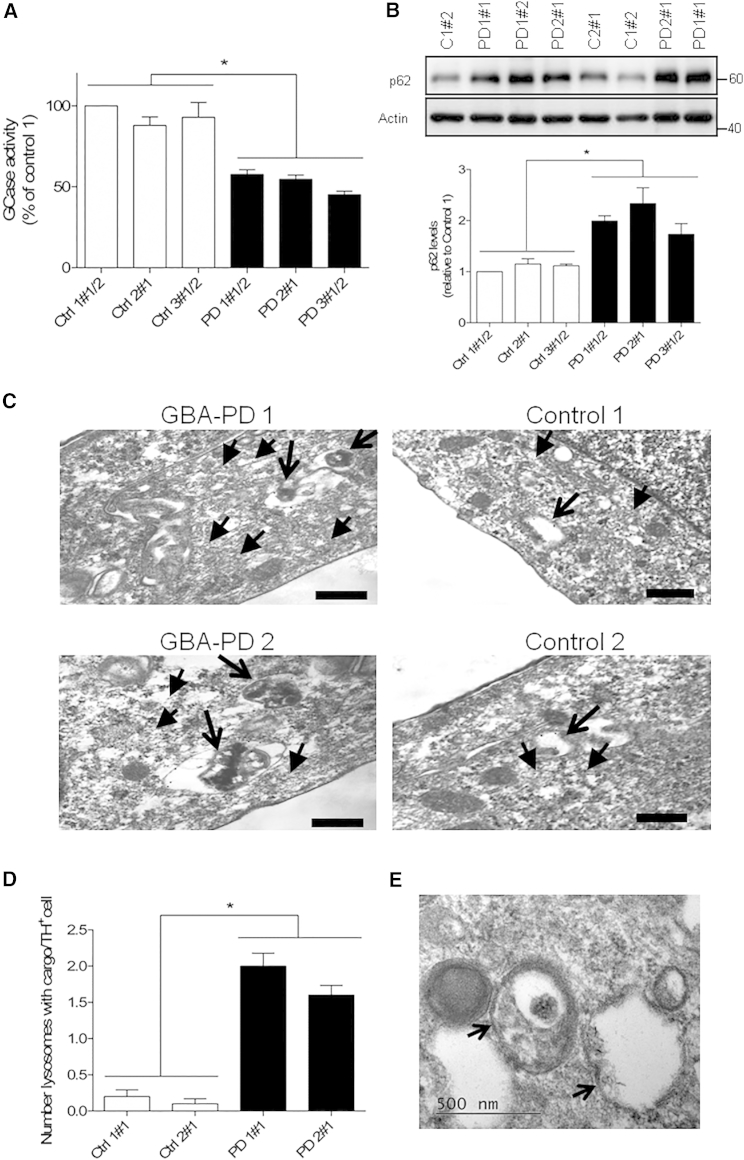
Accumulation of autophagosomes can also reflect a defect in lysosomal clearance (Klionsky et al., 2012). Since the GBA-N370S mutation is located in the catalytic domain of GCase (Wei et al., 2011), we determined the impact of the heterozygous GBA-N370S mutation on the activity of this lysosomal enzyme. Dopaminergic neuronal cultures from GBA-N370S PD patients had significantly reduced GCase activity (∼50% decrease) when compared with control individuals (Figure 5A). Since reduced GCase activity could impair the overall lysosomal degradation capacity of dopamine neurons, we analyzed the levels of p62/SQSTM1 that reflect autophagic-mediated degradation (Klionsky et al., 2012). p62 protein levels were increased specifically in GBA-N370S dopaminergic neuronal cultures (Figure 5B), which supports a defect of these cells in the clearance of autophagic vacuoles. To follow up the findings from GBA-N370S cultures, we next compared GBA-N370S and control dopamine neurons for ultrastructural alterations by electron microscopy (EM), specifically in identified TH-positive neurons. We found an accumulation of electron-dense debris within lysosomal structures in GBA-N370S mutant dopaminergic neurons in TH immunogold-labeled cells, (Figures 5C–5E), which likely represents undegraded cargo found in GBA-N370S dopamine neurons.
Figure 5.
Impaired Lysosomal Degradation Capacity in Heterozygous GBA-N370S PD Dopamine Neurons
(A) GCase enzyme activity in differentiated dopaminergic neuronal cultures was reduced to 50% when compared with controls. Student’s t test; ∗p < 0.0001.
(B) Increased p62 protein levels in heterozygous GBA-N370S dopaminergic cultures when compared with controls. Representative western blot analysis and respective quantification. In each case, data represent the mean ± SEM from performing at least three independent differentiation experiments per line each analyzed in triplicate. Student's t test; ∗p < 0.0001.
(C) Electron micrographs showing representative examples of undegraded cargo observed within lysosomal structures in TH immunogold-positive GBA-N370S-derived neurons. Arrows indicate LAMP1 positive structures of the lysosomal pathway; arrowheads indicate TH immunogold (5 nm). Scale bar, 200 nm.
(D) Quantification shows increased number of lysosomes with undegraded cargo per TH+ cell in GBA-N370S cultures compared with controls. Data represent the mean ± SEM of n = 20 cells per line from two independent differentiation experiments. Student's t test; ∗p < 0.001.
(E) A high magnification image reveals double-membrane vesicles corresponding to autophagolysosomes, with arrows indicating double membranes.
Enlarged Lysosomal Compartment in GBA-N370S Dopaminergic Neurons
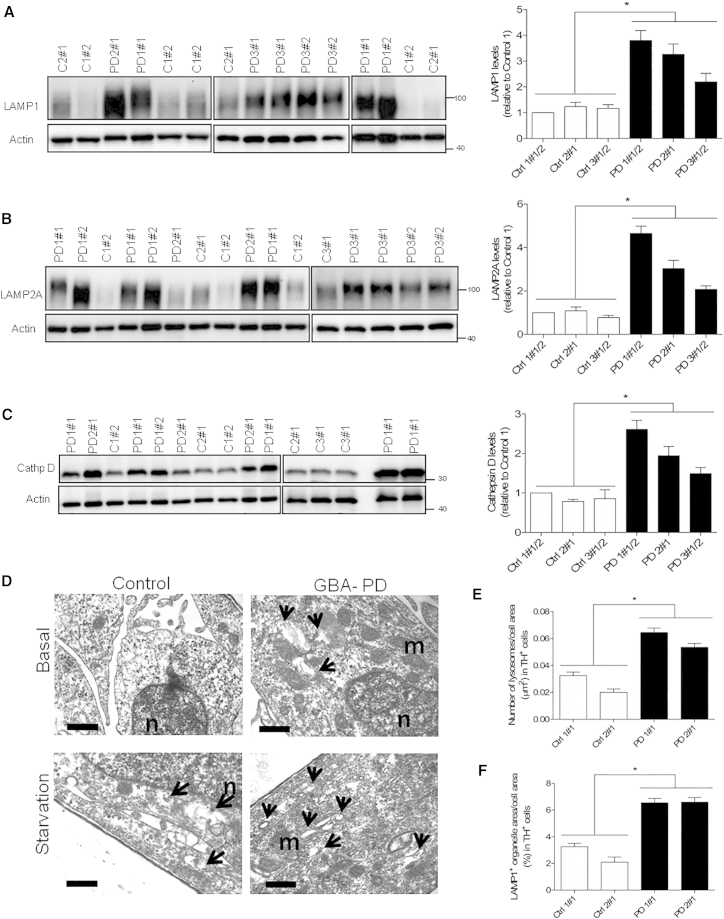
Following the previous observations suggesting impaired lysosomal degradation, the lysosomal compartment was analyzed in more detail. Dopaminergic neuronal cultures derived from GBA-N370S PD patients showed increased expression of the lysosomal markers LAMP1 (Figure 6A) and LAMP2A (Figure 6B) when compared with cultures derived from controls. The levels of cathepsin D, a major lysosomal enzyme involved in α-synuclein degradation (Sevlever et al., 2008) and associated with GCase deficiency (Vitner et al., 2010), was also found increased in dopaminergic neuronal cultures from GBA-N370S patients (Figure 6C).
Figure 6.
Enlargement of the Lysosomal Compartment in Dopamine Neurons Derived from PD Patients Carrying a Heterozygous GBA-N370S Mutation
(A–C) Increased protein levels of multiple lysosomal markers in heterozygous GBA-N370S PD dopaminergic cultures. Representative western blots and quantifications for the expression of: (A) LAMP1, (B) LAMP2A, and (C) cathepsin D. In each case, data represent the mean ± SEM from performing at least three independent differentiation experiments per line each analyzed in triplicate. Student's t test; ∗p < 0.001.
(D) Electron micrographs showing representative images of immunogold-labeled TH-positive neurons for differentiated controls and GBA-N370S neurons in basal and under starvation conditions. Arrows indicate LAMP1-positive structures of the lysosomal pathway. Scale bar, 200 nm.
(E) Quantification of EM data shows an increase number of lysosomes in basal conditions for TH neurons in GBA-N370S cultures compared with controls.
(F) The lysosomal compartment, represented as a percentage of the cell area occupied by LAMP1-labeled organelles, was also increased in size in TH-positive neurons in basal conditions in GBA-N370S cultures. In each case, data represent the mean ± SEM of n = 20 cells per line from two independent differentiation experiments. Student's t test; ∗p < 0.001.
See also Figure S5.
The enlarged lysosomal compartment was confirmed by EM, which demonstrated a ∼2-fold increase in the number of lysosomes in TH immunogold-labeled cells (Figures 6D and 6E). Furthermore, the area occupied by lysosomal organelles within TH-positive neurons detected by EM was increased by ∼2.5-fold in GBA-N370S cells compared with controls (Figures 6D and 6F), suggesting an enlargement of the lysosomal compartment. EM data also highlighted that the enlargement of the lysosomal compartment was specific to TH-positive dopaminergic neurons, as no differences were found in TH-negative cells (Figures S5A and S5B), emphasizing the importance of studying a disease-relevant cell type. The enlarged lysosomal compartment may reflect impaired lysosomal degradation capacity in dopamine neurons, which was not altered in TH-negative cells. GBA-N370S dopaminergic cultures were still capable of responding efficiently to situations of increased autophagic demand, as demonstrated by increased lysosomal numbers under starvation conditions determined by EM analysis (Figure S5C). Treatment with the V-ATPase inhibitor bafilomycin A1 also resulted in increased number of lysosomes in TH-positive neurons, which indicates that the process of lysosomal biogenesis is still inducible in GBA-N370S dopaminergic cultures (Figure S5C).
Increased Extracellular α-Synuclein in GBA-N370S Dopaminergic Neuronal Cultures
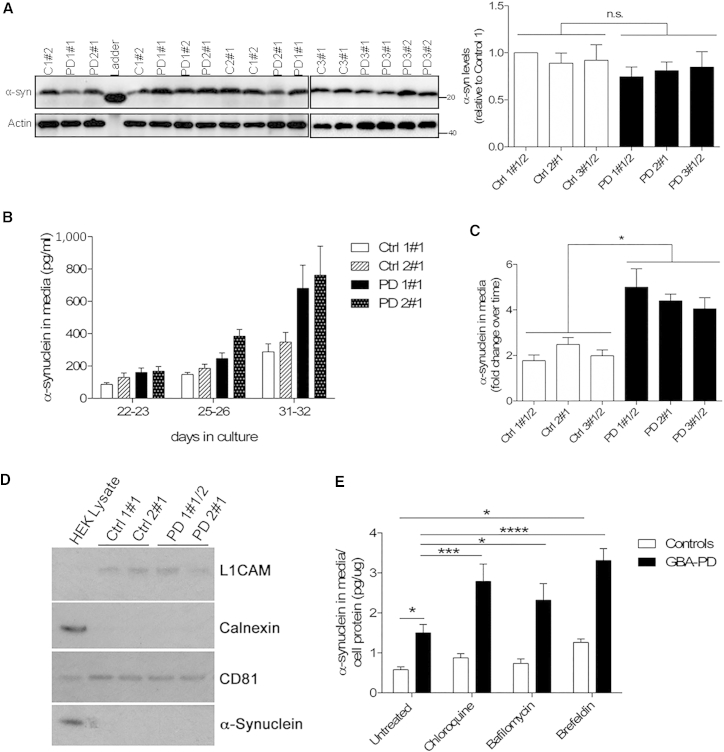
Accumulation of α-synuclein is the characteristic pathological marker of PD and can result from defective cellular clearance mechanisms. α-Synuclein is degraded by multiple pathways including autophagy and the autophagy-lysosome pathway (Cuervo et al., 2004), and α-synuclein levels have been shown to be altered in response to pharmacologically induced autophagic impairments (Klucken et al., 2012) and ER stress (Hoepken et al., 2008). Analysis of the intracellular α-synuclein content in dopaminergic neuronal cultures from GBA-N370S PD showed no differences when compared with controls (Figure 7A). However, recent reports have shown that impairments of the autophagic machinery can affect the release of α-synuclein in neuroblastoma cells, rat primary neurons, and mouse fibroblasts (Alvarez-Erviti et al., 2011, Ejlerskov et al., 2013, Emmanouilidou et al., 2010, Jang et al., 2010, Lee et al., 2013). We therefore investigated if the autophagic/lysosomal disturbances observed in GBA-N370S dopaminergic neuronal cultures could alter α-synuclein release in our human PD-specific cell model. Analysis by ELISA revealed the presence of α-synuclein in the culture media of differentiated dopaminergic cultures, which increased over time (Figure 7B). Importantly, the levels of α-synuclein in the media were found to be increased for mature dopaminergic neuronal cultures differentiated from GBA-N370S patients when compared with controls (Figure 7C), suggesting a possible link between the autophagic/lysosomal disturbances we report and the modulation of α-synuclein release.
Figure 7.
Increased Extracellular α-Synuclein in Heterozygous GBA-N370S-Derived Dopaminergic Neuronal Cultures
(A) There is no difference in the intracellular α-synuclein content in dopaminergic neuronal cultures from controls and GBA-N370S PD lines. Representative western blot and quantification for intracellular α-synuclein levels.
(B) Analysis of culture media by ELISA revealed the presence of α-synuclein, which increased over time.
(C) Levels of extracellular α-synuclein in culture media were higher for heterozygous GBA-N370S dopaminergic cultures when compared with controls, during maturation, measured by ELISA. Fold change of neurons at day 31–36 compared with early neurons at day 21. In each case, data represent the mean ± SEM from performing at least three independent differentiation experiments per line each analyzed in triplicate. Student's t test; ∗p < 0.001.
(D) Western blot analysis of culture media extracted exosomes confirmed the expression of CD81 and L1CAM exosomal markers in the absence of calnexin. Data represent the mean of replicates ± SEM from performing at least three independent differentiation experiments per line.
(E) Modulation of α-synuclein release in differentiated dopaminergic neurons from control and GBA-N370S dopaminergic cultures by treatment with CQ, bafilomycin A1, and brefeldin A, measured by the MesoScale Discovery platform. Each bar represents the mean ± SEM of differentiated lines from at least three independent individuals done in duplicate (n = 6–8). Two-way ANOVA; ∗p < 0.05; ∗∗∗p < 0.005; ∗∗∗∗p < 0.0001.
See also Figure S6.
The secretion of α-synuclein has been associated with exosomes in α-synuclein overexpressing neuroblastoma cells, following an unconventional secretory pathway (Emmanouilidou et al., 2010). We therefore examined if a similar mechanism could be associated with α-synuclein release in our PD patient model. Exosomes were isolated from dopaminergic neuronal cultures and analyzed by nanoparticle tracking analysis. This analysis did not reveal any significant difference in the nodal size of exosomes between control and GBA-N370S cultures (Figure S6). In addition, there was no difference in the concentration of exosomes secreted by control or GBA-N370S dopaminergic cultures (Figure S6). Western blot analysis of extracted exosomes confirmed the detection of generic (CD81) and neuron-specific (L1CAM) exosomal markers, and the absence of calnexin, an indicator of apoptotic bodies (Figure 7D). These data indicate that extracellular α-synuclein is not associated with neuron-derived exosomes. Accordingly, ELISA analysis showed that exosome depletion of conditioned media did not change the α-synuclein levels in the media and it was estimated that exosome-associated α-synuclein constitutes less than about 1.6% of the total extracellular fraction.
To further investigate the mechanisms resulting in α-synuclein release in dopaminergic cultures carrying GBA-N370S mutation, cells were treated with either chloroquine (CQ) or with bafilomycin A1, both of which cause impaired lysosomal function by neutralizing lysosomal pH (Juhasz, 2012). Each treatment resulted in a significant increase in released α-synuclein in GBA-N370S dopaminergic cultures (Figure 7E). Both treatments had a bigger impact on patient cells than controls, suggesting a role for lysosomal function in α-synuclein release in GBA-N370S dopaminergic cultures. To explore a possible role for the ER/Golgi-dependent classical export pathway in α-synuclein release, cultures were treated with brefeldin A, which blocks the trafficking between ER and Golgi. Brefeldin A treatment resulted in increased release of α-synuclein in all dopaminergic cultures (Figure 7E), more pronounced in GBA-N370S lines, suggesting that the ER/Golgi vesicle-mediated transport is also involved in α-synuclein release. Overall, these data confirm an increased release of α-synuclein in GBA-N370S PD dopaminergic neuronal cultures associated with an impairment of the autophagic/lysosome pathway, offering a possible therapeutic target.
Discussion
Using human iPSCs derived from PD patients carrying the heterozygous GBA-N370S mutation, we have identified relevant mechanisms by which a heterozygous GBA mutation associated with PD may increase cellular susceptibility to disease. Our results highlight an important role of heterozygous mutant GCase in the disruption of protein homeostasis in dopaminergic neurons, ultimately leading to increased α-synuclein release, which may be central for the early pathogenesis of PD. Differentiation of ten independent iPSC lines from three controls and three unrelated GBA-N370S PD patients into dopaminergic neurons allowed us to robustly investigate this genetic contribution in an appropriate human model of early PD pathology.
Our data suggest that the heterozygous GBA-N370S mutation results in the retention of GCase within the ER in PD iPSC-derived dopaminergic neuronal cultures. We found no evidence of a reduction of GCase protein levels in our multiple lines derived from three unrelated patients, indicating that the protein is not targeted for degradation. We have observed an overall increased expression of LIMP2, a GCase-specific lysosomal receptor, in dopaminergic cultures from GBA-N370S patients. Increased LIMP2 expression is likely to be part of a cellular response to GBA mutations, as it has been previously shown that LIMP2 overexpression increases transportation of ER-retained GCase toward the lysosomes (Reczek et al., 2007).
We observed the upregulation of multiple ER stress markers in GBA-N370S-derived dopaminergic neuronal cultures, which likely reflects a dysregulation of the ER homeostatic environment induced by misprocessing of misfolded GCase similar to a postmortem analysis of PD GBA patients showing UPR activation (Gegg et al., 2012).
We have also identified alterations in the autophagy pathway in our PD model. Levels of autophagosomes were increased in dopamine neurons from GBA-N370S lines, and we provide multiple lines of evidence to support an impairment of general autophagic vacuole clearance. In addition, multiple lysosomal markers were upregulated in GBA-N370S dopaminergic lines consistent with an impairment of lysosomal degradation capacity. Moreover, an enlargement of the lysosomal compartment was identified, specifically within TH immunogold-identified dopaminergic neurons derived from patient's lines, characterized by increased number and size of lysosomal structures. Lysosomal dysfunction is a key pathological event in PD (Dehay et al., 2010), and the specificity to dopamine neurons may explain the preferential vulnerability of these neurons.
Altered cellular lipid composition can be associated with ER disturbances (Basseri and Austin, 2012) and autophagic impairments (Koga et al., 2010). Although the mechanisms underlying cellular distribution of GlcCer species in neurons is not well understood, lipid contents are known to differ in membranes undergoing ER-Golgi maturation or fusion within various organelles (Halter et al., 2007). The different GlcCer species distribution observed in our neuronal cultures between control and GBA-N370S lines may be due to the different trafficking of GCase. As a result of altered trafficking, the mutant GCase enzyme may hydrolyze a species that might not have been degraded in normal cells, thereby decreasing total amounts of certain lipids (e.g., 30% reduction of C20:0 GlcCer levels in GBA-N370S neurons). On the other hand, reduced GCase concentration in particular sub-compartments may promote the accumulation of other species (e.g., ∼65% increase in C16:0 and C24:0 species) in GBA-N370S neurons.
It is known that α-synuclein is degraded in part by the autophagic/lysosomal pathway (Cuervo et al., 2004). We observed no differences for the intracellular α-synuclein levels in dopaminergic cultures derived from three patients carrying the GBA-N370S mutation, which is in line with the report of Schöndorf et al. (2014). Although postmortem studies have suggested the possibility of increased intracellular α-synuclein in brains from PD patients carrying GBA mutations, postmortem tissue likely reflects a much more advanced stage of disease pathology (Choi et al., 2012, Gegg et al., 2012).
Since recent reports have demonstrated that perturbations of the autophagic pathway can affect α-synuclein release in neuroblastoma cells and mouse fibroblasts, we decided to investigate if a similar mechanism operated in our physiological human model of PD. Interestingly, we observed increased levels of α-synuclein released in the culture media from GBA-N370S dopaminergic neuronal cultures, which may be a consequence of the autophagic/lysosomal defects in these cells. To further investigate the potential mechanisms by which GBA-N370S mutation results in increased α-synuclein release, dopaminergic cultures were treated with the lysosomal function inhibitors CQ or bafilomycin A1. Each treatment increased the levels of released α-synuclein, further suggesting the involvement of the lysosome in the release of α-synuclein in GBA-N370S dopaminergic cultures. These results suggest that the autophagic and lysosomal deficits we describe result in increased α-synuclein release in GBA-N370S dopaminergic cultures. The lack of significant increase in released α-synuclein in control cultures after induced lysosomal dysfunction suggests a difference in the way the lysosomes handle α-synuclein between PD and control cultures. In addition, although earlier reports showed that α-synuclein release was independent of the ER/Golgi classical export pathway (Emmanouilidou et al., 2010, Jang et al., 2010, Lee et al., 2005), recent studies have shown that in some models, including enteric neurons, α-synuclein release is dependent on ER/Golgi exocytosis (Chutna et al., 2014, Paillusson et al., 2013). Our results indicate that α-synuclein release is also sensitive to brefeldin A treatment, supporting a role for the ER/Golgi classical pathway in the release of α-synuclein in both GBA-N370S and control dopaminergic cultures. Taken together, our data suggest that α-synuclein release can occur via both conventional and unconventional pathways in dopaminergic neuronal cultures from GBA-N370S cells.
α-Synuclein release could be an important early event in the pathology of PD whereby uptake of α-synuclein by neighboring neurons could result in impaired cellular proteostasis (Desplats et al., 2009). α-Synuclein involvement in this context might be complex since it was also previously shown to cause ER stress, block ER-Golgi trafficking (Cooper et al., 2006), induce lysosomal rupture (Freeman et al., 2013), decrease lysosomal function (Cuervo et al., 2004), and inhibit GCase activity (Mazzulli et al., 2011).
Secreted α-synuclein has also been associated with exosomes in the context of neuroblastoma cells overexpressing α-synuclein (Alvarez-Erviti et al., 2011, Emmanouilidou et al., 2010). To expand on the observation of increased α-synuclein release in our model, we extracted and analyzed human iPSC-derived neuronal exosomes. We did not find a readily detectable association of α-synuclein with exosomes in our model, which is consistent with our recent proteomic analysis in serum-derived microvesicles from PD patients (Tomlinson et al., 2015).
Heterozygous GBA-N370S mutations are the strongest risk factor for PD and have also been associated with related Lewy body disorders, in particular with dementia with Lewy bodies, where they represent an important risk factor (Nalls et al., 2013). Dopaminergic neurons of the substantia nigra are lost in both PD and dementia with Lewy bodies, which may occur through the mechanisms of altered protein homeostasis we describe here. Interestingly, although a GBA mutation increases the relative risk for PD by 30-fold compared with the general population (McNeill et al., 2012), most GBA mutation carriers do not develop disease. This suggests that a GBA mutation confers strong susceptibility, but that a second hit, likely genetic or environmental, is required to drive pathology. Pharmacological rescue in PD GBA-N370S neurons using chemical chaperones such as isofagomine and ambroxol, or treatment with recombinant GCase, will allow further dissection of the mechanisms by which GBA mutations confer susceptibility to PD and may identify promising protective therapies.
Taken together, our findings suggest that in PD, the heterozygous GBA-N370S mutation leads to the misprocessing of GCase, ER stress upregulation, and autophagic/lysosomal dysfunction in dopaminergic neurons. We propose that this combination of disturbances impairs protein homeostasis in dopamine neurons, which leads to increased α-synuclein release. Such cellular events in combination may lead to preferential dopamine neuronal vulnerability in PD.
Experimental Procedures
Participant Recruitment
Participants were recruited to this study having given signed informed consent, which included mutation screening and derivation of hiPSC lines from skin biopsies (Ethics Committee: National Health Service, Health Research Authority, NRES Committee South Central, Berkshire, UK, who specifically approved this part of the study (REC 10/H0505/71).
Culture and Reprogramming of Primary Fibroblasts
Skin punch biopsies (4 mm in diameter) were obtained from participants and low passage fibroblast cultures established and transduced with reprogramming retroviruses (c-MYC, KLF4, SOX2, OCT3/4, and Nanog). Colonies displaying iPSC morphology were picked on day 28 and passaged on murine embryonic fibroblasts by manual dissection every 5–7 days. Full characterization information from the PD patient lines is given in the Supplemental Experimental Procedures. The control iPSC lines have been described fully elsewhere (Hartfield et al., 2014, van Wilgenburg et al., 2013).
RT-PCR, Immunocytochemistry, and Western Blot Analysis
These procedures were performed using standard methods and details are given in Supplemental Experimental Procedures. Representative western blots for antibodies used are shown Figure S7.
α-Synuclein Measurements
An in-house ELISA for the accurate quantification of α-synuclein concentration was developed by using two commercially available α-synuclein-specific antibodies: the monoclonal Syn-1 (BD Transductions) as the capture antibody and the polyclonal C-20 (Santa Cruz) as the detection antibody, which was used after its covalent conjugation with horseradish peroxidase (Emmanouilidou et al., 2011). MesoScale Discovery assays were used following the manufacturer's instructions. Further details are given in the Supplemental Experimental Procedures.
Acknowledgments
The work was supported by the Monument Trust Discovery Award from Parkinson's UK, The Oxford Martin School, which provides core support to the James Martin Stem Cell Facility (LC0910-004) and the Innovative Medicines Initiative Joint Undertaking under grant agreement number 115439, resources of which are composed of financial contribution from the European Union's Seventh Framework Program (FP7/2007e2013) and EFPIA companies' in kind contribution. The work was supported by the Monument Trust Discovery Award from Parkinson's UK. The OPDC Discovery cohort was also supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre based at Oxford University Hospitals NHS Trust and University of Oxford, and the Dementias and Neurodegenerative Diseases Research Network (DeNDRoN). H.J.R.F. was supported by a Fundacao para a Ciencia e Tecnologia (FCT) grant (SFRH/BD/65787/2009) and S.C. has been supported by a Wellcome Trust Career Re-Entry Fellowship (WT082260/Z/07/Z). We thank the High-Throughput Genomics Group at the Wellcome Trust Centre for Human Genetics, Oxford (funded by Wellcome Trust grant reference 090532/Z/09/Z and MRC Hub grant G0900747 91070) for the generation of Illumina genotyping and transcriptome data.
Published: February 18, 2016
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Supplemental Information includes Supplemental Experimental Procedures, seven figures, and one table and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2016.01.013.
Accession Numbers
The SNP datasets and the Illumina HT12v4 transcriptome array results have been deposited in Gene Expression Omnibus under accession number GEO: GSE53426.
Supplemental Information
References
- Alegre-Abarrategui J., Christian H., Lufino M.M., Mutihac R., Venda L.L., Ansorge O., Wade-Martins R. LRRK2 regulates autophagic activity and localizes to specific membrane microdomains in a novel human genomic reporter cellular model. Hum. Mol. Genet. 2009;18:4022–4034. doi: 10.1093/hmg/ddp346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Erviti L., Seow Y., Schapira A.H., Gardiner C., Sargent I.L., Wood M.J., Cooper J.M. Lysosomal dysfunction increases exosome-mediated alpha-synuclein release and transmission. Neurobiol. Dis. 2011;42:360–367. doi: 10.1016/j.nbd.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basseri S., Austin R.C. Endoplasmic reticulum stress and lipid metabolism: mechanisms and therapeutic potential. Biochem. Res. Int. 2012;2012:841362. doi: 10.1155/2012/841362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J.M., Kim W.C., Lyoo C.H., Kang S.Y., Lee P.H., Baik J.S., Koh S.B., Ma H.I., Sohn Y.H., Lee M.S. Association of mutations in the glucocerebrosidase gene with Parkinson disease in a Korean population. Neurosci. Lett. 2012;514:12–15. doi: 10.1016/j.neulet.2012.02.035. [DOI] [PubMed] [Google Scholar]
- Chutna O., Goncalves S., Villar-Pique A., Guerreiro P., Marijanovic Z., Mendes T., Ramalho J., Emmanouilidou E., Ventura S., Klucken J. The small GTPase Rab11 co-localizes with alpha-synuclein in intracellular inclusions and modulates its aggregation, secretion and toxicity. Hum. Mol. Genet. 2014;23:6732–6745. doi: 10.1093/hmg/ddu391. [DOI] [PubMed] [Google Scholar]
- Cooper A.A., Gitler A.D., Cashikar A., Haynes C.M., Hill K.J., Bhullar B., Liu K., Xu K., Strathearn K.E., Liu F. Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson's models. Science. 2006;313:324–328. doi: 10.1126/science.1129462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuervo A.M., Stefanis L., Fredenburg R., Lansbury P.T., Sulzer D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science. 2004;305:1292–1295. doi: 10.1126/science.1101738. [DOI] [PubMed] [Google Scholar]
- Dehay B., Bové J., Rodríguez-Muela N., Perier C., Recasens A., Boya P., Vila M. Pathogenic lysosomal depletion in Parkinson's disease. J. Neurosci. 2010;30:12535–12544. doi: 10.1523/JNEUROSCI.1920-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desplats P., Lee H.-J., Bae E.-J., Patrick C., Rockenstein E., Crews L., Spencer B., Masliah E., Lee S.-J. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc. Natl. Acad. Sci. USA. 2009;106:13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do C.B., Tung J.Y., Dorfman E., Kiefer A.K., Drabant E.M., Francke U., Mountain J.L., Goldman S.M., Tanner C.M., Langston J.W. Web-based genome-wide association study identifies two novel loci and a substantial genetic component for Parkinson's disease. PLoS Genet. 2011;7:e1002141. doi: 10.1371/journal.pgen.1002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejlerskov P., Rasmussen I., Nielsen T.T., Bergström A.-L., Tohyama Y., Jensen P.H., Vilhardt F. Tubulin polymerization-promoting protein (TPPP/p25α) promotes unconventional secretion of α-synuclein through exophagy by impairing autophagosome-lysosome fusion. J. Biol. Chem. 2013;288:17313–17335. doi: 10.1074/jbc.M112.401174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmanouilidou E., Melachroinou K., Roumeliotis T., Garbis S.D., Ntzouni M., Margaritis L.H., Stefanis L., Vekrellis K. Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J. Neurosci. 2010;30:6838–6851. doi: 10.1523/JNEUROSCI.5699-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmanouilidou E., Elenis D., Papasilekas T., Stranjalis G., Gerozissis K., Ioannou P.C., Vekrellis K. Assessment of α-synuclein secretion in mouse and human brain parenchyma. PLoS One. 2011;6:e22225. doi: 10.1371/journal.pone.0022225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman D., Cedillos R., Choyke S., Lukic Z., McGuire K., Marvin S., Burrage A.M., Sudholt S., Rana A., O'Connor C. Alpha-synuclein induces lysosomal rupture and cathepsin dependent reactive oxygen species following endocytosis. PLoS One. 2013;8:e62143. doi: 10.1371/journal.pone.0062143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegg M.E., Burke D., Heales S.J.R., Cooper J.M., Hardy J., Wood N.W., Schapira A.H.V. Glucocerebrosidase deficiency in substantia nigra of parkinson disease brains. Ann. Neurol. 2012;72:455–463. doi: 10.1002/ana.23614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goker-Alpan O., Schiffmann R., LaMarca M.E., Nussbaum R.L., McInerney-Leo A., Sidransky E. Parkinsonism among Gaucher disease carriers. J. Med. Genet. 2004;41:937–940. doi: 10.1136/jmg.2004.024455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goker-Alpan O., Giasson B.I., Eblan M.J., Nguyen J., Hurtig H.I., Lee V.M., Trojanowski J.Q., Sidransky E. Glucocerebrosidase mutations are an important risk factor for Lewy body disorders. Neurology. 2006;67:908–910. doi: 10.1212/01.wnl.0000230215.41296.18. [DOI] [PubMed] [Google Scholar]
- Halter D., Neumann S., van Dijk S.M., Wolthoorn J., de Maziere A.M., Vieira O.V., Mattjus P., Klumperman J., van Meer G., Sprong H. Pre- and post-Golgi translocation of glucosylceramide in glycosphingolipid synthesis. J. Cell Biol. 2007;179:101–115. doi: 10.1083/jcb.200704091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartfield E.M., Fernandes H.J.R., Vowles J., Cowley S.A., Wade-Martins R. Cellular reprogramming: a new approach to modelling Parkinson's disease. Biochem. Soc. Trans. 2012;40:1152–1157. doi: 10.1042/BST20120159. [DOI] [PubMed] [Google Scholar]
- Hartfield E.M., Yamasaki-Mann M., Ribeiro Fernandes H.J., Vowles J., James W.S., Cowley S.A., Wade-Martins R. Physiological characterisation of human IPS-derived dopaminergic neurons. PLoS One. 2014;9:e87388. doi: 10.1371/journal.pone.0087388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoepken H.-H., Gispert S., Azizov M., Klinkenberg M., Ricciardi F., Kurz A., Morales-Gordo B., Bonin M., Riess O., Gasser T. Parkinson patient fibroblasts show increased alpha-synuclein expression. Exp. Neurol. 2008;212:307–313. doi: 10.1016/j.expneurol.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Høyer-Hansen M., Jäättelä M. Connecting endoplasmic reticulum stress to autophagy by unfolded protein response and calcium. Cell Death Differ. 2007;14:1576–1582. doi: 10.1038/sj.cdd.4402200. [DOI] [PubMed] [Google Scholar]
- Jang A., Lee H.J., Suk J.E., Jung J.W., Kim K.P., Lee S.J. Non-classical exocytosis of alpha-synuclein is sensitive to folding states and promoted under stress conditions. J. Neurochem. 2010;113:1263–1274. doi: 10.1111/j.1471-4159.2010.06695.x. [DOI] [PubMed] [Google Scholar]
- Juhasz G. Interpretation of bafilomycin, pH neutralizing or protease inhibitor treatments in autophagic flux experiments: novel considerations. Autophagy. 2012;8:1875–1876. doi: 10.4161/auto.21544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiskinis E., Eggan K. Progress toward the clinical application of patient-specific pluripotent stem cells. J. Clin. Invest. 2010;120:51–59. doi: 10.1172/JCI40553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky D.J., Abdalla F.C., Abeliovich H., Abraham R.T., Acevedo-Arozena A., Adeli K., Agholme L., Agnello M., Agostinis P., Aguirre-Ghiso J.A. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8:445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucken J., Poehler A.-M., Ebrahimi-Fakhari D., Schneider J., Nuber S., Rockenstein E., Schlötzer-Schrehardt U., Hyman B.T., McLean P.J., Masliah E. Alpha-synuclein aggregation involves a bafilomycin A 1-sensitive autophagy pathway. Autophagy. 2012;8:754–766. doi: 10.4161/auto.19371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga H., Kaushik S., Cuervo A.M. Altered lipid content inhibits autophagic vesicular fusion. FASEB J. 2010;24:3052–3065. doi: 10.1096/fj.09-144519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.J., Patel S., Lee S.J. Intravesicular localization and exocytosis of alpha-synuclein and its aggregates. J. Neurosci. 2005;25:6016–6024. doi: 10.1523/JNEUROSCI.0692-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.-J., Cho E.-D., Lee K.W., Kim J.-H., Cho S.-G., Lee S.-J. Autophagic failure promotes the exocytosis and intercellular transfer of α-synuclein. Exp. Mol. Med. 2013;45:e22. doi: 10.1038/emm.2013.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzulli J.R., Xu Y.H., Sun Y., Knight A.L., McLean P.J., Caldwell G.A., Sidransky E., Grabowski G.A., Krainc D. Gaucher disease glucocerebrosidase and alpha-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell. 2011;146:37–52. doi: 10.1016/j.cell.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill A., Duran R., Hughes D.A., Mehta A., Schapira A.H. A clinical and family history study of Parkinson's disease in heterozygous glucocerebrosidase mutation carriers. J. Neurol. Neurosurg. Psychiatry. 2012;83:853–854. doi: 10.1136/jnnp-2012-302402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill A., Magalhaes J., Shen C., Chau K.-Y., Hughes D., Mehta A., Foltynie T., Cooper J.M., Abramov A.Y., Gegg M. Ambroxol improves lysosomal biochemistry in glucocerebrosidase mutation-linked Parkinson disease cells. Brain. 2014;137:1481–1495. doi: 10.1093/brain/awu020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller F.-J., Schuldt B.M., Williams R., Mason D., Altun G., Papapetrou E.P., Danner S., Goldmann J.E., Herbst A., Schmidt N.O. A bioinformatic assay for pluripotency in human cells. Nat. Methods. 2011;8:315–317. doi: 10.1038/nmeth.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalls M.A., Duran R., Lopez G., Kurzawa-Akanbi M., McKeith I.G., Chinnery P.F., Morris C.M., Theuns J., Crosiers D., Cras P. A multicenter study of glucocerebrosidase mutations in dementia with Lewy bodies. JAMA Neurol. 2013;70:727–735. doi: 10.1001/jamaneurol.2013.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paillusson S., Clairembault T., Biraud M., Neunlist M., Derkinderen P. Activity-dependent secretion of alpha-synuclein by enteric neurons. J. Neurochem. 2013;125:512–517. doi: 10.1111/jnc.12131. [DOI] [PubMed] [Google Scholar]
- Reczek D., Schwake M., Schröder J., Hughes H., Blanz J., Jin X., Brondyk W., Van Patten S., Edmunds T., Saftig P. LIMP-2 is a receptor for lysosomal mannose-6-phosphate-independent targeting of beta-glucocerebrosidase. Cell. 2007;131:770–783. doi: 10.1016/j.cell.2007.10.018. [DOI] [PubMed] [Google Scholar]
- Sardi S.P., Clarke J., Kinnecom C., Tamsett T.J., Li L., Stanek L.M., Passini M.A., Grabowski G.A., Schlossmacher M.G., Sidman R.L. CNS expression of glucocerebrosidase corrects alpha-synuclein pathology and memory in a mouse model of Gaucher-related synucleinopathy. Proc. Natl. Acad. Sci. USA. 2011;108:12101–12106. doi: 10.1073/pnas.1108197108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöndorf D.C., Aureli M., McAllister F.E., Hindley C.J., Mayer F., Schmid B., Sardi S.P., Valsecchi M., Hoffmann S., Schwarz L.K. iPSC-derived neurons from GBA1-associated Parkinson’s disease patients show autophagic defects and impaired calcium homeostasis. Nat. Commun. 2014;5:4028. doi: 10.1038/ncomms5028. [DOI] [PubMed] [Google Scholar]
- Sevlever D., Jiang P., Yen S.-H.C. Cathepsin D is the main lysosomal enzyme involved in the degradation of alpha-synuclein and generation of its carboxy-terminally truncated species. Biochemistry. 2008;47:9678–9687. doi: 10.1021/bi800699v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidransky E., Nalls M.A., Aasly J.O., Aharon-Peretz J., Annesi G., Barbosa E.R., Bar-Shira A., Berg D., Bras J., Brice A. Multicenter analysis of glucocerebrosidase mutations in Parkinson's disease. New Engl. J. Med. 2009;361:1651–1661. doi: 10.1056/NEJMoa0901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tofaris G.K. Lysosome-dependent pathways as a unifying theme in Parkinson's disease. Movement Disord. 2012;27:1364–1369. doi: 10.1002/mds.25136. [DOI] [PubMed] [Google Scholar]
- Tomlinson P.R., Zheng Y., Fischer R., Heidasch R., Gardiner C., Evetts S., Hu M., Wade-Martins R., Turner M.R., Morris J. Identification of distinct circulating exosomes in Parkinson's disease. Ann. Clin. Transl. Neurol. 2015;2:353–361. doi: 10.1002/acn3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wilgenburg B., Browne C., Vowles J., Cowley S.A. Efficient, long term production of monocyte-derived macrophages from human pluripotent stem cells under partly-defined and fully-defined conditions. PLoS One. 2013;8:e71098. doi: 10.1371/journal.pone.0071098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitner E.B., Dekel H., Zigdon H., Shachar T., Farfel-Becker T., Eilam R., Karlsson S., Futerman A.H. Altered expression and distribution of cathepsins in neuronopathic forms of Gaucher disease and in other sphingolipidoses. Hum. Mol. Genet. 2010;19:3583–3590. doi: 10.1093/hmg/ddq273. [DOI] [PubMed] [Google Scholar]
- Wei R.R., Hughes H., Boucher S., Bird J.J., Guziewicz N., Van Patten S.M., Qiu H., Pan C.Q., Edmunds T. X-ray and biochemical analysis of N370S mutant human acid β-glucosidase. J. Biol. Chem. 2011;286:299–308. doi: 10.1074/jbc.M110.150433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorimitsu T., Nair U., Yang Z., Klionsky D.J. Endoplasmic reticulum stress triggers autophagy. J. Biol. Chem. 2006;281:30299–30304. doi: 10.1074/jbc.M607007200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.